Abstract
The effects of Ba2+ (0.1–2 mM) on the component of the perineural voltage change associated with nerve terminal calcium currents (prejunctional Ca2+ currents) were compared with the effects of this ion to antagonize calcium-dependent acetylcholine (ACh) release. These experiments were made on isolated neuromuscular junctions of the frog.
In the presence of sufficient concentrations of K+ channel blockers to eliminate measurable prejunctional K+ currents, low concentrations of Ba2+ selectively antagonized prejunctional Ca2+ currents in normal Ca2+ solutions. Higher concentrations of Ba2+ also substantially reduced the Na+ component of the perineural waveform.
Ba2+ inhibited the prolonged prejunctional Ca2+ currents that developed in the presence of higher concentrations of K+ channel blockers.
Simultaneous measurements of the prejunctional Ca2+ currents and the electrophysiological correlates of ACh release (i.e. end-plate potentials, EPPs) were made under conditions of modest K+ channel blockade. Under these conditions, Ba2+ generally produced simultaneous decreases in both Ca2+ currents and EPP amplitudes. In some instances, a prolongation of prejunctional Ca2+ currents and a transient increase in EPP amplitudes preceded the decreases in both electrophysiological events.
These results suggest that Ba2+ ions can antagonize the entry of calcium into motor nerve endings and this effect is likely to be responsible for the inhibitory effects of Ba2+ on evoked ACh release.
Keywords: Calcium channels, neuromuscular junction, neurotransmitter release, barium
Introduction
Ca2+ currents in nerve endings couple depolarization of the nerve terminal to the synchronous release of neurotransmitter packets (for reviews see Katz, 1969; Silinsky, 1985; Südhof, 1995). In studies of Ca2+ currents in other systems, Ba2+ is frequently substituted for Ca2+ as the charge carrier as Ba2+ does not produce divalent-cation dependent inactivation of Ca2+ channels (Hess et al., 1984; Hille, 1992). Whilst this is a useful substitution for the purposes of studying Ca2+ currents in isolation from effects on transmitter release, Ba2+ is not an appropriate replacement for Ca2+ for studies of rapid stimulus-secretion coupling. Specifically, Ba2+ has been found not to support the synchronous release of multiple neurotransmitter quanta in response to a solitary nerve impulse (Silinsky, 1977; 1978a; McLachlan, 1977; Alvarez-Leefmans et al., 1979; Augustine & Eckert, 1984).
In contrast to its lack of effectiveness in supporting synchronous, evoked transmitter release, Ba2+ is highly efficacious in supporting the asynchronous release of transmitter in response to repetitive presynaptic stimulation (Silinsky, 1977; 1978a; McLachlan, 1977). At cholinergic synapses, this effect of Ba2+ is reflected as a dramatic increase in the frequency of miniature end-plate potentials (MEPPs); this increase in MEPP frequency is the electrophysiological correlate of the large outpouring of neurotransmitter release evoked in Ba2+ solutions as measured by either biochemical or bioassay methods (for review see Silinsky, 1985). The stimulatory effects of Ba2+ on asynchronous transmitter release are due to the entry of Ba2+ through voltage-gated calcium channels and the action of intracellular Ba2+ at a part of the secretory apparatus distinct from that which normally mediates synchronous release (Silinsky, 1978a; 1985; Südhof, 1995; Südhof & Rizzo, 1996).
Given that Ba2+ can enter through voltage-sensitive ionic channels in the nerve endings and is unable to support multiquantal evoked transmitter secretion, it is not surprising that under some conditions, Ba2+ can antagonize Ca2+-dependent neurotransmitter release (Silinsky, 1978b). Whilst other explanations were also proposed,it has been suggested that the inhibitory effect of Ba2+ on the evoked release of acetylcholine (ACh) release could reflect an antagonistic action of this ion at the level of the Ca2+ channel (Silinsky, 1978b). This suggestion at present remains untested.
The perineural recording technique for measuring the extracellular reflection of the nerve terminal Ca2+ current was first introduced by Gunderson et al. (1982) and a short time later used to relate specific currents in the axon membranes to the perineural voltage changes (Brigant & Mallart, 1982; Mallart, 1985a,1985b). This method was modified recently to allow for simultaneous electrophysiological measurements of the extracellular component of the prejunctional Ca2+ current and evoked neurotransmitter release (Redman & Silinsky, 1995). It would thus be of interest to use the perineural recording technique to examine the possibility that the inhibitory effect of Ba2+ on evoked ACh release is at the level of the Ca2+ channel. This paper describes such a study, choosing conditions to minimize the secondary effects of this alkaline earth cation.
Methods
General
Experiments were performed on the isolated cutaneous pectoris nerve-muscle preparation of the frog (Rana pipiens). Animals were humanely sacrificed by anaesthesia with 5% ether, followed by double pithing. Electrophysiological recordings of voltage changes in the perineural space induced by Ca2+ entry via voltage-gated Ca2+ channels were made using the perineural recording method (Brigant & Mallart, 1982; Mallart, 1985a,1985b; Redman & Silinsky, 1995). The perineural recording electrode (which when filled with normal Ringer solution had resistances of 3–10 MΩ), was first positioned under visual control near small axon bundles at the end of the myelin sheaths. In some experiments, intracellular recordings of end-plate potentials (EPPs) were made from end-plate regions of skeletal muscle simultaneously with perineural recordings. The intracellular recording electrodes were filled with 3 M KCl (resistances ranging from 10–20 MΩ) and positioned within 50 μm of the perineural recording electrode. The motor nerve (n. propialis) was stimulated via a polyethylene suction electrode at frequencies ranging from 0.01–0.3 Hz, depending upon the bathing solutions (see Figure legends). Interesting electrophysiological deflections (i.e. perineural waveforms and EPPs) were recorded using a conventional high-input impedance microelectrode preamplifier (Axoclamp 2A, Axon Instruments Inc.). Responses were averaged using an IBM AT-compatible microcomputer, TL-1 interface and pCLAMP software (Axon Instruments). Hard copy of the data were made by first importing the ASCII files to Sigma Plot (Jandel Scientific Inc.). Final lettering was made using Microsoft Power Point after scanning with a Umax Super Vista S-12 Scanner (Version 2.3, Eastman-Kodak Inc.).
