Abstract
The kinetics of open channel block of GluR2-containing and GluR2-lacking AMPA receptors (AMPAR) by dicationic compounds (IEM-1460, IEM-1754, and IEM-1925) have been studied in rat hippocampal neurones using whole-cell patch clamp recording and concentration-jump techniques. Neurones were isolated from hippocampal slices by vibrodissociation.
The dicationic compounds were approximately 100–200 times more potent as blockers of GluR2-lacking AMPAR than as blockers of GluR2-containing AMPAR. The subunit specificity of channel block is determined by the blocking rate constant of a dicationic compound, whereas differences in unblocking rate constants account for differences in potency.
Hyperpolarization may decrease the block produced by IEM-1460 and IEM-1754 block due to the voltage-dependence of the unblocking rate constants for these compounds. This suggests that dicationic compounds permeate the AMPAR channel at negative membrane potentials. The effect was particularly apparent for GluR2-lacking AMPAR. These findings indicate that the presence of GluR2-subunit(s) in AMPAR hinders the binding of the cationic compounds and their permeation through the channel.
The most potent compound tested was IEM-1925. The presence of a phenylcyclohexyl moiety instead of an adamantane moiety, as in IEM-1460 and IEM1754, is probably responsible for the higher potency of IEM-1925. Dicationic compounds are important not only as pharmacological tools, but also as templates for the synthesis of new selective AMPAR blockers which may be potential therapeutic agents.
Keywords: AMPA receptor, channel block, voltage dependence, kinetics, permeation, structure-activity relationships
Introduction
Excitatory synaptic transmission in the central nervous system is predominantly mediated by ionotropic glutamate receptors comprising α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPAR) and N-methyl-D-aspartate (NMDAR) subtypes. NMDAR channels, which are highly permeable to Ca2+, may be effectively blocked by Mg2+ ions and various organic compounds including MK-801, memantine- and phencyclidine-like drugs (Mayer et al., 1984; MacDonald et al., 1991; Chen et al., 1992; Wyllie et al., 1996; Sobolevsky & Koshelev, 1998). The ion channels of AMPAR exhibit lower Ca2+ permeability (Geiger et al., 1995) and are not antagonized by most NMDAR channel blockers (Ferrer-Montiel et al., 1998). It should be noted that the list of drugs that block AMPAR channels is very short. It includes different polyamine compounds like spermine, polyamine-containing neurotoxins isolated from wasp and spider venoms (Brackley et al., 1990; 1993; Blaschke et al., 1993; Herlitze et al., 1993; Bowie & Mayer, 1995; Koh et al., 1995; Kamboj et al., 1995; Washburn et al., 1997) and dicationic adamantane derivatives (Magazanik et al., 1997). The potency of these compounds strongly depends on the subunit composition of AMPAR, e.g. recombinant GluR2-containing AMPAR are relatively insensitive to block. It is noteworthy that presence of a GluR2 subunit in AMPAR reduces its Ca2+ permeability and its single channel conductance (Hume et al., 1991; Burnashev et al., 1992; Swanson et al., 1997). Molecular biology studies have revealed that the properties of AMPAR channels are governed by the residue at the Q/R site in its channel-lining M2 segments (see Dingledine et al., 1999 for review). The residues are controlled by site-selective RNA editing. The editing involves the replacement of a glutamine (Q) by an arginine (R) at the Q/R site of GluR2 subunit. Several data indicate that a reduction in edited GluR2 subunit expression with consequent formation of Ca2+-permeable AMPAR is likely to be a major factor contributing to the delayed neurodegeneration that follows global ischaemia and kainate-induced status epilepticus (see Pellegrini-Giampietro et al., 1997 for review). As such, drugs that selectively block GluR2-lacking AMPAR may be potential therapeutic agents.
The dicationic adamantane derivatives IEM-1754 and IEM-1460 are potent blockers of open channels of native ionotropic glutamate receptors including quisqualate-sensitive receptors in insect muscles (Magazanik et al., 1984; Samoilova et al., 1997), NMDAR in cultured rat cortical neurones (Antonov et al., 1995) and AMPAR in freshly isolated hippocampal cells (Magazanik et al., 1997). In the latter case, the potencies of these compounds depend on the cell type studied. It has been shown that hippocampal pyramidal and nonpyramidal cells exhibited low and high sensitivity to the blockers, respectively. This difference seems to be due to differential expression of GluR2-containing and GluR2-lacking AMPAR in these cells. This conclusion is supported by immunocytochemical, in situ hybridization and RT–PCR data (Bochet et al., 1994; Geiger et al., 1995; Gold et al., 1997; Petralia et al., 1997). It has recently been demonstrated that sensitivity to IEM-1460 block of neurones from different regions of rat brain is positively correlated with the relative Ca2+ permeability of AMPAR in these regions (Samoilova et al., 1999). Moreover, the potencies of IEM-1460 block of AMPAR expressed by hippocampal nonpyramidal cells and of recombinant homomeric GluR1 or GluR3 AMPAR expressed in Xenopus oocytes are similar. Low sensitivity to IEM-1460 block of AMPAR expressed by pyramidal cells resembles that of recombinant heteromeric GluR1+GluR2 AMPAR (Magazanik et al., 1997). Therefore, the effect of dicationic compounds on pyramidal and nonpyramidal cells may be referred to as the block of GluR2-containing and GluR2-lacking AMPAR, respectively. Use- and voltage-dependent action of dicationic adamantane derivatives at both GluR2-containing and GluR2-lacking AMPAR suggests an open-channel blocking mechanism with possible trapping of the drug in the closed channel. But some qualitative differences between AMPAR types have been revealed. In particular, IEM-1460 and IEM-1754 block of GluR2-containing AMPAR is enhanced by hyperpolarization in agreement with the classical single-exponential model. In contrast, the block of GluR2-lacking AMPAR is reduced by hyperpolarization.
