Abstract
Intracellular recording methods and immunostaining revealed the existence of functional group I metabotropic glutamate receptors (mGluRs) in submucous plexus neurons of guinea-pig ileum. Selective group I, but not groups II or III metabotropic glutamate receptor agonists induced concentration-dependent, slowly-activating depolarizing responses. Group I metabotropic glutamate receptor antagonism observed with (S)-4-carboxyphenylglycine (S-4-CPG) (100–600 μM) was competitive as determined by Schild analysis (pA2=3.81±0.02). Neither the group II and III metabotropic nor ionotropic glutamate receptor antagonists altered responses evoked by group I receptor agonists. Immunoreactivities for metabotropic glutamate 1α and 5 receptors were found to locate exclusively in neurons in the submucous plexus of guinea-pig ileum with the highest density around the cell bodies. The results suggest that group I metabotropic glutamate receptors are functionally expressed in the submucous plexus of guinea-pig small intestine.
Keywords: Metabotropic glutamate receptors, submucous plexus, enteric nervous system, small intestine, intestinal secretion
Introduction
L-glutamate is a major excitatory neurotransmitter in the central nervous system. Multiple glutamate receptors have been identified in the mammalian brain, including ligand-gated ion channels (i.e., ionotropic receptors) that are multimeric heteromers composed of distinct subunits. A second subfamily includes eight G-protein coupled, metabotropic receptors referred to as mGluRs. All mGluRs have a similar structure with seven transmembrane domains and a large amino terminal extracellular domain. Based on amino acid sequence similarity, agonist pharmacology and their signal transduction pathways, these receptors have been classified into three groups. Group I mGluRs stimulate inositol phosphate metabolism and mobilization of intracellular Ca2+, whereas group II and III are coupled to adenylyl cyclase (Conn & Pin, 1997). In the enteric nervous system, excessive exposure to glutamate, or related agonists, produces neurotoxicity and glutamate immunoreactivity is found in cholinergic enteric neurons co-localized with substance P and calbindin (Kirchgessner et al., 1997; Liu et al., 1997). Neural glutamatergic receptors are involved in intestinal motility and secretion (Jankovic et al., 1999; Rhoads et al., 1995). Nevertheless, no evidence for the existence of mGluRs in the enteric nervous system has been available. In the present study, we demonstrate localization of functional group I metabotropic receptors to submucous plexus neurons in the guinea-pig small intestine.
Methods
Tissue preparation
Male guinea-pigs (400–600 g) were stunned and exsanguinated from the cervical vessels according to protocols approved by the Ohio State University Laboratory Animal Care and Use Committee. Segments of small intestine were removed 10–20 cm proximal to the ileocecal junction. Preparations of submucous plexus were dissected, as described in detail elsewhere (Zafirov et al., 1993). The preparations were mounted in a 1.5 ml glass-bottomed recording chamber perfused at a rate of 10–15 ml min−1 with Krebs solution warmed to 37°C and gassed with 95% O2–5% CO2 to buffer at pH 7.3–7.4. Composition of the Krebs solution was (in mM) NaCl 120.9, KCl 5.9, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 14.4, CaCl2 2.5 and glucose11.5. For experiments involving low Na+ Krebs solution, external NaCl was substituted with equal molar N-methyl-D-glucamine (NMDG) or choline chloride. This substitution reduced the Na+ concentration of the bathing solution from 136.5 to 15.6 mM, or 11.4% of the standard Krebs solution. Preparations for immunohistochemistry were pinned flat in a sylgard-lined Petri dish and fixed for 4 h in 2% paraformaldehyde and 0.5% saturated picric acid in 0.1 M phosphate buffer (pH 7.4). After washing three times in phosphate buffer, whole mounts of the submucous plexus were obtained by removing the mucosa and careful dissection of the inner and outer muscle layers.