Solutions were delivered by superfusion with a peristaltic pump (Watson-Marlow) and removed by vacuum suction. All experiments were performed at room temperature.
Specific solutions and their electrophysiological correlates
The normal Ringer solution used for superfusion prior to experimentation contained (mM) NaCl, 115; KCl, 2; CaCl2 1.8, HEPES, 2 (pH 7.2–7.4), generally with 8–9 μM d-tubocurarine chloride to reduce EPPs below threshold for the generation of muscle action potentials. Perineural recording solutions were of the same basic composition but contained the K+ channel blockers 3,4,-diaminopyridine (DAP) and tetraethylammonium (TEA), and differing concentrations of divalent cations. Normal calcium current Ringer contained normal (1.8 mM) calcium, 39 μM d-tubocurarine, and concentrations of K+ channel blockers (100 μM DAP, 1 mM TEA) that: (i) eliminated measurable K+ currents, (ii) allowed a Ca2+ current component to be measured unencumbered by the opposing Ca2+-dependent K+ current and (iii) failed to generate a long-lasting Ca2+ current that may not be relevant to normal, phasic ACh release (see Silinsky & Solsona, 1992). Repetitive firing occurred in this solution and was employed as an additional index of the level of Ca2+ entry (see Results, Figures 1 and 2). In some experiments, for comparison with the results of others, high K+ channel blocker Ringer was employed. This solution contained normal calcium with 10 mM TEA, 200 μM DAP, and procaine (100 μM, to prevent repetitive firing) and generated a longer duration Ca2+ current (e.g. Figure 3).
Figure 1.
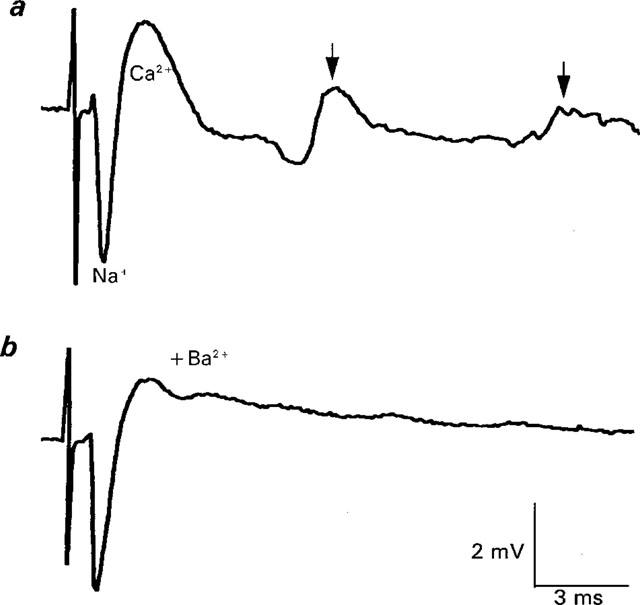
Prejunctional Ca2+ currents in normal Ca2+ current Ringer and its antagonism by Ba2+ (0.1 mM). The averaged control response shown (a) is characterized by a downward (inward) tetrodotoxin-sensitive Na+ current (Na+) and an upward (outward) deflection that largely reflects the prejunctional calcium current (Ca2+) that mediates ACh release. For the control record, the peak of the Ca2+ component ranged from 1.9–2.2 mV (mean±1 s.e.mean=2.1±0.04 mV, n=5 stimuli). For details of the repetitive firing, see text. After 15 min of superfusion with ringer containing 0.1 mM Ba2+, the peak of the prejunctional Ca2+ current was decreased without a change in the Na+ component. The peak of the Ca2+ component in (b) ranged from 1.3–1.6 mV (mean±1 s.e.mean=1.5±0.06 mV, n=5 stimuli). The decrease was highly statistically significant (Mann Whitney rank sum test, P<<0.01). Note also the elimination of repetitive firing (also indicative of a reduction in Ca2+ entry). Each trace is the averaged response to five stimuli delivered at 0.3 Hz.
Figure 2.
Ba2+ (2 mM) antagonizes prejunctional Ca2+ currents in normal Ca2+ current Ringer. (a) shows the control perineural trace. The mean Ca2+ component was 1.63 mV. (b) shows perineural records after 5 min in 2 mM Ba2+. Note the inhibition of the perineural Ca2+ current (mean amplitude=0.74 mV) without an accompanying effect on the Na+ component in this experiment. Each trace is the averaged response to 64 stimuli (0.3 Hz).