The present investigations were designed to further our understanding of the mechanism of channel block by dicationic compounds at AMPAR channels. Several questions have been addressed. Are differences in the potencies of the dicationic compounds at GluR2-containing and GluR2-lacking receptors due to differences in stability of the drug-receptor complexes or are they due to differences in accessibility to the binding sites on these receptors? Why are the voltage-dependencies of block of GluR2-lacking and GluR2-containing AMPAR opposite? We have also compared the blocking properties of two adamantane derivatives, IEM-1754 and IEM-1460, and IEM-1925, which has a phenylcyclohexyl moiety instead of adamantane. The chemical structures of these compounds are shown in Figure 1. IEM-1925, like IEM-1460 and IEM-1754, reversibly blocks ion channels of ionotropic glutamate receptors of insect muscles and mollusc neurones (Samoilova et al., 1997). The studies reported herein have been performed on visually identified pyramidal and nonpyramidal cells isolated from rat hippocampal slices without enzymatic treatment. A concentration-jump approach has been used to estimate apparent blocking and unblocking rate constants at different membrane potentials.
Figure 1.
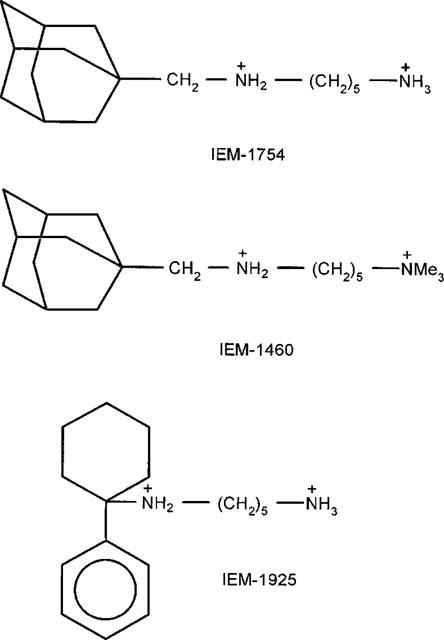
Chemical structures of the dicationic compounds studied. IEM-1754, 1-ammonio-5-(1-adamantane-methylammonio pentane) dibromide; IEM-1460, 1-trimethylammonio-5-(1-adamantane-methylammonio pentane) dibromide; IEM-1925, N-(5-aminopentyl)-1-phenylcyclohexylamine dibromide.
Methods
Experimental procedure
Young rats (aged 15–24 days) were anaesthetized with urethane before decapitation. The brains were removed rapidly and immediately cooled at 2–4°C in an ice bath. Transverse hippocampal slices (200–500 μm thick) were cut using a vibratome (Campden) and stored in a solution containing (in mM): NaCl 124, KCl 5, CaCl2 1.3, MgCl2 1.5, NaHCO3 20, NaH2PO4 1.24, D-glucose 10, bubbled with 95% O2 / 5% CO2 (pH 7.4–7.5) at 30–32°C. After 1–6 h incubation, the slices were transferred to the recording chamber. Neurones were freed from a slice by vibrodissociation at 50–120 Hz (without any enzymatic treatment of the tissue) (Vorobjev, 1991). The method allows a cell to be isolated from a local part of the slice under visual control using an inverted microscope. Pyramidal cells were isolated from stratum pyramidale. They had pyramidal-like somata and preserved apical and basal (in some neurones) dendrites. Nonpyramidal neurones, presumably interneurones, were isolated from stratum radiatum and stratum lacunosum moleculare. They varied in size and shape, but most of them were round-to-oval, in contrast to pyramidal neurones in these regions. The nonpyramidal cells appeared to be neurones rather than glial cells, as judged by the appearance of action potentials during application of depolarizing pulses under current clamp.
Whole-cell patch clamp recording techniques were used for recording currents induced by kainate. The current signals were amplified by an Axopatch 200A (Axon Instr.), filtered at 5 kHz, sampled and stored on a personal computer for ‘on-line' and ‘off-line' analysis. The extracellular solution contained (in mM): NaCl 143, KCl 5, CaCl2 2.7, D-glucose 10, HEPES 10 (pH was adjusted to 7.4 with NaOH). The pipette solution contained (in mM): CsF 100, CsCl 40, NaCl 5, CaCl2 0.5, EGTA 5, HEPES 10 (pH was adjusted to 7.2 with CsOH).
Drugs were applied using a fast perfusion technique (Vorobjev et al., 1996). The patch pipette, with an isolated neurone in the whole-cell configuration, was placed into the glass tube through which the controlled extracellular solution constantly flowed. When 100 μM kainate was added to the perfusion solution, sustained inward currents were recorded at negative membrane potentials. Co-application of kainate (100 μM) and cyclothiazide (100 μM) increased the amplitude of the current 2–4 times (data not shown). These data indicated that kainate-induced currents were due to the activation of AMPA rather than kainate receptors. A thin (0.2 mm diameter) glass capillary located in the glass tube was used to deliver dicationic compounds. The perfusion system was moved laterally so as to place the cell in the solution stream leaving the capillary and containing blocker plus 100 μM kainate. The solution half-exchange time measured from the time for the whole-cell current induced by 15 mM KCl was about 10 ms. The rapid and temporal (for 15–25 s) exposures were done under computer control. Figure 2a is an example of a current evoked in hippocampal cell by kainate. IEM-1460 applications decreased the amplitude of the current in a dose-dependent manner. The effect of 5–6 different concentrations of a dicationic compound were studied in the same cell. Block reached a steady state level within 5–15 s of blocker application. Percentage block was measured and used to construct concentration-inhibition curves. The data were fitted to the formula:
where I and Imax are agonist evoked whole-cell currents in the presence and in the absence of blocker, respectively, IC50 is the blocker concentration producing 50% inhibition, [B]=blocker concentration, and D is the percentage of the maximum inhibition. In the present study IC50 values were determined from each individual concentration-inhibition curve. The percentage of maximum inhibition induced by dicationic compounds varied from 70 to 97% in individual cells. The incomplete block may indicate that some portion of AMPAR channels in the cells studied cannot be blocked by dicationic compounds at concentrations used.
Figure 2.
