Abstract
We investigated how manipulations of the degree of activation of adenosine A1 and A2A receptors influences the action of the neuropeptide, calcitonin gene-related peptide (CGRP) on synaptic transmission in hippocampal slices. Field excitatory post-synaptic potentials (EPSPs) from the CA1 area were recorded.
When applied alone, CGRP (1–30 nM) was without effect on field EPSPs. However, CGRP (10–30 nM) significantly increased the field EPSP slope when applied to hippocampal slices in the presence of the A1 receptor antagonist, 1,3-dipropyl-8-cyclopenthyl xanthine (DPCPX, 10 nM), or in the presence of the A2A adenosine receptor agonist CGS 21680 (10 nM).
The A2A receptor antagonist, ZM 241385 (10 nM) as well as adenosine deaminase (ADA, 2 U ml−1), prevented the enhancement of field EPSP slope caused by CGRP (30 nM) in the presence of DPCPX (10 nM), suggesting that this effect of CGRP requires the concomitant activation of A2A adenosine receptors by endogenous adenosine.
The protein kinase-A inhibitors, N-(2-guanidinoethyl)-5-isoquinolinesulfonamide (HA-1004, 10 μM) and adenosine 3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-cAMPS, 50 μM), as well as the inhibitor of ATP-sensitive potassium (KATP) channels, glibenclamide (30 μM), prevented the facilitation of synaptic transmission caused by CGRP (30 nM) in the presence of DPCPX (10 nM), suggesting that this effect of CGRP involves both KATP channels and protein kinase-A.
It is concluded that the ability of CGRP to facilitate synaptic transmission in the CA1 area of the hippocampus is under tight control by adenosine, with tonic A1 receptor activation by endogenous adenosine ‘braking' the action of CGRP, and the A2A receptors triggering this action.
Keywords: Calcitonin gene-related peptide, neuropeptides, adenosine, A1 adenosine receptor, A2A adenosine receptor, neuromodulation, hippocampus, synaptic transmission, excitatory postsynaptic potentials
Introduction
Adenosine, by operating inhibitory A1 receptors or excitatory A2 receptors, has widespread modulatory actions in the nervous system (for a review see Sebastião & Ribeiro, 1996) and may interfere with the action of other neuromodulator/neurotransmitter substances (Ribeiro, 1999). Of particular interest in the context of the present study are the evidences that A2A receptor activation inhibits A1 receptor function in the hippocampus (Cunha et al., 1994) and that tonic activation of adenosine A2A receptors facilitates the action of other neuromodulators, such as that of the neuropeptide calcitonin gene-related peptide (CGRP) at motor nerve terminals (Correia-de-Sá & Ribeiro, 1994). Adenosine, through A1 receptor activation, may also interfere with CGRP release (Santicioli et al., 1993).
CGRP is a 37-amino acid peptide generated from the calcitonin gene by alternate tissue-specific splicing (Amara et al., 1982). It is widely present in the peripheral and in the central nervous system (for reviews see Ishida-Yamamoto & Tohyama, 1989; Poyner, 1992), including the hippocampus (Bulloch et al., 1996; Freund et al., 1997; Oliver et al., 1999), where it inhibits NMDA receptor-mediated actions (Bouchard et al., 1995) and may protect against injury (Bulloch et al., 1996; 1998).
The present work was designed to investigate how manipulations of the degree of activation of adenosine A1 and A2A receptors influences the action of CGRP on synaptic transmission in the hippocampus. Since the predominant action of endogenous adenosine on synaptic transmission in the hippocampus is inhibitory, through A1 receptor activation (e.g. Dunwiddie & Diao, 1994), we first evaluated how blockade of A1 receptors could influence the action of CGRP. Because synaptic transmission in the hippocampus can be influenced by both inhibitory A1 (Sebastião et al., 1990) and excitatory A2A (Sebastião & Ribeiro, 1992) adenosine receptors, we then investigated how the simultaneous blockade of these two adenosine receptor subtypes, and how removal of endogenous extracellular adenosine with adenosine deaminase, could influence the effect of CGRP. How A2A adenosine receptor activation by a selective agonist interferes with the action of CGRP on hippocampal synaptic transmission, was also evaluated. Since several CGRP-mediated action involve activation of protein-kinase A and of ATP-sensitive potassium channels (KATP channels) (e.g. Zang et al., 1994; Santicioli & Maggi, 1994; Wellman et al., 1998), we investigated how inhibition of PK-A with HA-1004 (Hidaka & Kobayahi, 1992) or with the cyclic AMP analogue, RpcAMPS (Rothermel & Parker-Bothelho, 1998) and how inhibition of KATP channels with glibenclamide (Schmid-Antomarchi et al., 1987) affects the action of CGRP on synaptic transmission in the hippocampus.