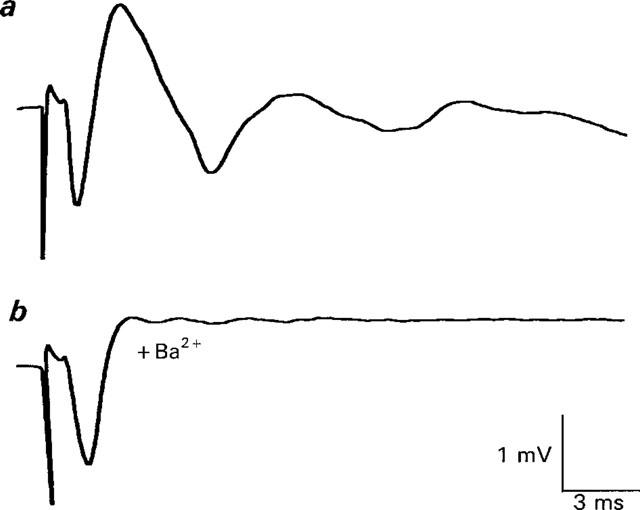
Figure 3.
Ba2+ antagonizes Ca2+ currents in the presence of higher concentration of K+ channel blockers. (a) shows control response. (b) shows response 6 min after the beginning of superfusion with 2 mM Ba2+. This solution contained normal calcium with 10 mM TEA, 200 μM DAP, and procaine (100 μM, to prevent repetitive firing) and generated a longer duration Ca2+ current. This effect of Ba2+ was reversible (data not shown, but see Figure 6). Each trace is the average of two responses (frequency of stimulation=0.01 Hz).
Whilst the calcium current solutions employed thus far allowed for reliable measurements of changes in perineural Ca2+ currents, stable measurements of EPPs could not be made in these solutions as a profound depression of ACh release occurs with even brief low frequency stimulation under these conditions (see Anderson et al., 1988; Redman & Silinsky, 1995 and unpublished observations). To make simultaneous measurements of perineural Ca2+ currents and evoked ACh release, I used a modified Ringer solution termed calcium current-ACh release Ringer. This Ringer, previously termed ‘Ca2+ current' Ringer (Redman & Silinsky, 1995), contained CaCl2 0.9 mM, MgCl2 10 mM, DAP 100 μM, TEA 250 μM, and d-tubocurarine (7.8–20 μM). The lower concentrations of TEA still allowed for reliable measurements of Ca2+ currents, comparable in many ways to those observed in normal Ca2+ current Ringer, but also permitted stable measurements of the electrophysiological correlate of evoked ACh release (i.e., EPPs) to be made simultaneously with an intracellular microelectrode (see e.g. Redman & Silinsky, 1995; and Figures 4, 5 and 7 in the Results).
Figure 4.
Ba2+ decreases perineural Ca2+ currents (upper traces) and evoked ACh release (EPPs) measured simultaneously (lower traces). Each trace is the averaged response to 21 stimuli in Ca2+ current ACh release Ringer (frequency of stimulation=0.05 Hz). Upper traces show perineural currents, lower traces show EPPs. (a) shows control data. (b) shows effects of 0.5 mM Ba2+. The average perineural Ca2+ waveform was reduced from a mean control level of 3.2 mV to 2.0 mV after 21 min in 0.5 mM Ba2+. The average EPP measured simultaneously was reduced from 3.7–1 mV. It should be noted that in this solution, repetitive firing often develops in low Ca2+ solutions (see b, lower trace) and, in contrast to the experiments of Figures 1 and 2, is not indicative of the degree of Ca2+ entry (see Redman & Silinsky, 1995, Figure 4).
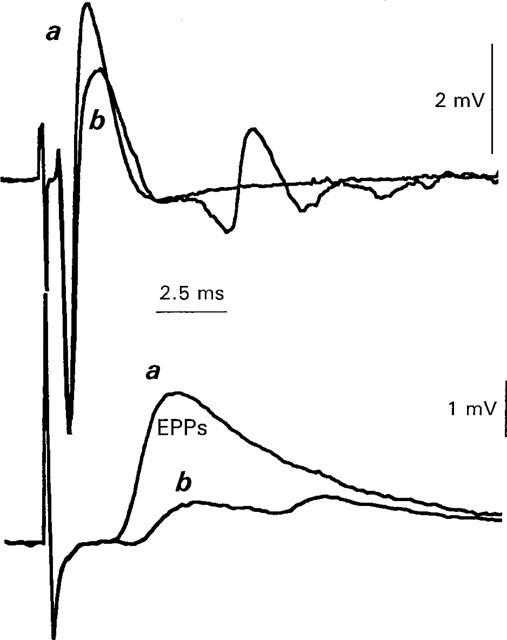
Figure 5.
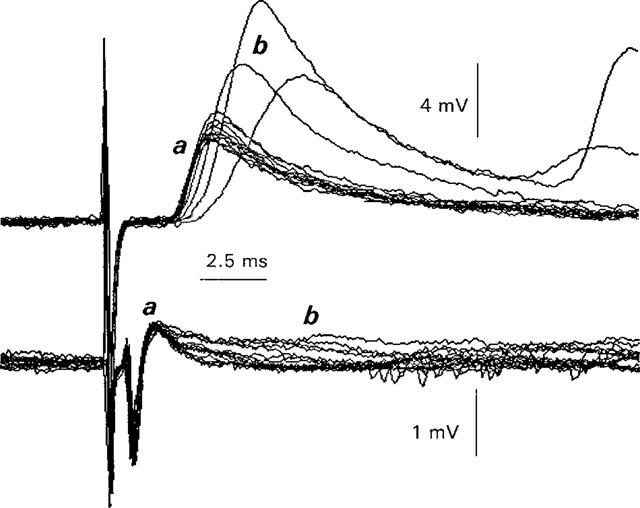
Increases in Ca2+ current duration and ACh release produced by Ba2+ when K+ channels are not fully blocked. Ringer solution was Ca2+ current-ACh release Ringer. Prior to superfusion with Ba2+, nine control perineural currents (lower trace, a) were recorded. The mean amplitude of the perineural Ca2+ voltage change=0.64±0.03 mV (mean±1 s.d., n=9, range 0.59–0.69 mV). Simultaneously, nine control EPPs were recorded (a, upper trace). The mean EPP amplitude=4.5±0.2 mV, mean±1 s.d., n=9, range 4.1–4.9 mV). The coefficient of variation (s.d.mean−1) was thus 0.5% for the perineural Ca2+ component and 0.6% for the EPPs, justifying (i) the use of the Rahamimoff (1967) statistical method to quantify the perineural recordings (see Methods) and (ii) the stability of both types of electrophysiological measurements in this solution. At (b), superfusion was begun with Ba2+ containing solution. Note the progressive prolongation of the perineural Ca2+ current (lower traces) and the increase in EPP amplitudes in Ba2+ to 7.2 mV (±1.0 mV) (mean±1 s.e.mean, n=6). Shortly after these records, EPPs were eliminated completely and both the Na2+ and Ca2+ components of the perineural traces were decreased (data not shown). Frequency of stimulation=0.05 Hz.