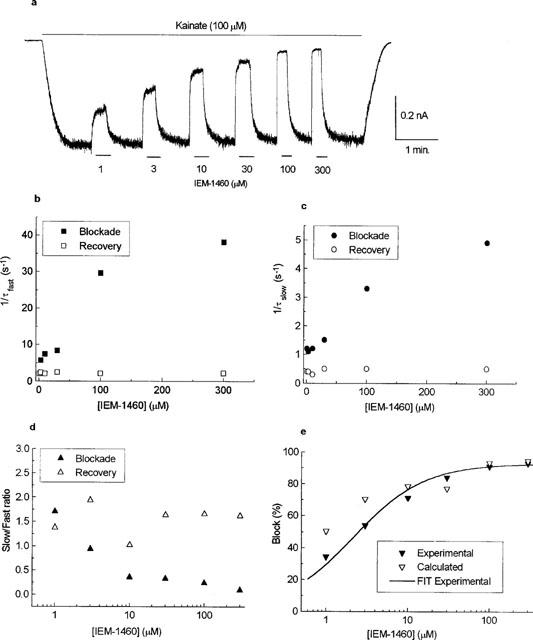
Concentration-dependence of IEM-1460 block. (a) Representative current induced by 100 μM kainate (long bar) in a rat hippocampal nonpyramidal cell (expressing GluR2-lacking AMPAR). The kainate-induced response is blocked in a concentration-dependent manner by applications (short bars) of progressively higher concentrations of IEM-1460 (1–300 μM). Membrane potential, −80 mV. (b) Rates of the fast components of IEM-1460 block (1/τ+fast) and recovery from the block (1/τ−fast) plotted against IEM-1460 concentration. (c) Rates of the slow components of IEM-1460 block (1/τ+slow) and recovery from the block (1/τ−slow) plotted against IEM-1460 concentration. (d) Plot of the ratio of amplitudes of slow and fast components of IEM-1460 block and recovery from the block versus IEM-1460 concentration. (e) Concentration curve for inhibition by IEM-1460; experimental data points are fitted to equation 1 assuming IC50 value of 2.7 μM and maximal inhibition of 93%. The percentages of block calculated using rate constants agree with experimentally measured ones.
Data analysis and statistics
Taking into account our previous finding that dicationic compounds act as open channel blockers and can be trapped in the closed channel (Magazanik et al., 1997), the kinetic scheme for the block should be:
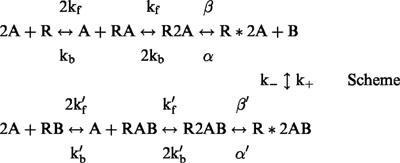 |
where A is the agonist molecule, R and R* are closed and open states of the receptor, respectively, B is the blocking molecule. Forward and backward rate constants of agonist binding are represented by kf and kb, respectively; rate constants of channel opening and closing are β and α, respectively; k+ and k− are blocking and unblocking rate constants of blocking reaction, respectively. Rate constants for the blocked channels are marked by ‘′'. In the absence of an allosteric effect of the blocker, the Scheme becomes symmetrical with kf′=kf, kb′=kb, α′=α, and β′=β. In this case, an equilibrium dissociation constant for block (KD) is equal to IC50.
The Scheme suggests that after a concentration jump, block and recovery from block must be a double-exponential process. The fast components of block and recovery reflect interaction of the blocker with the open channel, while the slow components correspond to multi-step reactions, i.e. the equilibration of blocker with the population of non-conductive channels. Time constants of block (τ+fast and τ+slow) and recovery from block (τ−fast and τ−slow) were analysed by the iterative fitting programme (PATCH9, D.B. Tikhonov).
We assume that the voltage-dependence of the block induced by a positively charged blocking molecule applied from the extracellular side is due to its transition through the membrane field and that the blocker binding and permeation may be described by the two-barrier model (Woodhull, 1973). For the impermeant blocking molecule the model predicts a simple equation describing the voltage dependence:
where V is the transmembrane potential, z is the valence of the blocking particle, δ is the fraction of the membrane field transversed by the blocking molecule moving from outside solution to binding site in the channel, F, R and T are the Faraday constant, gas constant, and absolute temperature, respectively. VD is the value of a change of membrane potential that induces an e-fold change of KD. Modification of the Woodhull model allows one to analyse the voltage dependence of blocking (k+) and unblocking (k−) rate constants separately and calculate position of energy barrier (δb), that determines the binding site accessibility (see Tikhonov & Magazanik, 1998 for review).
V+ and V− are the values of a change of membrane potential that induces an e-fold change of k+ and k−, respectively. The block acquires some additional properties if the blocker can pass through the channel. The rate of dissociation of the blocker to the external side and permeation to the inside of the membrane is expected to show an inverse dependence on membrane potential. Then the voltage-dependence of recovery from the block should be biphasic and the voltage dependent increase of k− allows one to determine the electrical distance between the energy minimum and the second barrier. The dependence of KD, k−, and k+ on membrane potential was approximated by one or two single-exponential functions using equations 2–4. Corresponding electrical distances were calculated for z=2 (the compounds studied are doubly charged at physiological pH).
The data are expressed as mean value±s.d. of n experiments. The significance of differences was evaluated by one-way-analysis of variance (ANOVA). The IEM compounds were synthesized at the Institute of Experimental Medicine RAMS (St. Petersburg, Russia). Kainate was purchased from Sigma.
Results
Blocking kinetics of IEM-1460
At concentrations of 10–3000 μM and of 1–300 μM, IEM-1460 causes a concentration-dependent block of kainate-induced inward currents in pyramidal and nonpyramidal cells, respectively. Figure 2 shows an example of a current recording obtained from a nonpyramidal cell and an analysis of IEM-1460 blocking kinetics. The rate constants of the fast (1/τ+fast) (Figure 2b) and slow (1/τ+slow) (Figure 2c) components of the block increased with increasing blocker concentration. The relative amplitude of the slow component of block decreased with increasing IEM-1460 concentration (Figure 2d). In contrast, the recovery kinetics (1/τ−fast and 1/τ−slow) were concentration-independent. Qualitatively similar results were obtained from nonpyramidal and pyramidal cells.
