Abstract
To study the role of interleukin (IL)-4 in the onset of contact hypersensitivity (CH) in mice, the effect of IL-4 gene-depletion and anti-IL-4 monoclonal antibody treatment on dinitrofluorobenzene (DNFB)-induced CH was examined. Simultaneously, to clarify the effect of background gene, DNFB-induced CH in BALB/c and C57BL/6 mice was compared.
Five repeated topical applications of DNFB to the ears of mice resulted in CH of the ears in terms of increases in ear thickness and histopathological changes. The magnitude of ear thickness increase in BALB/c mice was almost three times greater than that in C57BL/6 mice.
The CH in BALB/c mice was significantly suppressed by IL-4 gene-depletion and anti-IL-4 monoclonal antibody treatment. In contrast, the symptoms of dermatitis in C57BL/6 mice were slightly affected by the same treatment. These changes corresponded well to the production of specific IgE antibody.
Total IgE antibody production and the expression of productive Cε mRNA were dramatically suppressed by IL-4 gene-depletion and anti-IL-4 treatment in BALB/c and C57BL/6 mice. Neither total IgG nor IgM levels in either strain of mice was altered by depletion of IL-4.
The expression of IFN-γ in the skin lesion was dramatically suppressed by IL-4 gene-depletion in BALB/c mice, but not in C57BL/6 mice.
These findings indicate that IL-4 plays an important role in the onset of DNFB-induced CH in BALB/c mice, but not in C57BL/6 mice.
Keywords: IL-4, BALB/c, C57BL/6, contact hypersensitivity, gene depletion
Introduction
Our previous studies demonstrated that five repeated topical applications of 2,4-dinitrofluorobenzene (DNFB) to the ears of BALB/c mice resulted in contact hypersensitivity (CH) of the ears as well as significant elevations of dinitrophenyl (DNP) specific IgE antibody and total IgE antibody in the serum (Nagai et al., 1997a,1997b; 1999). Severe eczematic histopathological changes and high expression of interleukin (IL)-2 and interferon (IFN)-γ mRNAs were observed in ear lesions. Simultaneously, high expressions of IL-4, IL-5, and germline and productive Cε mRNAs were detected in the lymph nodes. These results suggest the participation of T helper (Th) 1 cells for the onset of dermatitis in the skin lesions, and the participation of Th2 cells for the production of IgE antibody in the lymph node.
As for the role of cytokines for the development of CH, some investigators have reported the main role of Th1 cell-derived cytokines including IL-12 and IFN-γ (Abe et al., 1996; Riemann et al., 1996; Xu et al., 1996; 1998). In addition, some other investigators pointed out the inhibitory effect of IL-10, a Th2 cell-derived cytokine, on the development of CH (Maguire et al., 1997; Meng et al., 1998). In addition to IL-10, the role of IL-4 in the development of CH was also reported, because IL-4 is well known as a key cytokine in the development of T cells, especially Th2 cells (Brown & Hural, 1997; Lewis et al., 1993; Tepper et al., 1990). But the results were inconstant. Gautam et al. (1992) reported the suppressive effect of IL-4 in the development of CH, but Salerno et al. (1995) and Weigman et al. (1996) have pointed out the phlogistic activity of IL-4.
The present study was, therefore, conducted to clarify the role of IL-4 in T cell-mediated CH by employing IL-4 gene-deficient mice and anti-IL-4 monoclonal antibody treatment.
Moreover, some researchers have pointed out that the disease susceptibility of gene-targeted mice is strongly affected by the background genes and mouse strain (Frankel, 1998; Fujii et al., 1997; Lohoff et al., 1998; Mulle et al., 1998; Postma et al., 1995; Petit-Frere et al., 1993; Schauwecker & Steward, 1997; Sjölander et al., 1998). Mice from two different backgrounds, BALB/c and C57BL/6 strains, were used to research the roles of IL-4 in the development of DNFB-induced CH.
Methods
Animals
Female BALB/c and C57BL/6 background IL-4 gene-deficient mice were purchased from Jackson Laboratory (U.S.A.). Female BALB/c and C57BL/6 mice were also purchased from Jackson Laboratory. Mice were housed in plastic cages in an air-conditioned room at 24°C, fed a standard laboratory diet, and given water ad libitum. All experiments were carried out following the guidelines for the care and use of experimental animals by the Japanese Association for Laboratory Animal Science in 1987.
Reagents and antibodies
DNFB was purchased from Nacalai Tesque Inc., Tokyo, Japan. Monoclonal anti-mouse IgE antibody (LO-ME-3, Serotec Co. Ltd., Oxford, U.K.), peroxidase-conjugated streptavidin (Dakopatts a/s, Glostrup, Denmark), bovine serum albumin (BSA for ELISA grade, Sigma Chemical Co. Ltd., St. Louis, MO, U.S.A.), DNP-BSA (LSL Co. Ltd., Tokyo, Japan), NHS-LC-Biotinylation kit (Pierce Chemical Co. Ltd., Rockford, IL, U.S.A.), peroxidase labelled anti-mouse IgE (Nordic Immunology Co. Ltd., Tilbug, Netherlands), goat anti-mouse IgG and IgM, peroxidase labelled goat anti-mouse IgG and IgM (Organon Teknika Co. Turnhour, Belgium), substrate kit (Sumitomo Bakelite Co. Ltd., Tokyo, Japan), ISOGEN (Nippon Gene Co. Ltd., Tokyo, Japan), PCR primers (β-action, IFN-γ, IL-4, IL-5, productive Cε; Stratagene Co. Ltd., La Jolla, CA, U.S.A.), 1st-STRANDED TM cDNA Synthesis kit (Clontech Lab, Inc., Palo Alto, CA, U.S.A.), and GeneAmp PCR Reagent kit with AmpliTaq DNA Polymerase (Perkin Elimer Japan Co. Ltd., Urayasu, Japan) were purchased.
