Abstract
In vitro and in vivo studies have demonstrated a dysfunctional nitrergic system in diabetes mellitus, thus explaining the origin of diabetic impotence. However, the mechanism of this nitrergic defect is not understood.
In the penises of streptozotocin (STZ)-induced diabetic rats, here, we show by immunohistochemistry that nitrergic nerves undergo selective degeneration since the noradrenergic nerves which have an anti-erectile function in the penis remained intact.
Nitrergic relaxation responses in vitro and erectile responses to cavernous nerve stimulation in vivo were attenuated in these animals, whereas noradrenergic responses were enhanced.
Activity and protein amount of neuronal nitric oxide synthase (nNOS) were also reduced in the penile tissue of diabetic rats.
We, thus, hypothesized that NO in the nitrergic nerves may be involved in the nitrergic nerve damage, since only the nerves which contain neuronal NO synthase underwent degeneration.
We administered an inhibitor of NO synthase, NG-nitro-L-arginine methyl ester (L-NAME), in the drinking water of rats for up to 12 weeks following the establishment of diabetes with STZ.
Here we demonstrate that this compound protected the nitrergic nerves from morphological and functional impairment. Our results show that selective nitrergic degeneration in diabetes is NO-dependent and suggest that inhibition of NO synthase is neuroprotective in this condition.
Keywords: Nitric oxide, nitrergic, diabetes mellitus, neurodegeneration, penile erection, impotence, erectile dysfunction, streptozotocin
Introduction
Penile erection comprises increased arterial inflow and restricted venous outflow from the penis, co-ordinated by relaxation of the penile corpus cavernosum (Lue & Tanagho, 1987). Relaxation of the cavernosal smooth muscle is regulated by local factors released from nerve fibres and the sinusoidal endothelium (Andersson & Wagner, 1995). The identity of the major mediator responsible for the relaxation of the corpus cavernosum has long been a subject for debate. Nitric oxide (NO) is now accepted as the most important relaxant mediator in the process of erection (Burnett et al., 1992; Andersson & Wagner, 1995) and other putative mediators like VIP, ATP or acetylcholine have been excluded (Wagner & Brindley, 1980; Andersson, 1993; Andersson & Wagner, 1995)
In the penile corpus cavernosum NO is produced in the endothelium and nitrergic nerves (Moncada et al., 1997) by endothelial NO synthase (eNOS) and neuronal NO synthase (nNOS) respectively. Although acetylcholine evokes endothelium-dependent relaxation of the isolated corpus cavernosum (Saenz de Tejada et al., 1988), erection is unlikely to involve release of acetylcholine from parasympathetic nerves and its subsequent diffusion to the endothelium to release NO, since neither atropine nor neostigmine inhibit erection when injected intracavernosally (Wagner & Brindley, 1980; Brindley, 1986; Burnett et al., 1992). Furthermore, relaxation of the corpus cavernosum by transmural stimulation does not require functional endothelium (Saenz de Tejada et al., 1988; Kim et al., 1991; Okamura et al., 1999). Thus the majority of NO eliciting relaxation in penile tissue seems to be derived from nNOS in nitrergic nerves.
In vivo and in vitro studies suggest that the nitrergic system is essential for penile erection (Andersson & Wagner, 1995). In anaesthetized animals, erection induced by stimulation of the cavernous nerve or spinal cord can be inhibited by NOS inhibitors (Holmquist et al., 1991; Burnett et al., 1992; Trigo-Rocha et al., 1993; Finberg et al., 1993; Andersson & Wagner, 1995). Corpus cavernosum obtained from patients relaxes to transmural stimulation in vitro, an effect that can be blocked by NOS inhibitors (Bush et al., 1992; Holmquist et al., 1992).
Erectile dysfunction is caused by many diseases, one of which is diabetes mellitus; the prevalence of impotence in diabetic patients is up to 50% (Rubin & Babbott, 1958). Since NO has been shown to play an important role in the physiology of penile erection, it is not surprising that the aetiology of impotence has been linked to impaired nitrergic neurotransmission. In vitro and in vivo studies in animals and in human strongly suggest that a nitrergic defect may underlie the pathophysiology of erectile dysfunction (Saenz de Tejada et al., 1989; Azadzoi & Saenz de Tejada, 1992; Vernet et al., 1995; Rehman et al., 1997). The mechanism by which the nitrergic defect occurs in diabetes is, however, unknown. We have, therefore, investigated morphological and functional changes in nitrergic nerves in penile tissue from experimental streptozotocin-induced diabetic rats.
Methods
Induction of diabetes in rats
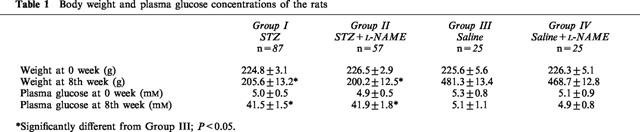
Male Wistar rats (200–250 g, n=320) were divided into four groups (Table 1). The first and second groups were injected with STZ (Rakieten et al., 1963) (75 mg kg−1, i.p.), the third and fourth groups were injected with sterile saline (1 ml kg−1, i.p.). The second and fourth groups were given L-NAME (0.05 mg ml−1 and 0.1 mg ml−1 respectively) in drinking water beginning 72 h (the time required for diabetes to be established) after the injection of STZ or saline. Diabetic rats were observed to drink approximately 100 ml day−1 whereas non-diabetic rats drank approximately 50 ml day−1; thus each animal in the second and fourth groups received 5 mg L-NAME each day. This dose has been shown to inhibit NO synthase activity but not to alter renal function or catecholamine and insulin concentrations in the rat (Navarro et al., 1994). Blood glucose and body weight were monitored weekly (Table 1). At the end of the first week any animal in the first and second group with a blood glucose concentration lower than 20 mM was excluded from the study. Another group of rats (weight-matched rats; 180–200 g; n=8) was also evaluated in pharmacological experiments; no significant difference was found between them and Group III (age-matched control rats). L-NAME was withdrawn 72 h prior to the experiments; this time period has been shown to reduce the L-NAME concentrations in the blood by 80–90% (Piotrovskij et al., 1993; Tabrizi-Fard & Fung, 1994).
Table 1.
Body weight and plasma glucose concentrations of the rats
All animal experiments were conducted according to the rules outlined by the Home Office, Animals (Scientific Procedures) Act 1986 (project number: 70/3842).