A preliminary account of this study has been presented to the 6th International Symposium of Adenosine and Adenine Nucleotides, in Ferrara, Italy, May 1998 (Sebastião et al., 1998) and to the British Pharmacological Society (Sebastião et al., 1999)
Methods
The experiments were performed on hippocampal slice preparations taken from male Wistar rats (5–6 weeks old) handled according to the European Community guidelines on Animal Care. The animals were decapitated under halothane anaesthesia and the hippocampus dissected free into ice-cold Krebs solution of the following composition (mM): NaCl 124, KCl 3, NaH2PO4 1.25, NaHCO3 26, MgSO4 1, CaCl2 2, glucose 10, previously gassed with 95% O2/5% CO2 (pH≈7.4). Slices (400 μm thick) were cut perpendicular to the long axis of the hippocampus with a McIlwain tissue chopper, and allowed to recover for at least 1 h in a chamber within the same gassed medium at room temperature (22–25°C). A slice was then transferred to a 1 ml (plus 2 ml dead volume) recording chamber for submerged slices and continuously superfused with gassed bathing solution at 30°C, at a flow rate of 3 ml min−1. Drugs were added to this superfusion solution.
Monopolar stimulation (rectangular pulse of 0.1 ms applied once every 10 s) was delivered through a concentric electrode placed on the Schaffer collateral/commissural fibres, in the stratum radium near the CA3/CA1 border. Evoked field excitatory post-synaptic potentials (field EPSPs) were recorded through an extracellular electrode (4 M NaCl, 3–5 MΩ resistance) placed in the stratum radiatum of the CA1 area. The intensity of the stimulus (80–400 μA) was initially adjusted to obtain a large field EPSP slope with a minimum population spike contamination. Recordings were obtained with an Axoclamp 2B amplifier coupled to a DigiData 1200 interface (Axon Instruments). Averages of eight consecutive responses were continuously monitored on a personal computer with the LTP program (Anderson & Collingridge, 1997), kindly supplied by W.W. Anderson (University of Bristol, U.K.). Responses were quantified as the slope of the initial phase of the averaged field EPSPs, since slope measures are considered a more accurate measure of field EPSP magnitude than the amplitude, due to contamination by population spike.
To minimise underestimation of the CGRP responses due to desensitization (see Aiyar et al., 1992), only one concentration of CGRP was applied to each slice, and thus, the concentration response curves showed in this paper were performed in a non-cumulative manner. CGRP was applied to each slice either alone or in the presence of adenosine receptor agonists and/or antagonists. Whenever the effect of CGRP was tested in the presence of other drugs, the neuropeptide was applied to the preparations only after a stable response to these drugs was observed and at least 20 min after starting their perfusion. Because CGRP is readily adsorbed onto plastic and glass, the chamber and all the connecting tubes were pre-treated with Sigmacote to minimise losses of the peptide (see Afonso et al., 1996).
Drugs
Rat calcitonin gene-related peptide (CGRP) and sigmacote were from Sigma. CGS 21680 (2-[p-(2-carboxyethylphenethylamino]-5′-N-ethylcarboxamide adenosine) and 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) were from Research Biochemicals Inc. (R.B.I.). ZM 241385 (4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5ylamino]ethyl)phenol), was a kind gift from ZENECA. DPCPX was made up in a 5 mM stock solution in 99% dimethylsuphoxide (DMSO)/1% NaOH1 M (v v−1). CGS 21680 and ZM 241385 was made up in 5 mM stock solutions in DMSO. CGRP was made up as a 1 mM stock solution in distilled water. Aliquots of the stock solutions were kept frozen at −20°C until use. In each experiment, one aliquot was thawed and diluted in Krebs solution.
Analysis of the data
The data are expressed as means±s.e.mean from n number of slices. Unless otherwise stated, the significance of the differences between the means was evaluated by the Student's t-test. When comparing more than two experimental groups (Figure 3), one-way repeated measures ANOVA was used followed by the Newman-Keuls test. Values of P<0.05 were considered to represent statistically significant differences.
Figure 3.

Influence of the blockade of A2A adenosine receptors with the selective antagonist, ZM 241385 (10 nM), upon the enhancement caused by calcitonin gene related peptide (CGRP, 30 nM) on the slope of field excitatory postsynaptic potentials (fEPSPs) recorded from the CA1 area of rat hippocampal slices. (A) Represents the results obtained in the experiments (n=3) in which ZM241385 was applied before CGRP and (B) represents the results obtained in the experiments (n=4) in which ZM241385 was applied after the full effect of CGRP. The presence (+) or absence (−) of each drug is indicated below each column. In each panel the sequence of drug application corresponds to the sequence of columns. The field EPSP slope in the absence of any drugs was 0.45±0.02 mV ms−1 in (A) and 0.48±0.11 mV ms−1 in (B). *P<0.05 (one-way repeated measures ANOVA followed by the Newman-Keuls test) as compared with the field EPSP slope recorded in the same slices before application of CGRP (first column in each panel). Note that ZM241385 prevented (A), but did not reverse (B), the excitatory effect of CGRP.