The monoclonal anti DNP IgE-producing cell line, EC1, was established by fusing spleen cells from a DNP-Ascaris extract-primed BALB/c mouse with P3X63-Ag8, 653 (8-azaguanine-resistant, IgG-nonsecreting). EC1 cells were maintained in a medium containing equal volumes of RPMI 1640 (Gibco, BRL, Grand Island, NY, U.S.A.) and DMEM (high glucose, Gibco) supplemented with 10% foetal bovine serum (Irvine Scientific, Santa Arta, CA, U.S.A.) 100 units ml−1 penicillin-G, and 100 μg ml−1 streptomycin in a humidified atmosphere of 5% CO2 at 37°C. After reaching a confluent state, cells were recovered by gentle flushing, mixed with 3–5 times volume of fresh medium, and seeded in new culture flasks. Under the culture condition, cells usually reached confluence in 3 days. Finally, culture supernatant was separated by centrifugation at 400 ×g for 10 min. The cell-free fluid was stored at −80°C and used as a source of IgE. The maximum dilution of the preparation that gave a positive passive cutaneous anaphylaxis in Wistar rats challenged with DNP-bovine serum albumin was 1:1024.
Procedure for allergic dermatitis by repeated painting with DNFB
Experiments were carried out by the method previously described (Nagai et al., 1997a,1997b; 1999). Mouse ears were painted with DNFB or vehicle (acetone:olive oil (3:1)) once each week for 5 weeks. Twenty-five microliters of 0.15% DNFB in vehicle was applied to each side of both ears. Ear thickness was measured using an engineering micrometer (R1-A, Ozaki MFG Co. Ltd., Tokyo, Japan) and expressed as the increase in thickness from time 0. Mouse ears were removed 24 h after the fifth painting and fixed with 10% neutral formalin. Ears were then cut into parasagittal slices, dehydrated, and embedded in paraffin by standard procedures. Paraffin sections were stained with hematoxylin and eosin and assessed by light microscopy. Individual inflammatory cell types were counted in high power fields (HPF; ×200).
Determination of hapten-specific IgE titer and immunoglobulin concentrations in mouse serum
Hapten-specific IgE (sIgE) and immunoglobulin concentrations (total IgE: tIgE, total IgG: tIgG and total IgM: tIgM) in mouse serum were measured using the enzyme-linked immunosorbent assay (ELISA) described below. To measure the concentration of each immunoglobulin, serum was obtained from the mice 24 h after each application of DNFB.
We measured the hapten sIgE titer by captured ELISA (Hirano et al., 1989; Hamid et al., 1994). Briefly, immunoplates (EIA II Plus Microtitration plate, Flow Laboratories, Inc., U.S.A.) were coated with monoclonal anti-mouse IgE antibodies and incubated at 4°C overnight. The plates were blocked with phosphate buffered saline (PBS) containing 1% BSA, incubated at room temperature for 1 h, and washed three times with PBS containing 0.2% Tween 20 (T-PBS). Monoclonal anti-DNP IgE antibody (monoclonal anti-DNP IgE, clone SPE-7, Sigma, MO, U.S.A.) was sequentially diluted as a standard. Diluted serum sample (100 μl) was added to each well and the plates were incubated at room temperature for 1 h. After washing with T-PBS, 100 μl diluted biotinylated DNP-BSA was added to each well and incubated at room temperature for 1 h. After extensive washing with T-PBS, 100 μl diluted peroxidase-conjugated streptavidin was added to each well. The enzymatic reaction was stopped by adding 100 μl stop solution after incubation at room temperature for 1 h. The optical density of the reaction mixture was read using an automatic ELISA plate reader (Titertek Multiscan MCC/340, Flow Laboratories, Inc., U.S.A.) at 450 nm.
To measure total immunoglobulin concentrations (tIgE, tIgG and tIgM), immunoplates were coated with diluted monoclonal anti-IgE, goat anti-mouse IgG, or goat anti-mouse IgM antibodies, respectively by incubating overnight at 4°C. The plates were blocked as described above and washed three times with T-PBS. Standard curves were generated as described above by employing standard IgE, standard mouse IgG (Miles Scientific, IL, U.S.A.) and standard IgM (Miles). Serum sample (100 μl) was added to each well and incubated at room temperature for 1 h. ELISA was performed using peroxidase-conjugated anti-mouse IgE, IgG and IgM. The ELISA data compared with the standards added to each plate were analysed using the DELTA Soft program for the Macintosh computer. The sIgE, tIgE, and tIgM titers are expressed in μg ml−1 based on laboratory-generated standards and appropriate commercial standards.
