Abstract
The adenosine receptor subtype mediating adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP) formation and the effect of its activation on endothelin-1 (ET-1) secretion were studied in primary cultures of tracheal epithelial cells.
Adenosine analogues showed the following rank order of potency (pD2 value) and intrinsic activity on the generation of cyclic AMP by tracheal epithelial cells: 5′-N-ethylcarboxyamidoadenosine (NECA, A1/A2A/A2B, pD2: 5.44±0.16)>adenosine (ADO, non selective, pD2: 4.99±0.09; 71±9% of NECA response) ⩾2-Cl-adenosine (2CADO, non selective, pD2: 4.72±0.14; 65±9% of NECA response)>>>CGS21680 (A2A; inactive at up to 100 μM).
Cyclic AMP formation stimulated by NECA in guinea-pig tracheal epithelial cells was inhibited by adenosine receptor antagonist with the following order of apparent affinity (pA2 value): Xanthine amine congeners (XAC, A2A/A2B, 7.89±0.22)>CGS15943 (A2A/A2B, 7.24±0.26)>ZM241385 (A2A, 6.69±0.14)>DPCPX (A1, 6.51±0.14)>3n-propylxanthine (weak A2B, 4.30±0.10). This rank order of potency is typical for A2B-adenosine receptor.
Adenosine decreased basal and LPS-stimulated irET production in a concentration-dependent manner. Moreover, NECA but not CGS21680 inhibited LPS-induced irET production.
The inhibitory effect of NECA on LPS-induced irET production was reversed by XAC (pA2=8.84±0.12) and DPCPX (pA2=8.10±0.22).
These results suggested that adenosine increased cyclic AMP formation and inhibited irET production/secretion by guinea-pig tracheal epithelial cells through the activation of a functional adenosine receptor that is most likely the A2B subtype. This adenosine receptor may be involved in the regulation of the level of ET-1 production/secretion by guinea-pig tracheal epithelial cells in physiological as well as in pathophysiological conditions.
Keywords: Adenosine, adenosine receptors, adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP), A2B-adenosine receptor, endothelin-1, airway, epithelium, trachea
Introduction
The function of the airway epithelium is modulated by the action of multiple molecules acting through specific receptors present on epithelial cells. One of these factors is adenosine which acts through a family of receptors called P1-purinoceptors. This family has been divided into four subclasses called A1, A2A, A2B and A3, all of which are seven-transmembrane domain proteins coupled to regulatory G-proteins that inhibit (A1 and A3 subtypes) or stimulate (A2A and A2B subtypes) adenosine 3′ :5 ′-cyclic monophosphate (cyclic AMP) formation (Fredholm et al., 1994). The latter classes of receptors may be distinguished on the basis of the potency of agonists and antagonists, and by the lack of activity of the selective A2A-adenosine receptor agonists and antagonists. The non-selective A1/A2A/A2B-adenosine receptor agonist, N-ethylcarboxyamidoadenosine (NECA) and a selective A2A-adenosine receptor agonist, CGS21680, have been widely used as tools to discriminate the effects mediated by the two Gs-coupled receptor (Feoktistov & Biaggioni, 1993; 1995; Alexander et al., 1996; Cooper et al., 1997). On human platelet A2A-adenosine receptors, CGS21680 and NECA showed similar potency in activating cyclic AMP formation (Feoktistov & Biaggioni, 1993). In contrast, on other cell preparations which express A2B-adenosine receptors, CGS21680 was inactive whereas NECA was shown to be a potent agonist (Feoktistov & Biaggioni, 1993; 1995; 1997; Alexander et al., 1996; Cooper et al., 1997). Selective A2A-adenosine receptor antagonists have been used to characterize the effects mediated by A2A-adenosine receptors, and to discriminate for the presence or absence of this receptor subtype. The lack of selective A2B-adenosine receptor antagonist has made difficult the characterization of this subtype, but the use of a number of adenosine agonists and antagonists has allowed an acceptable characterization of this receptor population (Alexander et al., 1996, Cooper et al., 1997). Furthermore, the antiasthmatic drug, enprofylline (3-n-propylxanthine), a weak A1/A2 receptor antagonist, was also shown to block the A2B-adenosine receptor (Casadó et al., 1992; Feoktistov & Biaggioni, 1993; 1997). Xanthine amine congener (XAC) and CGS15943 were also shown to be potent A2A/A2B-adenosine receptor antagonists (Fredholm et al., 1994; Peakman & Hill, 1994; Alexander et al., 1996; Cooper et al., 1997), whereas a selective A2A-adenosine receptor antagonist, ZM241385 (Poucher et al., 1995), and a selective A1-adenosine receptor antagonist, DPCPX (Poucher et al., 1995), appeared as poor antagonists at A2B-receptors and, therefore, the affinity of these antagonists can be useful to discriminate between effects mediated by A1, A2A or A2B-adenosine receptors.
The presence of adenosine receptors has been reported in various epithelia (Spinowitz & Zadunaisky, 1979; Dobbins et al., 1984; Pratt et al., 1986; Schwiebert et al., 1990; Barrett et al., 1990). In human airway epithelium, the presence of an A2-adenosine receptor which increases cyclic AMP generation and modulates chloride secretion was reported (Lazarowski et al., 1992) However, the subtype mediating this effect was not well characterized. In selected cell populations, the activation of Gs-coupled receptors has been shown to modulate the production of several mediators including that of endothelin-1 (ET-1) (Sakamoto et al., 1992; Durieu-Trautman et al., 1993; Prins et al., 1994; Patel et al., 1997). A better understanding of the regulation of the production of this peptide in the pulmonary system is of interest since ET-1 has been implicated in various physiological and pathological functions (reviewed by Battistini et al 1993). These include (1) modulating chloride secretion by tracheal epithelial cells (Plews et al., 1991; Satoh et al., 1992), (2) promoting mitogenesis of airway smooth muscle cells (Noveral et al., 1992; Glassberg et al., 1994), (3) inducing activation of alveolar macrophages, that subsequently increase eicosanoid release (Millul et al., 1991), and (4) increasing superoxide production (Haller et al., 1991). Moreover, ET-1 is recognized as a potent constrictor of airway smooth muscle (Inui et al., 1994) that has been implicated in pulmonary hypertension (Uchida et al., 1988; Cernacek & Stewart, 1989; Horgan et al., 1991), asthma (Boichot et al., 1991) and cystic fibrosis (Plews et al., 1991).
In the present investigation, the effects of adenosine on basal and LPS-induced irET production/secretion by guinea-pig tracheal epithelial cells were studied. The adenosine receptors mediating cyclic AMP formation and the inhibition of irET production/secretion by these cells were also characterized.