Immunohistochemistry
Rats were perfused through the left ventricle with 4% paraformaldehyde in 0.1 M phosphate buffer. Serial cryosections from the penises and anococcygeus muscles at 40 μm intervals were stained with a polyclonal antibody against nNOS (in order to visualize to the nitrergic nerves; 1:3000 dilution; prepared as described in Rodrigo et al., 1994) or with a polyclonal antibody against tyrosine hydroxylase (in order to visualize the noradrenergic nerves; 1:1000 dilution; Pel-Freez Biologicals, U.S.A.) or with a polyclonal antibody against nitrotyrosine (in order to visualize peroxynitrite-induced nitrotyrosine formation; 1:1000 dilution; prepared as described in Uttenthal et al., 1998) followed by detection with biotinylated secondary antibodies and with the avidin-biotin peroxidase complex and nickel-enhanced diaminobenzidine procedures. Because of the tortuous course of nitrergic nerve fibres in the penis, multiple exposures in different focal planes were obtained and then a photomontage technique was used to put the sequential images together on one plane as described previously (Rodrigo et al., 1994; 1997; 1998).
In vitro pharmacology
Bilateral anococcygeus muscles from the rats were placed in a horizontal superfusion chamber and superfused with modified Krebs' solution containing dexamethasone (5 μM) and indomethacin (5 μM) as described before (Kasakov et al., 1995). The parameters of the electrical field stimulation (EFS) were 50 V, 0.3 ms pulse duration, 1–25 Hz, for 5 s, every 2 min. Nitrergic relaxation responses to EFS were recorded after treatment of the tissues with scopolamine (5 μM) and guanethidine (5 μM) followed by elevation of the tone with phenylephrine (EC80) as described before (Kasakov et al., 1995); these were affirmed by total inhibition by 300 μM NG-nitro-L-arginine (L-NOARG) at the end of the experiment.
In vivo pharmacology
Anaesthesia was induced in the rats with sodium thiopentone (120 mg kg−1, i.p.) and was maintained by a subsequent injection of sodium pentobarbital (5 mg kg−1, i.p.) as required. The right external jugular vein and left carotid artery were cannulated for saline infusion (50 μl min−1) and for blood pressure monitoring, respectively. The penile shaft and cavernous nerve were exposed as described (Vernet et al., 1995). Intracavernous pressure (ICP) in the right crus of the penis was monitored using a 25-gauge cannula connected to a pressure transducer. The cavernous nerve was stimulated by bipolar platinum hook electrodes (0.5 mm in diameter). Square pulses of 10 Hz, 0.3 ms pulse duration, for 1–3 min, 0.05–0.5 mA were applied via a Grass stimulator coupled to a Grass constant current unit. Cavernous nerve stimulation-induced increase in ICP was completely blocked by L-NAME (10 mg kg−1, i.v.), an effect reversed by L-arginine (100 mg kg−1, i.v.) (not shown).
NO synthase activity assay
The whole penis and anococcygeus muscles of the rats were frozen in liquid nitrogen and pulverized in a stainless steel pestle and mortar. Homogenization was performed using HESD buffer (HEPES 20 mM, pH: 7.2, EDTA 1 mM, sucrose 0.2 M, dithiothreitol 5 mM, PMSF 0.1 mM, leupeptin and soya bean trypsin inhibitor 20 μg ml−1, 5 μg ml−1 each pepstatin A, E-64, bestatin, aprotinin and 3,4-dichloroisocoumarin) and centrifuging at 13,000 ×g for 30 min at 4°C. Endogenous L-arginine in the supernatant was removed using activated Dowex-50W resin. NO synthase was assayed in the supernatant by the formation of [U-14C]-citrulline from L-[U-14C]-arginine as described (Knowles & Salter, 1997).
Western-blot/densitometric analysis of nNOS
Equal amounts (39 μg lane−1) of the same homogenates described above were run on 7.5% polyacrylamide SDS gels, then transferred to nitrocellulose filters. The blots were incubated for 1 h with mouse anti-human nNOS (1:2000 dilution; Transduction Labs, U.K.) and anti-actin (1:2000 dilution; Boehringer-Mannheim, U.K.) monoclonal antibodies. Final incubation was performed for 1 h with horse-radish peroxidase-conjugated goat anti-mouse IgG (1:3000 dilution; Vectors Labs, U.K.). The reactive bands were detected with a luminol-based kit (ECL, Amersham, U.K.). The optimal X-ray exposure was selected, scanned and the density of each band was calculated by densitometry using a computer analysis program.
Statistical analysis
Results are expressed as the mean±s.e.mean. n denotes the number of experiments in each group. Student's t-test for unpaired observations was used to determine the significance of differences between the groups. P value less than 0.05 was considered significant.
Results
Selective nitrergic degeneration shown by immunohistochemistry
Immunohistochemical study of the penis from STZ-induced diabetic rats revealed that nitrergic nerves undergo degeneration, whereas noradrenergic nerves remain intact (Figures 1 and 2). We hypothesized that NO in the nitrergic nerves may be responsible for the nerve damage, since only the nerves which contain neuronal NO synthase underwent degeneration. We thus administered an inhibitor of NO synthase, NG-nitro-L-arginine methyl ester (L-NAME), in the drinking water of rats which had received an injection of STZ 72 h previously. After 4, 8, 12 and 16 weeks, animals were sacrificed and some were perfused with paraformaldehyde for immunohistochemical studies. The bilateral anococcygeus muscles and penises were isolated from some of the remaining animals for functional and biochemical studies; others were used for an in vivo study of cavernous nerve stimulation-induced penile erection. L-NAME was withdrawn from the drinking water 72 h before initiation of the in vitro and in vivo experiments to allow the drug and its effect to wear off. The experiments were then carried out in the absence of L-NAME.
Figure 1.