The above findings apparently agree with the predictions of the Scheme. It follows, therefore, that a linear fit to the plot of 1/τ+fast against. IEM-1460 concentration will give an estimate of the blocking rate constant (k+). However, in most cells at high blocker concentrations (exceeding IC50 values by more than one order of magnitude) 1/τ+fast was so fast that a reliable estimation could not be obtained. Accordingly, its dependence on the blocker concentration deviated from linearity (Figure 2b). This restricted the use of this approach for quantitatively analysing k+. 1/τ−fast may be used as an approximate measure of the unblocking rate constant (k−). The validity of this assumption has been tested by comparing experimental data with the percentage of block calculated as 100% (1−τ+fast/τ−fast). Figure 2e shows a reasonable agreement between the calculated and experimental values. This allowed us to use 1/τ−fast, together with the experimentally-obtained value for KD, for quantitative analysis of the blocking kinetics. As outlined above, 1/τ−fast was independent of IEM-1460 concentration. Therefore, in each cell the values of 1/τ−fast obtained at different IEM-1460 concentrations were pooled and averaged. This averaged value was used as a measure of k−. The value of k+ was calculated from a ratio of k− and KD.
The equilibrium dissociation constants and blocking rate constants for IEM-1460 block of AMPAR in hippocampal pyramidal cells (GluR2-containing AMPAR) (n=18) and in hippocampal nonpyramidal cells (GluR2-lacking AMPAR) (n=11) were estimated. The results are summarized in Table 1. At −80 mV, the KD for IEM-1460 block of GluR2-containing AMPAR is 210 times higher than that of GluR2-lacking AMPAR. The unblocking rate constants for block of GluR2-containing and GluR2-lacking receptors do not significantly (P>0.1) differ, suggesting that subunit specificity of IEM-1460 block is due to differences in the blocking rate constants for this compound.
Table 1.
Kinetics and voltage dependence of AMPAR block by IEM-1460
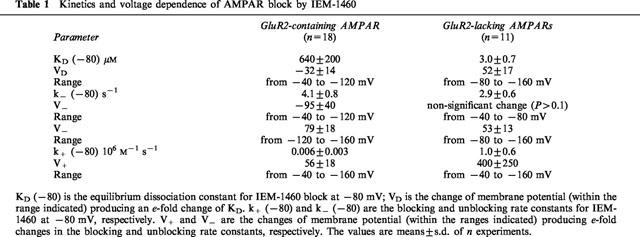
The voltage-dependence of IEM-1460 block of the two types of AMPAR were studied within a wide range of membrane potentials. In a good agreement with our previous observations, the dependencies of IEM-1460 block of GluR2-lacking and GluR2-containing AMPAR on membrane potential were greatly different. Representative experiments are illustrated in Figure 3, which demonstrates that the block of GluR2-containing AMPAR increased with hyperpolarization from −40 to −160 mV, whereas the block of GluR2-lacking AMPAR decreased at membrane potentials more negative than −80 mV. Such changes were characteristic of both types of AMPAR. However, quantitative analysis of the voltage-dependence of IEM-1460 block was complicated by variations in sensitivity to the blocker exhibited by individual cells. Mean values for the equilibrium dissociation constant (KD) for each membrane potential were corrected for each group of cells, by multiplying each individual value (K′D) by the ratio KD(−80)/K′D(−80) which are mean and individual value of the parameter at −80 mV, respectively. Individual unblocking rate constant values were corrected in the same way. The results of a comparative analysis of the kinetics of IEM-1460 block of the two AMPAR types are illustrated in Figure 4 and summarized in Table 1. For block of GluR2-containing AMPAR, KD decreased exponentially with hyperpolarization from −40 to −120 mV (Figure 4a). The data points in Figure 4a are reasonably well-fitted by equation 3, assuming a KD at 0 mV of 5500 μM. The fit indicates an e-fold decrease in KD per 32 mV hyperpolarization. At more negative potentials, the KD for IEM-1460 became almost independent of membrane potential. The unblocking rate constant was minimal at −120 mV. It exhibited an e-fold decrease per 95 mV hyperpolarization from −40 to −120 mV, but further hyperpolarization to −160 mV induced an e-fold enhancement of k− per 79 mV (Figure 4b). The calculated value for the blocking rate constant monotonously increased with hyperpolarization from −40 to −160 mV, exhibiting an e-fold change per 53 mV (Figure 4c).
Figure 3.
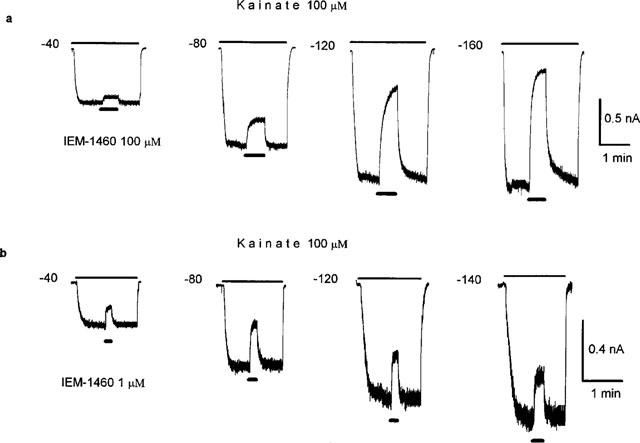
Voltage-dependence of IEM-1460 block. (a) In the same hippocampal pyramidal cell (expressing GluR2-containing AMPAR) 100 μM IEM-1460 produces block of the responses to 100 μM kainate recorded at four different membrane potentials indicated by figures. Block of GluR2-containing AMPAR increases with hyperpolarization. (b) In the same hippocampal nonpyramidal cell (expressing GluR2-lacking AMPAR) 1 μM IEM-1460 produces block of the responses to 100 μM kainate at four different membrane potentials indicated by figures. Block of GluR2-lacking AMPAR decreases at membrane potentials more negative than −80 mV.
Figure 4.