Methods
Animals
Male Dunkin Hartley guinea-pigs (300–350 g) were obtained from Charles River Laboratory (St-Constant, Québec, Canada). The animals were killed by cervical dislocation according to the guidelines of the Canadian Council on Animal Care. The trachea was harvested under sterile conditions and dissected into Krebs-Heinseleit physiological solution.
Isolation and cell culture
Guinea-pig tracheal epithelial cells were obtained following a 1 h incubation of the trachea at 37°C with a solution of 0.15% protease type XXIV in Krebs-Heinseleit buffer, according to a previously described procedure (White et al., 1993). The cells were then mechanically removed from the mucosal surface of the trachea by gentle scraping with a policeman. They were centrifuged and washed twice with 5 ml of culture medium, DMEM-F12, containing 10% foetal bovine serum (FBS), penicillin (100 u·ml−1), streptomycin (0,08%) and fungizone (1%). The cells were resuspended in 10 ml of medium, counted and seeded at a concentration of 4–5×105 cells·ml−1·well−1 in a 24 wells culture plate or 1.2–1.5×106 cells··ml−1··well−1 in 6 wells culture plate. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. The medium was renewed after 48 h with the same medium and changed every 24 h with a serum free medium until the cells reached confluency after 5 days. Confluent cells were used in all experiments.
irET production/secretion
Before the experiments, the DMEM-F12 medium was aspirated from the 24-well plates and 1 ml of fresh medium was added. The cells were incubated at 37°C with the selected pharmacological agents in a humidified atmosphere of 5% CO2 for 24 h. At the end of the incubation period, the culture media were collected and stored at −20°C until measurement of immunoreactive-ETs (irETs). Triton X-100 (0.1%; 0.5 ml) was added to each well and, following an overnight incubation at 4°C, total proteins were assayed in the Triton X-100 (0.1%) using the Bio-Rad protein assay.
Measurement of immunoreactive-ETs by radioimmunoassay
The concentrations of ir-ETs were measured by radioimmunoassay (RIA) using a commercially available kit as previously described (Laporte et al., 1996). Briefly, fixed amounts of samples or standards, antiserum, and tracer (125I-ET-3) were mixed together. The assay is based on the competition between unlabelled ETs (sample) and a fixed quantity of 125I-ET-3 for a limited number of binding sites on an ET-specific antibody. The amount of radioactive ligand bound by the antibody is inversely proportional to the concentration of added non-radioactive ligand. The antibody- bound fraction was separated with magnetic beads. The detection limit of the assay was 0.5 fmol··tube−1. The ET-1 antiserum cross-reacts with ET-1 (100%), ET-2 (144%), ET-3 (52%), and big ET-1 (0.04%). The amounts of each aliquot was expressed in picograms per milligram of total proteins.
Cyclic AMP formation
Intracellular cyclic AMP levels were determined by measuring the conversion of [3H]-ATP into [3H]-cyclic AMP as described by Weiss et al., (1985). Briefly, after 5 days of culture in 6 well plates, cells were washed and incubated for 2 h at 37°C in DMEM-F12 medium containing 2 μCi [3H]-Adenine·ml-1. The cells were washed twice with Hanks Buffered salt solution (HBSS) containing 1M glucose and pre-incubated in the presence or absence of selected antagonists and inhibitors for 15 min in a solution of HBSS-Bovine serum albumin (BSA) containing 10 μM Rolipram, a selective phosphodiesterase IV inhibitor. Adenosine and congeners were then added to the incubation medium for 5 min at 37°C. The reaction was ended by the addition of 100 μl of ice-cold 50% Perchloric acid (PCA) (final concentration of 5%) and putting the 6 well plates on ice. Cells were scraped with a rubber policeman and 100 μl of ice-cold solution of ATP and cyclic AMP (5 mM of each) were added to the cellular extract. The samples were vortexed and centrifuged 15 min at 3000 r.p.m. at 4°C. The supernatants were sequentially chromatographed on Dowex and alumina columns allowing the separation of [3H]-ATP from [3H]-cyclic AMP. Cyclic AMP formation was expressed as: precent of conversion of [3H]-ATP to [3H]-cyclic AMP and calculated using the following formula ([3H]-cyclic AMP/ ([3H]−ATP+[3H]-cyclic AMP))×100.
Protein assay
Guinea-pig tracheal epithelial cells in each well were disrupted overnight with 0.1% Triton detergent. A 10 μl aliquot was mixed with 200 μl of the Bio-Rad protein assay reagent and incubated for 15 min at room temperature to evaluate the concentration of cell proteins. The concentration of proteins was determined by measuring the absorbance at 590 nm, using a standard curve of bovine serum albumin (25–400 μg··ml−1).
Chemicals and drugs
The following chemical and drugs were used: Culture medium (DMEM-F12), fetal bovine serum (FBS) and antibiotics (Penicillin, Streptomycin and fungizone) (Gibco, New-York, U.S.A.); Protease Type XXIV, Adenosine, ATP, cyclic AMP, Bovine serum albumin, Adenosine deaminase, Imidazol and Alumina (Sigma Chemicals Co., St-Louis, MO, U.S.A.); The Bio-Rad protein assay kit, Dowex 1×8 (100–200 mesh) (Bio-Rad, Mississauga, ON, Canada); [3H]-Adenine, ETs Radioimmunoassay kits (Amersham, Oakville, ON, Canada); Rolipram, 2-Cl-adenosine (2CADO), Xanthine amine congener (XAC), CPA (N6-cyclopenthyladenosine), CGS15943 (5-amino - 9 - chloro - 2 - (2 - furyl)1,2,4 - triazol[1,5-c]quinazoline), NECA (5′-N-ethylcarboxamidoadenosine), DPCPX (8-cyclopentyl-1,3-diprylxanthine), and enprofylline (3-n-propylxanthine) (RBI, Natik, CA, U.S.A.). CGS21680 (2-(p-(-carboxyethyl)-phenethylamino)-5′-N-ethylcarboxamidoadenosine) (generously given by Dr Arco Y. Jeng from Novartis, U.S.A.); ZM241385 (4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)phenol) (generously given by Dr Simon M. Poucher from Zeneca Pharmaceuticals., U.K.).
Adenosine receptor antagonists were dissolved to 10 mM in DMSO. NECA and CGS21680 were also dissolved in DMSO to 50 and 100 mM respectively. Adenosine and 2 CADO were dissolved in medium at 10 mM.
Data analysis and statistics
Sigmoidal curves were fitted to concentration-response data, following the substraction of the basal levels of cyclic AMP, by the use of the computer programs Microcal™ (Microcal software, MA, U.S.A.) to generate estimates of EC50 and Emax values: Response=(Emax Xn)/(EC50+Xn), where X is the agonist concentration, Emax is the maximal response, n is the Hill coefficient and EC50 is the concentration of agonist producing half maximal stimulation. Antagonist IC50 values were calculated from the same equation to fit a curve for data points of increasing concentrations of antagonists in the presence of a fixed concentration of NECA (10 μM). Antagonist pA2 values were calculated from the IC50 by the use of the null method described by Lazareno & Roberts (1987) and commonly used by other research groups (Alexander et al., 1996; Cooper et al., 1997): pA2=−log[IC50/(C/C′−1)], where C is the NECA concentration (i.e. 10 μM) and C′ is the NECA concentration evoking 50% of the response achieved at C, in the absence of antagonist.