Analysis of cytokine messenger RNA (mRNA) expression in mouse auricular lymph nodes and ears by RT–PCR
Changes in cervical lymph node- and ear-derived cytokine mRNA levels were assessed by reverse transcriptase-polymerase chain reaction (RT–PCR) using a thermal cycler (Bio Metra Trio-Thermoblock, Bio Metra Co. Ltd., Germany). Using ISOGEN, total RNA was prepared from the ears and lymph nodes of mice 4 h after five applications of either vehicle or DNFB. The amount of total RNA in each sample was measured spectophotometrically at 260 nm and quality was checked by electrophoresis. RT–PCR was performed as follows: total RNA (500 ng) was reverse-transcribed for 60 min at 42°C using the 1st-STRAND TM cDNA Synthesis kit. Each cDNA sample was amplified in a total volume of 100 μl containing 0.5 μM of each primer (RT–PCR primers set from the GeneAmp PCR Reagent kit). The internal control was β-actin. The mixture was overlaid with mineral oil and put in the thermal cycler for 35 cycles. RT–PCR was performed on β-actin, IFN-γ, IL-4 and IL-5. The PCR products were resolved by electrophoresis and stained with ethidium bromide. Samples were obtained from two mice whose ears and cervical lymph nodes were removed 4 h after the fifth application of DNFB. RT–PCR was semi-quantified by densitometrically scanning photo negatives taken with a Polaroid camera (Polaroid 665 film, Polaroid Corp., Cambridge, MA, U.S.A.). For semiquantification, the densitometry value of each cytokine was normalized to that of the house keeping gene, β-actin, which was not affected by the DNFB concentrations applied in this study. In addition, a linear correlation between RNA input and PCR product was examined. Fair linearity was obtained between the density value of PCR products and RNA input. All PCR amplifications were performed at least twice with multiple sets of experimental RNAs.
Analysis of productive Cε gene expression by RT–PCR
The productive Cε mRNA is expressed prior to IgE antibody production, and the ε heavy chain of IgE antibody is translated from productive Cε mRNA (Stavnezer-Nandgren & Sirlin, 1986; Jabara et al., 1990; Thyphronitis et al., 1993). RT–PCR was performed as described above to assess the changes in cervical lymph node-derived productive Cε mRNA levels. RNA (500 ng) was reverse-transcribed for 60 min at 42°C using the 1st STRAND cDNA synthesis kit. The cDNA samples were amplified in a total volume of 100 μl containing 5′ and 3′ primers (primers sequences for productive Cε, 5′ primer j4: TGGACTACTGGGGTCAAGG, 3′ primer Cε2: AGCGATGAATGGAGTAGC, Thyphronitis et al., 1993) with GeneAmp PCR reagents (GeneAmp PCR Reagent kit with AmpliTaq DNA polymerase).
The mixture underwent a 5 min denaturation at 94°C, 5 min annealing at 60°C, and then 30 cycles of 1.5 min at 72°C, 1.5 min at 94°C, and 1.5 min at 60°C, with a final extension of 10 min at 72°C. RT–PCR was performed on β-actin and productive Cε. The internal control was β-actin. Each PCR product was resolved by electrophoresis and visualized with ethidium bromide. Auricular lymph nodes were obtained 4 h after the fifth applications with DNFB.
IgE-mediated biphasic cutaneous reaction
This method was described previously (Nagai et al., 1995; 19971997). In brief, mice received two i.v. injections (5 min-interval) of 0.5 ml monoclonal IgE preparation. Twenty-four hours after sensitization, a skin reaction was elicited by applying 25 μl of 0.15% DNFB acetone-olive oil solution to both sides of both ears. The reaction was assessed by measuring ear thickness using an engineering micrometer (Ozaki) at different times after challenge. Data were exposed as an increase in ear thickness after antigen challenge by subtracting the value measured immediately before challenge. Because we had previously confirmed that the vehicle for the monoclonal IgE preparation did not affect the skin reaction in this experimental protocol, we injected saline into control mice instead of the IgE preparation.
Statistical analysis
Results are expressed as the mean±s.e.mean. To define statistical significant differences between two groups were analysed by Student's t-test and three groups or more were subjected one-way analysis of variance (ANOVA) followed by Dunnett's multiple range test. P values less than 0.05 were considered significant.
Results
CH in IL-4 gene-deficient mice
Repeated topical applications of DNFB to the ears provoked typical contact dermatitis. Figure 1A shows the time course of the changes in ear thickness, which increased in proportion to the number of applications of DNFB in BALB/c and C57BL/6 mice. Ear thickness at time 0 was 22.5±0.19×10−2 mm in BALB/c and 23.3±0.15×10−2 mm in C57BL/6 mice. The increase in ear thickness 24 h after the fifth application was 19.5±0.21×10−2 mm in BALB/c mice and 7.2±0.11×10−2 mm in C57BL/6 mice. As shown in Figure 1B, when the increase in ear thickness was calculated by the area under the curve (AUC) over 5 weeks, BALB/c mice showed an almost 3 fold increase over C57BL/6 mice. The increase in AUC of ear thickness was dramatically decreased in IL-4 gene-deficient BALB/c mice when compared to the response in BALB/c wild type mice (Figure 2A). In C57BL/6 mice, the increase in ear thickness was not affected by depletion of IL-4 gene. Similar results were obtained by treatment with anti-IL-4 monoclonal antibody (Figure 2B). Treatment with anti-IL-4 monoclonal antibody (2 mg) clearly suppressed the increase in ear thickness in BALB/c mice, but not in C57BL/6 mice. Figure 3 shows the histopathological changes in the mice skin lesion 24 h after the fifth application of DNFB. Marked infiltration of inflammatory cells such as monocytes, eosinophils and neutrophils, and epidermal hypertrophy were evident in DNFB-treated wild type BALB/c mice (Figure 3B) compared to vehicle-treated mice (Figure 3A). In IL-4 gene-deficient mice, only a slight infiltration of inflammatory cells and epidermal hypertrophy were observed after the treatment with DNFB (Figure 3D). Regarding eosinophils, no infiltration was observed in IL-4 gene-deficient BALB/c mice whereas evident infiltration was observed in wild type after five repeated DNFB treatments. In C57BL/6 mice, marked infiltration of cells, mainly monocytes, and epidermal hypertrophy were observed after the fifth application of DNFB. Moderate histopathological changes were observed in IL-4 gene-deficient C57BL/6 mice after the application of DNFB. No significant change was observed by the treatment with vehicle in both BALB/c and C57BL/6 wild type mice (Figure 3A,E). Moderate infiltration of inflammatory cells and epidermal hypertrophy were observed in IL-4 gene deficient BALB/c and C57BL/6 mice (Figure 3D,H). Treatment with anti-IL-4 monoclonal antibodies shows similar results (data not shown).
