Abstract
T-butyl-bicyclo-phosphorothionate (TBPS) is a prototypical representative of the cage-convulsants which act through a use-dependent block of the GABAA-receptor-ionophore complex. Using current recordings from cultured neurones of rat striatum the manner was investigated in which two antagonists, bicuculline and penicillin, presumably acting at the agonist binding site and in the ionic channel, respectively, modify the rate of block by TBPS.
Penicillin (5 or 10 mM) did not slow the rate of block by TBPS, but produced a significant enhancement of block rate, which, however, was inversely related to the degree of antagonism by penicillin of the GABA-induced current.
Bicuculline (10 μM) reduced the rate of block by TBPS. However, this effect was 3 fold weaker than its GABA-antagonistic action. The slowing of block rate and the current antagonism exhibited a biphasic, positive-negative relationship. Co-application of bicuculline (100 μM) in a concentration that produced nearly complete antagonism and TBPS (10 μM) resulted in a marked (∼40%) reduction of subsequent GABA response amplitudes compatible with a direct, bicuculline-induced conformational change in the receptor required for the binding of and block by TBPS.
The lack of protection afforded by the channel blocker penicillin as well as the lack of correlation between bicuculline antagonism of the Cl−-current and its efficiency in protecting against TBPS block is evidence against an open channel blocking mechanism for TBPS. TBPS does, therefore, not appear to gain access to its binding site via the open pore but through alternative routes regulated from the agonist binding site.
Keywords: GABAA-receptor, t-butyl-bicyclo-phosphorothionate, convulsant, bicuculline, penicillin, striatum, voltage-clamp
Introduction
The GABAA-receptor is richly endowed with binding sites for various drugs and endogenous substances. Among these, a so-called convulsant site has been identified that binds a class of structurally related antagonists (Ticku, 1986), called cage-convulsants. The best known of these is picrotoxin (with its main active constituent picrotoxinin) the action of which on the GABA-meditated Cl−-conductance has been studied electrophysiologically for more than 30 years in a variety of preparations (Takeuchi & Takeuchi, 1969; Constanti, 1978; Smart & Constanti, 1986; Yakushiji et al., 1987).
T-butyl–bicyclo-phosphorothionate (TBPS) is a potent convulsant (Bowery et al., 1977) and a structural analogue of picrotoxinin. Because of the high specificity and affinity of its binding to GABAA-receptors (Squires et al., 1983), it is widely used in a 35S-labelled form for binding studies on the GABAA-receptor. These have shown TBPS to bind and unbind in a manner dependent on the activity of the GABAA-receptor. Thus, depending on the experimental protocol, both binding and unbinding are promoted by agonists such as GABA and muscimol, or by positive allosteric modulators (Ticku, 1986). This activity-dependence of interaction together with a number of observations indicating that the convulsant binding site is closely associated with the Cl− ion channel (Havoundjian et al., 1986; Sigel et al., 1989; Zhang et al., 1994) has led to the hypothesis that the mechanism of convulsant action is by physically plugging the pore, with the channel open state being the requirement for the drugs both to enter and to leave it (Gee, 1988; Sieghart, 1992; McKernan & Whiting, 1996).
Such a mechanism would be analogous to the action of the anticonvulsant MK801 on N-methyl-D-aspartate receptors described by Huettner & Bean (1988) and would account for many of the features of convulsant binding to the GABAA-receptor. However, electrophysiological investigations into the mechanism of the blocking action of both picrotoxin and TBPS on ensemble and single channel currents have not produced unequivocal evidence supporting this view (Constanti, 1978; Barker et al., 1983; Smart & Constanti, 1986; Van Renterghem et al., 1987; Inoue & Akaike, 1988; Twyman et al., 1989; Newland & Cull Candy, 1992; Yoon et al., 1993; Zhang et al., 1994; Macdonald & Olsen, 1994; Dillon et al., 1995). In fact, a majority of investigators have concluded that more complex effects, involving modification of channel gating and/or binding of the drug to the liganded receptor rather than the open channel, are more likely to underlie the block of GABAA-receptor function by convulsants than is simple open channel block.
However, there is a widespread consensus that block of the GABAA-receptor by convulsants is strongly sensitive to the activity of the GABA receptor, i.e. that it is use-dependent (Yakushiji et al., 1987; Akaike et al., 1987; Inoue & Akaike, 1988; Dillon et al., 1995). In principle, therefore, these compounds are of potential interest as tools for the study of inhibitory synapses in a manner analogous to glutamatergic synapses, where MK801 has been used to assess variables such as NMDA-receptor open probability (Jahr, 1992) and release probability (Hessler et al., 1993; Rosenmund et al., 1993; Murthy et al., 1997). Further investigation into the mechanism of action of cage-convulsants is, therefore, warranted.