Electrophysiological recording
Our methods for intracellular recording are described in detail elsewhere (Zafirov et al., 1993). Recording electrodes were glass micropipettes filled with 4% biocytin in 2 M KCl containing 0.05 M Tris buffer (pH 7.4, resistances of the electrodes were 80–120 MΩ). WPI M-707 amplifiers (World precision Instruments, Saratoga, FL, U.S.A.) were used in the bridge circuit mode to record transmembrane potentials and to inject electrical current.
Immunostaining
The tissues were rinsed in PBS and pre-incubated for 20 min in 0.1% H2O2 and 20 min in 4% normal goat serum and 1% Triton-X-100 prior to incubation at room temperature overnight with the primary antibody (1 : 500–1 : 1000) corresponding to amino acids of rat mGluR1α (Calbiochem, La Jolla, CA, U.S.A.) or mGluR5 sequences (Chemicon, Temecula, CA, U.S.A.). Three 10 min washes in PBS were done after each step. Then the second antibody (goat anti-rabbit IgG) diluted in PBS was applied for 30 min at room temperature. The preparations were reacted with avidin-biotin complex (ABC) (vector, elite ABC) for 30 min and carried through the DAB color-developing reaction, then dehydrated in alcohol and mounted in Canada balsam. Immunostaining of biocytin-injected neurons was the same as described previously (Christofi & Wood, 1993).
Drug application
All drugs were purchased from Tocris Cookson Inc. (Ballwin, MO, U.S.A.) except (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo [a, d] cyclohepten-5,10-imine / dizocilpine (MK-801). D(-)-2-Amino-5-phosphonopentanoic acid (D-AP5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and tetrodotoxin (TTX) were from RBI (Natick, MA, U.S.A.). Actions of the drugs were studied either by micropressure ejection or by application in the superfusion solution. Dilutions were made in Krebs solution. Data are expressed as means±standard error; n values refer to numbers of neurons. EC50 values were obtained from the pooled data by a sigmoid fit to V=Vmax [1+(EC50 C−1)nH]−1, where V is the observed membrane depolarization, Vmax is the maximal response, C is the corresponding drug concentration, EC50 is the concentration which induces the half-maximal response, and nH is the apparent Hill coefficient using Sigmaplot software (SPSS Inc., Chicago, IL, U.S.A.)
Results
Pharmacological profile of metabotropic glutamate group I receptor agonists and antagonists
Most of the submucous plexus neurons examined (23/25, 92%) were depolarized by bath application of the group I mGluRs agonists. All of the responding neurons examined were S/type 1 neurons with uniaxonal morphology. The only two AH/Dogiel type 2 neurons examined were insensitive to glutamate. The enteric neurons were classified according to previously described criteria (Wood, 1994). Figure 1A shows how the selective group I mGluRs agonist, 3,5-dihydroxyphenylglycine (3,5-DHPG), evoked concentration-dependent, slowly-activating depolarizing responses associated with increased neuronal excitability and decreased