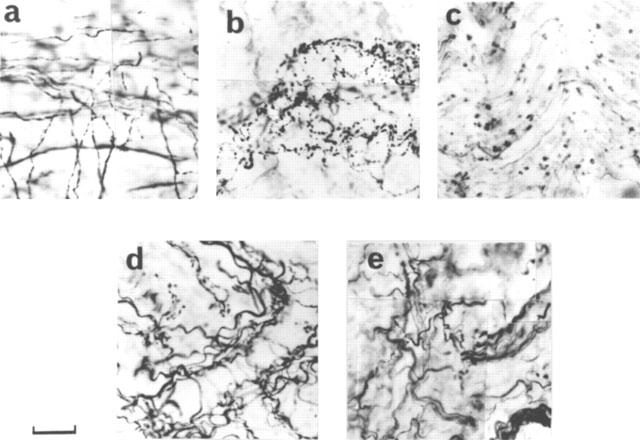
Immunohistochemical demonstration of nitrergic degeneration in the corpus cavernosum of diabetic rats. nNOS immunostaining in a control rat (a), in a 4 week diabetic rat (b, note the varicosity formation and breakage in the nerve fibres), in an 8 week diabetic rat (c, note that the nitrergic nerves became very sparse), in an 8 week diabetic rat treated with L-NAME (d, note that the structure and number of the nitrergic nerve fibres are preserved during diabetes) and in a rat which was treated with L-NAME for 8 weeks (e, note that the structure and number of nerve fibres are not different from a). Scale is 33 μm for c, 43 μm for others. The results are typical of those observed in 6–12 animals in each group.
Figure 2.
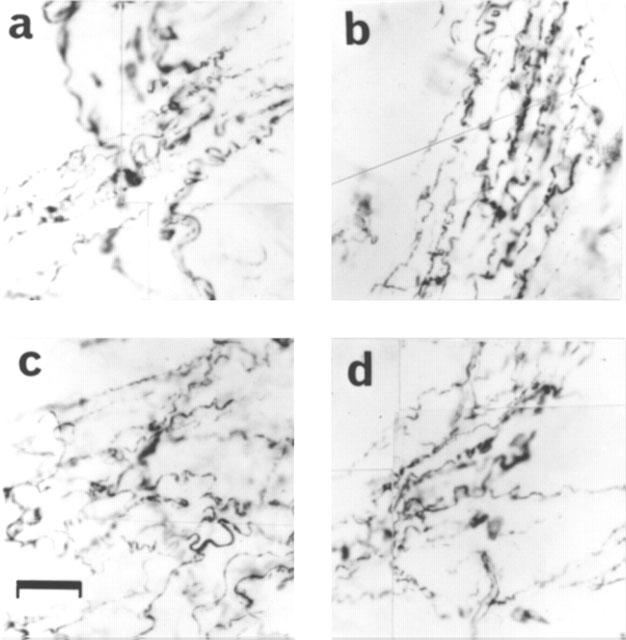
Tyrosine hydroxylase immunostaining in a control rat (a), an 8 week diabetic rat (b, note that the morphology and density of noradrenergic nerves are unchanged), an 8 week diabetic rat treated with L-NAME (c) and a control rat treated with L-NAME for 8 weeks (d). Scale bar is 40 μm. The results are typical of those observed in 6–12 animals in each group.
L-NAME treatment protects the nitrergic nerves against degeneration
Immunohistochemical study of the penis and anococcygeus muscles from 4 week diabetic rats showed varicosity formation and breakage in the nitrergic nerve fibres (Figure 1b). This degenerative process was more pronounced in the tissues from 8 week diabetic animals, thus leading to a decreased number of nitrergic nerve fibres (Figure 1c). Noradrenergic nerve fibres were intact, showing no degeneration even at 8 weeks (Figure 2b). In tissues obtained from diabetic rats which had received L-NAME, however, the degenerative process was not observed (Figure 1d).
Functional in vitro studies with diabetic rats
Our morphological findings were supported with functional studies which were performed on the anococcygeus muscles of the diabetic animals. Noradrenergic contractions, which are known to be modulated by nitrergic neurotransmission (Li & Rand, 1989; Cellek & Moncada, 1997), were enhanced in the tissues from 4 week diabetic animals. This enhancement was more pronounced at 8 weeks (Figure 3a). Moreover, nitrergic relaxant responses were significantly decreased at 8 weeks (Figure 3b). In tissues from diabetic animals treated with L-NAME, however, the noradrenergic contractile and nitrergic relaxant responses were not significantly different from control animals (Figure 3a,b). Defective nitrergic relaxation was not due to changes in the responsiveness of the smooth muscle to NO since concentration-response curves to exogenous sodium nitroprusside (SNP) in control and diabetic animals were indistinguishable (Figure 4a). Concentration-response curves to exogenous noradrenaline were also indistinguishable between control and diabetic animals (Figure 4b), which excludes the possibility that the enhanced noradrenergic contraction might be due to hyperresponsiveness of the smooth muscle to noradrenaline. These results suggest that nitrergic neurotransmission becomes defective due to morphological changes in the nitrergic nerves which is prevented when the animals are treated with L-NAME.
Figure 3.
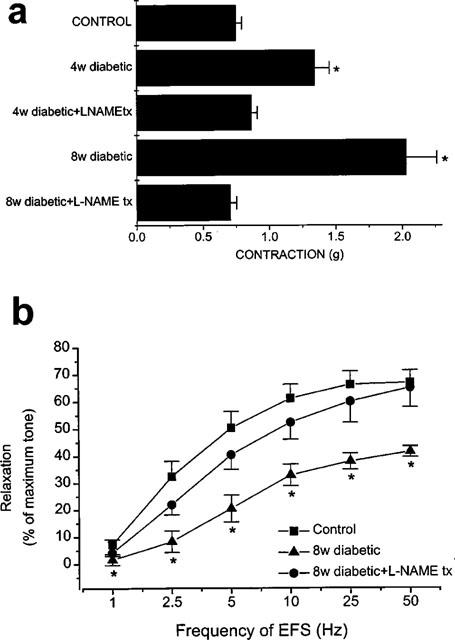
Effect of diabetes and L-NAME treatment during diabetes on the noradrenergic and nitrergic responses of the rat anococcygeus muscle. (a) Noradrenergic contractions elicited by EFS (5 Hz) were enhanced in the anococcygeus muscle of diabetic rats but not of the diabetic rats treated with L-NAME. (*significantly different from control; P<0.001; n=6–12) (b) EFS-induced nitrergic relaxations in the anococcygeus muscle of control, of 8 week diabetic rats and of 8 week diabetic rats treated with L-NAME. Note that L-NAME administration was stopped 72 h before the experiments. (*significantly different from control; P<0.05; n=6–12).
Figure 4.