Results
Influence of A1 receptor blockade upon the effect of calcitonin gene-related peptide (CGRP) on hippocampal synaptic transmission
To prevent A1 receptor activation by endogenous adenosine, we used the A1 receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX) at a concentration (10 nM) 20 times higher its affinity value for the A1 receptors in the hippocampus (see Sebastião et al., 1990). At this concentration, DPCPX increased the slope of the field EPSPs by 21±4.2% (n=20, P<0.05), which is consistent with its ability to prevent the tonic inhibition of synaptic transmission caused by endogenous adenosine.
When applied to hippocampal slices in the absence of DPCPX, CGRP in low nanomolar concentrations (1–30 nM) was virtually devoid of effect on the field EPSPs. However, as illustrated in Figure 1, when CGRP (30 nM) was applied to an hippocampal slice after a stable response to DPCPX was recorded, the neuropeptide caused a consistent and reversible increase (17±1.3%, n=19, P<0.05) in the slope of the field EPSPs. The maximal effect of the neuropeptide was obtained 15–20 min after starting CGRP perfusion and was reversed within 15–20 min after stopping CGRP perfusion (Figure 1).
Figure 1.

Enhancement caused by calcitonin gene-related peptide (CGRP, 30 nM) on the slope of field excitatory postsynaptic potentials (fEPSPs) recorded from the CA1 area of an hippocampal slice in the presence of the A1 adenosine receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX, 10 nM). The upper panel shows the time course of the effect of CGRP. Each point, in the ordinates, corresponds to the slope of the average of eight consecutive field EPSPs, and in the abscissae to the start of averaging. The time of perfusion of CGRP is indicated by the horizontal bar, and DPCPX (10 nM) was always present. The lower panel shows recording of field EPSPs (each an average of eight consecutive responses) obtained (from left to right): before perfusion of CGRP, at the maximum effect of CGRP (30 nM), and after washing out CGRP; the field EPSPs are preceded by the synaptic volley and the stimulus artefact.
In Figure 2 are compared the concentration-response curves for the effects of CGRP on the slope of field EPSPs recorded in the absence and in the presence of DPCPX (10 nM). In the presence of this A1 receptor antagonist, but not in its absence, CGRP concentration-dependently increased the slope of the field EPSPs when tested in concentrations up to 30 nM. At an higher concentration (100 nM) CGRP inhibited the slope of the field EPSPs by 15±6.2% (n=3), an action virtually not modified when A1 receptors were blocked by DPCPX (10 nM), the average inhibition caused by CGRP under this condition being 13±3.9% (n=3) (Figure 2).
Figure 2.
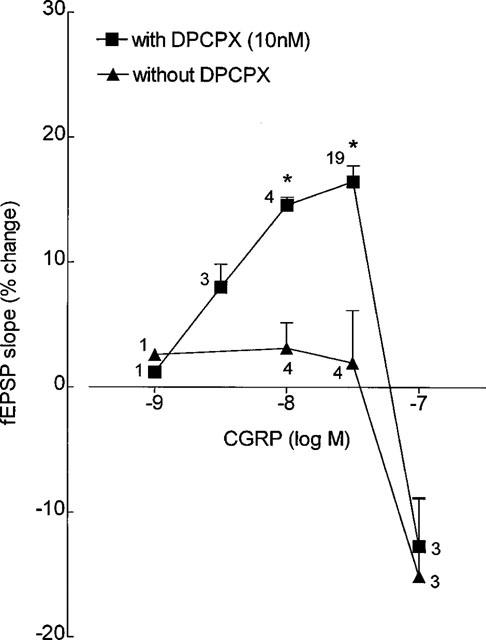
Concentration-response curves for the effects of calcitonin gene-related peptide (CGRP) on the slope of field excitatory postsynaptic potentials (fEPSPs) recorded from the CA1 area of the rat hippocampal slices in the absence and in the presence of the A1 adenosine receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), as indicated by the symbols. Each point represents the mean±s.e.mean results obtained in n slices, as indicated close to each symbol. The concentration-response curves were obtained in a non-cumulative way, and in each slice CGRP was applied only once. The field EPSP slope before CGRP (0%) was 0.41±0.05 mV ms−1 (n=12) in the experiments without DPCPX and 0.48±0.03 mV ms−1 (n=30) in the experiments with DPCPX. *P<0.05 (Student's t-test) as compared with the effect of CGRP in the absence of DPCPX.