input resistance in a S/type 1 submucous plexus neuron with uniaxonal morphology (Figure 3D). The EC50 values for the different agonists were: 2.3±0.4 μM; 4.0±0.4 μM; 7.3±1.5 μM; 9.6±2.3 μM; 9.6±0.9 μM; 11±1.5 μM and 14±2.2 μM for quisqualate (QA); 3,5-dihydroxyphenylglycine (3,5-DHPG); glutamate; 1S,3R-1-amino-1,3-cyclopentanedicarboxylate (ACPD); ibotenate; (2S,1′S,2′S)-2-(carboxycyclopropyl)glycine (L-CCG-I); 3-hydroxyphenylglycine and (3-HPG), respectively (Figure 1B,C). The agonists potency order was QA>3,5-DHPG>glutamate>ACPD=ibotenate>L-CCG-I>3-HPG. This order is similar to the pharmacological profile of the group I mGluRs in other nervous tissues (Conn & Pin, 1997). Therefore, the selective competitive group I mGluRs antagonist, S-4-CPG (100–600 μM), was used to study antagonism of the depolarizing response evoked by QA (0.03–100 μM). The nature of the antagonism was determined by generating a series of concentration-effect curves for QA-induced membrane depolarization in the presence of different concentrations of S-4-CPG. Concentration-response curves for QA were shifted rightward in concentration-dependent manner by S-4-CPG (Figure 2C). Schild analysis confirmed that the antagonism produced by S-4-CPG was competitive with a pA2 of 3.85 (Figure 2D). The pA2 value was 3.81±0.02 (n=3). QA-evoked depolarization was also suppressed by AIDA (500 μM, n=5), a selective and potent group I mGluR antagonist (Conn & Pin, 1997). The selective mGluR5 receptor agonist, (RS) - 2 - chloro - 5 - hydroxy phenyl glycine (CHPG) (300 μM–10 mM) also evoked depolarizing responses that were suppressed by S-4-CPG (300 μM) (n=3) (not shown). The selective group II agonist (2S, 1′R, 3′R) -2 -(carboxycyclopropyl) glycine (DCG-IV) (10 nM-300 μM) (n=5) or the group III agonist L (+)-2amino-4-phosphonobutryic acid (L-AP4) (10 nM–300 μM) (n=8) evoked no response. The antagonists of ionotropic glutamate receptors, D-AP5 (100 μM, n=6), MK801 (100 μM, n=5), CNQX (30 μM, n=7) and the selective group II or III mGluR antagonists, (RS)-1-amino-5-phosphonoindan-1-carboxylic acid ((RS)-APICA) (1 mM) (n=7) or (RS)-α-Cyclopropyl-4-phosphonophenylglycine (CPPG) (1 mM) (n=5), did not affect QA-induced depolarizing responses.
Figure 1.
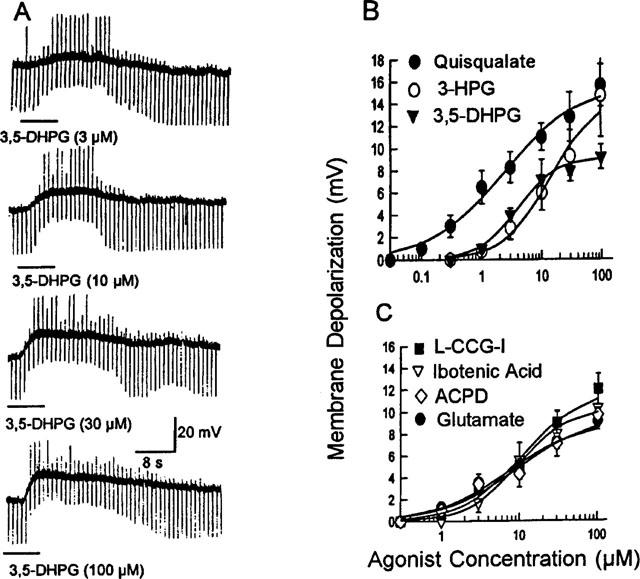
Effect of metabotropic glutamate group I receptors agonists on the submucous plexus neurons. (A) 3,5-DHPG (1–100 μM) induced dose-dependent membrane depolarization associated with increased neuronal excitability and decreased input resistance in an S/type 1 submucous neuron. The increased excitability was reflected by the appearance of anodal break excitation at the offset of hyperpolarizing current pulses. Morphology of this neuron appears in Figure 3D. (B) and (C) Concentration-response curves for agonists of group I mGluRs. The graphs in the figures were drawn by averaging results from all experiments and fitting a single concentration-response curve to the pooled data. Each point represents 5 to 12 neurons.
Figure 3.