Figure 1.
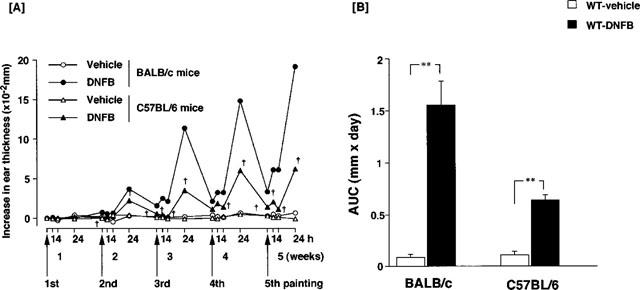
Ear swelling in BALB/c and C57BL/6 mice caused by repeated applications of dinitrofluorobenzene (DNFB). Mice received a topical application of 0.15% DNFB in acetone and olive oil or vehicle on the ears once a week for 5 weeks. Each point indicates the mean of five or six mice. (A) Time course for an increase in ear thickness. †Significant difference from BALB/c mice at P<0.01. h: Time after each painting. (B) Area under the curve (AUC) of an increase in ear thickness over 5 weeks. **Significant difference from DNFB treated mice at P<0.01.
Figure 2.
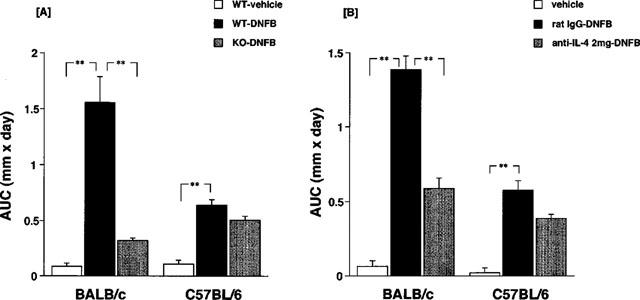
Effect of IL-4 gene depletion (A) and anti-IL-4 (B) monoclonal antibody treatment on contact hypersensitivity (CH) caused by repeated applications of DNFB. Each column consists of the mean±s.e.mean of six or seven animals. **(A) Significant difference from DNFB treated wild type mice at P<0.01. (B) Significant difference from rat IgG and DNFB treated mice.
Figure 3.
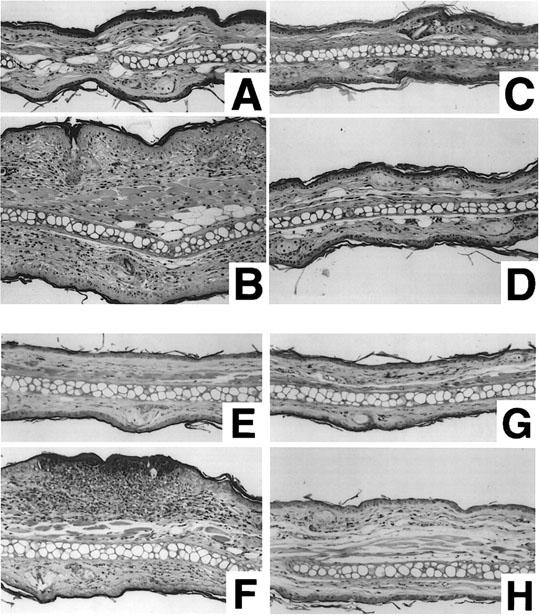
Histopathological changes of mice ear lesion. Each sample was obtained 24 h after the fifth application and fixed with 10% neutral formalin. Sections were stained with hematoxylin and eosin (× 200). (A) vehicle-treated wild type BALB/c mice, (B) DNFB-treated wild type BALB/c mice, (C) vehicle-treated IL-4 gene-depleted BALB/c mice, (D) DNFB-treated IL-4 gene-depleted BALB/c mice, (E) Vehicle-treated wild type C57BL/6 mice, (F) DNFB-treated wild type C57BL/6 mice, (G) Vehicle-treated IL-4 gene-depleted C57BL/6 mice, (H) DNFB treated IL-4-depleted C57BL/6 mice.