An important piece of evidence for open channel block as the mechanism for the use-dependent action of MK801 is the fact that the NMDA-receptor is protected from block by Mg2+-ions (Huettner & Bean, 1988), which produce a fast (flicker-type) block of the open NMDA-receptor channel (Nowak et al., 1984; Mayer & Westbrook, 1985; Ascher & Nowak, 1988). The antibiotic, penicillin, is known to produce a similar kind of block of the open GABAA-receptor Cl−-channel (Chow & Mathers, 1986; Twyman et al., 1992). It was, therefore, relevant to investigate whether penicillin would afford a similar protection against block of the GABAA-receptor by TBPS. If, on the other hand, the agonist-enhanced TBPS binding is not conditional on the channel being open, but rather on the agonist binding site being occupied, an antagonist that reduces GABA-receptor agonist occupancy, like bicuculline, would be expected to protect against block by TBPS. Experiments to answer these questions were performed using whole-cell voltage clamp recordings of GABAA-receptor mediated Cl− currents in cultured neurones of rat striatum.
Methods
Cell culture methods have been described previously (Rumpel & Behrends, 1999). Briefly, following deep ether anaesthesia, pregnant rats were decapitated and the uterus dissected out and placed on an ice-cold glass dish. Embryos (E17) were removed and their brains placed into ice-cold culture medium (Eagles MEM). The lateral ganglionic eminences were dissected and subjected to mechanical dissociation. Cultures were grown in Eagles MEM supplemented with 10% horse serum, 0.5 mM glutamine and 37.5 μg ml−1 insuline and 2 mg ml−1 glucose. Arabinoside-C (10 μM) was applied for 12 h on day 4 in vitro to stop glial proliferation. Cultures were used for recordings from 12 to 22 days in vitro. For whole-cell recordings, borosilicate pipettes (see below) were filled with a solution containing (mM): KCl 110, MgCl2 5, ethylene glycol-bis-(β-aminoethyl ether) N,N,N′,N′-etetraacetic acid 0.6, N-2-hydoxyethylpiperazine-N′etraacetic acid 0.6, N-2- hydroxyethylpiperazine-N′2-ethanesulphonic acid 10, Na-adenosine-5′trisphosphate 2, Na-guanosine-5′ trisphosphate 1 (pH set to 7.3 with KOH, 230 mOsm). The control bath solution was composed of (mM): NaCl 125, KCl 1, MgCl2 1, N-2-hydroxyethylpiperazine-N′2-ethanesulphonic acid 20, glucose 10, CaCl2 2 (pH set to 7.35 with NaOH, 270 mOsm). All bath solutions were prepared by adding glucose and CaCl2 to a stock solution containing all other constituents. All chemicals except t-butyl-bicyclophosphorothionate (TBPS, Research Biochemicals Inc., Natick, MA, U.S.A.) were purchased from Sigma Germany (Munich). TBPS was dissolved in dimethyl sulphoxide (DMSO) and diluted in external solution to 3 or 10 μM. Final DMSO concentration was, therefore, 0.03 or 0.1% which has no effect on GABA-receptor mediated Cl−-currents (Rumpel & Behrends, 1999).
Patch-clamp experiments were performed at room temperature (23–25°C) under direct visual control on a Zeiss IM 35 inverted microscope. Borosilicate pipettes with an outer tip diameter of 2–3 μm and an open tip resistance of 2–5 MΩ were used. In all experiments cells were continuously superfused with standard or test solutions, employing a gravity-driven local application system (Y-tube) that allowed for complete exchange of solution around a cell in <100 ms. GABAA-receptor-mediated responses were elicited every 5 s using a pressure pipette outer diameter 3 μm) containing GABA at 100 μM in normal extracellular solution and placed in the immediate vicinity of the cell (<10 μm). A potential problem between applications was continuous activation of receptors by GABA leaking from the pipette. This was usually prevented by the rapid continuous flow of background solution from the Y-tube. If not, this was immediately detectable as an inward current associated with increased background noise and pipette was moved further from the cell. However, the position of the puffer pipette was kept constant for each trial.
It should, furthermore, be noted that neither the concentration of GABA at the receptor nor the time course of its delivery were controlled in these experiments, as is also indicated by the considerable variability of antagonist efficacy observed from experiment to experiment (see Results). This is also due to the fact that the pressure-application of GABA physically displaces and dilutes the antagonist-containing solution.
An EPC-7 patch clamp amplifier (HEKA-Electronics, Darmstadt, Germany) was used for recording without series resistance compensation (<15 MΩ). Output signals were filtered at 3 kHz and digitized on-line at 24 kHz with a National Instruments (Austin, TX, U.S.A.) NB-MIO 16L 14-bit AD-converter in a Macintosh 8100/100 computer.
The amplitudes of current responses were automatically measured off-line using custom-developed routines written in LabView (National Instruments). Kinetics of block by TBPS under various conditions were determined using the built-in curve fitting routine of IGORPro (Wavemetrics, Lake Oswego, OR, U.S.A.) to approximate an exponential function of the form y(t)=A*exp(−x/k) to the decay phase, where A is the control amplitude of the response and x the ordinal number of successive test applications of GABA. The waveforms of individual responses were averaged (n=5–20) and their integrals, i.e. the total charge transfer during the response, computed using routines of IgorPro in order to determine the total channel open probability.