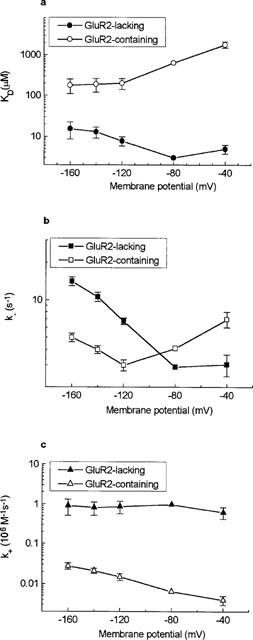
Voltage-dependence of equilibrium dissociation constant and kinetic rate constants for IEM-1460 block. (a) Equilibrium dissociation constants (KD) for IEM-1460 block of GluR2-lacking and GluR2-containing AMPAR plotted against membrane potential. (b) Plot of unblocking rate constants (k−) for IEM-1460 block versus membrane potential. The unblocking rate constants for block of GluR2-containing and GluR2-lacking AMPAR are minimal at −120 and −80 mV, respectively, and increase with both depolarization and hyperpolarization. (c) Plot of blocking rate constants for IEM-1460 block versus membrane potential. The blocking rate constant for block of GluR2-containing AMPAR monotonously increases with hyperpolarization; in the case of GluR2-lacking AMPAR the blocking rate constant is voltage-independent. Data are means±s.d. of 18 (GluR2-containing AMPAR) and 11 (GluR2-lacking AMPAR) experiments.
For IEM-1460 block of GluR2-lacking AMPAR, KD was minimal at −80 mV. Hyperpolarization from −80 to −160 mV induced an e-fold enhancement of KD per 52 mV (Figure 4a). There was also a slight relief from block when a cell was depolarized from −80 to −40 mV. KD and k− for IEM-1460 block were similarly dependent on membrane potential (Table 1), suggesting that k+ was voltage-independent (Figure 4c). The increase of the unblocking rate constant with hyperpolarization can be explained by the two-barrier model of channel block as a voltage-dependent leak of blocking molecules from the binding site in the channel into the cell cytoplasm. Thus, IEM-1460 may be characterized as a permeant blocker of the GluR2-lacking AMPAR. Evidence for permeation of the blocker through the GluR2-containing AMPAR is less strong, except at membrane potentials more negative than −120 mV.
Quantitative data on the voltage-dependence of the rate constants for block and unblock allows one to calculate the positions of barriers and a minimum in the membrane electric field using equations 2–4. The proposed energy profiles are shown in Figure 5. The voltage-independence of the blocking rate constant for the block of GluR2-lacking AMPAR suggests that the first energy barrier determining k+ is located at the outer edge of the membrane field. The lower blocking rate for GluR2-containing AMPAR indicates the presence of a higher barrier which, according to the voltage-dependence of k+, has an apparent electrical depth of 0.23. The positions of the minimum and the second barrier for GluR2-containing AMPAR correspond to electrical distances of about 0.4 and 0.6, respectively. The positions of the minimum and the second barrier for block of GluR2-lacking AMPAR cannot be calculated from our data. However, the V− value at high negative potentials does not depend significantly (P>0.1) on AMPAR type (Table 1), suggesting that the electrical distances between the energy minimum and the second barrier are about 0.2 for both GluR2-containing and GluR2-lacking AMPAR. Accordingly, the main difference between the energy profiles of these receptors is the higher free energies of the barriers and the minimum for GluR2-containing AMPAR.
Figure 5.
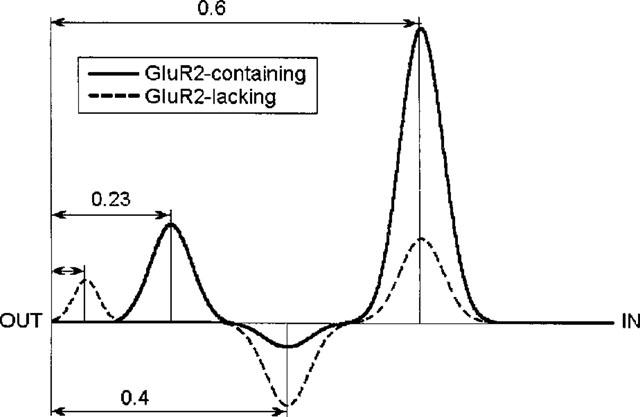
Proposed energy profiles for IEM-1460 block. For both AMPAR channel types, the positions of the well and second barrier coincide. The first energy barrier determining k+ is located deeper in GluR2-containing channels. The free energies of both barriers and the intervening minimum is higher for GluR2-containing channels.
Peculiarities of the action of IEM-1754 and IEM-1925
Previously, it has been shown that the effect of the two dicationic adamantane derivatives on AMPAR do not differ qualitatively (Magazanik et al., 1997). We have now shown that the mechanism of action of the structurally-related compound IEM-1925 on currents induced by kainate in hippocampal cells is similar to that of the two dicationic adamantane derivatives. IEM-1925 antagonizes AMPAR in a use- and voltage-dependent manner and selectively blocks GluR2-lacking AMPAR (Figure 6). At −80 mV, the KD for IEM-1925 block of GluR2-containing AMPAR is 210 times higher than that of GluR2-lacking AMPAR (Table 2).
Figure 6.