The results are expressed as the mean±s.e.mean of n determinations made with different cell preparations. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by a Dunnet's multiple comparison test when comparing two or more treatments vs control or Bonferroni post test when comparing two or more treatments. P values less than 0.05 were considered significant.
Results
Effect of adenosine deaminase on the generation of cyclic AMP
To determine the effect of the endogenous adenosine on the cyclic AMP formation, the effect of adenosine deaminase (ADA; 1.5 ug·ml−1) on the generation of cyclic AMP was assayed. ADA decreased basal formation of cyclic AMP by 15% (0.19±0.01% conversion compared to 0.16±0.01% in the presence of ADA). Since a very small decrease was observed, the subsequent experiments were performed without ADA unless where indicated.
Effect of adenosine and adenosine analogues on the cyclic AMP formation
The production of cyclic AMP by tracheal epithelial cells increased in the presence of increasing concentrations of adenosine (Figure 1a). The basal generation of cyclic AMP in the presence of Rolipram (1×10−5 M) was 0.068±0.003%. Following stimulation of tracheal epithelial cells with adenosine at concentrations of 1×10−7 to 1×10−3 M, a concentration dependent increase was noted and the maximal effect was observed at the concentration of 1×10−3 M where the conversion of [3H]-ATP to [3H]-cyclic AMP reached 0.195±0.04%. 2CADO and NECA (1×10−7 to 1×10−3 M), two adenosine analogues, also produced concentration-dependent increases in the conversion of [3H]-ATP to [3H]-cyclic AMP whereas the selective A2A receptor agonist, CGS 21680, was inactive at concentrations up to 100 μM (Figure 1b). The rank order of pD2 values for these compounds on cyclic AMP formation was: NECA (5.44 ± 0.16) > adenosine (4.9 ± 0.09) ⩾ 2CADO (4.72 ± 0.14) > > > CGS21680 (>100 μM) and the maximal effect of adenosine and 2CADO was 71 ± 9% and 65 ± 9% of the maximal effect produced by NECA (Figure 1b).
Figure 1.
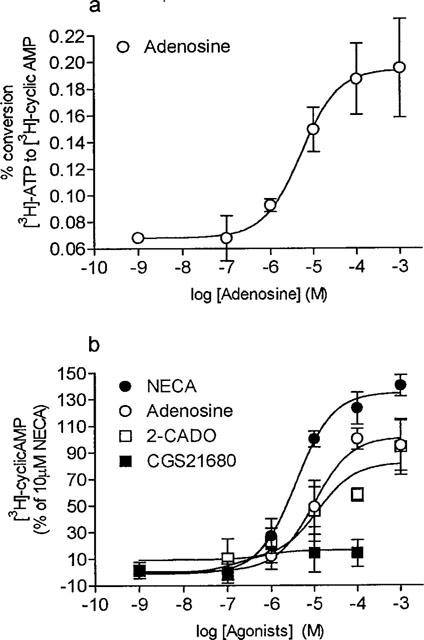
Cyclic AMP accumulation in guinea-pig tracheal epithelial cells in the presence of adenosine and adenosine analogues. Cultured cells were preincubated in the presence of Rolipram (10 μM) for 15 min and thereafter stimulated for 5 min with increasing concentrations of (a) adenosine. Each point represents the mean±s.e.mean of four determinations made with different cell preparations. (b) Cultured cells were preincubated in the presence of Rolipram (10 μM) for 15 min and thereafter stimulated for 5 min with increasing concentrations of NECA, 2CADO, adenosine and CGS21680. Values are the mean±s.e.mean of 4–8 determinations made with different cell preparations, expressed as a percentage of the response to 100 μM adenosine. See Methods for further details.
Effect of adenosine receptor antagonists on the production of cyclic AMP evoked by NECA
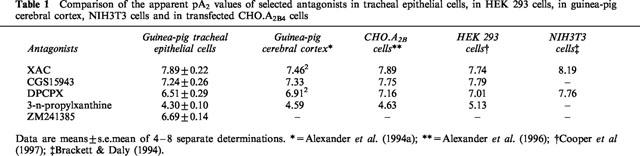
The cyclic AMP accumulation in guinea-pig tracheal epithelial cells by sub-maximal concentrations of NECA (10 μM) were inhibited in the presence of selective and non-selective antagonists of A1, A2A and A2B-adenosine receptors (Figure 2). The rank order of potency based on apparent pA2 values was XAC (7.89±0.22)>CGS15943 (7.24±0.26)>ZM241385 (6.69±0.14)>DPCPX (6.51±0.29)>3-n-propylxanthine (4.30±0.10) (Table 1). A strong correlation was found between the apparent pA2 values obtained for these antagonists in guinea-pig tracheal epithelial cells and the pA2 obtained in other published studies with cells expressing the A2B-adenosine receptors (Table 1).
Figure 2.
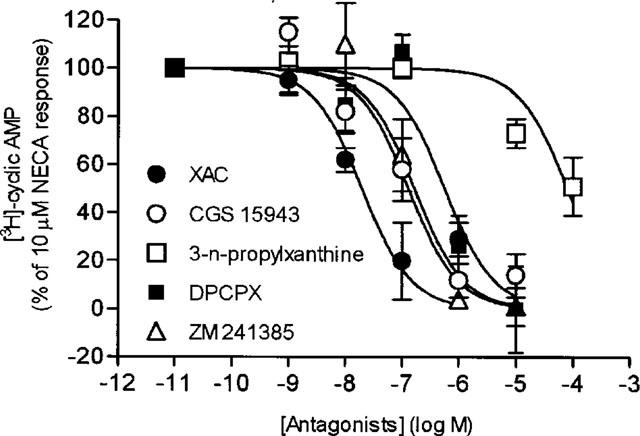
Effect of selective and non-selective adenosine receptor antagonists on the NECA evoked production of cyclic AMP. Cultured cells were preincubated 15 min in the presence of increasing concentrations of XAC, CGS15943, DPCPX, ZM241385 and 3-n-propylxanthine and thereafter stimulated with NECA (10 μM) for 5 min and the reaction was ended as described in Methods. Each point represents the mean±s.e.mean of 4–8 determinations made in different cell preparations, expressed as a percentage of the response of 10 μM NECA.
Table 1.