Statistical analysis was done by paired or unpaired t-test, as applicable, using StatView software (Abacus Concepts, Berkeley, CA, U.S.A.). Mean values are given±the standard error of the mean.
Results
The effect of penicillin on TBPS block
Pressure-driven application of GABA (100 μM, 20 ms) resulted in a whole-cell inward current with a rise time of typically <100 ms and peak amplitudes of 1–3 nA (Figure 1A), which returned to baseline with a roughly monoexponential time course with time constants on the order of 100–200 ms. Application of penicillin (5 mM) resulted in a reduction of peak amplitude accompanied by enhanced current noise and a drastic change in the kinetics of current decay which now exhibited two exponential components (Figure 1B). This is a typical finding with open channel blockers that prevent normal closing of the channel, classically described for local anaesthetics acting on the endplate acetylcholine-receptor response (Steinbach, 1968; Katz & Miledi, 1975; Adams, 1976; Beam, 1976; Ruff, 1977; Neher & Steinbach, 1978). The intention in this experiment was to use the open-channel block by penicillin to determine whether TBPS requires a conducting open channel to reach its binding site. If this is the case, then the parameter that has to be affected by a drug with protective action is the total channel open time, which is given by the integral of the current, rather than its peak value. This distinction is relevant here, because penicillin induces, in addition to the depression in the peak response, a kinetic change that will tend to prolong total channel open time and, hence, reduce a possible protective effect against block by TBPS. Therefore, the actual change in total channel open time produced by penicillin, i.e. in the current integral, was determined for each experiment. Inset b in Figure 1B shows a superimposition of the current integrals computed from the responses under control conditions and in penicillin. Exponential extrapolation of the time courses of the integrals was used to prolong them to full steady state.
Figure 1.
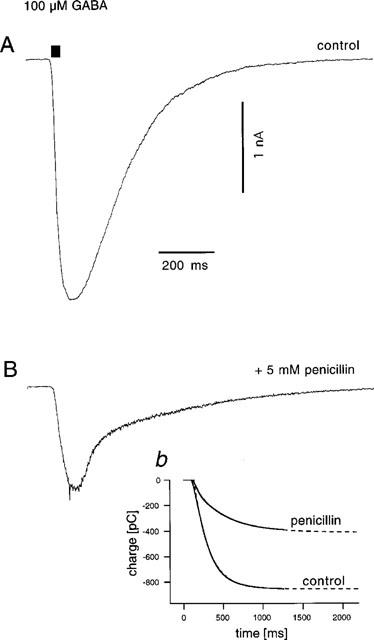
Effect of penicillin on amplitude and kinetics of GABA-responses. (A) Control response to a 20 ms pressure application from a pipette containing GABA at 100 μM. (B) Response obtained immediately following perfusion of the cell with a solution containing penicillin (5 mM). Note the slower rising phase and the early rapid and late slow decay phases, as well as the enhanced current noise characteristic of simple open channel block (see text). Inset b: The superimposition of the current integrals indicates that despite the slow decay, the total open probability time (i.e. Po*t) of the Cl−-channels during the response is reduced by >50% by the action of penicillin.
In the experiment shown in Figure 1, penicillin produced a decrease in current integral (i.e. total open probability) to 48% of control, an effect only slightly smaller than the decrease in peak amplitude (to 44% of control) confirming that penicillin had indeed produced a substantial reduction in total channel open time.
Figure 2 shows a sample experiment performed to test for a protective effect of penicillin-induced open channel block against TBPS, which was applied twice to the same cell: first in the presence of penicillin and a second time after wash-out. As can be appreciated from the graph of amplitudes vs time, TBPS was effective in blocking GABA-induced currents both in the presence and in the absence of penicillin. In fact, quantitative analysis of the time course of the block reveals that block with penicillin was 20% faster than block without, requiring 1.66 vs 2.08 applications for an e-fold reduction in peak amplitude. In none of six similar experiments (5 mM penicillin, n=3, 10 mM penicillin, n=3) a relative slowing of TBPS-induced block by penicillin was observed. On average, the time constant of block by TBPS was 2.27±0.32 applications in penicillin and 3.74±0.39 applications after washout (P=0.001). However, the relative amount of current block by TBPS in penicillin and after wash-out was not significantly different (79.4±2.8 and 81.6±3.0%, respectively, P=0.22, one-tailed, paired t-test). There was also no obvious effect of penicillin on the speed of current recovery after washout of TBPS. After five pulses of GABA in the absence of TBPS, on average 31.2±7.9% of the block was removed in the presence and 31.0±5.5% was removed in the absence of penicillin. A paired t-test revealed no significant difference (P=0.49). Figure 3 shows a summary of these experiments as a plot of the relative change in block rate vs the relative change in current integral from penicillin to control conditions. Interestingly, this graph suggests a relationship between the two variables such that the enhancement by penicillin of TBPS block rate became less important with greater efficiency of current block by penicillin (see Discussion).