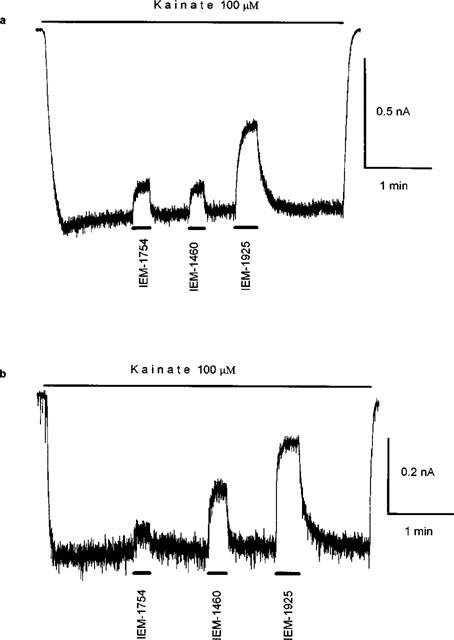
Comparison of the blocking effects of three dicationic compounds. (a) Representative current induced by 100 μM kainate (long bar) in a rat hippocampal pyramidal cell. IEM-1460, IEM-1754, and IEM-1925 applied at the same concentration (100 μM) induce reversible block of the current. IEM-1925 is the strongest blocker, while IEM-1460 and IEM-1754 are nearly equipotent at GluR2-containing AMPAR. Membrane potential −80 mV. (b) Representative current induced by 100 μM kainate (long bar) in a rat hippocampal nonpyramidal cell. IEM-1754, IEM-1460, and IEM-1925 applied at the same concentration (1 μM) reversibly reduced the current amplitude. The order of dicationic compound potencies at GluR2-lacking AMPAR are IEM-1925>IEM-1460>IEM-1754. Membrane potential is −80 mV.
Table 2.
Comparison of the blocking kinetics of three dicationic compounds
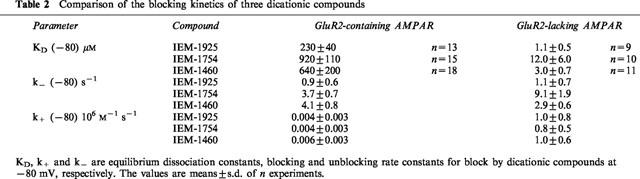
At −80 mV, the KD for IEM-1460 and IEM-1754 block of GluR2-containing AMPAR and the rate constants for block were not significantly different (P>0.1). The KD for IEM-1925 block differs significantly (P<0.05) from those for IEM-1460 and IEM-1754. IEM-1925 is the most potent antagonist of GluR2-containing AMPAR due to slow unblocking rate. The voltage-dependence of IEM-1925 and IEM-1754 action is similar to that of IEM-1460, suggesting that these compounds also behave as weakly-permeant blockers of GluR2-containing AMPAR.
At −80 mV, the ratios for the KD for IEM-1754, IEM-1460 and IEM-1925 block of GluR2-lacking AMPAR were 4.0 : 1.0 : 0.4. The corresponding ratios for k− were 3.0 : 1.0 : 0.4, suggesting that the blocking rate constants are equal. The voltage-dependence of block of GluR2-lacking AMPAR by IEM-1754, IEM-1460 and IEM-1925 are markedly different. KD for IEM-1754 block increases monotonously with hyperpolarization. KD for IEM-1460 block has a minimum at −80 mV, increasing with both depolarization and hyperpolarization. KD for IEM-1925 block decreases with hyperpolarisation from −40 to −120 mV. For all three compounds, the equilibrium dissociation constants and unblocking rate constants exhibited a similar dependence on membrane potential, suggesting that the rate constants for block of GluR2-lacking AMPAR are voltage-independent.
Discussion
The present study demonstrates that the equilibrium dissociation constants for block by dicationic compounds of GluR2-lacking AMPAR are approximately two orders of magnitude lower than those of GluR2-containing AMPAR (Tables 1 and 2). Because the unblocking rate constants for block of both AMPAR types are of the same order of magnitude, the strong dependence of the potencies of dicationic compounds on the subunit composition of AMPAR is obviously due to the difference in the blocking rate constants. In other words, the subunit composition of AMPAR affects mainly the accessibility of blocker to its binding site on AMPAR but not the stability of the blocker-channel complex. The selectivity of dicationic compounds for GluR2-lacking AMPAR can be explained by the structural properties of AMPAR channels. The glutamine to arginine substitution at the Q/R site in the channel-lining M2 segment of GluR2-containing AMPAR is well-known (see Dingledine et al., 1999 for review). The presence of GluR2 subunits with positively-charged arginine residues at the selectivity filter of AMPAR hinders the interaction of the channel with permeant cations and positively charged blockers. This accords with the suggestion of Brackley et al., (1993) who studied the actions of polyamine-containing compounds on recombinant AMPAR and NMDAR. Our data suggest that electrostatic repulsion between this site and a positively-charged blocking molecule affects not only the energy of equilibrium binding but also increases the energy of the barriers (see Figure 5).
At negative potentials, the unblocking rate constant for IEM-1460 and IEM-1754 block of AMPAR increases with hyperpolarization. Based on the two-barrier model of voltage-dependent open channel block, such behaviour is interpreted as possible dissociation of the blocking molecule from its binding site to the cell cytoplasm. In this study, the permeability of the blocking molecules has not been measured directly, but qualitative comparisons of the blocker permeation through different AMPAR channels can be surmised on the basis of electrophysiological data. In comparison with GluR2-lacking AMPAR, the unblocking rate constant for IEM-1460 block of GluR2-containing AMPAR reverses at more negative potentials, suggesting that the presence of GluR2 subunits diminishes permeation. It is well-known that the incorporation of GluR2 subunit(s) also decreases the channel conductance and relative Ca2+ permeability (Hume et al., 1991; Burnashev et al., 1992; Geiger et al., 1995; Swanson et al., 1997). In the case of GluR2-lacking AMPAR, the permeabilities of the three dicationic compounds are correlate with the size of these molecules. The unblocking rate constant for the smallest compound, IEM-1754, increased with hyperpolarization from −40 mV. In contrast, that for IEM-1460 increased with hyperpolarization from −80 mV. Finally, there was no minimum for the unblocking rate constant for IEM-1925, which is the largest of the three molecules.
Differences in drug-channel complex stability (unblocking rate constant) are responsible for differences in the potencies of dicationic compounds. IEM-1925 is the most potent blocker of both AMPAR types. Its phenylcyclohexyl moiety provides more effective binding than the adamantane moiety present in the other two compounds, indicating that the hydrophobic region of these molecules is an important potency determinant. At −80 mV, IEM-1460 and IEM-1754 are equipotent at GluR2-containing AMPAR, but IEM-1460 is four times more active than IEM-1754 at GluR2-lacking AMPAR. We suppose that the permeation properties of the compounds account for these differences. Both IEM-1754 and IEM-1460 are weakly permeant through GluR2-containing AMPAR channels. In the case of GluR2-lacking AMPAR channels, IEM-1754, unlike IEM-1460, can pass through the channel at −80 mV. This increases the unblocking rate constant for IEM-1754.