Comparison of the apparent pA2 values of selected antagonists in tracheal epithelial cells, in HEK 293 cells, in guinea-pig cerebral cortex, NIH3T3 cells and in transfected CHO.A2B4 cells
Forskolin-stimulated cyclic AMP generation
Since A1 adenosine receptors are commonly associated with inhibition of adenylyl cyclase, the possible functional linkage of this type of receptor with the regulatory Gi protein in guinea-pig tracheal epithelial cells was investigated using an adenosine analogue that selectively activates A1 adenosine receptors (N6-cyclopentyladenosine: CPA) and forskolin as a stimulus for cyclic AMP formation. When the cells are stimulated with 10 μM forskolin in the presence of ADA (1,5 ug·ml−1), a 4 fold increase in cyclic AMP generation was observed (data not shown). In the presence of CPA (1×10−6 M), a further significant increase of cyclic AMP was observed in the cells (124±6% of forskolin responses P<0.05) but no effect was noted at concentration of 1×10−7M (Figure 3).
Figure 3.
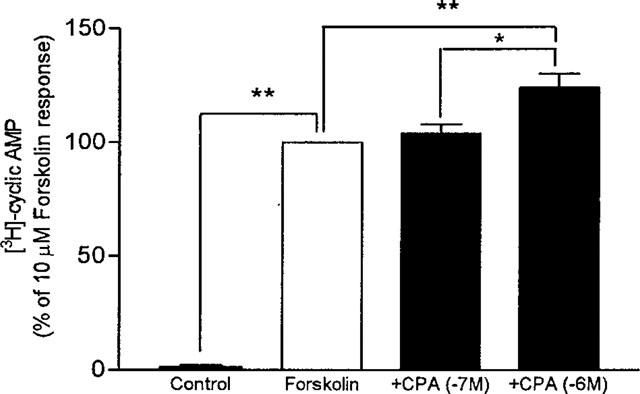
Effect of N6-cyclopentyladenosine (CPA) on Forskolin-stimulated cyclic AMP accumulation in guinea-pig tracheal epithelial cells. Cells were preincubated 15 min in the presence of Rolipram (10 μM) and adenosine deaminase (1.5 u·ml−1) and thereafter stimulated for 5 min in the absence (Control) or in the presence of 10 μM forskolin alone (Forskolin) or with forskolin and increasing concentrations of CPA (+CPA). Values are the mean±s.e.mean of four determinations made with different cell preparations, expressed as percentage of the response to 10 μM forskolin. *P<0.05 and **P<0.01.
Effect of adenosine and adenosine analogues on basal and LPS-induced irET production/secretion
Basal production/secretion of irET by guinea-pig tracheal epithelial cells reached 12000 pg·mg−1 of total proteins after a 24 h incubation period (Figure 4a). In the presence of adenosine (1×10−8 to 1×10−3 M), a concentration-dependent decrease in the production/secretion of irET was observed and the maximal effect was reached at a concentration of 1×10−3 M where the production/secretion was 7345± 366 pg·mg−1 of total proteins, which corresponded to a decrease of 38% of the basal level (P<0.05, ANOVA followed by Bonferroni post test). A strong correlation between the inhibitory effect of adenosine on irET production/secretion and the stimulatory effect of adenosine on cyclic AMP generation was noted with a slope of 0.96 and a r coefficient of 0.98 (data not shown). Basal production/secretion of irET by guinea-pig tracheal epithelial cells (12769±1320 pg·mg−1 of total proteins) increased by 2.5 fold in the presence of LPS (10 μg·ml−1) during a 24 h incubation period and reached 32973±4248 pg·mg−1 of total proteins. In the presence of adenosine, a concentration-dependent inhibition of the LPS-stimulated irET production/secretion was observed and at the concentration of 100 μM, adenosine abolished the effect of LPS on the irET production/secretion (Figure 4b). NECA, but not CGS21680, inhibited LPS-stimulated irET production/secretion in a concentration-dependent manner with an IC50 value of 36±27 nM (Figure 5a,b). Its maximal effect was observed at a concentration of 1 μM and it abolished the LPS-induced irET production/secretion.
Figure 4.

Effect of adenosine on basal (a) and lipopolysaccharide-induced (b) immunoreactive ET-1 production/secretion by tracheal epithelial cells. Cultured guinea-pig tracheal epithelial cells were pretreated or not (Control) with LPS (10 μg·ml−1) during 30 min and therefore incubated 24 h in the presence or absence (control, LPS) of increasing concentrations of adenosine and the irET was measured as described in Methods. Values are the means±s.e.mean of four determinations made with different cell preparations. (f P<0.05 vs LPS, **P<0.01 vs Control).
Figure 5.
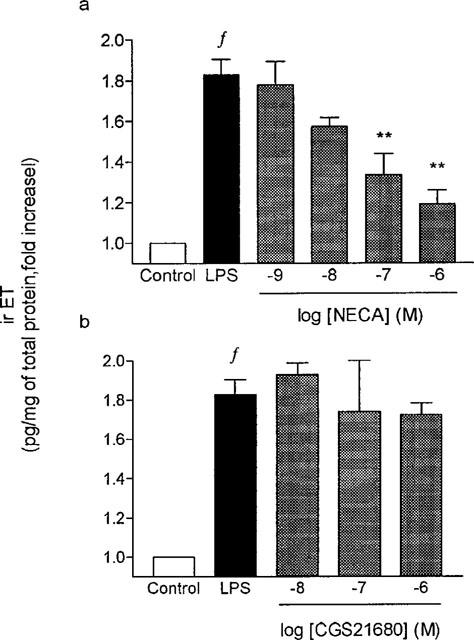
Effect of adenosine analogues on LPS-induced irET production/secretion by guinea-pig tracheal epithelial cells. Cultured cells were preincubated in the presence or absence of increasing concentrations of NECA (a) or CGS21680 (b) during 30 minutes and then incubated with or without LPS (10 ug·ml−1 LPS) for 24 h and the irET was measured as described in methods. Values are means±s.e.mean of 3–4 determinations made in different cell preparations. (**P<0.01 vs LPS and f P<0.01 vs Control).
Effect of adenosine receptor antagonists on NECA-induced inhibition of irET production/secretion
The inhibition of LPS-induced irET production/secretion evoked by NECA was reversed by the presence of increasing concentrations of XAC and DPCPX (1×10−8 to 1×10−6 M). XAC is more potent than DPCPX with apparent pA2 values of 8.36±0.29 and 7.58±0.39 (Figure 6a,b). CGS15943 (1×10−7 M) also reversed the inhibitory effect of NECA and seemed to be more potent than DPCPX (data not shown).
Figure 6.