Figure 2.
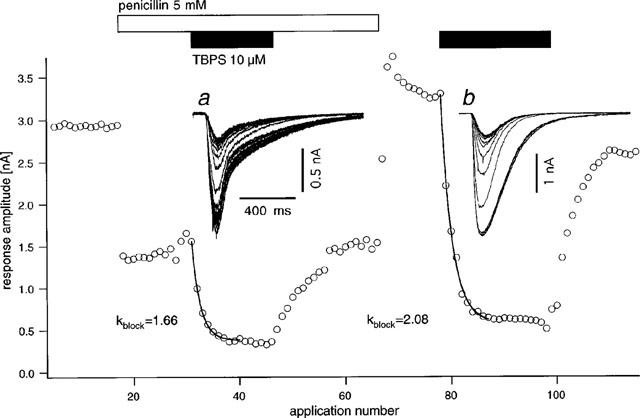
Effect of penicillin on the rate of block of GABA-responses by TBPS. Graph of the current amplitude vs sequential number of test GABA-applications (delivered 1/5 s). Upon application of penicillin (5 mM, open bar) response amplitude is immediately reduced to <50% of control. Application of TBPS (10 μM, closed bar) produces a rapid exponential block with an e-fold reduction in amplitude after 1.66 applications of GABA. Upon washout of TBPS, a slower, exponential recovery to control values is observed. After washout of penicillin and recovery of control amplitudes, TBPS is applied again inducing again a rapid decline in current amplitudes with an e-fold reduction in amplitude after 2.08 GABA-applications, i.e. it is slower than that observed in the presence of penicillin. Insets a and b show superimposed original current traces from the respective portions of the experiment: four traces recorded before TBPS-application are shown together with those during development of the block to the steady state. Data from the same experiment as in Figure 1.
Figure 3.
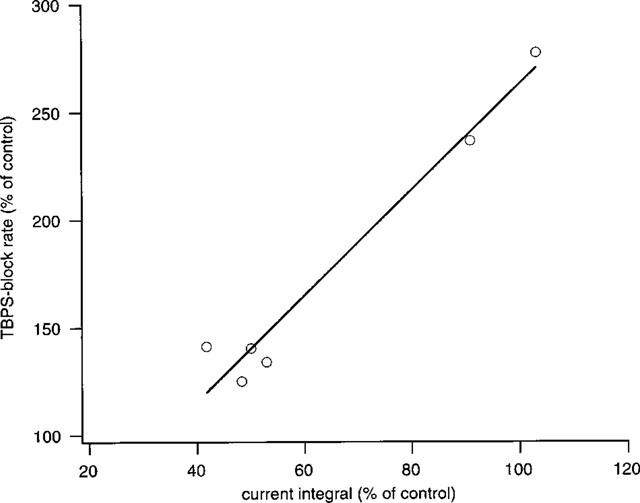
Summary of results of six experiments with penicillin and TBPS. The graph shows relative change in block rate plotted against the change in current integral (i.e. total channel open time) produced by penicillin (5 or 10 mM). Note that all values for change in block rate are >100%, indicating that penicillin invariably produced an enhancement of block. However, this graph suggests a negative correlation (solid line, correlation coefficient: 0.93) with the total reduction in charge transfer produced by penicillin (see text).
Effect of bicuculline-antagonism on the rate of block by TBPS
A similar set of experiments was undertaken in order to investigate whether bicuculline is able to protect against block by TBPS. As shown in Figure 4, bicuculline at a concentration of 10 μM does indeed slow the block by TBPS (3 μM). This finding was established in eight identical experiments, where blocking rates were reduced on average to 64.8±3.1% of control (P<0.0005, one-tailed, paired t-test). Mean recovery of block after five GABA-applications following washout of TBPS was 11.6±4.4% in the presence and 22.9±8.12% in the absence of bicuculline (P=0.03, one-tailed, paired t-test). While the blocking rate was thus reduced by a factor of ∼1.6, the total channel open channel time as estimated by the current integral was, on average, reduced to 20.0±2.3% of control, i.e. by a factor of ∼5, indicating a dichotomy between the efficiency of bicuculline as an antagonist of channel opening on the one hand and of the block by TBPS on the other. In order to further explore the nature of this relationship, Figure 5 shows the data from the bicuculline experiments in a manner analogous to Figure 3 for penicillin. The graph shows that the efficiency of bicuculline in protecting from the block of TBPS slowly increases with its efficiency in preventing channel opening up to an 80% suppression of the current integral but appears to decrease thereafter.
Figure 4.