Figure 7.
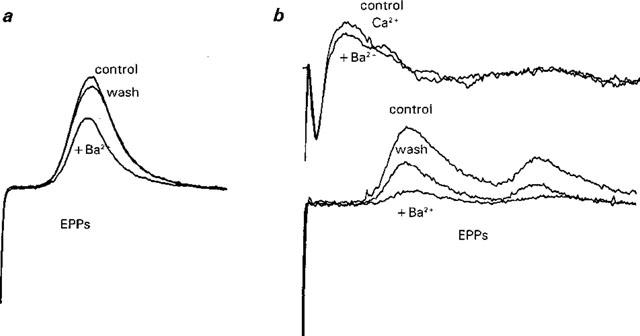
Reversible decreases in EPPs produced by brief exposure to Ba2+ (0.2 mM) in Ca2+ current-ACh release Ringer. Traces in (a) are averaged EPPs in control solution, after 2.5 min in 0.2 mM Ba2+, and after washing in control solution for 3 min. Each trace is the averaged response to 5 stimuli (frequency of stimulation=0.05 Hz). An analysis of variance followed by multiple comparisons revealed highly statistically-significant differences between Ba2+-containing solutions and control conditions. No statistically significant difference was observed between control and wash after Ba2+. Traces in (b) illustrates another experiment in which both perineural Ca2+ currents (upper traces) and EPPs (lower traces) were recorded. Note the concomitant decrease in the average Ca2+ component of the control perineural current and the EPP (n=11 stimuli). The effect on the EPPs was partly reversible (post Ba2+ wash). Recovery of the Ca2+ current also occurred (data not shown but see Figure 6).
A note on terminology of the perineural electrical waveforms
It should be stressed that the perineural deflections described herein reflect the voltage change produced across the resistance of the perineural sheath surrounding several small axon bundles by currents flowing between the myelinated portion of the axons and the nerve terminals. These extracellular perineural currents are proportional to the difference in potential between the nerve endings and the nodes of Ranvier. Whilst these waveforms are not, in the strictest sense, membrane ionic currents, they are highly related to membrane conductance changes that occur both at nerve endings and at last nodes of Ranvier. With respect to the N-type Ca2+ current that initiates transmitter release, the conductance change that initiates this Ca2+ current is localized to the nerve endings (Mallart, 1985b; Robitaille et al., 1990). The Ca2+ current in the nerve endings generates a proportional current in the perineural space; this current flows upstream to the recording site where it is detected as an outward (upward-going) deflection. For the sake of clarity, therefore, I will term the upward-going voltage change that is antagonized by N-type Ca2+ channel blockers the ‘prejunctional Ca2+ current' as this voltage change is generated by Ca2+ currents (ICa) in the nerve ending flowing across the resistance of the perineurium (Rp, i.e. an ICa Rp voltage ‘drop'). The calcium waveform recorded in this way is of reverse polarity to that recorded from the nerve ending because the inward currents in the nerve endings flow back to the axon bundles in which the recording electrode is situated and is detected as outward currents at the recording site. For similar reasons, perineural voltage changes attributable to Na+ and K+ currents in the presynaptic element will be termed perineural Na+ currents and perineural K+ currents respectively as they are reflective of the membrane permeability changes associated with Na+ and K+ currents. Perineural deflections are quantified as the magnitude of the extracellular voltage change as measured from the baseline to the peak of the current (see Silinsky & Solsona, 1992; Redman & Silinsky, 1995 for further details).
Statistical methods
Statistical methods were similar to those described previously (see Silinsky & Solsona, 1992; Silinsky, 1984). In most experiments, appropriate numbers of evoked responses were averaged to reduce the coefficient of variation (i.e., the standard deviation/mean) to less than 5% and to make statistically significant differences at P<0.01. (Rahamimoff, 1967; Silinsky, 1984). For justification of this method for both perineural currents and EPPs measured simultaneously, see legend to Figure 5. Generally, the responses to 5–20 stimuli were averaged. When smaller numbers (5–10) of stimuli were averaged, the individual data traces were first tested for normality and then comparisons between control and Ba2+-treated neuromuscular junctions made by either parametric statistics (e.g. A Student's paired t-test) or non-parametric statistics (Mann-Whitney rank sum test, see Glantz, 1992 and legend to Figure 1). In the few instances when more than two groups were compared, an analysis of variance for the normally distributed data was followed by multiple comparisons using the Bonferroni inequality. This method is reasonable when three–four groups are compared and is the most conservative of the multiple comparisons procedures (see Glantz, 1992). Generally, there is so little variation in perineural currents and quantal ACh release in Ca2+ current-ACh release Ringer that if changes in the ionic composition of the bathing fluid produce detectable changes in electrophysiological behaviour, then these changes are generally highly significant (Redman & Silinsky, 1995; for further justification see legend to Figure 5). Changes in EPPs in the presence of Ba2+ in this study are due to changes in ACh release and not to changes in postjunctional sensitivity to ACh in the presence of Ba2+ (see Silinsky, 1978a).