Influence of combined A1 and A2A adenosine receptors blockade, and of removal of endogenous adenosine, on the effect of CGRP on synaptic transmission
In a first set of experiments we applied CGRP to hippocampal slices where A1 and A2A adenosine receptors have been blocked. To antagonize A2A receptors we used the selective antagonist, ZM 241385, which has been shown to prevent adenosine A2A receptor-mediated actions in the hippocampus (Cunha et al., 1997); CGRP was tested in the concentration (30 nM) that caused maximal enhancement of the field EPSPs in the presence of DPCPX (10 nM). As illustrated in Figure 3A, CGRP (30 nM) was virtually devoid of effect on the field EPSPs when applied to slices where both A1 and A2A adenosine receptors have been previously blocked with DPCPX (10 nM) and ZM 241385 (10 nM). By itself, ZM (10 nM), applied in the presence of DPCPX (10 nM), was virtually devoid of effect on the slope of EPSPs (per cent change of the field EPSP slope: 0.2±2.5%, n=3), which confirms previous observations (Cunha et al., 1997). The simultaneous perfusion of ZM 241385 (10 nM) and DPCPX (10 nM) increased EPSP slope by 25±1.2% (n=3), an increase that was not significantly different (P>0.05) from that obtained (21±4.2%, n=20) with DPCPX (10 nM) alone.
In another set of experiments we applied the A2A receptor antagonist only after the full excitatory action of CGRP (30 nM) in the presence of the DPCPX (10 nM) had been observed. Under these conditions, CGRP (30 nM) increased the field EPSP slope by 18±5.4% (n=4, P<0.05) and ZM 241385 (10 nM) was unable to reverse this excitatory effect of the neuropeptide. (Figure 3B).
To further evaluate how A2A receptor activation by endogenous adenosine was needed for the enhancement of synaptic transmission caused by CGRP upon A1 receptor blockade, experiments were designed to test the influence of adenosine deaminase, an enzyme that inactivates extracellular adenosine into inosine, in that action of CGRP. Adenosine deaminase (2 U ml−1) was applied to hippocampal slices in the presence of DPCPX (10 nM) and, as expected from the lack of effect of the A2A antagonist on field EPSPs, the enzyme was also virtually devoid of effect (per cent change of field EPSP slope: −2.5±2.3%, n=4, after 20 min application) on field EPSP slope. In these slices, the subsequent application of CGRP (30 nM) did not appreciable affect the field EPSPs (per cent change of the slope: 1.3%±2.4, n=4). As expected, in parallel slices from the same hippocampus, GCRP (30 nM) applied in the presence of DPCPX (10 nM) but in the absence of adenosine deaminase, increased the slope of the EPSPs by 15±2.3% (n=4, P<0.05)
Influence of A2A receptor activation upon the effect of CGRP on hippocampal synaptic transmission
The results obtained with the simultaneous blockade of A1 and A2A receptors suggested that the induction of the excitatory effect of CGRP in the presence of DPCPX requires tonic activation of A2A receptors by endogenous adenosine. To further evaluate how A2A receptor activation interferes with the action of CGRP on synaptic transmission in the hippocampus, we tested the action of this neuropeptide in slices where the A2A adenosine receptors have been activated by the A2A agonist, CGS 21680. By itself, CGS 21680 (10 nM) increased the slope of field EPSPs by 8.7±1.7% (P<0.05) in seven out of 12 experiments. In five experiments CGS 21680 was virtually devoid of effect on the field EPSPs. This variability in the effect of the A2A receptor agonist in the hippocampus has been previously reported (Sebastião & Ribeiro, 1992; Li & Henry, 1998).
As illustrated in Figure 4A, CGRP (30 nM) caused a marked and reversible increase in the slope of the field EPSPs when applied to hippocampal slices in the presence of the A2A agonist, CGS 21680 (10 nM). The facilitation of synaptic transmission caused by CGRP (3–30 nM) in the presence of CGS 21680 (10 nM) was concentration-dependent (Figure 4B), 30 nM CGRP increasing the slope of the field EPSPs by 36±8.2% (n=5, P<0.05), an effect which is significantly larger (P<0.05) than that observed in other experiments using the same concentration of CGRP but in the presence of 10 nM DPCPX (17±1.3%, n=19). Interestingly, the ability of the A2A agonist to unmask the facilitatory effect of CGRP on synaptic transmission does not appear to depend on its ability to facilitate synaptic transmission, since in the hippocampal slices where CGS 21680 (10 nM) did not increase the field EPSP slope, CGRP (3–30 nM) applied in the presence of CGS 21680 did facilitate synaptic transmission.
Figure 4.