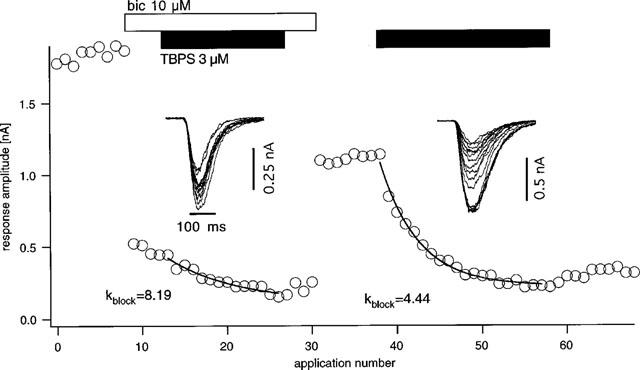
Effect of bicuculline on block rate of GABA-responses by TBPS. The graph shows the response amplitude plotted against the application number. Application of bicuculline (10 μM, open bar) produces a strong reduction in inward current responses. Addition of TBPS at 3 μM (closed bar) shows a slow, exponential block with an e-fold reduction of amplitude occurring after 8.19 GABA-pulses and washout of TBPS produces little recovery. Upon washout of bicuculline, TBPS is applied again at 3 μM and produces a faster block with e-fold amplitude reduction after 4.44 pulses.
Figure 5.
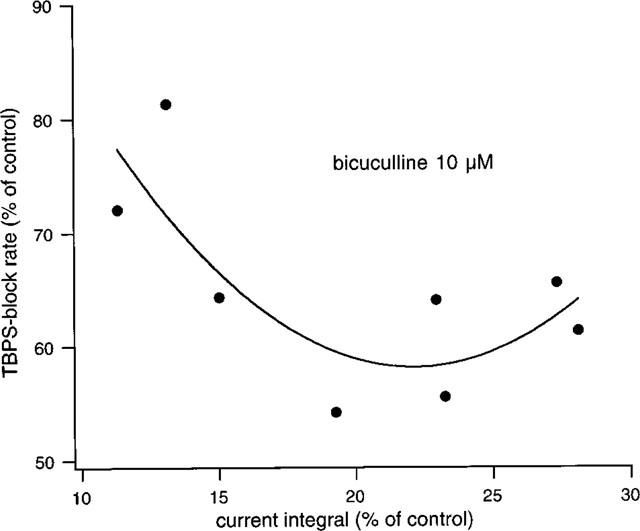
Summary of eight experiments with bicuculline (10 μM) and TBPS. The graph shows the relationship between change in the blocking rate in TBPS and the decrease in current integral obtained with 10 μM bicuculline. Note that there is a biphasic relationship. The solid line is a quadratic fit. The protective effect of bicuculline increases up to an 80% decrease in current integral but declines thereafter (see text).
The reason for the considerable variation in relative antagonist efficiency of bicuculline in these experiments lies in the variable concentrations reached and time courses of delivery of GABA at the receptors due to variations in the distance of the drug delivery pipette to the cell (see Methods) leading to different degrees of displacement of bicuculline. The varying extents of relative reduction in the current integral produced by bicuculline are, therefore, likely to represent various ratios of mean receptor occupancy by GABA and bicuculline. Consequently, the relationship shown in Figure 5 indicates that an occupancy ratio leading to a decrease in current integral to 20% of control is associated with a maximal slowing of the block by 40%, while further shifting the occupancy ratio in the favour of bicuculline produces less, not more, protection against the block by TBPS. This behaviour might indicate that bicuculline can act directly to promote TBPS binding.
In order to address more directly the possibility that bicuculline has a direct permissive action with respect to the binding of TBPS, a series of experiments using 100 μM bicuculline was performed (Figure 6). This concentration of bicuculline depressed GABA-responses by more than 95%; yet this effect was rapidly reversible upon washout. Despite this near-total block of the GABA-response, following a 1 min period of co-application of 10 μM TBPS+100 μM bicuculline, removal of both substances revealed a depression of the GABA response to 56.2±5.4% of control (n=5). Surprisingly, no appreciable recovery from this effect could be obtained by further GABA-applications.
Figure 6.
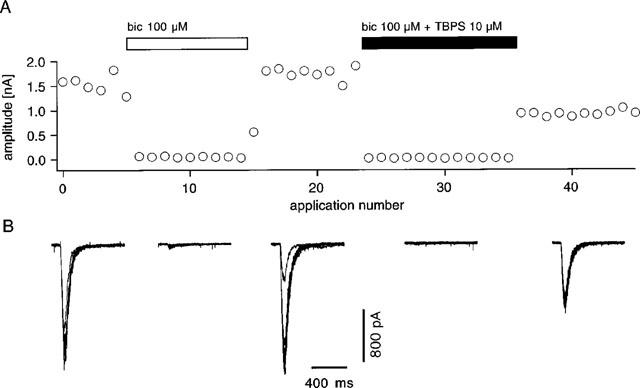
Effect of co-application of TBPS with a near-maximal concentration of bicuculline. (A) Response amplitude is plotted against number of GABA-applications. Application of 100 μM bicuculline (open bar) produces a near complete suppression of the GABA response. This effect is readily reversible upon washout. Co-application of TBPS (10 μM) with bicuculline (closed bar) produces immediate total block of the response. Upon washout of both drugs, the response is found to be suppressed without a clear tendency for recovery. (B) Superimposed original traces coresponding to A. Similar results were obtained in four other cells.