Chemicals
TEA and DAP were obtained from the Sigma Chemical Company. D-tubocurarine was obtained from Research Biochemicals International.
Results
Effects of Ba2+ on Ca2+ currents in normal Ca2+ current Ringer
Figure 1a shows the typical control perineural waveform recorded under conditions whereby K+ currents were blocked by 1 mM TEA and 100 μM DAP (normal calcium current Ringer – see Methods). In these traces, the negative deflection (Figure 1a, Na+) corresponds to the inward Na+ current generated by the opening of tetrodotoxin-sensitive Na+ channels in the latter part of the myelinated axons and the first segment of the non-myelinated terminal (Mallart, 1985a; Anderson et al., 1988). A significant portion of the upward-going deflection (Figure 1a, Ca2+) represents the prejunctional Ca2+ current that mediates ACh release via N-type Ca2+ channels (see Methods for justification of terminology). This current is inward at the nerve endings but leaves the membrane as an outward current back at the recording site (for details of Ca2+ current polarity and possible contaminants, see Methods, Discussion, and Silinsky & Solsona, 1992).
The repetitive firing of the Na+ and Ca2+ currents in this solution (Figure 1a, arrows) is generally attributed to the persistent depolarization at the last node of Ranvier due to the prolonged Ca2+ current at the nerve endings (Molgo & Mallart, 1985). Hence the magnitude of repetitive firing in normal Ca2+ current Ringer provides an additional sensitive indicator of the level of prejunctional Ca2+ entry, with a decrease or elimination of repetitive firing and a decrease in the peak Ca2+ current both being associated with a blockade of Ca2+ entry (Silinsky & Solsona, 1992).
Figure 1b shows the effect of a low concentration of Ba2+ (100 μM) on the averaged perineural waveforms; note the reduction in the prejunctional Ca2+ current by 29% and the elimination of repetitive firing without a significant change in the amplitude of the perineural Na+ current (compare with Figure 1a). This effect of Ba2+ on the peak of the perineural Ca2+ currents is highly significant statistically (see legend to Figure 1) and reversible (data not shown, but see Figure 6). Figure 2 illustrates the effect of a higher concentration of Ba2+ (2 mM) in another experiment. Note the more substantial reduction in the Ca2+ component (by 54%, see Figure 2b) without a change in the amplitude of the Na+ component of the averaged record.
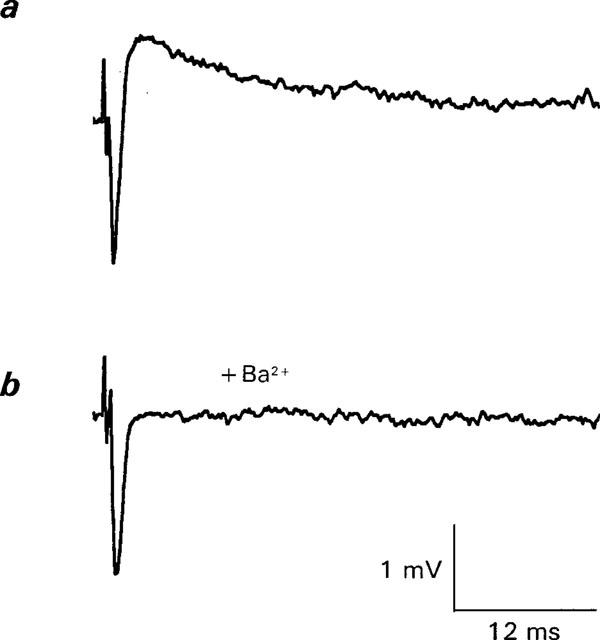
Figure 6.
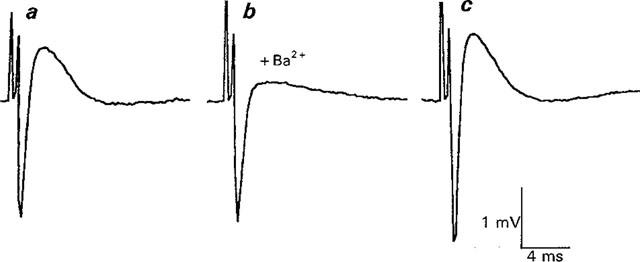
Reversible effects of Ba2+ on Ca2+ currents in Ca2+ current-ACh release Ringer. (a) shows control response, (b) shows response after 18 min in 1 mM Ba2+ ringer. Note reduction in Ca2+ current without an effect on Na+ current. In (c) and Ca2+ current is restored after 16 min in Ba2+ free solution. The increase in Ca2+ response after Ba2+ treatment is likely to be due to an increase in the Na+ current, possibly produced by a small shift in the position of the recording electrode. EPPs were eliminated during this part of the experiment (see text). Each trace is the average response to 64 stimuli (0.3 Hz).