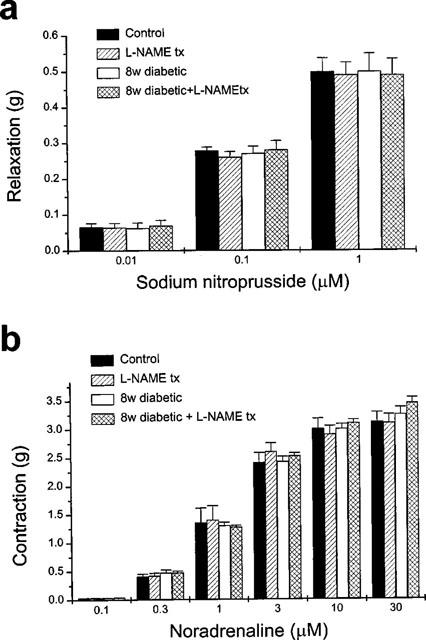
Responses of the rat anococcygeus muscle to exogenous sodium nitroprusside or noradrenaline were not affected by diabetes with or without L-NAME treatment. (a) Relaxation responses of the rat anococcygeus muscle to exogenous sodium nitroprusside (0.01–1 μM) after treatment with guanethidine and after elevation of the tone with phenylephrine were similar in control, L-NAME-treated, 8 week diabetic and 8 week diabetic+ L-NAME-treated animals (n=6). (b) Contractions elicited by exogenous noradrenaline (0.1–30 μM) were not affected by L-NAME treatment or diabetes with or without L-NAME treatment (n=6). Note that L-NAME administration was stopped 72 h before the experiments.
Effect of L-NAME treatment on non-diabetic animals
In the penile tissues from non-diabetic rats which were treated with L-NAME, there was no detectable degeneration either in the nitrergic nerves or noradrenergic nerves (Figures 1e and 2d). In some experiments, we used some animals from which L-NAME had been withdrawn only 1 h before. Those experiments revealed that nitrergic responses (both in vivo and in vitro) were inhibited 60–75% in acutely withdrawn animals (not shown). The control animals which received L-NAME but which were withdrawn from L-NAME treatment 72 h prior to the experiments did not show altered nitrergic response either in vitro or in vivo.
The activity of constitutive NO synthase and the amount of nNOS in diabetic rats
The nitrergic defect was confirmed by performing a NO synthase activity assay in a crude extract of anococcygeus muscle of STZ-induced diabetic rats. Constitutive NO synthase activity declined significantly during the course of the 12 week period of diabetes starting from the second week (Figure 5a). In tissues from diabetic animals which were treated with L-NAME and studied 72 h after termination of the drug treatment, the decrease in the NO synthase activity was not observed (Figure 5a). Inducible NO synthase (iNOS) activity was not detectable in any of the groups (not shown).
Figure 5.
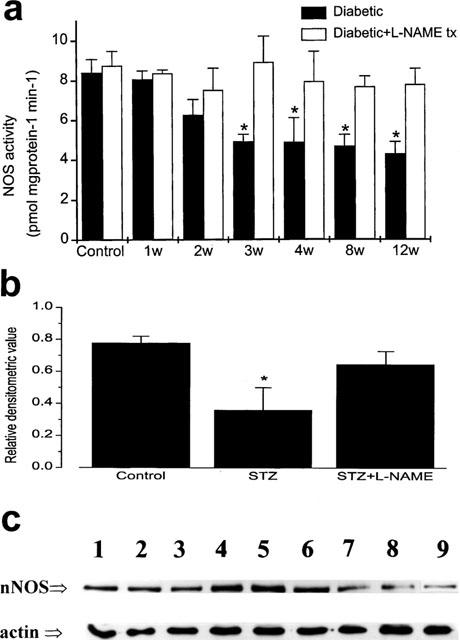
Constitutive NO synthase activity and nNOS protein decreases during diabetes; this effect is not seen in L-NAME-treated diabetic animals. (a) Constitutive (Ca2+-dependent) NO synthase activity decreased gradually during the course of diabetes in the anococcygeus muscle of rats but did not change in the L-NAME-treated diabetic animals. The columns of the control group represent the results from saline-injected animals and saline-injected animals which were treated with L-NAME for 8 weeks respectively. Note that L-NAME was withdrawn 72 h before the experiments. (*significantly different from control; P<0.05; n=6–20). (b) Western-blot densitometry of crude extracts of the penis of control, 12 week diabetic and 12 week diabetic treated with L-NAME rats. The graph represents the relative densitometric values as calculated for the intensities of the main 160K bands on the blots divided by the corresponding intensity of the actin bands (*significantly different from control; P<0.05; n=3). (c) a reprensentative Western blot showing the bands corresponding to nNOS and actin as indicated. Lanes 1–3 are from control rats, lanes 4–6 are from 12 week diabetic rats treated with L-NAME and lanes 7–9 are from 12 week diabetic rats.
Our biochemical results were further supported by Western-blot/densitometric analysis of the crude extract of penises from STZ-induced diabetic animals. nNOS protein was visualized using a monoclonal antibody specific against the enzyme. The densitometric analysis of the main band with an Mr of 160 K revealed a significant 48% reduction of the band intensity in the 12 week-diabetic rats; this effect was not observed in the diabetic animals treated with L-NAME (Figure 5b,c).
Erectile function in vivo
We investigated the protective effect of L-NAME treatment on the erectile function of the diabetic rats by studying the effects of in vivo electrical stimulation of the cavernous nerve. Nerve stimulation elicited an increase in intracavernous pressure (ICP) which was sustained during the stimulation period in the anaesthetized control rats (Figure 6). In the diabetic rats, however, the increase in ICP was not maintained, falling to the basal pressure within 2–3 min, during stimulation (Figure 6). This defective erectile function was not observed in the diabetic animals 12 weeks after treatment with L-NAME (Figure 6). These experiments clearly showed that L-NAME could provide protection of the erectile function in vivo.
Figure 6.
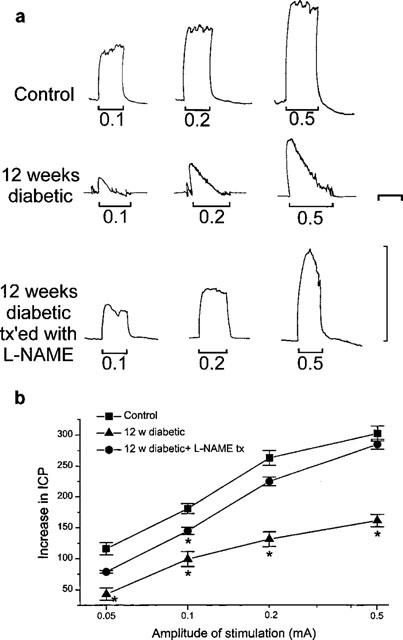
Effect of diabetes and L-NAME treatment during diabetes on the erectile function of the rats in vivo. (a) typical traces from a control rat (upper trace), a 12 week diabetic rat (middle trace; note that the increase in intracavernous pressure (ICP) could not be maintained during the stimulation period) and a 12 week diabetic rat treated with L-NAME (lower trace). Note that L-NAME was withdrawn 72 h before the experiments. The vertical scale corresponds to 100 cmH2O ICP. The horizontal scale corresponds to 2 min. (b) The increase in ICP (measured as the area under the curve and expressed as arbitrary units) is plotted against the amplitude of the electrical stimulation of the cavernous nerve in the control rats, 12 week diabetic rats and 12 week diabetic rats treated with L-NAME (*significantly different from control; P<0.05; n=6–10).