Discussion
The present experiments show that antagonism by bicuculline, but not penicillin, has a protective action on GABAA-receptors against the block by TBPS in rat striatal neurones in culture. Because penicillin acts by an open channel block (Chow & Mathers, 1986; Twyman et al., 1992) the lack of protective effect is not easily reconciled with the hypothesis that the open channel is the route TBPS is required to take towards its binding site. At the same time, the protective action by bicuculline is far weaker (by a factor of ∼3) than its depressant action on channel opening. If the channel is required to be open in order for TBPS to reach its target, one would expect a linear relationship with a slope near unity between the ability of an antagonist to reduce total channel open time and its protective efficacy against TBPS-induced channel block. This finding must, therefore, equally be regarded as evidence against open channel block as a mechanism of action for TBPS.
Furthermore, the fact that the protective action of bicuculline showed a biphasic relationship with its antagonist activity may indicate that bicuculline itself is able to induce (albeit with small efficiency) a conformational transition that allows TBPS to bind to its receptor. This scenario is in line with results of binding studies showing that bicuculline is able to modulate the specific binding of TBPS to rat brain membranes in the absence of GABA (Trifiletti et al., 1984; Liljequist & Tabakoff, 1993; Luddens & Korpi, 1995). In one of these studies using recombinant GABAA-receptors, a biphasic interaction between the effect of GABA and bicuculline on TBPS-binding was also described (c.f. Figure 5 in Lüddens & Korpi, 1995).
The block of the GABA response obtained using co-application of TBPS and a near-maximal concentration of bicuculline (c.f. Figure 6) appears to corroborate this hypothesis. However, the fact that this effect did not show the usual use-dependent recovery with further applications of GABA is surprising. This might indicate that the type of binding of TBPS to the GABA receptor that is promoted by bicuculline is of a fundamentally different nature from that promoted by GABA, maybe preferentially affecting receptors of a specific subunit composition that are less able to recover from TBPS block (c.f. Lüddens & Korpi, 1995). In the experiments testing for protective effects, recovery from block was also conspicuously slower in the experiments where bicuculline had been applied than in those where penicillin had been tested (compare Figures 2 and 4 and the values given in Results). However, these experiments were not designed to follow the process of recovery and some caution is appropriate in interpreting these results. Clearly, further study is needed to resolve this issue.
In the present series of experiments, penicillin enhanced TBPS block rate, rather than showing a protective effect. This enhancement, however, was inversely proportional to penicillin's inhibitory effect on total charge transfer, i.e. the open probability of the channels. The established kinetic scheme for open channel block by penicillin (Twyman et al., 1992) predicts that the channel will be unable to enter the normal closed state as long as penicillin is bound. This, in turn, might imply by the sequential scheme of channel opening that the agonist (GABA) is unable to unbind unless penicillin has first left the channel. By preventing normal channel closure, penicillin might thus promote prolonged binding of GABA and, therefore, association of TBPS with its target site. While this reasoning may well explain the fact that penicillin enhances TBPS block rate, it seems confusing that it should do so more efficiently when the decrease in current integral is small than when it is larger. This however, is suggested by the relationship between change in block rate and change in current integral produced by penicillin (c.f. Figure 3). This finding might be explained if penicillin, could in addition to blocking the open channel, act through a different mechanism. For example, displacement of GABA from its binding site by high concentrations of penicillin, as described in the literature (Antoniadis et al., 1980) may be responsible for this intriguing observation. It is equally conceivable that penicillin, by prolonging the binding of GABA would promote desensitization of the receptor, which could also affect TBPS binding.
While the present data argue strongly against the hypothesis that TBPS selectively binds to the open state of the GABAA-receptor or reaches its binding site through the open channel, the results reported here do not affect the likelihood that the convulsant binding site is in the channel or closely associated with it. In addition to the evidence linking the convulsant receptor to an anion recognition site (Havoundjian et al., 1986) it has been shown that channels consisting exclusively of β1-subunits of the GABA-receptor do not require gating by GABA to open, yet are blocked by picrotoxin (Sigel et al., 1989). Furthermore, a single point mutation in the dieldrin resistance gene of Drosophila has been shown to alter single channel conductance and at the same time confer resistance to picrotoxin block (Zhang et al., 1994). Thus, it remains likely that the binding site of convulsants is in the channel. It remains also possible that TBPS acts by physically plugging the channel rather than through a modification of channel gating. However, as shown here, an open channel is not required for TBPS to reach its target. The relative hydrophobicity of TBPS and picrotoxinin allows the speculation that the binding site might be reached (as well as left) through a ‘hydrophobic pathway' (Hille, 1977) through the lipid phase of the membrane and the channel ‘wall', which would become accessible through a conformational changes gated by binding of agonists as well as other effectors.