Similar results to those of Figures 1 and 2, in which the prejunctional Ca2+ current was reduced by Ba2+ without a change in the Na+ current, were observed in a total of seven experiments in this solution in the concentration range of 0.1–2 mM external Ba2+. Unfortunately, many other experiments using 1–2 mM concentrations of Ba2+ were accompanied by a large and generally reversible decrease in the Na+ component, either simultaneously with a decrease in the Ca2+ component or with a somewhat delayed time course (data not shown, see also Mallart, 1985a, Figure 5). The results shown in Figures 1 and 2 however, confirm previous suggestions that an experimental window is available at low Ba2+ concentrations or early during exposure to higher Ba2+ concentrations to isolate the effects of Ba2+ on ACh release and the associated Ca2+ currents that initiates ACh release whilst minimizing the effects of Ba2+ on univalent ionic currents (Silinsky, 1977; 1978a,1978b). The remaining experiments described in this paper will focus on the selective effects Ba2+ on prejunctional Ca2+ currents.
Effects of Ba2+ on prejunctional Ca2+ currents in high K+ channel blocker Ringer
Many studies of perineural Ca2+ currents are performed in the presence of higher concentrations of K+ channel blockers (high K+ channel block Ringer–see e.g. Molgo et al., 1991; Redman & Silinsky, 1995; Katz et al., 1995). Whilst the prolonged Ca2+ currents in this solution may or may not be relevant to the Ca2+ current that promotes ACh release (Anderson et al., 1988; Redman & Silinsky, 1995; Katz et al., 1995), such high concentrations of K+ channel blockers; (i) minimize the possibility of any secondary effects of Ba2+ on unblocked K+ channels and (ii) eliminate the confounding effects of Ba2+ on residual outward current contaminants as such contaminants are rare in the presence of these high concentrations of K+ channel blockers (Katz et al., 1995–see also Silinsky & Solsona, 1992 and Discussion). As Figure 3b shows, Ba2+ (2 mM) fully antagonizes the Ca2+ currents in high K+ channel block solutions, leaving no net outward current in the perineural traces, and does so without antagonizing the Na+ currents in this experiment (n=3 experiments). This effect of Ba2+ is reversible (data not shown, but see Figure 6).
Effects of Ba2+ on prejunctional Ca2+ currents and ACh release (EPPs) measured simultaneously
Whilst the results thus far suggest that Ba2+ antagonizes prejunctional Ca2+ currents, the conditions of these experiments preclude reliable measurements of EPPs simultaneously with the Ca2+ currents as EPPs in these solutions rapidly decline to unmeasurable level (Redman & Silinsky, 1995; Anderson et al., 1988). It would thus be of interest to examine the relationship between the effects of Ba2+ on prejunctional Ca2+ currents and EPPs under conditions where simultaneous measurements of both electrophysiological phenomena could be made, i.e., in Ca2+ current ACh release Ringer. Figure 4 shows a representative experiment made in such solutions (n=6). Note that 0.5 mM Ba (Figure 4b) antagonizes the peak Ca2+ current (upper traces) and EPPs (lower traces) measured concomitantly. Indeed, the effects of Ba2+ on both Ca2+ currents and EPPs in this experiment are remarkably similar to the published experimental records depicting a reduction in extracellular Ca2+ concentrations by greater than 50% of control (see e.g. Figure 4 in Redman & Silinsky, 1995).
In this solution, a fraction of the K+ current termed IKCa2+, remains uninhibited (Mallart, 1985b). It might thus be expected, at least during the early part of Ba2+ exposure, that an increase in the duration of the Ca2+ current due to a further inhibition IKCa2+ by Ba2+, might be observed and that this effect would be associated with an increase in ACh release (e.g. Katz et al., 1995). Figure 5 shows this to be the case. Note that the control Ca2+ current tracings (lower records, a) were increased in duration within 1 min after the beginning of superfusion with Ringer containing 2 mM Ba2+ (b, lower records) and this effect was associated with a transient increase in ACh release (b, upper records) over control (a, upper records). After this enhancement of both Ca2+ current duration and evoked ACh release by Ba2, repetitive firing of action potentials and EPPs rapidly developed (b, upper records) and ACh release was reduced and then eliminated (n=3 experiments, data not shown–see Silinsky, 1978a for details).
Despite the reduced concentrations of K+ channel blockers in Ca2+ current-ACh release Ringer, accurate assessments of Ca2+ currents may be made in this solution from the peak of the perineural deflection, even when EPPs are no longer detectable. Figure 6 illustrates this aspects of the method and shows the reversible inhibition by Ba2+ (1 mM) of the Ca2+ current in Ca2+ current ACh release Ringer (n=7 experiments). It thus appears that the predominant effect of Ba2+ in these experiments is a reduction in the prejunctional Ca2+ current and, when measurable, a parallel reduction in evoked ACh release.
It still remains to demonstrate that the decrease in evoked ACh release produced by Ba2 can be reversed by washing in Ba2−-free solutions, as is the case with the prejunctional Ca2+ current (Figure 6). These experiments present a number of obstacles, most notably, the profound run-down of EPPs that occurs in Ba2+ solutions (see Silinsky, 1978a). To attempt to reduce the rundown of ACh release, experiments were conducted using brief (2–13 min) exposure to a low concentration of Ba2+ (0.2 mM). Under these conditions it was possible to demonstrate a reversible decrease in EPPs produced by Ba2+ in Ca2+ current-ACh release Ringer in seven experiments. Figure 7a shows an experiment in which the average control EPP (7.9±0.2 mV, n=5), was reduced by 2.5 min of exposure to 0.2 mM Ba, (5.7±0.1 mV, n=5) and this effect was fully reversible after a 3 min wash in Ba2+-free Ringer (post Ba2+ control=7.5±0.1 mV, n=5). In the experiment shown in Figure 7b, simultaneous measurements of prejunctional Ca2+ currents (upper traces) and EPPs (lower traces) were made. After 3 min in 0.2 mM Ba2+, highly statistically significant differences in the average Ca2+ current and EPPs were observed. This effect was reversible with only the partial reversal of the EPPs shown for convenience see e.g. Figure 6 for reversible effects of Ba2+ on Ca2+ currents in this solution). It thus appears that low concentrations of Ba2+ produce reversible decreases in ACh release and this is associated with reversible changes in the prejunctional Ca2+ current.