Nitrotyrosine immunostaining
Immunostaining with anti-nitrotyrosine antibody showed a basal level of nitrotyrosine formation located in the nerve structures in the anococcygeus muscle and penis from control rats (Figure 7a). Detailed analysis of these tissues using electron microscopy revealed that nitrotyrosine was confined to the Schwann cells and was not observed in the axons (not shown). Although a similar basal level of nitrotyrosine staining was observed in diabetic animals with or without L-NAME treatment (Figure 7b,c), these results did not allow us to conclude that nitrotyrosine staining was not changed during diabetes with or without L-NAME treatment.
Figure 7.
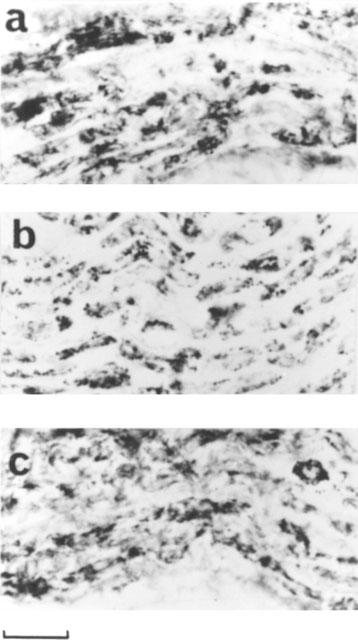
Nitrotyrosine immunostaining in the penis of a control rat (a), an 8 week diabetic rat (b) and an 8 week diabetic rat treated with L-NAME (c). Note that the morphology and density of nitrotyrosine staining are unchanged. Scale bar is 40 μm. The results are typical of those observed in 3–4 animals in each group
Discussion
Peripheral nerves have been shown to undergo a degenerative process in the penis of diabetic men (Faerman et al., 1974). These early studies did not, however, specify the type of the nerves involved. Shortly after the discovery of the L-arginine-NO pathway (Palmer et al., 1988) and the recognition of the importance of nitrergic neurotransmission in the physiology of penile erection (Burnett et al., 1992), nitrergic relaxations were shown to be deficient in the corpus cavernosum of diabetic impotent men (Saenz de Tejada et al., 1989) and in the corpus cavernosum of alloxan-induced diabetic rabbits (Azadzoi & Saenz de Tejada, 1992). Neurogenic erectile function has been shown to be diminished in diabetic rats in which diabetes was induced either by STZ or subtotal pancreatectomy (Rehman et al., 1997). The activity and amount of nNOS protein have been shown to be reduced in the penis of spontaneously diabetic BB/WORdp rats (Vernet et al., 1995). El-Sakka et al. (1999) have shown a decrease in the number of NOS-containing (NADPH-diaphorase-positive) nerve fibres, a decrease in intracavernous pressure to cavernous nerve stimulation and down-regulation of nNOS mNRA and protein expression in the penis of diabetic rats. Our study confirms these previous reports and demonstrates that the reduction in nNOS activity and protein amount is due to selective nitrergic degeneration.
There is a report showing an increase in NOS activity in the penis of diabetic rats (Elabbady et al., 1995) which disagrees with our results. That study, however, did not investigate which isoform of NOS might be responsible for this elevation in enzyme activity. The authors observed signs of inflammation in the penis of diabetic rats which may be related to induction of iNOS, which we did not observe in our experiments.
We have shown in this study that noradrenergic nerves remain intact during diabetes. In previous studies, a decrease in noradrenaline content in the corpus cavernosum of diabetic men has been reported (Melman & Henry, 1979; Lincoln et al., 1987). It is difficult to compare these studies with ours since we are working with animals early in their diabetic process and we do not know whether sympathetic neuropathy would have occurred if we had kept the animals diabetic for longer periods. Our results clearly demonstrate a difference between nitrergic and sympathetic neuropathy in diabetes. This may explain the clinical phenomenon of earlier occurrence of parasympathetic neuropathy than the sympathetic neuropathy in diabetes (Clarke et al., 1979) since ∼85% of the parasympathetic nerves innervating the penis are nitrergic (Schirar et al., 1994).
We did not investigate whether or not sensory nerves remain intact during diabetes mellitus. Our unpublished observations suggest that sensory nerves are not involved in nitrergic relaxation of rabbit corpus cavernosum or rat and rabbit anococcygeus muscles. This is in accordance with Teixeira et al. (1998) who have shown that capsaicin-induced relaxations are not inhibited by L-NAME in the rabbit corpus cavernosum. Davies et al. (1998) showed that capsaicin elicits NO-dependent relaxation in the rat anococcygeus muscle only when the concentration of calcium in the medium is kept low. Further research is needed to elucidate whether sensory nerves are involved in erectile nitrergic response and whether or not they are affected in diabetes mellitus.
Nitrergic control of noradrenergic responses in the human corpus cavernosum (Cellek & Moncada, 1997) suggests a key role for this interaction in the pathophysiology of erectile dysfunction. Indeed, a nitrergic-noradrenergic imbalance in favour of the anti-erectile noradrenergic system has been implicated in penile tissues from patients with erectile dysfunction (Taub et al., 1993; Christ et al., 1995). However, the mechanism of this imbalance is not fully understood. Our results with diabetic rat anococcygeus muscle suggest that the nitrergic defect causes removal of nitrergic modulation of the noradrenergic system which leads to an apparent overactive noradrenergic response.