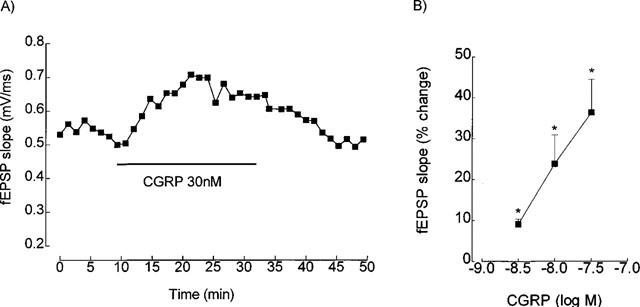
Enhancement caused by calcitonin gene-related peptide (CGRP) of the slope of field excitatory postsynaptic potentials (fEPSPs) recorded from the CA1 area of the rat hippocampal slices in the presence of the A2A receptor agonist, CGS 21680 (10 nM). (A) Shows the time course of the effect of CGRP obtained in one slice. Each point, in the ordinates, corresponds to the slope of the average of eight consecutive field EPSPs, and in the abscissae to the start of averaging. The time of perfusion of CGRP (30 nM) is indicated by the horizontal bar and CGS 21680 (10 nM) was always present. (B) Shows the percentage enhancement of the field EPSP slope caused by different concentrations of GCRP. Each point represents the mean±s.e.m. results obtained in three to five slices obtained from different animals. The concentration-response curves were obtained in a non-cumulative way, and in each slice CGRP was applied only once, and after a stable response to CGS 21680 (10 nM) was obtained. Average field EPSP slope in control conditions (0%, presence of 10 nM CGS 21680): 0.49±0.04 mV ms−1 (n=12). *P<0.05 as compared with 0% (Student's t-test).
Influence of PK-A blockade or KATP channels blockade on the effect of CGRP on synaptic transmission
To evaluate the influence of the activity of protein kinase-A (PK-A) on the effect of CGRP we tested whether HA-1004 or RpcAMPS, two PK-A inhibitors (Hidaka & Kobayashi, 1992; Rothermel & Parker-Bothelho, 1998), affected the ability of CGRP to enhance the slope of field EPSPs. When applied to hippocampal slices in the presence of DPCPX (10 nM), neither HA-1004 (10 μM) nor RpcAMPS (50 μM) caused appreciable changes in the slope of the field EPSPs (per cent change: −3.5±3.2%, n=5, for HA-1004 and −2.4±4.7%, n=3, for RpcAMPS); however, the subsequent application of CGRP (30 nM) to these slices failed to increase the slope of the field EPSPs (1.8±1.7%, n=5, in the HA-1004 experiments, and −0.76±3.2%, n=3, in the RpcAMPS experiments). As illustrated in Figure 5, CGRP (30 nM) increased the slope of the field EPSPs (16±1.4%, n=6, P<0.05) when applied to parallel slices in the presence of DPCPX (10 nM) but in the absence of the PK-A inhibitors.
Figure 5.

Blockade by the protein kinase A inhibitor, HA-1004 (10 μM), and by the inhibitor of KATP channels, glibenclamide (30 μM), of the facilitatory action of calcitonin gene related peptide (CGRP, 30 nM) on field excitatory postsynaptic potentials (EPSPs) recorded from the CA1 area of rat hippocampal slices. The recordings were obtained in the same experiment, using three hippocampal slices from the same hippocampus. Each panel shows two superimposed field EPSPs (each an average of eight consecutive responses) from the same slice obtained immediately before (control, C) and 20 min after CGRP perfusion, as indicated by the arrows. The effect of CGRP was tested first in the absence (upper panel), than in the presence of HA-1004 (middle panel) or glibenclamide (lower panel). The adenosine A1 receptor antagonist, DPCPX (10 nM) was present throughout the experiment. In this experiment, the per cent modification of the field EPSP slope caused by HA-1004 (10 μM) and glibenclamide (30 μM) was −0.5% and −6%, respectively.
In Figure 5 is also illustrated the ability of the inhibitor of ATP dependent potassium channels (KATP channels), glibenclamide, to prevent the excitatory effect of CGRP on synaptic transmission. By itself, and when applied to hippocampal slices in the presence of DPCPX (10 nM), glibenclamide (30 μM) caused a decrease (15±4.9%, n=7, P<0.05) of the slope of the field EPSPs. In six experiments using glibenclamide (30 μM) plus DPCPX (10 nM), CGRP (30 nM) failed to modify the EPSP slope in the presence of glibenclamide (per cent change: 1.7±0.7%) but increased EPSP slope (17±5%) in the absence of the KATP channels inhibitor.
Discussion
To observe an exciatory action of CGRP on synaptic transmission in the CA1 area of the hippocampus it was necessary to block A1 adenosine receptors with its selective antagonist, DPCPX or to activate A2A adenosine receptors with its selective agonist, CGS 21680. The enhancement of synaptic transmission caused by CGRP upon blockade of A1 adenosine receptors requires activation of excitatory A2A adenosine receptors by endogenous adenosine, since the selective A2A receptor antagonist ZM 241385, or adenosine deaminase, applied before CGRP, could prevent the facilitatory effect of this neuropeptide. Interestingly, when applied after the full effect of CGRP, ZM 241385 was unable to revert the excitatory action of the neuropeptide. This precludes the remote possibility that the A2A agonist was directly interfering with the CGRP receptors and suggests that tonic activation of A2A adenosine receptors is required for the induction but not for the maintenance of the excitatory action of CGRP on hippocampal synaptic transmission.