Discussion
The results described herein support the suggestion that Ba2+ blocks evoked ACh release in amphibia as a consequence of its inhibitory effect on N-type Ca2+ channels in motor nerve endings (Silinsky, 1978a). It is noteworthy that Ba2+ antagonizes prejunctional Ca2+ currents in all solutions used, including those that allow for simultaneous measurements of ACh release together with prejunctional Ca2+ currents (Redman & Silinsky, 1995) and those that minimize the outward current contamination of prejunctional Ca2 currents (Katz et al., 1995). Furthermore, when the effects of Ba2+ on ACh release were measured in normal Ca2 ringer without K+ channel blockers, the inhibitory effect of Ba2+ on EPPs was similar to the potency of Ba2+ as an inhibitor of Ca2+ currents in normal Ca2+ current Ringer (n=3, data not shown, see also Silinsky, 1978b).
The mechanism by which Ba2+ inhibits Ca2+ currents is unknown. One possibility suggested by results on cloned Ca2+ channels (Bourinet et al., 1996) is that Ba2+ could travel more slowly through its conductance pathway than Ca2+ (see also Silinsky, 1978a), thus impeding the entry of Ca2+. Whilst the change in time to peak of the Ca2+ current shown in Figure 4 is in support of this notion, it must be viewed as speculation at this juncture.
To my knowledge, there are no published experiments examining the inhibitory effects of Ba2+ on N-type Ca2+ channels. In preliminary experiments, however, we have found similar effects of Ba2+ to those shown in Figures 4, 5, 6 and 7 when studied on N-type Ca2+ currents in sympathetic neurons under similar conditions to these present experiments. These preliminary results, whilst including increases in Ca2+ currents, also revealed inhibitory effects of Ba2+ on Ca2+ currents in sympathetic neurons (T.J. Searl & E.M. Silinsky, unpublished experiments using whole cell patch clamp on acutely dissociated celiac neurons from the guinea-pig–see e.g. Searl et al., 1998). Furthermore, antagonism of Ba2+ currents by Ca2+ has been reported (Hess et al., 1984). Finally Ba2+, when substituted for Ca2+, could not support measurable Ca2+ currents in preliminary experiments when the nerve was stimulated at low frequencies (n=2).
It might be argued that the early effects of Ba2+ at low concentrations were due to a local depolarization at the nerve ending by an unspecified mechanism. This depolarization, if it occurred, would in turn reduce the local circuit current required to bring the potential of the nerve ending to that of the node of Ranvier, and hence reduce the current flowing between the nerve terminal and its parent axon. (Mallart, 1985b). The likelihood of such a mechanism is reduced by separate experiments in which simultaneous electrophysiological recordings were made of the perineural univalent cation signals emanating from the last node of Ranvier (e.g. see Redman et al., 1997; Figure 2) and of the extracellular currents flowing at the nerve ending using a patch electrode for focal recording (see e.g. Silinsky, 1984). Using this dual recording technique, concentrations of Ba2+ as low as 200 μM reduced EPPs without changing the Na+ component of either waveform. If Ba2+ were producing a local depolarization at either region at the nerve ending, and hence reducing perineural current flow by mechanisms unrelated to Ca2+ channels then this would have been reflected as a change in the size of one or both Na+ components during the early inhibitory effects on EPPs. This result was not observed (n=3, data not shown).
When higher concentrations of Ba2+ were employed, an inhibition of both Na+ channels and K+ channels ensued (see also Mallart, 1985b). Such additional inhibitory effects precluded careful measurements of the relationship between Ba2+ and Ca2+ at the level of perineural ionic currents, although relationship between Ba2+ and Ca2+ for EPPs can be modelled on the assumption of a competitive antagonism for a common site in the ion channel (Silinsky, 1978b).
The results provide further support for the view that Ba2+ is capable of producing complex effects on both ionic channels and the secretory apparatus in nerve endings. In addition to its effects on the Na+, K+ and Ca2+ channels that precede secretion described here, Ba2+ is a selective agonist at sites in the nerve ending associated with asynchronous transmitter release (Silinsky 1978a; 1985). The mechanism of this agonist effect is likely to be by binding selectively to synaptotagmin III or to a related Ca2+ sensor; synaptotagmin III is a divalent cation-binding protein that mediates asynchronous but not physiologically-functional synchronous neurotransmitter release (Südhof, 1995; Südhof & Rizzo, 1996). This property would make Ba2+ an unsuitable alkaline earth cation to act as the physiological link between nerve terminal depolarization and evoked ACh. Indeed the barrage of asynchronous electrical activity (i.e. MEPPs) evoked by repetitive nerve stimulation in Ba2+ solutions is reminiscent of the electrophysiological effects produced by the active ingredient of black widow spider venom (compare Silinsky, 1978a with Ceccarelli & Hurlbut, 1980). The antagonism by Ba2+ of Ca2+ entry into nerve endings described here would also render this divalent cation unsuitable as an initiator of rapid excitatory synaptic events.
Acknowledgments
I am deeply indebted to Dr T.J. Searl for dissections, preparation of solutions and, the fabrication of electrodes. I also thank Drs Searl, R.S. Redman, and J.K. Hirsh for their helpful comments on the manuscript. This work was supported by a research grant (NS12782) from the NIH (CSP #1).