IgE production
As indicated in Table 1, the levels of hapten specific IgE significantly increased in the serum of BALB/c mice after five applications of DNFB. No hapten specific IgE was recognized in the serum of C57BL/6 wild type mice after the DNFB application. The elevation of total IgE level in IL-4-deficient and anti-IL-4-treated mice were significantly low compared to that of those in wild type mice. In C57BL/6 mice, the elevation of total IgE level by DNFB was almost one third of that to BALB/c wild type mice. tIgG and tIgM levels were not changed significantly by the sensitization with DNFB and depletion of IL-4 in either strains of mice. The expression of productive Cε in lymph nodes by DNFB sensitization was dramatically decreased by IL-4 gene-depletion (Figure 4), and anti-IL-4 monoclonal antibody treatment (data not shown).
Table 1.
Quantitative analysis in immunoglobulin levels in IL-4 gene-depletion and anti-IL-4 monoclonal antibody treated BALB/c and C57BL/6 mice

Figure 4.
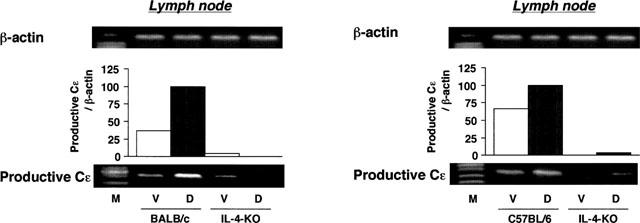
Expression of productive Cε mRNA in cervical lymph nodes of BALB/c and C57BL/6 IL-4 gene-depleted and wild type mice. Mice received a topical application of 0.15% of DNFB in acetone and olive oil (vehicle) on the ears once a week for 5 weeks. Cervical lymph nodes were obtained 4 h after the fifth application. The expression of productive Cε mRNA was examined using RT–PCR. M: size marker, V: vehicle, D: DNFB.
The expression of cytokine mRNAs in lymph nodes and skin lesions
To investigate the mechanism of differing susceptibility to IL-4-depletion of BALB/c and C57BL/6 mice, the antigen-induced expression of cytokine mRNAs in aulicular lymph nodes and skin lesions were examined. The expression of IFN-γ and IL-13 mRNAs in lymph nodes, and IFN-γ mRNA in the ears were indicated in Figure 5. Semiquantitative analysis of the expression of mRNAs was indicated in Table 2. The expression of each cytokine mRNA in C57BL/6 was significantly lower than that in BALB/c mice (Table 2). The expression of TH1 cytokines (IFN-γ and IL-2) mRNA was slightly changed whereas Th2 cytokine mRNAs expression was significantly decreased in DNFB-treated IL-4 gene-deficient mice compared to those of wild type mice. The magnitude of the IFN-γ mRNA expression in ear lesions was dramatically decreased by IL-4 gene-depletion in BALB/c mice, but not in C57BL/6 mice.
Figure 5.
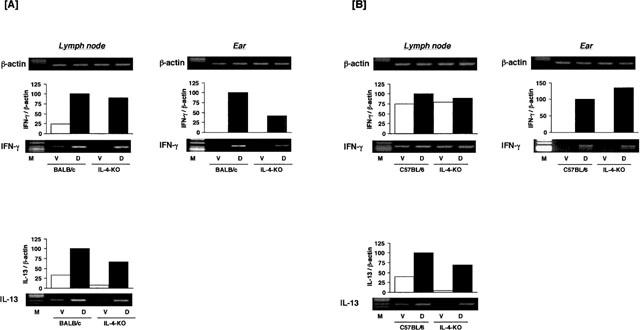
Expression of cytokine mRNAs in the ears and cervical lymph nodes of BALB/c and C57BL/6 IL-4 gene-depleted and wild type mice treated with DNFB. Mice received a topical application of 0.15% DNFB in acetone and olive oil (vehicle) on the ears once a week for 5 weeks. The ears and cervical lymph nodes were obtained 4 h after the fifth application. The expression of cytokine mRNA was examined using RT–PCR. M: size marker, V: vehicle, D: DNFB.
Table 2.
Semiquantitative analysis of the expression of cytokine mRNAs in lymph nodes and skin lesions
IgE antibody-mediated biphasic cutaneous reaction
Mice were sensitized by intravenous injections of anti-DNP IgE monoclonal antibody and challenged with an antigen epicutaneously. Results of a time-course study are shown in Figure 6. Intravenous administration of IgE and the subsequent epicutaneous antigen (DNFB) challenge induced a biphasic cutaneous reaction in wild type and IL-4-deficient mice of BALB/c and C57BL/6 strains. A significant increase in ear thickness was observed at 1 and 24 h after antigen challenge in sensitized BALB/c mice (Figure 6A). The magnitude of the responses at 1 and 24 h after antigen challenge was identical in wild type and IL-4-deficient mice. In C57BL/6 mice, responses at 1 and 4 h after antigen challenge were not observed, whereas a clear increase in ear thickness appeared at 24 h (Figure 6B). The magnitude of the response at 24 h was almost identical between wild type and IL-4-deficient mice for both strains.
Figure 6.