The ability of DPCPX and of CGS 21680 to trigger an excitatory action of CGRP on synaptic transmission in the hippocampus does probably not result solely from their facilitatory action on synaptic transmission, since (1) the simultaneous perfusion of DPCPX and ZM 241385 also caused an increase in the field EPSP slope but, under these conditions, CGRP was unable to facilitate synaptic transmission and (2) CGS 21680 was able to trigger an excitatory action of CGRP even in the experiments where the A2A agonist was unable to facilitate synaptic transmission.
A schematic diagram of the probable interplay between CGRP and A1 or A2A receptors is represented in Figure 6. It is known that the main tonus of endogenous adenosine in the hippocampus, at least in the experimental conditions used, is inhibitory (e.g. Dunwiddie & Diao, 1994; Cunha et al., 1996). Thus, the absence of effect of CGRP alone and its effect in the presence of DPCPX are highly suggestive that endogenous adenosine, by activating A1 receptors, is tonically restraining the action of CGRP. Relieve of this inhibition by DPCPX allows the initiation of the action of CGRP providing that the A2A receptors are operative to be activated by endogenous adenosine. Instead of blockade of A1 receptors, activation of A2A receptors with its agonist, CGS 21680, was also able to trigger an excitatory action of CGRP on synaptic transmission. It is known that A2A adenosine receptor activation by CGS 21680 inhibits A1 receptor functioning in the hippocampus (Cunha et al., 1994). Thus, the A1 adenosine receptors are less operative in the presence of CGS 21680 and this may contribute to the ability of the A2A agonist to trigger the facilitatory action of CGRP on synaptic transmission. However, this A2A/A1 adenosine receptor interaction is probably not the only mechanism by which A2A receptor activation induces the ability of CGRP to enhance synaptic transmission, since the effect of this neuropeptide was greater in the presence of the A2A agonist, CGS 21680, than in the presence of the A1 antagonist, DPCPX.
Figure 6.

Schematic diagram of the model proposed for the interactions between adenosine (ADO) and CGRP in the hippocampus. Tonic activation of A1 adenosine receptors by endogenous adenosine is indicated by the double arrow. An inhibitory interaction is indicated by (−) and a facilitatory interaction is indicated by (+).
CGRP receptors are positively coupled to adenylate cyclase in a variety of tissues (for a review see Wimalawansa, 1996), and evidence has been provided that stimulation of adenylate cyclase, increased production and accumulation of cyclic AMP, and activation of PK-A, mediates activation of KATP channels by GCRP (Wellman et al., 1998). The present observations that the excitatory action of CGRP on synaptic transmission is prevented by inhibitiors of PK-A, as well as by a KATP channel blocker, is also consistent with an involvement of PK-A and KATP channels in the action of CGRP in the hippocampus. A2A adenosine receptors are in most cases positively coupled to adenylate cyclase (see Sebastião & Ribeiro, 1996). It is conceivable that A2A receptor activation, by causing local enhancement of cyclic AMP levels, acts as a primer of the transducing system operated by CGRP in the hippocampus, which might explain the presently observed positive interaction between A2A receptors activation and the action of CGRP on synaptic transmission.
The predominant inhibitory tonus by endogenous adenosine in the hippocampus (Dunwiddie & Diao, 1994), might explain why CGRP by itself is unable to affect synaptic transmission. This contrasts with what occurs at motor nerve terminals, where endogenous adenosine tonically activates A2A receptors (Correia-de-Sá et al., 1996), and CGRP by itself facilitates neurotransmitter release (Correia-de-Sá & Ribeiro, 1994). Interestingly, as it occurs in the hippocampus (present results), blockade of A2A receptors prevents the facilitatory action of CGRP at motor nerve terminals (Correia-de-Sá & Ribeiro, 1994).
In conclusion, the results presented in this paper show that the ability of CGRP to facilitate synaptic transmission in the CA1 area of the hippocampus is under tight control by adenosine, with the A1 receptors ‘braking' the action of CGRP and the A2A receptors ‘triggering' this action. The need of A2A receptor activation by endogenous adenosine to reveal the excitatory action of CGRP on synaptic transmission in the hippocampus, taken together with the observation that the induction of the excitatory action of CGRP on synaptic transmission caused by CGS 21680 was more evident and consistent than the enhancement of synaptic transmission caused by the A2A receptor agonist itself, suggests that a main role A2A adenosine receptors in the hippocampus is to modulate the action of other neuromodulators and, by this process, to contribute for a sophisticated ‘fine tune' of hippocampal functioning.
Acknowledgments
Work supported by Fundação para a Ciência e Tecnologia and European Union. The authors acknowledge Dr. W.W. Anderson (University of Bristol, U.K.) the kind gift of the data analysis (LTP) program. The gift of ZM 241385 by ZENECA is also acknowledged.