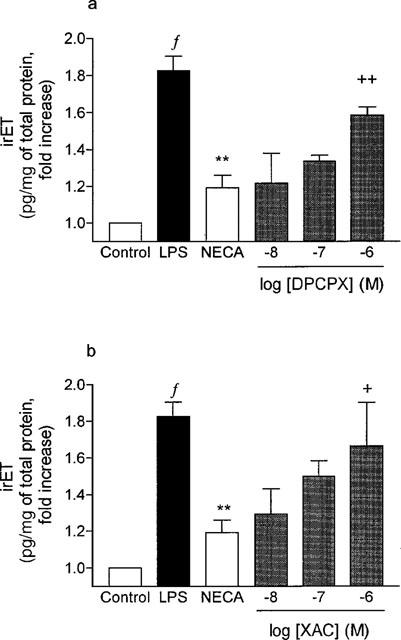
Effects of selective adenosine receptor antagonists on the inhibition of LPS-induced irET production/secretion evoked by NECA. Cultured guinea-pig tracheal epithelial cells were first incubated in the presence of (a) DPCPX (1×10−8 to 1×10−6 M) or (b) XAC (1×10−8 to 1×10−6 M) for 30 min. Thereafter, the cells were incubated in the presence or absence (Control, LPS) of NECA (1 μM) for another 15 min before being stimulated or not (Control) by LPS (LPS, NECA and −8, −7, −6) during 24 h. IrET was measured as described in Methods. Values are means±s.e.mean of 3–4 determinations made in different cell preparations. (f P<0.01 vs Control, **P<0.01 vs LPS treated cells and +P<0.05 and ++P<0.01 vs NECA).
Discussion
In this investigation, cyclic AMP generation and inhibition of irET production/secretion by adenosine were studied in guinea-pig tracheal epithelial cells maintained in tissue culture. The results presented show that adenosine increases the cyclic AMP formation, most likely through the activation of epithelial A2B-adenosine receptors. The first line of evidence was given by the observation that adenosine increases the generation of cyclic AMP in a concentration-dependent manner and the rank order of potency of adenosine analogues in stimulating the generation of adenylyl cyclase was NECA > adenosine ⩾ 2CADO>>>CGS21680 (ineffective at concentrations up to 100 μM). These findings are consistent with previous studies where the same rank order of potency was observed for the human A2B-adenosine receptor that was cloned and transfected in CHO cells, the A2B-receptor present in guinea-pig cerebral cortex or the one endogenously expressed in HEK293 cells (Alexander et al., 1996; Cooper et al., 1997). Furthermore, the lack of activity of the selective A2A receptor agonist CGS21680 (Lupica et al., 1990; Feoktistov & Biaggioni, 1993) is an accepted indication of the absence of an A2A-adenosine receptor which is the only other form of adenosine receptor coupled to a regulatory Gs-protein (Fredholm et al., 1994).
Characterization of receptors based on agonist affinity is far from ideal since the response elicited is not only dependent of the ligand binding to the receptors but also on multiple processes involved in the transduction pathway (Kenakin, 1987). The second line of evidence which suggested that adenosine acts through an A2B-receptor is given by the rank order of potency of adenosine receptor antagonists on the cyclic AMP accumulation evoked by NECA. The rank order of potency found in our study i.e. XAC>CGS15943>ZM241385>DPCPX>>3-n-propylxanthine is the same as those observed in previous studies characterizing the A2B-adenosine receptor in other experimental models (Alexander et al., 1996; Cooper et al., 1997). The relatively high affinity of xanthine derivatives at antagonizing cyclic AMP accumulation evoked by NECA indicated that the receptors present in the tracheal epithelial cells were not related to A3-adenosine receptors since these compounds have low affinities at A3 receptors (Fredholm et al., 1994). The low affinity of the selective A1-adenosine receptor antagonist, DPCPX (Bruns et al., 1987), also suggested that the receptor present in tracheal epithelial cells was not of the A1 subtype. Furthermore, the low affinity of ZM241385, a selective A2A-adenosine receptor antagonist (Poucher et al., 1995), also suggested that the receptors expressed in these cells were not A2A-receptors. The selective but of low affinity A2B-adenosine receptor antagonist, 3-n-propylxanthine (Feoktistov & Biaggioni, 1995), antagonized the NECA-evoked cyclic AMP formation with a relatively low affinity in our experimental model, which is also a characteristic feature of this receptor.
Our results showed that the non selective adenosine receptor agonist NECA had the highest potency for stimulating cyclic AMP generation by tracheal epithelial cells (pD2 value 5.44). Similar values were reported for the A2B-adenosine receptor in guinea-pig cerebral cortex and for the putative A2B-adenosine receptor cloned from human brain (5.9; Alexander et al., 1996), on HEK293 (5.29; Cooper et al., 1997), in rat primary astrocytes (6.0; Peakman & Hill, 1994), in guinea-pig cerebellum (6.22; Brackett & Daly, 1994), in guinea-pig cerebral cortex (5.5; Alexander et al., 1994b), in NIH3T3 cells (6.34; Peakman & Hill, 1994) and finally on guinea-pig aorta (6.16; Alexander et al., 1994b). Comparable results were observed with the two other non selective adenosine receptor agonists, adenosine and 2CADO. The pD2 values of these two agonists were shown to be 4.19 and 4.41 on HEK 293 endogenously expressing the A2B-adenosine receptor (Cooper et al., 1997), 5.69 and 5.27 in CHO cells expressing the A2B-adenosine receptor cloned from human brain (Alexander et al., 1996), 3.99 and 4.35 in guinea-pig cerebral cortex (Alexander et al., 1994a), 4.88 and 5.22 on guinea-pig cerebellum (Hernandez et al., 1993). In many experiments reported on A2B-adenosine receptors, the selective A2A agonist, CGS21680, was inactive (Casadó et al., 1992; Hernandez et al., 1993; Peakman & Hill, 1994; Feoktistov et al., 1994; Brackett & Daly, 1994; Alexander et al., 1994b, 1996; Strohme et al., 1995; Feoktistov & Biaggioni, 1995; Fiebich et al., 1996; Dubey et al., 1996; Cooper et al., 1997).
Linear regression of estimated apparent pA2 values obtained from tracheal epithelial cells (Table 1) compared to the estimated apparent pA2 of the guinea-pig cerebral cortex provided a slope of 1.14 and a r coefficient of 0.98 indicating the high degree of similarity between these two receptors. A good correlation was also observed with pA2 apparent affinities obtained in our experiments and on the HEK293 cells endogenously expressing human A2B adenosine receptor, and a slope of 1.23 and a r coefficient value of 0.98 were obtained.