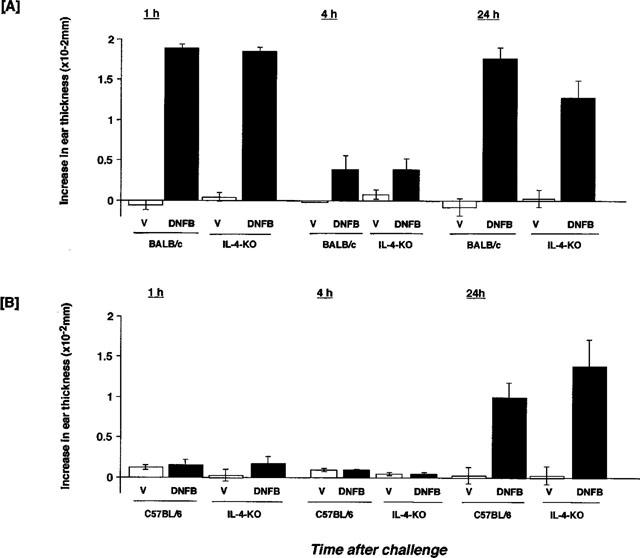
IgE-mediated biphasic cutaneous reaction in BALB/c and C57BL/6 IL-4-gene-depleted mice. Mice received an intravenous injection of 1.0 ml of anti-DNP IgE antibody 24 h before application of 0.15% DNFB. Each value represents the mean±s.e.mean of four or five mice.
Discussion
In this study, we demonstrated that IL-4 plays an important role in the development of DNFB-induced CH in BALB/c mice but not in C57BL/6 mice. Simultaneously, the present findings indicate that gene related responsibility to the IgE production may correlate well to the susceptibility of DNFB-induced CH in mice.
Strain related differences in susceptibility to some experimental diseases in mice have been widely reported (Frankel 1998; Fujii et al., 1997; Lohoff et al., 1998; Mulle et al., 1998; Petit-Frere et al., 1993; Postma et al., 1995; Schauwecker & Steward, 1997; Sjölander et al., 1998). In the present study, C57BL/6 mice showed low responsiveness to DNFB-induced IgE production and CH. BALB/c mice are high responders in IgE antibody production against nematode infection, whereas C57BL/6 mice are low responders (Lohoff et al., 1998; Sjölander et al., 1998). Similar results were obtained in the present study. BALB/c mice showed high IgE responses after five applications of DNFB, but low total IgE and no antigen specific IgE responses were observed in C57BL/6 mice. Shanker & Titus (1995) reported that resisting the production of IgE antibodies against nematode infection in C57BL/6 mice is dependent on both T cell and non-T cell compartments. In the present study, clear expression of productive Cε mRNA and elevation of total IgE antibody in the serum were detected in C57BL/6 mice after five applications of DNFB, although hapten specific IgE was not detected in C57BL/6 mice sera. Therefore, the responsiveness of T and B cells in IgE production might be normal but low in C57BL/6 mice under the present experimental conditions. The reason why C57BL/6 mice showed low responsibility of DNFB-induced IgE production is still obscure. Since C57BL/6 mice lacks the production of IL-9 is probably one of the reasons, because IL-9 is an evident potentiating factor in IgE production (Nicolaides et al., 1997). The experiment to study the role of IL-9 in DNFB-induced CH will be necessary. Since the difference in CH response between wild type and IL-4-deficient BALB/c and C57BL/6 mice is well matched to the antigen specific IgE response, different susceptibility to IL-4-depletion may be closely related to the responsibility of antigen specific IgE production in both strains. The C57BL/6 strain is reported to be deficient in low molecular weight secretory phospholipase A2, and mast cell protease as well as IL-9 (Murakami et al., 1997). The mast cell dysfunction may be one of another reason in low responsiveness to CH in C57BL/6 mice. Further experiments are necessary to analyse the genetic components to clarify the low responsiveness of C57BL/6 mice in IgE antibody production and CH. Additionally, the genetic components that mediate the high responsiveness of BALB/c mice to IgE responses will also be investigated.
Regarding the role of IL-4 in CH, conflicting results have been reported (Gautam et al., 1992; Salerno et al., 1995; Weigmann et al., 1996). Whereas in studies by Gautam et al. (1992), IL-4 inhibited the development of CH, and treatment of mice with anti-IL-4 monoclonal antibody augmented the response, the reverse was reported by Salerno et al. (1995). In addition, Weigmann et al. (1996) have reported that IL-4 gene-deficient mice lack the late phase reaction in CH caused by DNFB. The present findings indicate that role of IL-4 in CH is dependent on the genetic background of mice. Previous studies to indicate the conflicting data may be due to the experiments employing a different kind of background mice.
In addition, Berg et al. (1995) reported that IL-4 transgenic mice with C57BL/6 background showed a reduced CH-induced ear swelling response 48 and 72 h after antigen challenge. In their experiments, oxazolone was used as the contact antigen. In general, DNFB-induced CH is more severe than that of oxazolone. The different contact antigen may be another reason leading to conflicting findings.
Regarding the expression of cytokine mRNAs, IL-4 gene-depletion clearly suppressed the expression of Th2 cytokine mRNAs in both C57BL/6 and BALB/c mice. Dramatic suppression was observed in the expression of IFN-γ mRNA in the ears of BALB/c mice by depletion of IL-4 gene. This may be related to the significant decrease in CH response in IL-4 gene-deficient mice. Unfortunately, the reason for the low expression of IFN-γ mRNA in BALB/c IL-4 gene depleted mice ear is not yet clear. Dieli et al. (1994) have reported that IL-4 is an important mediator in CH reactions, and γ/δ+ cells are one of the targets of IL-4 action. Further experiments to clarify the relationship among γ/δ+ cells, IL-4 and IFN-γ are necessary.