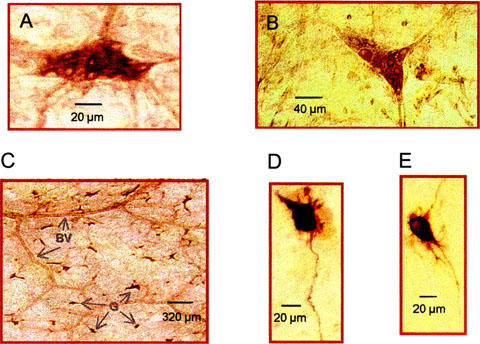
Histochemical localization of glutamate receptors in the submucous plexus. Immunohistochemical detection of the mGluR1α (A) and mGluR5 (B) receptors revealed by the peroxidase method at the light microscopic level. The majority of submucosal perikarya display immunoreactivities, whereas the dendritic staining for both receptor types is weaker. (C) Low-power field shows that staining of mGluR1α was localized to submucous ganglia (G), BV refers to blood vessels. (D)–(E) Morphology of biocytin-filled submucous plexus neurons recorded in Figures 1A and 2C, respectively.
Figure 2.
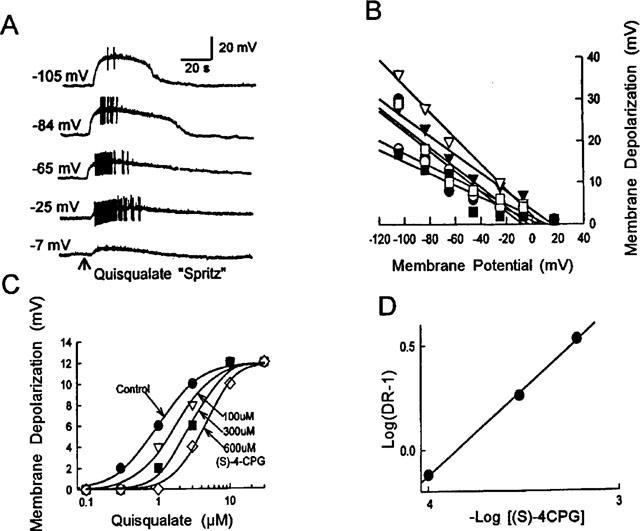
Reversal potential and antagonism of QA-evoked membrane depolarization. (A) The amplitude of QA-induced depolarization increased when the neuron was hyperpolarized and decreased when it was depolarized. QA was applied by micropressure ejection (100 μM, 40 ms). Morphology of this neuron appears in Figure 3E. (B) Quantitative data for the relationship of QA-induced depolarization and membrane potential for 6 different neurons. (C) Concentration-response curves obtained in absence and presence of different concentrations of S-4-CPG in a submucosal neuron. (D) Schild plots derived from graphs in C had a pA2 of 3.85.
The depolarizing responses induced by QA persisted in medium containing 300 nM TTX or low Ca2+ (0.5 mM)/high Mg2+ (12 mM, n=3). Input resistance was decreased by QA in most of the neurons examined (15/25, 60%). In 32% of the cells examined (8/25), the input resistance remained unchanged and in two neurons (2/25, 8%) we observed increased input resistance upon QA application. The amplitude of the QA-induced depolarization associated with decreased input resistance increased when the cells were hyperpolarized by current injection and decreased when the cells were depolarized (n=7, Figure 2A). The reversal potential for QA-induced depolarizing responses with decreased input resistance was 6.0±4.0 mV (range −8 to 20 mV, n=7) (Figure 2B). When low Na+ solution was in the bath, the amplitude of QA-induced depolarization was reduced by 58.4±9.9% (n=4).
Immunohistochemical evidence
The mGluR1α- and mGluR5-immunoreactivities were located exclusively on submucous neurons; other types of cells were devoid of staining (Figure 3C). In general, the immunolabelling was cytoplasmic, filling the perikarya and occasionally the proximal dendrites. In addition, the staining intensity of the cell bodies varied, with some being very intensely stained while others were more lightly stained. (Figure 3A,B). Immunoreactivities for mGluR1α and mGluR5 were also found in submucous plexus neurons of the jejunum and colon (not shown).