The results presented herein also suggested that the A1-adenosine receptor was not present on tracheal epithelial cells since no inhibitory effect on cyclic AMP was produced by CPA at concentrations that are reported to stimulate this receptor (Jacobson et al., 1992; Alexander et al., 1994a). Inhibition of forskolin-induced cyclic AMP generation was reversed by CPA at concentrations of 1×10−8 M, 1×10−7 and 1×10−6 M in guinea-pig cerebral cortex and rat primary astrocytes (Peakman & Hill, 1994; Alexander et al., 1994a). In contrast, our results suggested that the stimulatory effect of CPA at the concentration of 1×10−6 M was probably due to the activation of A2B-adenosine receptor. Activation of cyclic AMP generation by A1-adenosine receptor agonists was also reported in guinea-pig cerebral cortex where high concentrations of CPA, 2-chloro-N6-cyclopentyladenosine (CCPA) and R-N6-phenylisopropyladenosine (R-PIA) stimulated the cyclic AMP generation presumably through the A2B-adenosine receptor (Alexander et al., 1994a). However, since potent and selective A2B-adenosine receptor antagonists are not available, it was not possible to assess unequivocally that these effects are mediated by this receptor.
Our results also showed that adenosine inhibited in a concentration-dependent manner the basal and stimulated irET production/secretion. The non-selective adenosine receptor agonist, NECA, also inhibited the effect of LPS on irET production/secretion whereas CGS21680 failed. The lack of activity of CGS21680 is an accepted indication of the absence of an A2A-adenosine receptor (Lupica et al., 1990; Feoktistov & Biaggioni, 1993). Furthermore, our results showed that A1 adenosine receptor subtype is absent in our cell preparation. These pieces of evidence strongly suggest a role for the A2B receptor subtype. The antagonist profile suggested similar conclusion. The non-selective A2A/A2B adenosine receptor antagonist, XAC, and the selective A1-adenosine receptor antagonist, DPCPX, both reversed the inhibitory effect of NECA, but DPCPX is less potent than XAC. This is consistent with cyclic AMP assays presented in this article and other investigations of A2B receptor subtypes (Brackett & Daly, 1994, Alexander et al., 1994b; 1996; Cooper et al., 1997; Peakman & Hill, 1994. In several studies, Gs-coupled receptor was also shown to decrease ET-1 production/secretion (Sakamoto et al., 1992; Prins et al., 1994; Razandi et al., 1996).
Finally, our findings showed that adenosine stimulated the generation of cyclic AMP through an endogenously expressed A2B-adenosine receptor in guinea-pig tracheal epithelial cells. Activation of this receptor also lead to the inhibition of irET production/secretion. These pieces of evidence suggest a role for a cyclic AMP dependent pathway in the inhibition of irET production/secretion, by adenosine in guinea-pig tracheal epithelial cells. Vanio et al., 1996 demonstrated that adenosine increases the production/secretion of ET-1 through the activation of an A1-adenosine receptor in FRLT-5 thyroid cells. This observation is consistent with our findings since increasing the level of cyclic AMP reduced the production/secretion of the peptide and the activation Gi-coupled receptors can be responsible for mediating the increase of production/secretion of this peptide. These authors also showed that the A1-adenosine receptors also lead to an increase of intracellular calcium and the production/secretion of ET-1. The relationship between the generation of cyclic AMP and the inhibition of irET production/secretion were also reported in some other cell population such as rat mesengial cells and airway epithelial cells (Sakamoto et al., 1992; Yang et al., 1997). Interestingly, studies also suggested that Gs-coupled receptors mediated the decrease in ET-1 production/secretion in endothelial cells but through the activation of cyclic GMP and independently of the cyclic AMP formation (Prins et al., 1994; Razandi et al., 1996). In contrast, other reports suggested that the activation of Gs-coupled receptors, phosphodiesterase inhibitors and cyclic AMP analogues stimulated the production/secretion of ET-1 (Durieu-Trautman et al., 1993; Patel et al., 1997). In our model, PGE2, acting through the EP4 receptor subtype (a Gs coupled receptor), also inhibit basal production/secretion of irET without affecting cyclic GMP generation (unpublished results). Furthermore, both adenosine and PGE2 mediated inhibition of basal irET production/secretion are reversed by the presence of 100 μM of Rp-cAMP-S, a competitive and selective protein kinase A (PKA) inhibitor (Schaap et al., 1993) which strongly suggests the involvement of PKA in adenosine or PGE2 induced-inhibition of irET production/secretion.
In conclusion, we reported that A2B-adenosine receptors are expressed on guinea-pig tracheal epithelial cells. The activation of this receptor leads to the increase in cyclic AMP generation and concomitantly inhibits the basal and LPS-stimulated release of ET-1 by these cells. This suggests that adenosine, through the activation of A2B receptors, can play the role of an anti-inflammatory mediator since it reduces LPS-stimulated ET-1 release from intact epithelium.
Acknowledgments
The authors would like to thank the Medical Research Council of Canada (MRCC), the ‘Fond de la Recherche en Santé du Québec' and Pierre Pelletier for their financial support. The authors also thank Ms Solange Cloutier for excellent technical assistance, grateful help and enriching discussion.