Abbreviations
- ADA
adenosine deaminase
- CGRP
calcitonin gene-related peptide
- CGS 21680
2-[p-(2-carboxyethylphenethylamino]-5′-N-ethylcarboxamide adenosine
- DPCPX
1,3-dipropyl-8-cyclopenthyl xanthine
- EPSP
excitatory post-synaptic potential
- HA-1004
N-(2-guanidinoethyl)-5-isoquinolinesulfonamide (HA-1004)
- KATP channels
ATP-sensitive potassium channels
- PK-A
protein kinase-A
- Rp-cAMPS
adenosine 3′,5′-cyclic monophosphorothioate
- ZM 241385
(4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5ylamino]ethyl) phenol)
References
- AFONSO F., SEBASTIÃO A.M., PINHO M.S., FERNANDES P., RIBEIRO J.A., MATA L.R., GULBENKIAN S. Calcitonin gene-related peptide in the hamster seminal vesicle and coagulating gland. An immunohistochemical, autoradiographical and pharmacological study. Peptides. 1996;17:1189–1195. doi: 10.1016/s0196-9781(96)00183-0. [DOI] [PubMed] [Google Scholar]
- AIYAR N., GRIFFIN E., ALBRIGHTSON-WINSLOW C., FEUERSTEIN G., NAMBI P. Homologous desensitization of calcitonin gene-related peptide response in rat glomerular mesangial cells in culture. Mol. Cell Biochem. 1992;113:17–23. doi: 10.1007/BF00230881. [DOI] [PubMed] [Google Scholar]
- AMARA S.G., JONAS V., ROSENFELD M.G., ONG E.S., EVANS R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- ANDERSON W.W., COLLINGRIDGE G.L. A data acquisition program for online analysis of long-term potentiation and long-term depression. Neurosci. Abst. 1997;23:665. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- BOUCHARD P., MONNET F., BERGERON R., ROMAN F., JUNIEN J.-L., DE MONTIGNY C., DEBONNEL G., QUIRION R. In vivo modulation of sigma receptor sites by calcitonin gene-related peptide in the mouse and rat hippocampal formation: radioligand binding and electrophysiological studies. Eur. J. Neurosci. 1995;7:1952–1962. doi: 10.1111/j.1460-9568.1995.tb00718.x. [DOI] [PubMed] [Google Scholar]
- BULLOCH K., MILNER T.A., PRASAD A., HSU M., BUZASAKI G., MCEWEN B.S. Induction of calcitonin gene-related peptide-like immunoreactivity in hippocampal neurons following ischaemia: a putative regional modulator of the CNS injury/immune response. Exp. Neurol. 1998;150:195–205. doi: 10.1006/exnr.1997.6765. [DOI] [PubMed] [Google Scholar]
- BULLOCH K., PRASAD A., CONRAD C.D., MCEWEN B.S., MILNER T.A. Calcitonin gene-related peptide level in the rat dentate gyrus increases after damage. Neuro Report. 1996;7:1036–1040. doi: 10.1097/00001756-199604100-00016. [DOI] [PubMed] [Google Scholar]
- CORREIA-DE-SÁ P., RIBEIRO J.A. Potentiation by tonic A2a-adenosine receptor activation of CGRP-facilitated [3H]ACh release from rat motor nerve endings. Br. J. Pharmacol. 1994;111:582–588. doi: 10.1111/j.1476-5381.1994.tb14777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORREIA-DE-SÁ P., TIMÓTEO M.A., RIBEIRO J.A. Presynaptic A1-inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J. Neurophysiol. 1996;76:3910–3919. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., CONSTANTINO M.D., RIBEIRO J.A. ZM is an antagonist of the facilitatory responses produced by the A2A adenosine receptor agonists CGS21680 and HENECA in the rat hippocampus. Br. J. Pharmacol. 1997;122:1279–1284. doi: 10.1038/sj.bjp.0701507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA R.A., CORREIA-DE-SÁ P., SEBASTIÃO A.M., RIBEIRO J.A. Preferential activation of excitatory adenosine receptors at rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br. J. Pharmacol. 1996;119:253–260. doi: 10.1111/j.1476-5381.1996.tb15979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA R.A., JOHANSSON B., VAN DER PLOEG I., SEBASTIÃO A.M., RIBEIRO J.A., FREDHOLM B.B. Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res. 1994;649:208–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- DUNWIDDIE T.V., DIAO L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J. Pharmacol. exp. Ther. 1994;268:537–545. [PubMed] [Google Scholar]
- FREUND T.F., HÁJOS N., ACSÁDY L., GÖRCS T.J., KATONA I. Mossy cells of the rat dentate gyrus are immunoreactive for calcitonin gene-related peptide (CGRP) Eur. J. Neurosci. 1997;9:1815–1830. doi: 10.1111/j.1460-9568.1997.tb00748.x. [DOI] [PubMed] [Google Scholar]