Discussion
The results provide strong support for the existence of functional mGluR1α and 5 on submucous plexus neurons of guinea-pig small intestine. The evidence consists of: (1) The selective mGluR1α agonists, 3,5-DHPG and 3-HPG and the selective mGluR5 agonist, CHPG but not the group II or III mGluRs agonists evoked similar slowly activating depolarization. The potency order of the agonists was similar to that of group I mGluRs. (2) The depolarization evoked by QA persisted in Krebs solution containing TTX (300 nM) or low Ca2+/high Mg2+, thereby excluding the involvement of synaptic release of other excitatory neurotransmitters. (3) The QA-evoked depolarizing responses were competitively suppressed by the selective group I mGluRs antagonist, S-4-CPG, but not antagonists of the groups II and/or III mGluRs or ionotropic glutamate receptors. (4) Immunohistochemistry revealed the existence of mGluR1α-and mGluR5-immunoreactivities localized to the neurons.
The depolarizing response induced by QA was associated with decreased input resistance in most of the neurons examined. The reversal potential was ≈6 mV. In addition, QA-evoked responses were suppressed by decreasing Na+ in the bathing solution. This evidence suggests that the responses mediated by the group I metabotropic receptors are primarily due to opening of a non-selective cation conductance. This ionic mechanism was similar to excitatory responses mediated by other G-protein coupled receptors in the submucous plexus (Shen & Surprenant, 1993). Activation of group I mGluRs in CA1 pyramidal neurons also generates a long-lasting calcium-activated nonselective cationic current (Congar et al., 1997). Nevertheless, the input resistance was either not changed or was increased by QA in some neurons, suggesting the involvement of more complex ionic mechanisms besides opening of a non-selective ionic conductance in QA-evoked responses.
Subsets of glutamate-immunoreactive neurons are observed in both myenteric and submucous plexus neurons with more neurons being stained in the submucous plexus (Liu et al., 1997). This is consistent with our findings that most submucous plexus neurons are endowed with mGluR1α- and mGluR5-immunoreactivities and sensitivity to glutamate. Another finding of potential significance is that the neurons sensitive to glutamate were exclusively S/type 1 neurons with uniaxonal morphology. A subpopulation of S/type 1 neurons is considered to be secretomotor neurons in the submucous plexus (Wood, 1994). When secretomotor neurons fire, they release vasoactive intestinal polypeptide or acetylcholine at the neuroepithelial junctions and this stimulates the secretion of water and electrolytes into the crypt lumen. Both asparagine and glutamate are stimulants of Cl− secretion in the piglet intestine. Glutamate stimulates Cl− secretion by a neuronal mechanism because TTX abolishes the response (Rhoads et al., 1995). Consideration that activation of group I receptors following excitotoxic insult (i.e., experimental or ischaemia-related) appears to contribute to cell death suggests that the glutamate excitotoxicity observed in the enteric nervous system (Kirchgessner et al., 1997) may also be mediated by the group I mGluRs found in our study.
It has been suggested that glutamate is a neurotransmitter in the enteric nervous system. L-glutamate is located in cells of the enteric nervous system and released in a Ca2+-dependent manner during depolarization evoked by high K+ (Kirchgessner et al., 1997; Liu et al., 1997; Okuma et al., 1996; Sinsky & Donnerer, 1998). The high affinity neuronal glutamate transporter is also present in cultured enteric ganglia (Kirchgessner et al., 1997; Liu et al., 1997). The present study has provided evidence for the existence of functional group I mGluRs on neurons of the guinea-pig submucous plexus. The electrophysiological and morphological evidence supports the hypothesis that glutamate is a neurotransmitter that acts through the group I mGluRs in the enteric nervous system.
Acknowledgments
This work was supported by National Institute of Health grants RO1 DDK-37238 and DDK-46941. We appreciate Ms Liangyan Fan' s technical help with the immunostaining.