In summary, the present results do not appear compatible with the idea that the open state of the channel is required for TBPS to access its binding site. In addition, however, they point to a considerable degree of complexity regarding the regulation of the binding of this drug to the GABAA-receptor complex. In particular, the nature of bicuculline-promoted TBPS binding may be an important issue, especially in view of recent evidence that this antagonist has properties of an allosteric effector (Ueno et al., 1997).
Acknowledgments
This work was performed in the laboratory of Professor G. ten Bruggencate, to whom I am grateful for his constant support and encouragement. I thank L. Kargl and A. Grünewald for valuable technical assistance.
Abbreviations
- GABA
γ-aminobutyric acid
- GABAA-receptor
γ-aminobutyric acid type A receptor
- MK801
(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]-cyclohepten-5,10-imine maleate
- NMDA
N-methyl-D-aspartic acid
- TBPS
t-butyl-bicyclo-phosphorothionate
References
- ADAMS P.R. Drug blockade of open end-plate channels. J. Physiol. 1976;260:531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKAIKE N., YAKUSHIJI T., TOKUTOMI N., CARPENTER D.O. Multiple mechanisms of antagonism of gamma-aminobutyric acid (GABA) responses. Cell. Mol. Neurobiol. 1987;7:97–103. doi: 10.1007/BF00734993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTONIADIS A., MULLER W.E., WOLLERT U. Inhibition of GABA and benzodiazepine receptor binding by penicillins. Neurosci. Lett. 1980;18:309–312. doi: 10.1016/0304-3940(80)90302-x. [DOI] [PubMed] [Google Scholar]
- ASCHER P., NOWAK L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J. Physiol. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER J.L., MCBURNEY R.N., MATHERS D.A. Convulsant-induced depression of amino acid responses in cultured mouse spinal neurones studied under voltage clamp. Br. J. Pharmacol. 1983;80:619–629. doi: 10.1111/j.1476-5381.1983.tb10051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAM K.G. A quantitative description of end-plate currents in the presence of two lidocaine derivatives. J. Physiol. 1976;258:301–322. doi: 10.1113/jphysiol.1976.sp011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWERY N.G., COLLINS J.F., HILL R.G., PEARSON S. t-Butyl bicyclo phosphate: a convulsant and GABA antagonist more potent than bicuculline [proceedings] Br. J. Pharmacol. 1977;60:275P–276P. [PMC free article] [PubMed] [Google Scholar]
- CHOW P., MATHERS D. Convulsant doses of penicillin shorten the lifetime of GABA-induced channels in cultured central neurones. Br. J. Pharmacol. 1986;88:541–547. doi: 10.1111/j.1476-5381.1986.tb10234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANTI A. The ‘mixed' effect of picrotoxin on the GABA dose/conductance relation recorded from lobster muscle. Neuropharmacology. 1978;17:159–167. doi: 10.1016/0028-3908(78)90095-3. [DOI] [PubMed] [Google Scholar]
- DILLON G.H., IM W.B., CARTER D.B., MCKINLEY D.D. Enhancement by GABA of the association rate of picrotoxin and tert-butylbicyclophosphorothionate to the rat cloned alpha 1 beta 2 gamma 2 GABAA receptor subtype. Br. J. Pharmacol. 1995;115:539–545. doi: 10.1111/j.1476-5381.1995.tb16368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEE K.W. Steroid modulation of the GABA/benzodiazepine receptor-linked chloride ionophore. Mol. Neurobiol. 1988;2:291–317. doi: 10.1007/BF02935636. [DOI] [PubMed] [Google Scholar]
- HAVOUNDJIAN H., PAUL S.M., SKOLNICK P. The permeability of gamma-aminobutyric acid-gated chloride channels is described by the binding of a ‘cage' convulsant, t-butylbicyclophosphoro[35S]thionate. Proc. Natl. Acad. Sci. U.S.A. 1986;83:9241–9244. doi: 10.1073/pnas.83.23.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESSLER N.A., SHIRKE A.M., MALINOW R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- HILLE B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUETTNER J.E., BEAN B.P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE M., AKAIKE N. Blockade of gamma-aminobutyric acid-gated chloride current in frog sensory neurons by picrotoxin. Neurosci. Res. 1988;5:380–394. doi: 10.1016/0168-0102(88)90024-7. [DOI] [PubMed] [Google Scholar]
- JAHR C.E. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992;255:470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. The effect of procaine on the action of acetylcholine at the neuromuscular junction. J. Physiol. 1975;249:269–284. doi: 10.1113/jphysiol.1975.sp011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILJEQUIST S., TABAKOFF B. Bicuculline-produced regional differences in the modulation of 35S-TBPS binding by GABA, pentobarbital and diazepam in mouse cerebellum and cortex. J. Pharmacol. Exp. Ther. 1993;264:638–647. [PubMed] [Google Scholar]