We used anococcygeus muscle instead of penile corpus cavernosum for in vitro pharmacology experiments to assess the functional changes in the nitrergic neurotransmission during diabetes. In the rat, penile corpus cavernosum and anococcygeus muscles receive motor sympathetic innervation via the lumbar sympathetic nerves and parasympathetic innervation via the sacral parasympathetic nerves, thus sharing a common origin for nitrergic and noradrenergic pathways. Moreover, the anococcygeus muscle, unlike the penile corpus cavernosum, is a pure smooth muscle preparation containing very few blood vessels, which makes pharmacological experiments easier to interpret.
The non-selective NOS inhibitor, L-NAME, was administered to diabetic rats after the diabetes was established. L-NAME administration was not initiated during the first 72 h after STZ injection since NOS inhibitors have been shown to interfere with the pancreatic β-cell destruction following STZ injection (Kolb et al., 1991). L-NAME treatment did not affect the progression of the diabetes. This was evaluated by two parameters; the blood glucose and the body weight. The diabetic animals had similar blood glucose and similar rate of failure to gain weight in both L-NAME-treated and -untreated groups (Table 1).
L-NAME was withdrawn 72 h prior to experiments to allow enough time to wash the drug out of the system of animals. We performed some experiments in which the animals were not withdrawn from L-NAME, in order to check the effectiveness of L-NAME treatment. These experiments showed a 60–75% decrease in nitrergic function in vitro and in vivo.
Our finding that L-NAME administration prevents development of nitrergic dysfunction suggests that NO production during diabetes has a role in the nitrergic degeneration. We have not identified which isoform of NO synthase is responsible for the generation of the NO involved in this process. It is unlikely that the source of NO responsible for the selective nitrergic degeneration is iNOS, since neither iNOS enzyme activity nor iNOS positive immunohistochemistry could be detected in the penis of diabetic animals. Whether eNOS or nNOS is responsible for the degeneration requires further studies. However, we favour the hypothesis that nNOS is involved because of the specific localization of damage to the nitrergic nerves.
The mechanism by which NO produces nerve degeneration also needs to be clarified. We did not observe any increase in expression of eNOS or nNOS protein at any stage of diabetes; in fact we observed a decrease in nNOS protein as early as 1 week following the development of diabetes. Often, NO-dependent damage has been associated with increased generation of NO (Moncada et al., 1991; Dawson et al., 1991), although we cannot exclude the possibility that a decreased amount of enzyme might be generating increased amounts of NO. A further possibility is that this selective and self-destructive degeneration of nitrergic nerves might be the result of increased oxidative stress, known to occur in diabetes (Baynes, 1991; Honing et al., 1998), which in the presence of NO might generate the powerful pro-oxidant agent peroxynitrite. Indeed, the formation of peroxynitrite in diabetes has been suggested before (Lyall et al., 1998). We could not evaluate whether peroxynitrite was increased in the penis of diabetic animals due to the unusual finding that there is basal nitrotyrosine formation in the Schwann cells around the neuronal axons. The reason for this is at present unclear, however a basal level of nitrotyrosine immunostaining has recently been reported in other tissues (Bian et al., 1999; Nava et al., 1999). Further research will be needed to clarify whether indeed peroxynitrite is formed and its role in physiological conditions as well as its role during oxidative stress which may be involved in nitrergic degeneration in diabetes mellitus.
In summary, we demonstrate that erectile dysfunction in diabetes is due to a selective defect in the nitrergic system. This defect is a result of a morphological and functional damage of the nitrergic nerves. Our results also show that this damage is NO-dependent since it can be prevented by an inhibitor of NO synthase. This observation may have a significant therapeutic value in diabetes mellitus in humans.
Acknowledgments
The authors thank Mrs Annie Higgs for help in preparation of the manuscript. The authors are grateful to Dr G. Christ and his group (Albert Einstein College of Medicine, New York, U.S.A.) for help in setting-up the in vivo experiments. The staff of Biological Services Unit, University College London are acknowledged for their professional assistance in taking care of the diabetic animals. The authors also thank M.L. Bentura for help in processing the photographical material, J. Davidson for helpful advice on computer-based densitometric analysis of Western blots and J. Monkhouse for help in in vivo experiments.
Abbreviations
- EFS
electrical field stimulation
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- STZ
streptozotocin
References
- ANDERSSON K.E. The pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ANDERSSON K.E., WAGNER G. Physiology of penile erection. Physiol. Rev. 1995;75:191–235. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- AZADZOI K.M., SAEZ DE TEJADA I. Diabetes mellitus impairs neurogenic and endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J. Urol. 1992;148:1587–1591. doi: 10.1016/s0022-5347(17)36975-6. [DOI] [PubMed] [Google Scholar]
- BAYNES J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- BIAN K., DAVIS K., KURET J., BINDER L., MURAD F. Nitrotyrosine formation with endotoxin-induced kidney injury detected by immunohistochemistry. Am. J. Physiol. 1999;277:F33–F40. doi: 10.1152/ajprenal.1999.277.1.F33. [DOI] [PubMed] [Google Scholar]