Estimations of rate constants from integrated currents are based on several assumptions. In particular, the simplified kinetic scheme used in the present work implies that the blocking molecule cannot dissociate from non-conductive states of the receptor and that binding of the blocker does not affect channel kinetics. The use of the fast component of recovery from block as a measure of the unblocking rate constant, as well as the limited time resolution of the experimental system, possibly introduces additional errors. These factors limit the accuracy of our quantitative estimates. However, they do not markedly influence our main conclusions, which are of a comparative nature (i.e. analysis of voltage-dependence and structure-function relationships). Recently, a kinetic analysis of philanthotoxin-343 (PhTX-343) action at the homomeric GluR6(Q) kainate receptor has been done by Bahring & Mayer (1998) using a similar approach to the one adopted herein. The mechanism of AMPAR channel block by dicationic compounds presented herein and of kainate receptor channel block by PhTX-343 are similar. In both cases, it involves open channel block with trapping of the blocking molecule. Like the three dicationic compounds, PhTX-343 can seemingly pass through the glutamate receptor channels at highly negative membrane potentials (Usherwood & Blagbrough, 1989; Usherwood, 1991; Bahring & Mayer 1998).
IEM-1754 and IEM-1460 are also potent blockers of NMDAR channels (Antonov et al., 1995), but evidence for permeation of these blockers has not been revealed even at high negative potentials (up to −140 mV) (Antonov & Johnson, 1996), which contrast with our finding for AMPAR channels. This difference may be accounted for by differences in the diameters of NMDA and AMPAR channels estimated from the dimensions of the largest permeant organic cations. The diameter of the NMDA channel is about 5.5 Å (Villarroel et al., 1995), i.e. not enough to let the adamantane group which has the dimensions of 6.5 Å pass through. Contrary, the adamantane moiety can pass AMPAR channel which has a diameter of about 7.5 Å (Burnashev et al., 1996).
All known potent blockers of AMPAR channels like spermine and PhTX-343 have a polycationic structure, carrying a positively charge on each nitrogen atom at physiological pH (Jackson & Usherwood; 1988; Bowie & Mayer, 1995; Bahring & Mayer, 1998). On the other hand, monocationic analogues of IEM-1925 and IEM-1754 (phencyclidine and memantine, respectively) are very weak blockers of AMPAR channels (Ferrer-Montiel et al., 1998). These observations indicate that the presence of at least two positively charged groups is necessary for effective block of AMPAR channels and suggest that the binding site of dicationic compounds in AMPAR channels consists of at least two distant subsites interacting with both cationic groups of the blockers. The prominent dependence of the potencies of the compounds on the presence of the GluR2 subunit in AMPAR complexes indicates that the Q/R site forms one subsite. We suppose that the hydrophobic moiety binds to Q/R site, while the distant ammonium group penetrates deeper and reaches an additional nucleophylic zone in the channel. Electrophysiological studies, combined with site-directed mutagenesis, can reveal the nature of the ancillary subsite. Also, by varying the length of the carbon chain between two nitrogen atoms of the dicationic compounds it may be possible to determine the distance between the subsites. Dicationic compounds possessing a hydrophobic moiety and a charged ‘tail' may be good structures around which to design new potent blockers of AMPAR channels. These drugs are potentially useful as pharmacological tools for the study of specific receptor channel complexes and as neuroprotectants in a number of neurodegenerative disorders.
Acknowledgments
This work was supported by grants 99-04-49759 from RFBR and 95-1011 from INTAS-RFBR to L.G. Magazanik, and by a grant to S.L. Buldakova from the Wellcome Trust. We are thankful to prof. P.N.R. Usherwood for careful reading of the paper and valuable comments.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor
- NMDAR
N-methyl-D-aspartate receptor
- PhTX-343
philanthotoxin-343
References
- ANTONOV S.M., JOHNSON J.W. Voltage-dependent interaction of open-channel blocking molecules with gating of NMDA receptors in rat cortical neurons. J. Physiol. 1996;493:425–445. doi: 10.1113/jphysiol.1996.sp021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTONOV S.M., JOHNSON J.W., LUKOMSKAYA N.Y., POTAPYEVA N.N., GMIRO V.E., MAGAZANIK L.G. Novel adamantane derivatives act as blockers of open ligand-gated channels and as anticonvulsants. Mol. Pharmacol. 1995;47:558–567. [PubMed] [Google Scholar]
- BAHRING R., MAYER M.L. An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. J. Physiol. 1998;509:635–650. doi: 10.1111/j.1469-7793.1998.635bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKE M., KELLER B.U., RIVOSECCHI R., HOLLMANN M., HEINEMANN S., KONNERTH A. A single amino acid determines the subunit-specific spider toxin block of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor channels. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6528–6532. doi: 10.1073/pnas.90.14.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCHET P., AUDINAT E., LAMBOLEZ B., CREPEL F., ROSSIER J., IINO M., TSUZUKI K., OZAWA S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- BOWIE D., MAYER M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- BRACKLEY P.T.H., BELL D.R., CHOI S.K., NAKANISHI K., USHERWOOD P.N.R. Selective antagonism of native and cloned kainate and NMDA receptors by polyamine-containing toxins. J. Pharmacol. Exp. Ther. 1993;266:1573–1580. [PubMed] [Google Scholar]
- BRACKLEY P., GOODNOW R., JR, NAKANISHI K., USHERWOOD P.N.R. Spermine and philanthotoxin potentiate excitataory amino acid responses of Xenopus oocytes injected with rat and chick brain RNAs. Neurosci. Lett. 1990;114:51–56. doi: 10.1016/0304-3940(90)90427-b. [DOI] [PubMed] [Google Scholar]