- LUDDENS H., KORPI E.R. GABA antagonists differentiate between recombinant GABAA/benzodiazepine receptor subtypes. J. Neurosci. 1995;15:6957–6962. doi: 10.1523/JNEUROSCI.15-10-06957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD R.L., OLSEN R.W. GABAA receptor channels. Annu. Rev. Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- MAYER M.L., WESTBROOK G.L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J. Physiol. 1985;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKERNAN R.M., WHITING P.J. Which GABAA-receptor subtypes really occur in the brain? [see comments] Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- MURTHY V.N., SEJNOWSKI T.J., STEVENS C.F. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- NEHER E., STEINBACH J.H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J. Physiol. 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWLAND C.F., CULL CANDY S.G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J. Physiol. 1992;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWAK L., BREGESTOVSKI P., ASCHER P., HERBET A., PROCHIANTZ A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- ROSENMUND C., CLEMENTS J.D., WESTBROOK G.L. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- RUFF R.L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J. Physiol. 1977;264:89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUMPEL E., BEHRENDS J.C. Evoked asynchronous transmission rat striatal inhibitory synapses. J. Physiol. 1999;514:447–458. doi: 10.1111/j.1469-7793.1999.447ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGHART W. GABAA receptors: ligand-gated Cl- ion channels modulated by multiple drug-binding sites. Trends Pharmacol, Sci. 1992;13:446–450. doi: 10.1016/0165-6147(92)90142-s. [DOI] [PubMed] [Google Scholar]
- SIGEL E., BAUR R., MALHERBE P., MOHLER H. The rat beta 1-subunit of the GABAA receptor forms a picrotoxin-sensitive anion channel open in the absence of GABA. FEBS Lett. 1989;257:377–379. doi: 10.1016/0014-5793(89)81576-5. [DOI] [PubMed] [Google Scholar]
- SMART T.G., CONSTANTI A. Studies on the mechanism of action of picrotoxinin and other convulsants at the crustacean muscle GABA receptor. Proc. R. Soc. Lond. B. 1986;227:191–216. doi: 10.1098/rspb.1986.0019. [DOI] [PubMed] [Google Scholar]
- SQUIRES R.F., CASIDA J.E., RICHARDSON M., SAEDERUP E. [35S]t-butylbicyclophosphorothionate binds with high affinity to brain-specific sites coupled to gamma-aminobutyric acid-A and ion recognition sites. Mol. Pharmacol. 1983;23:326–336. [PubMed] [Google Scholar]
- STEINBACH A.B. Alteration by xylocaine (lidocaine) and its derivatives of the time course of the end plate potential. J. Gen. Physiol. 1968;52:144–161. doi: 10.1085/jgp.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J. Physiol. 1969;205:377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TICKU M.K.Convulsant binding sites on the benzodiazepine/GABA Receptor Benzodiazepine/GABA Receptors and Chloride Channels: Structural and Functional Properties 1986Alan R. Liss: New York; 195–207.In: Olsen, R.W. & Venter, J.C. (eds.) [Google Scholar]
- TRIFILETTI R.R., SNOWMAN A.M., SNYDER S.H. Anxiolytic cyclopyrrolone drugs allosterically modulate the binding of [35S]t-butylbicyclophosphorothionate to the benzodiazepine/gamma-aminobutyric acid-A receptor/chloride anionophore complex. Mol. Pharmacol. 1984;26:470–476. [PubMed] [Google Scholar]
- TWYMAN R.E., GREEN R.M., MACDONALD R.L. Kinetics of open channel block by penicillin of single GABAA receptor channels from mouse spinal cord neurones in culture. J. Physiol. 1992;445:97–127. doi: 10.1113/jphysiol.1992.sp018914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TWYMAN R.E., ROGERS C.J., MACDONALD R.L. Pentobarbital and picrotoxin have reciprocal actions on single GABAA receptor channels. Neurosci. Lett. 1989;96:89–95. doi: 10.1016/0304-3940(89)90248-6. [DOI] [PubMed] [Google Scholar]
- UENO S., BRACAMONTES J., ZORUMSKI C., WEISS D.S., STEINBACH J.H. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J. Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN RENTERGHEM C., BILBE G., MOSS S., SMART T.G., CONSTANTI A., BROWN D.A., BARNARD E.A. GABA receptors induced in Xenopus oocytes by chick brain mRNA: evaluation of TBPS as a use-dependent channel-blocker. Brain Res. 1987;388:21–31. doi: 10.1016/0169-328x(87)90017-9. [DOI] [PubMed] [Google Scholar]
- YAKUSHIJI T., TOKUTOMI N., AKAIKE N., CARPENTER D.O. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987;22:1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]
- YOON K.W., COVEY D.F., ROTHMAN S.M. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J. Physiol. 1993;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H.G., FFRENCH CONSTANT R.H., JACKSON M.B. A unique amino acid of the Drosophila GABA receptor with influence on drug sensitivity by two mechanisms. J. Physiol. 1994;479:65–75. doi: 10.1113/jphysiol.1994.sp020278. [DOI] [PMC free article] [PubMed] [Google Scholar]