Regarding the participation of IgE-mediated late phase cutaneous reaction in the oedema caused by repeated antigen application, we compared the IgE antibody-mediated biphasic cutaneous reactions in wild type and IL-4 gene-depleted mice in BALB/c and C57BL/6 mice. C57BL/6 mice showed a lack of immediate cutaneous reaction. This may be due to the dysfunction of mast cells in C57BL/6 mice, a lack of protease or others. However, the late phase response which appeared 24 h after antigen application had the same magnitude in both strains. This means the susceptibility of late phase allergic cutaneous response does not differ between the two strains. Immunologic responses including IgE production and IL-4 susceptibility may be the first cause of the difference in magnitude of dermatitis between the two strains of mice. Complex components will be affected by the different susceptibilities to dermatitis.
In conclusion, this study indicated the different roles of IL-4 in the onset of allergic dermatitis, and the participation of antigen specific IgE production in BALB/c and C57BL/6 mice.
Abbreviations
- AUC
area under the curve
- BSA
bovine serum albumin
- CH
contact hypersensitivity
- DNFB
dinitrofluorobenzene
- DNP
dinitrophenyl
- ELISA
enzyme-linked immunosorbent assay
- IFN
interferon
- IL
interleukin
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- Th
T helper
References
- ABE M., KONDO T., XU H., FAIRCHILD R.L. Interferon-gamma inducible protein (IP-10) expression is mediated by CD8+T cells and is regulated by CD4+T cells during the elicitation of contact hypersensitivity. J. Invest. Dermatol. 1996;107:360–366. doi: 10.1111/1523-1747.ep12363337. [DOI] [PubMed] [Google Scholar]
- BERG D.J., LEACH M.W., KÜHN R., RAJEWSKY K., MULLER W., DARIDSON N., RENNICK D. Interleukin-10 but not interleukin-4 is a natural suppressant of cutaneous inflammatory responses. J. Exp. Med. 1995;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M.A., HURAL J. Function of IL-4 and control of its expression. Crit. Rev. Immunol. 1997;17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- DIELI F., ASHERSON G.L., ROMANO G.C., SIRECI G., GERVASI F., SALERNO A. IL-4 is essential for the systemic transfer of delayed hypersensitivity by T cell lines. J. Immunol. 1994;152:2698–2704. [PubMed] [Google Scholar]
- FRANKEL W.N. Mouse strain backgrounds: More than black and white. Neuron. 1998;20:183. doi: 10.1016/s0896-6273(00)80447-x. [DOI] [PubMed] [Google Scholar]
- FUJII M., HARA H., MENG W., VONSATTEL J.P., HUANG Z., MOSKOWITZ M.A. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57BL/6 mice. Stroke. 1997;28:1805–1810. doi: 10.1161/01.str.28.9.1805. [DOI] [PubMed] [Google Scholar]
- GAUTAM S.C., CHIKKALA N.F., HAMILTON T.A. Anti-inflammatory action of IL-4. J. Immunol. 1992;148:1411–1415. [PubMed] [Google Scholar]
- HAMID Q., BOGUNIEWICZ M., LEUNG D. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J. Clin. Invest. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRANO T., YAMAKAWA N., MIYAJIMA H., MAEDA K., TAKAI S., UEDA A., TANIGUCHI O., HASHIMOTO H., HIROSE S., OKUMURA K., OVARY Z. An improved method for the detection of IgE antibody of defined specificity by ELISA using monoclonal anti-IgE antibody. J. Immunol. Methods. 1989;119:145–150. doi: 10.1016/0022-1759(89)90391-8. [DOI] [PubMed] [Google Scholar]
- JABARA H.H., SCHNEIDER L.C., SHAPIRA S.K., ALFIERI C., MOODY C.T., KIEFF E., GEHA R.S., VERCELLI D. Induction of germ-line and mature Cε transcripts in human B cells stimulated with rIL-4 and EBV. J. Immunol. 1990;145:3468–3473. [PubMed] [Google Scholar]
- LEWIS D.B., LIGGITT H.D., EFFMENN E.L., MOTLEY S.T., TEITELBAUM S.L., JEPSEN K.J., GOLDSTEIN S.A., BONADIO J., CARPENTER J., PERLIMUTTER R.M. Osteoporosis induced in mice by overproduction of interleukin 4. Proc. Natl. Acad. U.S.A. Sci. 1993;90:11618–11622. doi: 10.1073/pnas.90.24.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOHOFF M., GESSNER A., BOGDAN C., RÖLLINGHOFF M. The Th1/Th2 paradigm and experimental murine leishmaniasis. Int. Arch. Allergy Immunol. 1998;115:191–202. doi: 10.1159/000023900. [DOI] [PubMed] [Google Scholar]
- MAGUIRE H.C., Jr, KETCHA K.A., LATTIME E.C. Neutralizing anti-IL-10 antibody upregulates the induction and elicitation of contact hypersensitivity. J. Interferon Cytokine Res. 1997;17:763–768. doi: 10.1089/jir.1997.17.763. [DOI] [PubMed] [Google Scholar]