- BURNASHEV N., MONYER H., SEEBURG P.H., SAKMANN B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- BURNASHEV N., VILLARROEL A., SAKMANN B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J. Physiol. 1996;496:165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN H.S., PELLEGRINI J.W., AGGARWAL S.K., LEI S.Z., WARACH S., JENSEN F.E., LIPTON S.A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLEDINE R., BORGES K., BOWIE D., TRAYNELIS S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:8–61. [PubMed] [Google Scholar]
- FERRER-MONTIEL A.V., MERINO J.M., PLANELLS-CASES R., SUN W., MONTAL M. Structural determinants of the blocker binding site in glutamate and NMDA receptor channels. Neuropharmacology. 1998;37:139–147. doi: 10.1016/s0028-3908(98)00007-0. [DOI] [PubMed] [Google Scholar]
- GEIGER J.R., MELCHER T., KOH D.S., SAKMANN B., SEEBURG P.H., JONAS P., MONYER H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- GOLD S.J., AMBROS-INGERSON J., HOROWITZ J.R., LYNCH G., GALL C. M. Stoichiometries of AMPA receptor subunit mRNAs in rat brain fall into discrete categories. J. Comp. Neurol. 1997;385:491–502. doi: 10.1002/(sici)1096-9861(19970908)385:4<491::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- HERLITZE S., RADITSCH M., RUPPERSBERG J.P., JAHN W., MONYER H., SCHOEPFER R., WITZEMANN V. Argiotoxin detects molecular differences in AMPA receptor channels. Neuron. 1993;10:1131–1140. doi: 10.1016/0896-6273(93)90061-u. [DOI] [PubMed] [Google Scholar]
- HUME R.I., DINGLEDINE R., HEINEMANN S.F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- JACKSON H., USHERWOOD P.N.R. Spider toxins as tools for dissecting elements of excitatory amino acid transmission. Trends Neurosci. 1988;11:278–283. doi: 10.1016/0166-2236(88)90112-9. [DOI] [PubMed] [Google Scholar]
- KAMBOJ S.K., SWANSON G.T., CULL-CANDY S.G. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J. Physiol. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH D.S., BURNASHEV N., JONAS P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J. Physiol. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD J.F., BARTLETT M.C., MODY I., PAHAPILL P., REYNOLDS J.N., SALTER M.W., SCHNEIDERMAN J.H., PENNEFATHER P.S. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J. Physiol. 1991;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGAZANIK L.G., ANTONOV S.M., GMIRO V.E.Kinetics and pharmacological blockade of glutamate-activated postsynaptic ion channels Biologicheskie Membrany 19841130–140.(in Russian) [Google Scholar]
- MAGAZANIK L.G., BULDAKOVA S.L., SAMOILOVA M.V., GMIRO V.E., MELLOR I.R., USHERWOOD P.N. Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J. Physiol. 1997;505:655–663. doi: 10.1111/j.1469-7793.1997.655ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER M.L., WESTBROOK G.L., GUTHRIE P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- PELLEGRINI-GIAMPIETRO D.E., GORTER J.A., BENNETT M.V., ZUKIN R.S. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disoders. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- PETRALIA R.S., WANG Y.X., MAYAT E., WENTHOLD R.J. Glutamate receptor subunit 2 – selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among population of neurons. J. Comp. Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- SAMOILOVA M.V., BULDAKOVA S.L., VOROBJEV V.S., SHARONOVA I.N., MAGAZANIK L.G. Open channel blocking drug, IEM-1460, reveals functionally distinct (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in rat brain neurons. Neuroscience. 1999;94:261–268. doi: 10.1016/s0306-4522(99)00326-7. [DOI] [PubMed] [Google Scholar]
- SAMOILOVA M.V., FROLOVA E.V., POTAPJEVA N.N., FEDOROVA I.M., GMIRO V.E., MAGAZANIK L.G. Channel blocking drugs as tools to study glutamate receptors in insect muscles and molluscan neurons. Invert. Neurosci. 1997;3:117–126. [Google Scholar]
- SOBOLEVSKY A., KOSHELEV S. Two blocking sites of amino-adamantane derivatives in open N-methyl-D-aspartate channels. Biophys. J. 1998;74:1305–1308. doi: 10.1016/S0006-3495(98)77844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANSON G.T., KAMBOJ S.K., CULL-CANDY S.G. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J. Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIKHONOV D.B., MAGAZANIK L.G. Voltage dependence of open channel blockade: onset and offset rates. J. Membr. Biol. 1998;161:1–8. doi: 10.1007/s002329900309. [DOI] [PubMed] [Google Scholar]
- USHERWOOD P.N.R.Natural toxins and glutamate transmission Excitatory Amino Acids 1991New York: Raven Press; 329–395.In Meldrum, B.S., Moroni, E., Simon, R.P. & Woods, J.R. eds [Google Scholar]
- USHERWOOD P.N.R., BLAGBROUGH I.S.Antagonism of insect muscle glutamate receptors – with particular reference to arthropod toxins Insecticide Action. From Molecule to Organism 1989New York: Plenum; 13–31.In Narahashi, T. & Chambers, J.E. eds [Google Scholar]
- VILLARROEL A., BURNASHEV N., SAKMANN B. Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophys. J. 1995;68:866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOROBJEV V.S. Vibrodissociation of sliced mammalian nervous tissue. J. Neurosci. Methods. 1991;38:145–150. doi: 10.1016/0165-0270(91)90164-u. [DOI] [PubMed] [Google Scholar]
- VOROBJEV V.S., SHARONOVA I.N., HAAS H.L. A simple perfusion system for patch-clamp studies. J. Neurosci. Methods. 1996;68:303–307. doi: 10.1016/0165-0270(96)00097-0. [DOI] [PubMed] [Google Scholar]
- WASHBURN M.S., NUMBERGER M., ZHANG S., DINGLEDINE R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J. Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODHULL A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYLLIE D.J., BEHE P., NASSAR M., SCHOEPFER R., COLQUHOUN D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc. R. Soc. Lond. B. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]