Abbreviations
- ADA
adenosine deaminase
- Ado
adenosine
- 2CADO
2-Cl-adenosine
- CCPA
2-chloro-N6-cyclopentyladenosine
- CGS15943
5-amino-9-chloro-2-(2-furyl)1,2,4-triazol[1,5-c]quinazoline
- CGS21680
2-(p-(-carboxyethyl)-phenethylamino)-5′-N-ethylcarboxamidoadenosine
- CPA
N6-cyclopenthyladenosine
- cyclic AMP
adenosine 3′ : 5′-cyclic monophosphate
- DPCPX
8-cyclopentyl-1,3-diprylxanthine
- ET-1
endothelin-1
- irET
immunoreactive endothelin
- LPS
lipopolysaccharide
- NECA
5′-N-ethylcarboxamidoadenosine
- R-PIA
R-N6-phenylisopropyladenosine
- XAC
xanthine amine congener
- ZM241385
4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)phenol
References
- ALEXANDER S.P.H., COOPER J., SHINE J., HILL S.J. Characterization of the human brain putative A2B adenosine receptor expressed in chinese hamster ovary (CHO.A2B4) cells. Br. J. Pharmacol. 1996;119:1286–1290. doi: 10.1111/j.1476-5381.1996.tb16035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., CURTIS A.R., KENDALL D.A., HILL S.J. A1 adenosine receptor inhibition of cyclic AMP formation and radioligand binding in the guinea-pig cerebral cortex. Br. J. Pharmacol. 1994a;113:1501–1507. doi: 10.1111/j.1476-5381.1994.tb17166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., LOSINSKI A., KENDALL D.A., HILL S.J. A comparison of A2 adenosine receptor-induced cyclic AMP generation in cerebral cortex and relaxation of pre-contracted aorta. Br. J. Pharmacol. 1994b;111:185–190. doi: 10.1111/j.1476-5381.1994.tb14042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRETT K.E., COHN J.A., HUOTT P.A., WASSERMAN S.I., DHARMSATHAPHORN K. Immune-related intestinal chloride-secretion. II. Effect of adenosine on P84 cell line. Am. J. Physiol. 1990;258:C902–C912. doi: 10.1152/ajpcell.1990.258.5.C902. [DOI] [PubMed] [Google Scholar]
- BATTISTINI B., D'ORLÉANS-JUSTE P., SIROIS P. Endothelins: circulating plasma levels and presence in other biologic fluids. Lab. Invest. 1993;68:600–628. [PubMed] [Google Scholar]
- BOICHOT E., CARRE C., LAGENTE V., PONS F., MENCIA-HUERTA J.M., BRAQUET P. Endothelin-1 and bronchial hyperresponsiveness in the guinea pig. J. Cardiovasc. Pharm. 1991;17:S329–S331. doi: 10.1097/00005344-199100177-00094. [DOI] [PubMed] [Google Scholar]
- BRACKETT I.E., DALY J.W. Functional characterization of the A2B adenosine receptor in NIH 3T3 fibroblasts. Biochem. Pharmacol. 1994;47:801–814. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- BRUNS R.F., FERGUS J.H., BADGER E.W., BRISTOL J.A., SANTAY L.A., HARTMAN J.D., HAYS S.J., HUANG C.C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat membranes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;335:59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- CASADÓ M.V., CASILLAS T., MALLOL J., CANELA E.I., LLUIS C., FRANCO R. The adenosine receptors present on the plasma membrane of chromaffin cells are of the A2B subtype. J. Neurochem. 1992;59:425–431. doi: 10.1111/j.1471-4159.1992.tb09388.x. [DOI] [PubMed] [Google Scholar]
- CERNACEK P., STEWART D.J. Immunoreactive endothelin in human plasma: marked elevations in patients in cardiogenic shock. Biochem. Bioph. Res. Commun. 1989;161:562–567. doi: 10.1016/0006-291x(89)92636-3. [DOI] [PubMed] [Google Scholar]
- COOPER J., HILL S.J., ALEXANDER S.P.H. An endogenous A2B adenosine receptor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells. Br. J. Pharmacol. 1997;122:546–550. doi: 10.1038/sj.bjp.0701401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBBINS J.W., LAURENSON J.P., FORREST J.N.J. Adenosine and adenosine analogues stimulate adenosine cyclic 3′,5′-monophosphate-dependent chloride secretion in the mammalian ileum. J. Clin. Invest. 1984;74:929–935. doi: 10.1172/JCI111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBEY R.K., GILLESPIE D.G., OSAKA K., SUZUKI F., JACKSON E.K. Adenosine inhibits growth of rat aortic smooth muscle cells. Possible role of A2B receptor. Hypertension. 1996;27:786–793. doi: 10.1161/01.hyp.27.3.786. [DOI] [PubMed] [Google Scholar]
- DURIEU-TRAUTMAN O., FÉDÉRICI C., CRÉMINON C., FOIGNANT-CHAVEROT N., ROUX F., CLAIRE M., STROSBERG A.D., COURAUD P.O. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J. Cell. Physiol. 1993;155:104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- FEOKTISTOV I., BIAGGIONI I. Characterization of adenosine receptors in human erythroleukemia cells and platelets: further evidence for heterogeneity of adenosine A2 receptor subtypes. Mol. Pharmacol. 1993;43:909–914. [PubMed] [Google Scholar]
- FEOKTISTOV I., BIAGGIONI I. Adenosine A2B receptors evoke interleukin-8 secretion in human mast cells. J. Clin. Invest. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEOKTISTOV I., BIAGGIONI I. Adenosine A2B receptors. Pharmacol. Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- FEOKTISTOV I., MURRAY J.J., BIAGGIONI I. Positive modulation of intracellular Ca2+ levels by adenosine A2B receptors, prostacyclin, and prostaglandin E1 via cholera toxin-sensitive mechanism in human erythroleukemia cells. Mol. Pharmacol. 1994;45:1160–1167. [PubMed] [Google Scholar]
- FIEBICH B.L., BIBER K., GYUFKO K., BERGER M., BAUER J., VAN CALKER D. Adenosine A2B receptors mediate an increase in Interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J. Neurochem. 1996;66:1426–1431. doi: 10.1046/j.1471-4159.1996.66041426.x. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- GLASSBERG M.K., ERGUL A., WANNER A., PUETT D. Endothelin-1 promotes mitogenesis in airway smooth muscle cells. Am. J. Resp. Crit. Care. 1994;10:316–321. doi: 10.1165/ajrcmb.10.3.7509612. [DOI] [PubMed] [Google Scholar]
- HALLER H., SCHABERG T., LINDSCHAU C., LODE H., DISTLER A. Endothelin increases [Ca2+]i, protein phosphorylation, and O2− production human alveolar macrophages. Am. J. Physiol. 1991;261:L478–L484. doi: 10.1152/ajplung.1991.261.6.L478. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ F., KENDALL D.A., ALEXANDER S.P.H. Adenosine receptor-induced second messenger production in adult guinea-pig cerebellum. Br. J. Pharmacol. 1993;110:1085–1090. doi: 10.1111/j.1476-5381.1993.tb13925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORGAN M.J., PINHEIRO J.M.B., MALIK A.B. Mechanism of endothelin-1-induced pulmonary vasoconstriction. Circ. Res. 1991;69:157–164. doi: 10.1161/01.res.69.1.157. [DOI] [PubMed] [Google Scholar]