- BRINDLEY G.S. Pilot experiments on the action of drugs injected into the human corpus cavernosum penis. Br. J. Pharmacol. 1986;87:495–500. doi: 10.1111/j.1476-5381.1986.tb10191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNETT A.L., LOWENSTEIN C.J., BREDT D.S., CHANG T.S.K., SNYDER S.H. Nitric oxide: a physiological mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- BUSH P.A., ARONSON W.J., BUGA G.M., RAJFER J., IGNARRO L.J. Nitric oxide is a potent relaxant of human and rabbit corpus cavernosum. J. Urol. 1992;147:1650–1655. doi: 10.1016/s0022-5347(17)37671-1. [DOI] [PubMed] [Google Scholar]
- CELLEK S., MONCADA S. Nitrergic control of peripheral sympathetic responses in the human corpus cavernosum: A comparison with other species. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8226–8231. doi: 10.1073/pnas.94.15.8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRIST G.J., KIM D.C., TAUB H.C., GONDRE C.M., MELMAN A. Characterization of nitroglycerine-induced relaxation in human corpus cavernosum smooth muscle: implications to erectile physiology and dysfunction. Can. J. Physiol. Pharmacol. 1995;73:1714–1726. doi: 10.1139/y95-735. [DOI] [PubMed] [Google Scholar]
- CLARKE B.F., EWING D.J., CAMPBELL I.W. Diabetic autonomic neuropathy. Diabetologia. 1979;17:195–212. doi: 10.1007/BF01235856. [DOI] [PubMed] [Google Scholar]
- DAVIES R.E., BASHFORT P.M., DOCHERTY R.J. A comparison of the effects of capsaicin with inhibitory nerve stimulation in the rat anococcygeus muscle in vitro. Eur. J. Pharmacol. 1998;355:195–202. doi: 10.1016/s0014-2999(98)00499-3. [DOI] [PubMed] [Google Scholar]
- DAWSON V.L., DAWSON T.M., LONDON E.D., BREDT D.S., SNYDER S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical culture. Proc. Natl. Acad. Sci. U.S.A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELABBADY A.A., GAGNON C., HASSOUNA M.M., BEGIN L.R., ELHILALI M.M. Diabetes mellitus increases nitric oxide synthase in penises but not in major pelvic ganglia of rats. Br. J. Urol. 1995;76:196–202. doi: 10.1111/j.1464-410x.1995.tb07674.x. [DOI] [PubMed] [Google Scholar]
- EL-SAKKA A.I., LIN C.S., CHUI R.M., DAHIYA R., LUE T.F. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int. J. Impot. Res. 1999;11:123–132. doi: 10.1038/sj.ijir.3900392. [DOI] [PubMed] [Google Scholar]
- FAERMAN I., GLOCER L., FOX D., JADZINSKY M.N., RAPAPORT M. Impotence and diabetes: Histological studies of the autonomic nervous fibers of the corpora cavernosa in important diabetic males. Diabetes. 1974;23:971–976. doi: 10.2337/diab.23.12.971. [DOI] [PubMed] [Google Scholar]
- FINBERG J.P.M., LEVY S., VARDI Y. Inhibition of nerve stimulation-induced vasodilatation in corpora cavernosa of the pithed rat by blockade of nitric oxide synthase. Br. J. Pharmacol. 1993;108:1038–1042. doi: 10.1111/j.1476-5381.1993.tb13502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMQUIST F., HEDLUND H., ANDERSSON K.E. Characterization of inhibitory neurotransmission in the isolated corpus cavernosum from rabbit and man. J. Physiol. 1992;449:295–311. doi: 10.1113/jphysiol.1992.sp019087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMQUIST F., STIEF C.G., JONAS U., ANDERSSON K.E. Effects of the nitric oxide synthase inhibitor NG-nitro-L-arginine on the erectile response to cavernous nerve stimulation in the rabbit. Acta Physiol. Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- HONING M.L., MORRISON P.J., BANGA J.D., STROES E.S., RABELINK T.J. Nitric oxide availability in diabetes mellitus. Diabetes. Metab. Rev. 1998;14:241–249. doi: 10.1002/(sici)1099-0895(1998090)14:3<241::aid-dmr216>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- KASAKOV L., CELLEK S., MONCADA S. Characterization of nitrergic neurotransmission during short- and long-term electrical stimulation of the rabbit anococcygeus muscle. Br. J. Pharmacol. 1995;115:149–1154. doi: 10.1111/j.1476-5381.1995.tb15017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM N., AZADOZOI K.M., GOLDSTEIN I., SAENZ DE TEJADA I. A nitric oxide-like factor mediates non-adrenergic non-cholinergic neurogenic relaxation of penile corpus cavenosum smooth muscle. J. Clin. Invest. 1991;88:112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOWLES R.G., SALTER M.Measurement of NOS activity by conversion of radiolabeled arginine to citrulline using ion-exchange separation Methods in Molecular Biology 1997Humana Press: Totowa; 67–73.Titheradge, M.A. (ed.) [DOI] [PubMed] [Google Scholar]
- KOLB K., KIESEL U., KRONCKE K.D., KOLB-BACHOFE V. Suppression of low dose streptozotocin-induced diabetes in mice by administration of a nitric oxide synthase inhibitor. Life Sci. 1991;49:PL213–PL217. doi: 10.1016/0024-3205(91)90296-n. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin. Exp. Pharmacol. Physiol. 1989;16:933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- LINCOLN J., CROWE R., BLACKLAY P.F., PRYOR J.P., LUMLEY J.S., BURNSTOCK G. Changes in the vipergic, cholinergic and adrenergic innervation of human penile tissue in diabetic and non-diabetic impotent males. J. Urol. 1987;137:1053–1059. doi: 10.1016/s0022-5347(17)44358-8. [DOI] [PubMed] [Google Scholar]
- LUE T., TANAGHO E. Physiology of erection and pharmacological management of impotence. J. Urol. 1987;137:829–836. doi: 10.1016/s0022-5347(17)44267-4. [DOI] [PubMed] [Google Scholar]
- LYALL F., GIBSON J.L., GREER I.A., BROCKMAN D.E., EIS A.L., MYATT L. Increased nitrotyrosine in the diabetic placenta: evidence for oxidative stress. Diabetes Care. 1998;21:1753–1758. doi: 10.2337/diacare.21.10.1753. [DOI] [PubMed] [Google Scholar]
- MELMAN A., HENRY D.P. The possible role of catecholamines of the corporal bodies in the penile erection. J. Urol. 1979;121:419–421. doi: 10.1016/s0022-5347(17)56805-6. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HIGGS E.A., FURCHGOTT R.F. International Union of Pharmacology Nomenclature in Nitric Oxide Research. Pharmacol. Rev. 1997;49:137–142. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NAVA E., SALAZAR F.J., BENTURA M.L., MURILLO R.M., FERNANDEZ A.P., SERRANO J., RODRIGO J. Intrarenal distribution of constitutive nitric oxide synthases and nitrotyrosine in spontaneous hypertension. Acta Physiol. Scand. 1999;167 Suppl:34. [Google Scholar]