- MENG X., SAWAMURA D., TAMAI K., HANADA K., ISHIDA H., HASHIMOTO I. Keratinocyte gene therapy for systemic diseases. Circulating interleukin 10 released from gene-transferred keratinocytes inhibits contact hypersensitivity at distant areas of the skin. J. Clin. Invest. 1998;101:1462–1467. doi: 10.1172/JCI1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLE C., SALIER A., PÉREZ-OTOÑO I., DICKINSON-ANSON H., CASTILLO P.E., BUREAU I., MARON C., GAGE F.H., MANN J.R., BETTLER B., HEINEMANN S.F. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., NAKATANI Y., ATSUMI G., INOUE K., KUDO I. Regulatory functions of Phospholipase A2. Crit. Rev. in Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- NAGAI H., HIYAMA H., MATSUO A., UEDA Y., INAGAKI N., KAWADA K. FK-506 and cyclosporin A potentiate the IgE antibody production by contact sensitization with hapten in mice. J. Pharmacol. Exp. Ther. 1997a;283:321–327. [PubMed] [Google Scholar]
- NAGAI H., MATSUO A., HIYAMA H., INAGAKI N., KAWADA K. Immunoglobulin E production in mice by means of contact sensitization with a simple chemical hapten. J. Allergy Clin. Immunol. 1997b;100:S39–S44. doi: 10.1016/s0091-6749(97)70003-4. [DOI] [PubMed] [Google Scholar]
- NAGAI H., SAKURAI T., INAGAKI N., MORI H. An immunopharmacological study of the biphasic allergic skin reaction in mice. Biol. Pharm. Bull. 1995;18:239–245. doi: 10.1248/bpb.18.239. [DOI] [PubMed] [Google Scholar]
- NAGAI H., UEDA Y., TANAKA H., HIRANO Y., NAKAMURA N., INAGAKI N., TAKATSU K., KAWADA K. Effect of overproduction of interleukin 5 on dinitrofluorobenzene-induced allergic cutaneous response in mice. J. Pharmacol. Exp. Ther. 1999;288:43–50. [PubMed] [Google Scholar]
- NICOLAIDES N.C., HOLROYD K.J., EWART S.L., ELEFF S.M., KISER M.B., DRAGWA C.R., SULLIVAN C.D., GRASSO L., ZHANG L.Y., MESSLER C.J., ZHOU T., KLEEBERGER S.R., BUETOW K.H., LEVITT R.C. Interleukin 9: A candidate gene for asthma. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETIT-FRERE C., DUGAS B., BRAQUET P., MENCIA-HUERTA J.M. Interleukin-9 potentiates the interleukin-4 induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146–151. [PMC free article] [PubMed] [Google Scholar]
- POSTMA D.S., BLEECKER E.R., AMELUNG P.J., HOLROYS K.J., XU J., PANHUYSEN C.I.M., MEYERS D.A., LEVITT R.C. Genetic susceptibility to asthma-bronchial hyperresponsiveness coinherited with a major gene for atopy. N. Eng. J. Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- RIEMANN H., SCHWARZ A., GRABBE S., ARAGANE Y., LUGER T.A., WYSOCKA M., KUBIN M., TRINCHIERI G., SCHWARZ T. Neutralization of IL-12 in vivo prevents induction of contact hypersensitivity and induce hapten-specific tolerance. J. Immunol. 1996;156:1799–1803. [PubMed] [Google Scholar]
- SALERNO A., DIELI F., SIRECI G., BELLAVIA A., ASHERSON G.L. Interleukin-4 is a critical cytokine in contact sensitivity. Immunology. 1995;84:404–409. [PMC free article] [PubMed] [Google Scholar]
- SCHAUWECKER P.E., STEWARD O. Genetic determinants of susceptibility to excitotoxic cell death: Implications for gene targeting approaches. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANKAR A.H., TITUS R.G. T cell and non-T cell compartments can independently determine resistance to Leishmanis major. J. Exp. Med. 1995;181:845–855. doi: 10.1084/jem.181.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJÖLANDER A., BALDWIN T.M., CURTIS J.M., HANDMAN E. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J. Immunol. 1998;160:3949–3957. [PubMed] [Google Scholar]
- STAVNEZER-NORDGEN J., SIRLIN S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986;5:95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPER R.I., LEVINSON D.A., STANGER B.Z., CAMPOS-TORRES J., ABBAS A.K., LEDER P. IL-4 induced allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- THYPHRONITIS G., KATONA I.M., GAUSE W.C., FINKELMAN F.D. Germline and productive Cε gene expression during in vivo IgE responses. J. Immunol. 1993;151:4128–4136. [PubMed] [Google Scholar]
- WEIGMANN B., SCHWING J., HUBER H., ROSS R., MOSSMANN H., KNOP J., RESKE-KUNZ A.B. Diminished contact hypersensitivity response in IL-4 deficient mice at a late phase the elicitation reaction. Scand. J. Immuno. 1996;45:308–314. doi: 10.1046/j.1365-3083.1997.d01-402.x. [DOI] [PubMed] [Google Scholar]
- XU B., AOYAMA K., KITANI A., YU S., MATSUYAMA T., MATSUSHITA T. Interleukin-12 enhances contact hypersensitivity by modulating the in vivo cytokine pattern in mice. J. Interferon Cytokine Res. 1998;18:23–31. doi: 10.1089/jir.1998.18.23. [DOI] [PubMed] [Google Scholar]
- XU H., DIIULIO N.A., FAIRCHILD R.L. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+T cells. J. Exp. Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]