Abbreviations
- DAP
3,4-diaminopyridine
- EPPs
end-plate potentials
- MEPPs
miniature end-plate potentials
- TEA
tetraethylammonium
References
- ALVAREZ-LEEFMANS F.J., DE SANTIS A., MILEDI R. Effects of some divalent cations on synaptic transmission in frog spinal neurones. J. Physiol. 1979;294:387–406. doi: 10.1113/jphysiol.1979.sp012936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON A.J., HARVEY A.L., ROWAN E.G., STRONG P.N. Effects of charybdotoxin, a blocker of calcium-activated potassium channels, on motor nerve terminals. Br. J. Pharmacol. 1988;95:1329–1335. doi: 10.1111/j.1476-5381.1988.tb11772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUGUSTINE G.J., ECKERT R. Divalent cations differentially support transmitter release at the squid giant synapse. J. Physiol. 1984;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOURINET E., ZAMPONI G.W., STEA A., SOONG T.W., LEWIS B.A., JONES L.P., YUE D.T., SNUTCH T.P. The alpha-1E calcium channel exhibits permeation properties similar to low-voltage activated calcium channels. J. Neurosci. 1996;16:4983–4993. doi: 10.1523/JNEUROSCI.16-16-04983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIGANT J.L., MALLART A. Presynaptic currents in mouse motor endings. J. Physiol. 1982;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CECCARELLI B., HURLBUT W.P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol. Rev. 1980;69:396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- GLANTZ S.A. Primer of Biostatistics. McGraw Hill Inc: New York, NY; 1992. [Google Scholar]
- GUNDERSON C.B., KATZ B., MILEDI R. The antagonism between botulinum toxin and calcium in motor nerve terminals. Proc. Roy. Soc. 1982;216:369–376. doi: 10.1098/rspb.1982.0080. [DOI] [PubMed] [Google Scholar]
- HESS P., LANSMAN J.B., TSIEN R.W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- HILLE B. Ionic Channels of Excitable Membranes. Sinauer Associates Inc: Sunderland, MA, U.S.A.; 1992. [Google Scholar]
- KATZ B. The Release of Neural Transmitter Substances. University Press: Liverpool; 1969. [Google Scholar]
- KATZ E., FERRO P.A., CHERKSEY B.D., SUGIMORI M., LLINAS R., UCHITEL O.D. Effects of calcium channel blockers on transmitter release and presynaptic currents at the frog neuromuscular junction. J. Physiol. 1995;486:695–706. doi: 10.1113/jphysiol.1995.sp020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLART A. Electric current flow inside perineural sheaths of mouse motor nerves. J. Physiol. 1985a;368:565–575. doi: 10.1113/jphysiol.1985.sp015876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLART A. A calcium-activated potassium current in motor nerve terminals of the mouse. J. Physiol. 1985b;368:577–591. doi: 10.1113/jphysiol.1985.sp015877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLACHLAN E.M. The effects of strontium and barium ions at synapses in sympathetic ganglia. J. Physiol. 1977;267:497–518. doi: 10.1113/jphysiol.1977.sp011823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLGO J., DEL POZO E., BANOS J.E., ANGAUT-PETIT D. Changes of quantal transmitter release caused by gadolinium ions at the frog neuromuscular junction. Br. J. Pharmacol. 1991;104:133–138. doi: 10.1111/j.1476-5381.1991.tb12397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLGO J., MALLART A. Effects of Anemonia sulcata toxin II on presynaptic currents and evoked transmitter release at neuromuscular junctions of the mouse. Pflugers Archiv. Eur. J. Physiol. 1985;405:349–353. doi: 10.1007/BF00595687. [DOI] [PubMed] [Google Scholar]
- RAHAMIMOFF R. The use of the Biomac 500 computer for estimating facilitation at single end-plates. J. Physiol. 1967;191:12P–13P. [PubMed] [Google Scholar]
- REDMAN R.S., SEARL T.J., HIRSH J.K., SILINSKY E.M. Opposing effects of phorbol esters on transmitter release and calcium currents at frog motor nerve endings. J. Physiol. 1997;501:41–48. doi: 10.1111/j.1469-7793.1997.041bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDMAN R.S., SILINSKY E.M. On the simultaneous electrophysiological measurements of neurotransmitter release and perineural calcium currents from frog motor nerve endings. J. Neurosci. Meth. 1995;57:151–159. doi: 10.1016/0165-0270(94)00133-2. [DOI] [PubMed] [Google Scholar]
- ROBITAILLE R., ADLER M., CHARLTON M.P. Strategic location of calcium channel at transmitter release sites of frog neuromuscular junctions. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- SEARL T.J., REDMAN R.S., SILINSKY E.M. Mutual occlusion of P2X ATP receptors and nicotinic receptors on sympathetic neurons of the guinea-pig. J. Physiol. 1998;510:783–791. doi: 10.1111/j.1469-7793.1998.783bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. Can barium support the release of acetylcholine by nerve impulses. Br. J. Pharmacol. 1977;59:215–217. doi: 10.1111/j.1476-5381.1977.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J. Physiol. 1978a;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. Enhancement by an antagonist of transmitter release from frog motor nerve terminals. Br. J. Pharmacol. 1978b;63:485–493. doi: 10.1111/j.1476-5381.1978.tb07802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J. Physiol. 1984;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILINSKY E.M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol. Rev. 1985;37:81–131. [PubMed] [Google Scholar]
- SILINSKY E.M., SOLSONA C.S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J. Physiol. 1992;457:315–328. doi: 10.1113/jphysiol.1992.sp019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SÜDHOF T.C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- SÜDHOF T.C., RIZZO J. Synaptotagmins:C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]