- NAVARRO J., SANCHEZ A., SÁIZ J., RUILOPE L.M., GARCIA-ESTAN J., ROMERO J.C., MONCADA S., LAHERA V. Hormonal, renal and metabolic alterations during hypertension induced by chronic inhibition of NO in rats. Am. J. Physiol. 1994;267:R1516–R1521. doi: 10.1152/ajpregu.1994.267.6.R1516. [DOI] [PubMed] [Google Scholar]
- OKAMURA T., AYAJIKI K., FUJIOKA H., TODA M., FUJIMIYA M., TODA N. Effects of endothelial impairment by saponin on the responses to vasodilators and nitrergic nerve stimulation in isolated canine corpus cavernosum. Br. J. Pharmacol. 1999;127:802–808. doi: 10.1038/sj.bjp.0702623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.M.J., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PIOTROVSKIJ V.K., KALLAY Z., KREJCY K., HORECKY J., TRNOVEC T., RABERGER G. NG-nitro-L-arginine pharmacokinetics in rats after a single intravascular or oral dose: an appearance of secondary concentration time peaks. Drug. Metab. Dispos. 1993;21:962–964. [PubMed] [Google Scholar]
- RAKIETEN N., RAKIETEN M.L., NADKARNI M.V. Studies on the diabetogenic action of streptozotocin (NSC-37917) Cancer Chemother. Rep. 1963;29:91–98. [PubMed] [Google Scholar]
- REHMAN J., CHENVEN E., BRINK P., PETERSON B., WALCOTT W., WEN Y.P., MELMAN A., CHRIST G. Diminished neurogenic but not pharmacological erections in the 2- to 3-month experimentally diabetic F-344 rat. Am. J. Physiol. 1997;272:H1960–H1971. doi: 10.1152/ajpheart.1997.272.4.H1960. [DOI] [PubMed] [Google Scholar]
- RODRIGO J., RIVEROS-MORENO V., BENTURA M.L., UTTENTHAL L.O., HIGGS E.A., FERNANDEZ A.P., POLAK J.M., MONCADA S., MARTINEZ-MURILLO R. Subcellular localization of nitric oxide synthase in the cerebral ventricular system, subfornical organ, area postrema, and blood vessels of the rat brain. J. Comp. Neurol. 1997;378:522–534. doi: 10.1002/(sici)1096-9861(19970224)378:4<522::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- RODRIGO J., SPRINGALL D.R., UTTENTHAL O., BENTURA M.L., ABADIA-MOLINA F., RIVEROS-MORENO V., MARTINEZ-MURILLO R., POLAK J.M., MONCADA S. Localization of nitric oxide synthase in adult rat brain. Phil. Trans. R. Soc. Lond. B. 1994;345:175–221. doi: 10.1098/rstb.1994.0096. [DOI] [PubMed] [Google Scholar]
- RODRIGO J., UTTENTHAL L.O., PEINADO M.A., ESTEBAN F.J., FERNANDEZ A.P., SERRANO J., MARTINEZ DE VELASCO J., SANTACANA M., BENTURA M.L., MARTINEZ-MURILLO R., PEDROSA J.A. Distribution of nitric oxide synthase in the esophagus of the cat and monkey. J. Auton, Nerv. Syst. 1998;70:164–179. doi: 10.1016/s0165-1838(98)00053-8. [DOI] [PubMed] [Google Scholar]
- RUBIN A., BABBOTT D. Impotence and diabetes mellitus. J.A.M.A. 1958;168:498–500. doi: 10.1001/jama.1958.03000050010003. [DOI] [PubMed] [Google Scholar]
- SAENZ DE TAJADA I., BLANCO R., GOLDSTEIN I., AZADZOI K., DE LAS MORANES, KRANE R.J., COHEN R.A. Cholinergic neurotransmission in human corpus cavernosum. I. Responses of the isolated tissue. Am. J. Physiol. 1988;254:H468–H472. doi: 10.1152/ajpheart.1988.254.3.H459. [DOI] [PubMed] [Google Scholar]
- SAENZ DE TEJADA I., GOLDSTEIN I., AZADZOI K.M., KRANE R.J., COHEN R.A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. New Engl. J. Med. 1989;320:1025. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- SCHIRAR A., GIULIANO F., RAMPI O., ROUSSEAU J.P. A large proportion of pelvic neurons innervating the corpora cavernosa of the rat penis exhibit NADPH-diaphorase activity. Cell Tissue Res. 1994;278:517–525. doi: 10.1007/BF00331369. [DOI] [PubMed] [Google Scholar]
- TABRIZI-FARD M.A., FUNG H.L. Pharmacokinetics, plasma protein binding and urinary excretion of NG-nitro-L-arginine in rats. Br. J. Pharmacol. 1994;111:394–396. doi: 10.1111/j.1476-5381.1994.tb14747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUB H.C., LERNER S.E., MELMAN A., CHRIST G.J. Relationship between contraction and relaxation in human and rabbit corpus cavernosum. Urology. 1993;42:698–704. doi: 10.1016/0090-4295(93)90538-l. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA C.E., BENTO A.C., LOPES-MARTINS R.A., TEIXEIRA S.A., VON EICKESTEDT V., MUSCARA M.N., ARANTES E.C., GIGLIO J.R., ANTUNES E., DE NUCCI G. Effect of Tityus serrulatus scorpion venom on the rabbit isolated corpus cavernosum and the involvement of NANC nitrergic nerve fibres. Br. J. Pharmacol. 1998;123:435–442. doi: 10.1038/sj.bjp.0701623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRIGO-ROCHA F., ARONSON W.J., HOHENFELLNER L.J., IGNARRO L.J., RAJFER L.J., RAJFER J., LUE T.F. Nitric oxide and cGMP: mediators of pelvic nerve-stimulated erection in dogs. Am. J. Physiol. 1993;264:H419–H422. doi: 10.1152/ajpheart.1993.264.2.H419. [DOI] [PubMed] [Google Scholar]
- UTTENTHAL L.O., ALONSO D., FERNANDEZ A.P., CAMPBELL R.D., MORO M.A., LEZA J.C., LISAZOAIN I., ESTEBAN F.J., BARROSO J.B., VALDERRAMA R., PEDROSA J.A., PEINADO M.A., SERRANO J., RICHARD A., BENTURA M.L., SANTACANA M., MARTINEZ-MURILLO R., RODRIGO J. Neuronal and inducible nitric oxide synthase and nitrotyrosine immunoreactivities in the cerebral cortex of the aging rat. Microsc. Res. Tech. 1998;43:75–88. doi: 10.1002/(SICI)1097-0029(19981001)43:1<75::AID-JEMT11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- VERNET D., CAI L., GARBAN H., BABBITT M.L., MURRAY F.T., RAJFER J., GONZALEZ-CADAVID N.F. Reduction of penile nitric oxide synthase in diabetic BB/WORdp (Type I) and BBZ/WORdp (Type II) rats with erectile dysfunction. Endocrinology. 1995;136:5709–5717. doi: 10.1210/endo.136.12.7588327. [DOI] [PubMed] [Google Scholar]
- WAGNER G., BRINDLEY G.S.The effect of atropin and α- and β-blockers on human penile erection: a controlled pilot study Vasculogenic Impotence 1980Thomas: Springfield, IL; 77–81.Zorgiotti, A.W. & Rossi, G. (eds.) [Google Scholar]