- INUI T., JAMES A.F., FUJITANI Y., TAKIMOTO M., OKADA T., YAMAMURA T., URADE Y. ETA and ETB receptors on single smooth muscle cells cooperate in mediating guinea pig tracheal contraction. Am. J. Physiol. 1994;266:L113–L124. doi: 10.1152/ajplung.1994.266.2.L113. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., VAN GALEN P.J.M., WILLIAMS M. Adenosine receptors: pharmacology, structure-activity relationships and therapeutic potential. J. Med. Chem. 1992;35:407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P. Pharmacological Analysis of drug-receptor interaction. Raven Press: New York; 1987. Drug antagonism. [Google Scholar]
- LAPORTE J., D'ORLÉANS-JUSTE P., SIROIS P. Guinea-pig Clara cells secrete endothelin-1 through a phosphoramidon-sensitive pathway. Am. J. Resp. Cell Mol. 1996;14:356–362. doi: 10.1165/ajrcmb.14.4.8600940. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., ROBERTS F.F. Measuring muscarinic antagonist potency using stimulated phosphoinositide breakdown in rat cortical slices. Br. J. Pharmacol. 1987;92:677P. [Google Scholar]
- LAZAROWSKI E.R., MASON S.J., CLARKE L., HARDEN T.K., BOUCHER R.C. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br. J. Pharmacol. 1992;106:774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUPICA C.R., CASS W.A., ZAHNISER N.H., DUNWEDDIE T.V. Effect of the selective adenosine A2 receptor agonist CGS21680 on in vitro electrophysiology, cAMP formation and dopamine release in rat hipocampus and striatum. J. Pharmacol. Exp. Ther. 1990;252:1134–1141. [PubMed] [Google Scholar]
- MILLUL V., LAGENTE V., GILLARDEAUX O., BOICHOT E., DUGAS B., MENCIA-HUERTA J.-M., BÉRÉZIAT G., BRAQUET P., MASLIAH J. Activation of guinea pig alveolar macrophages by endothelin-1. J. Cardiovasc. Pharm. 1991;17:S233–S235. doi: 10.1097/00005344-199100177-00067. [DOI] [PubMed] [Google Scholar]
- NOVERAL J.P., ROSENBERG S.M., ANBAR R.A., PAWLOWSKI N.A., GRUNSTEIN M. Role of endothelin-1 in regulating proliferation of cultured rabbit airway smooth muscle cells. Am. J. Physiol. 1992;263:L317–L324. doi: 10.1152/ajplung.1992.263.3.L317. [DOI] [PubMed] [Google Scholar]
- PATEL K.V., SHETH H.G., SCHREY M.P. Stimulation of endothelin-1 secretion by human breast cancer cells through protein kinase A activation: a possible novel paracrine loop involving breast fibroblast-derived prostaglandin E2. Mol. Cell Endocrinol. 1997;126:143–151. doi: 10.1016/s0303-7207(96)03983-4. [DOI] [PubMed] [Google Scholar]
- PEAKMAN M.-C., HILL S.J. Adenosine A2B-receptor-mediated cyclic AMP accumulation in primary rat astrocytes. Br. J. Pharmacol. 1994;111:191–198. doi: 10.1111/j.1476-5381.1994.tb14043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLEWS P.I., ABDEL-MALEK Z.A., DOUPNIK C.A., LEIKAUF G.D. Endothelin stimulates chloride secretion across canine tracheal epithelium. Am. J. Physiol. 1991;261:L188–L194. doi: 10.1152/ajplung.1991.261.2.L188. [DOI] [PubMed] [Google Scholar]
- POUCHER S.M., KEDDIE J.R., SINGH P., STOGALL S.M., CAULKETT P.W.R., JONES G., COLLINS M.G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine, A2A selective adenosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRATT A.D., CLANCY G., WELSH M.J. Mucosal adenosine stimulates chloride secretion in canine tracheal epithelium. Am. J. Physiol. 1986;251:C167–C174. doi: 10.1152/ajpcell.1986.251.2.C167. [DOI] [PubMed] [Google Scholar]
- PRINS B.A., HU R.-M., NAZARIO B., PEDRAM A., FRANK H.J.L., WEBER M.A., LEVIN E.R. Prostaglandin E2 and prostacyclin inhibit the production and secretion from cultured endothelial cells. J. Biol. Chem. 1994;269:11938–11944. [PubMed] [Google Scholar]
- RAZANDI M., PEDRAM A., RUBIN T., LEVIN E.R. PGE2 and PGI2 inhibit ET-1 secretion from endothelial cells by stimulating particulate guanylate cyclase. Am. J. Physiol. 1996;270:H1342–H1349. doi: 10.1152/ajpheart.1996.270.4.H1342. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO H., SASAKI S., NAKAMURA Y., FUSHIMI K., MARUMO F. Regulation of endothelin-1 production in cultured rat mesangial cells. Kidney Int. 1992;41:350–355. doi: 10.1038/ki.1992.48. [DOI] [PubMed] [Google Scholar]
- SATOH M., SHIMURA S., ISHIARA H., NAGAKI M., SASAKI H., TAKISHIMA T. Endothelin-1 stimulates chloride secretion across canine tracheal epithelium. Respiration. 1992;59:145–150. doi: 10.1159/000196045. [DOI] [PubMed] [Google Scholar]
- SCHAAP P., VAN MENTS-COHEN M., SOEDE R.D.M., BRANDT R., FIRTEL R.A., DOSTMAN W., GENIESER H.-G., JASTORFF B., VAN HAASTERT P.J.M. Cell-permeable non-hydrolyzable cAMP derivatives as tools for analysis of signaling pathways controlling gene regulation in Dictyostelium. J. Biol. Chem. 1993;268:6323–6331. [PubMed] [Google Scholar]
- SCHWIEBERT E.M., LIGHT D., DIETEL P., FEJES-TOTH G., NARAY-FEJES-TOTH A., STANTON B. Protein kinase C and G protein, α1-3, regulate a Cl− channel in cortical collecting duct (CCD) Kidney Int. 1990;37:216. [Google Scholar]
- SPINOWITZ B.S., ZADUNAISKY J.A. Action of adenosine on chloride active transport of isolated frog cornea. Am. J. Physiol. 1979;237:F121–F127. doi: 10.1152/ajprenal.1979.237.2.F121. [DOI] [PubMed] [Google Scholar]
- STROHME G.R., REPPERT S.M., WAYNE I.L., MADARA J.L. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J. Biol. Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- UCHIDA Y., NINOMIYA H., SAOTOME M. Endothelin, a novel vasoconstrictor peptide, a potent bronchoconstrictor. Eur. J. Pharmacol. 1988;154:227–238. doi: 10.1016/0014-2999(88)90106-9. [DOI] [PubMed] [Google Scholar]
- VANIO M., SAIJONMAA O., FYHRQUIST F., TORNQUIST K. Purinergic agonists stimulate the secretion of endothelin-1 in rat thyroid FRLT-5 cells. J. Cell. Physiol. 1996;169:583–543. doi: 10.1002/(SICI)1097-4652(199612)169:3<538::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- WEISS S., SEBBEN M., BOCKAERT J. Corticotropin-peptide regulation in intracellular cyclic AMP production in cortical neurons in primary cultures. J. Neurochem. 1985;45:869–874. doi: 10.1111/j.1471-4159.1985.tb04074.x. [DOI] [PubMed] [Google Scholar]
- WHITE S.R., SIGRIST K.S., SPAETHE S.M. Prostaglandin secretion by guinea-pig tracheal epithelial cells caused by eosinophil major basic protein. Am. J. Physiol. 1993;265:L234–L242. doi: 10.1152/ajplung.1993.265.3.L234. [DOI] [PubMed] [Google Scholar]
- YANG Q., LAPORTE J., BATTISTINI B., SIROIS P. Effect of dexamethasone on the basal and cytokine-stimulated release of endothelin-1 from guinea-pig cultured tracheal epithelial cells. Can. J. Physiol. Pharm. 1997;75:576–581. doi: 10.1139/cjpp-75-6-576. [DOI] [PubMed] [Google Scholar]