Abbreviations
- ACPD
1S,3R-1-amino-1,3-cyclo-pentanedicarboxylate
- AIDA
(RS)-2-aminoindan-1,5-dicarboxylic acid
- CHPG
(RS)-2-chloro-5-hydroxyphenylglycine
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CPPG
(RS)-α-cyclopropyl-4-phosphonophenylglycine
- DAB
3,3′-diaminobenzidine
- D-AP5
D(-)-2-amino-5-phosphonopentanoic acid
- DCG-IV
(2S,1′R,3′R)-2-(carboxycyclopropyl)glycine
- 3,5-DHPG
3,5-dihydroxyphenylglycine
- 3-HPG
3-hydroxyphenylglycine
- L-AP4
L(+)-2-amino-4-phosphonobutryic acid
- L-CCG-I
(2S, 1′S,2′S)-2-(carboxycyclopropyl)glycine
- mGluRs
metabotropic glutamate receptors
- MK801
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclo-hepten-5,10-imine/dizocilpine
- NMDG
N-methyl-D-glucamine
- PBS
phosphate-buffered saline
- QA
quisqualate
- (RS)-APICA
(RS)-1-amino-5-phosphonoindan-1-carboxylic acid
- S-4-CPG
(S)-4-carboxyphenylglycine
- TTX
tetrodotoxin
References
- CHRISTOFI F.L., WOOD J.D. Effects of PACAP on morphologically identified myenteric neurons in guinea-pig small bowel. Am. J. Physiol. 1993;264:G414–G421. doi: 10.1152/ajpgi.1993.264.3.G414. [DOI] [PubMed] [Google Scholar]
- CONGAR P., LEINEKUGEL X., BEN-ARI Y., CREPEL V.A. Long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J. Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONN P.J., PIN J.-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- JANKOVIC S.M., MILOVANOVIC D., MATOVIC M., IRIC-CUPIC V. The effects of excitatory amino acids on isolated gut segments of the rat. Pharmacol. Res. 1999;39:143–148. doi: 10.1006/phrs.1998.0422. [DOI] [PubMed] [Google Scholar]
- KIRCHGESSNER A.L., LIU M.-T., ALCANTARA F. Excitotoxicity in the enteric nervous system. J. Neurosci. 1997;17:8804–8815. doi: 10.1523/JNEUROSCI.17-22-08804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU M.-T., ROTHSTEIN J.D., GERSHON M.D., KIRCHGESSNER A.L. Glutamatergic enteric neurons. J. Neurosci. 1997;17:4764–4784. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUMA Y., YOKOTANI K., NAKAMURA K, OSUMI Y. Calcium-dependent release of endogenous glutamate from vascularly perfused rat stomach in vitro. J. Neurosci. Res. 1996;44:507–511. doi: 10.1002/(SICI)1097-4547(19960601)44:5<507::AID-JNR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- RHOADS J.M., ARGENZIO R.A., CHEN W., GOMEZ G.G. Asparagine stimulates piglet intestinal Cl secretion by a mechanism requiring a submucosal glutamate receptor and nitric oxide. J Pharmacol. Exp. Ther. 1995;274:404–412. [PubMed] [Google Scholar]
- SHEN K.Z., SURPRENANT A. Common ionic mechanisms of excitation by substance P and other transmitters in guinea-pig submucosal neurones. J. Physiol. (London) 1993;462:483–501. doi: 10.1113/jphysiol.1993.sp019565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINSKY M., DONNERER J. Evidence for a neurotransmitter role of glutamate in guinea-pig myenteric plexus neurons. Neurosci. Lett. 1998;258:109–112. doi: 10.1016/s0304-3940(98)00866-0. [DOI] [PubMed] [Google Scholar]
- WOOD J.D.Physiology of the enteric nervous system Physiology of the gastrointestinal tract 1994New York: Raven; 423–482.Ed 3. Johnson LR, Alpers DH, Jacobson ED, Walsh JH, (eds) [Google Scholar]
- ZAFIROV D.H., COOKE H.J., WOOD J.D. Elevation of cAMP facilitates noradrenergic transmission in submucous neurons of guinea-pig ileum. Am. J. Physiol. 1993. pp. G442–G446. [DOI] [PubMed]