- HIDAKA H., KOBAYASHI R. Pharmacology of protein kinase inhibitors. Annu. Rev. Pharmacol. Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- ISHIDA-YAMAMOTO, TOHYAMA M. Calcitonin gene-related peptide in the nervous system. Prog. Neurobiol. 1989;33:355–386. doi: 10.1016/0301-0082(89)90006-3. [DOI] [PubMed] [Google Scholar]
- LI H., HENRY J.L. Adenosine A2 receptor mediation of pre- and postsynaptic excitatory effects of adenosine in rat hippocampus in vitro. Eur. J. Pharmacol. 1998;347:173–182. doi: 10.1016/s0014-2999(98)00105-8. [DOI] [PubMed] [Google Scholar]
- OLIVER K.R., WAINWRIGHT A., KINSEY A.M., HEAVENS R.P., SIRINATHSINGHJI D.J., HILL R.G. Regional and cellular localization of calcitonin gene-related peptide-receptor component protein mRNA in the guinea-pig central nervous system. Mol. Brain Res. 1999;66:105–210. doi: 10.1016/s0169-328x(99)00036-4. [DOI] [PubMed] [Google Scholar]
- POYNER D.R. Calcitonin gene-related peptide: multiple actions, multiple receptors. Pharmacol. Ther. 1992;56:23–51. doi: 10.1016/0163-7258(92)90036-y. [DOI] [PubMed] [Google Scholar]
- RIBEIRO J.A. Adenosine A2A receptor interactions with receptors for other neurotransmitters and neuromodulators. Eur. J. Pharmacol. 1999;375:101–113. doi: 10.1016/s0014-2999(99)00230-7. [DOI] [PubMed] [Google Scholar]
- ROTHERMEL J.D., PARKER-BOTHELHO L.H. A mechanistic and kinetic analysis of the interactions of the diasterioisomers of adenosine 3′,5′-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem. J. 1998;251:757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTICIOLI P., DEL BIANCO E., MAGGI C.A. Adenosine A1 receptors mediate the presynaptic inhibition of calcitonin gene-related peptide release by adenosine in the rat spinal cord. Eur. J. Pharmacol. 1993;231:139–142. doi: 10.1016/0014-2999(93)90695-e. [DOI] [PubMed] [Google Scholar]
- SANTICIOLI P., MAGGI C.A. Inhibitory transmitter action of calcitonin gene-related peptide in guinea-pig ureter via activation of glibenclamide-sensitive K channels. Br. J. Pharmacol. 1994;113:588–592. doi: 10.1111/j.1476-5381.1994.tb17030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID-ANTOMARCHH, DE WEILLE J., FOSSET M., LAZDUNSKI M. The receptor for the antidiabetic sulfonylureas controls the activity of the ATP-modulated K+-channel in insulin-secreting cells. J. Biol. Chem. 1987;262:15840–15844. [PubMed] [Google Scholar]
- SEBASTIÃO A.M., MACEDO M.P., RIBEIRO J.A. ‘Fine tune' modulation of neurotransmission in the hippocampus by adenosine. Drug Development Res. 1998;43:59. [Google Scholar]
- SEBASTIÃO A.M., MACEDO M.P., CUNHA-REIS D., RIBEIRO J.A. Tonic adenosine A1 and A2a receptors activation modulate facilitatory actions of neuropeptides on transmission in the rat hippocampus. Br. J. Pharmacol. 1999;127:140P. [Google Scholar]
- SEBASTIÃO A.M., RIBEIRO J.A. Evidence for the presence of excitatory A2 adenosine receptors in the rat hippocampus. Neurosci. Letts. 1992;138:41–44. doi: 10.1016/0304-3940(92)90467-l. [DOI] [PubMed] [Google Scholar]
- SEBASTIÃO A.M., RIBEIRO J.A. A2 receptor mediated excitatory actions of adenosine in the nervous system. Prog. Neurobiol. 1996;48:167–189. doi: 10.1016/0301-0082(95)00035-6. [DOI] [PubMed] [Google Scholar]
- SEBASTIÃO A.M., STONE T.W., RIBEIRO J.A. On the inhibitory adenosine receptor at the neuromuscular junction and hippocampus of the rat: antagonism by 1,3,8-substituted xanthines. Br. J. Pharmacol. 1990;101:453–459. doi: 10.1111/j.1476-5381.1990.tb12729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLMAN G.C., QUAYLE J.M., STANDEN N.B. ATP-sensitive K+-channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J. Physiol. 1998;507:117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIMALAWANSA S.J. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocrine Rev. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- ZANG L., BONEV A.D., MAWE G.M., NELSON M.T. Protein kinase A mediates activation of ATP-sensitive K+-currents by CGRP in gallbladder smooth muscle. Am. J. Physiol. 1994;267:G494–G499. doi: 10.1152/ajpgi.1994.267.3.G494. [DOI] [PubMed] [Google Scholar]


