Abstract
Nitroaspirin (2.5–50 mg kg−1, i.p. or 2.5–100 mg kg−1, p.o.) and aspirin (2.5–100 mg kg−1, i.p. or p.o.) exhibit anti-inflammatory activity in the carrageenan-induced hindpaw oedema model in the rat. When administered i.p., nitroaspirin was a more effective anti-oedema agent than aspirin particularly in the ‘early' phase (i.e. up to 60 min) of the response. The ED50 values for nitroaspirin and aspirin as inhibitors of the ‘late' phase response (measured at 180 min) were 64.3 μmol kg−1 and >555 μmol kg−1, respectively. When administered p.o., neither nitroaspirin nor aspirin exhibited significant anti-inflammatory activity in the ‘early' phase and were of similar potency in the ‘late' phase. Thus, at the highest dose used (100 mg kg−1, 360 min) orally administered nitroaspirin (aspirin in parenthesis) inhibited oedema formation by 46.9±1.6% (47.2±3.8%, both n=6, P<0.05).
Nitroaspirin and aspirin (25–200 mg kg−1, p.o.) caused dose-related inhibition of the hyperalgesia to mechanical stimulation following intraplantar injection of carrageenan in the rat. ED50 values were 365 μmol kg−1 and 784 μmol kg−1, respectively. Neither drug influenced the threshold for mechanical stimulation in the contralateral (i.e. untreated) hindpaw.
Nitroaspirin and aspirin (2.5–100 mg kg−1, p.o.) caused dose-related inhibition of acetic acid induced abdominal constrictions in the mouse (ED50 values of 154.7 μmol kg−1 and 242.8 μmol kg−1, respectively).
Nitroaspirin and aspirin (>200 mg kg−1, p.o.) reduced the ‘late' phase (but not the ‘early' phase) of the formalin-induced hindpaw licking assay in the mouse. Similarly, nitroaspirin and aspirin (>50 mg kg−1, p.o.) prolonged tail withdrawal latency following application of a noxious heat stimulus in the mouse.
Keywords: Nitroaspirin, aspirin, carrageenan, hyperalgesia, oedema, formalin, acetic acid
Introduction
Aspirin and like non-steroidal anti-inflammatory drugs (NSAID) have been extensively employed in the clinic for their anti-inflammatory and analgesic activity. The mechanism of action is widely believed to involve inhibition of cyclo-oxygenase (COX) mediated biosynthesis of prostacyclin (PGI2) and prostaglandin E2 (PGE2, Moncada et al., 1973). At the site of inflammation, these prostanoids exhibit both pro-inflammatory and pro-nociceptive activity and potentiate the effect of other pro-inflammatory mediators such as histamine and 5-hydroxytryptamine. Unfortunately, the side effects of aspirin in the gastrointestinal tract (e.g. haemorrhage, ulceration), resulting from reduced formation of vasodilator and cytoprotective prostanoids in the gastric mucosa, limit the usefulness of these compounds in the clinic (Wallace, 1997).
In recent years, a number of strategies have been adopted in an attempt to reduce the gastrointestinal side effects of aspirin and like NSAID. For example, the realization that COX exists as two separate isoforms viz. COX-1 and COX-2 (reviewed by Vane et al., 1998) has led to the development of a number of selective COX-2 inhibitors, which exhibit anti-inflammatory activity (COX-2 inhibition) but with reduced gastrointestinal toxicity (COX-1 inhibition) (for review, see Fung & Kirschenbaum, 1999).
An alternative approach to the development of less toxic NSAID is provided by the nitro-NSAID (NO-NSAID) class of compounds including nitroaspirin. To date, experiments in animals have revealed that, compared with aspirin, oral administration of nitroaspirin is associated with a marked reduction in gastrointestinal damage (Wallace et al., 1994a). Furthermore, nitroaspirin protects the stomach against injury following haemorrhagic shock (Wallace et al., 1997) and HCl/ethanol (Takeuchi et al., 1998) and additionally promotes the healing of pre-existing ulcers (Ukawa et al., 1997). Other NO-NSAID including nitrodiclofenac (Wallace et al., 1994b) and nitronaproxen (Davies et al., 1997) also cause reduced gastrointestinal damage when compared with the corresponding parent NSAID. In each case, nitro-NSAID most probably ‘spare' the gastrointestinal tract by the local release of nitric oxide (NO) resulting in increased stomach mucosal blood flow.
The improved gastrointestinal tolerability of nitro-NSAID (c.f. NSAID) suggest that these compounds may prove of value in the clinic. However, whilst the effect of nitroaspirin on gastrointestinal function has been extensively investigated (see above), its ability to reduce oedema formation and to modify algesia/hyperalgesia in experimental animals has, perhaps surprisingly, received relatively little attention. Indeed, to the best of our knowledge, there has been no systematic assessment of the ability of nitroaspirin vis-à-vis aspirin to exert anti-oedema and anti-nociceptive activity in a range of animal models and using a range of doses and by different routes of administration. To this end, we have now compared the ability of nitroaspirin and aspirin to influence, (i) carrageenan-induced hindpaw oedema and mechanical hyperalgesia in the rat and (ii) formalin-induced hindpaw licking, acetic acid induced abdominal constriction and tail flick latency to thermal stimulation in the mouse. Some of these results have been presented previously in preliminary form (al-Swayeh et al., 1999).
Methods
Rats (male, Wistar, 80–240 g, Tucks Ltd., U.K.) and mice (male, LACA, 22–35 g, Tucks Ltd., U.K.) were purchased from accredited suppliers and maintained in the Biological Services Unit of this College until required. All experiments were carried out ‘blind' in that the observer was not aware of the identity or dose of drugs administered to individual animals and were conducted under the conditions of the Animals Scientific Procedures Act, U.K. (1986).
Measurement of carrageenan-induced hindpaw oedema formation in the rat
Rats (male, Wistar, 160–240 g) were used in this study. Experiments were conducted essentially as described previously (Handy & Moore, 1998a). Animals were housed for 1 week prior to use and thereafter acclimatized in the laboratory for 1 h prior to commencement of the experiment. Hindpaw volume (ml) was determined as a measure of oedema formation, before, and at timed intervals (30 min–6 h), after intraplantar carrageenan injection. For this purpose, animals were held firmly and the volume of the injected hindpaw determined by plethysmography using a commercially available device (Ugo Basile Ltd.). Nitroaspirin (2.5–50 mg kg−1), aspirin (2.5–100 mg kg−1) or vehicle (0.5% w v−1 carboxymethylcellulose, CMC, 1 ml kg−1) were administered i.p. or p.o. 15 min prior to intraplantar injection of a standard volume (100 μl) of carrageenan (2% w v−1). At the end of the experiment, animals were killed by a blow to the head and exsanguination. Results are mean±s.e.mean, n=6.
Measurement of carrageenan-induced hindpaw hyperalgesia
Rats (male, Wistar, 80–120 g) were used in this study. Experiments were conducted essentially as described previously (Handy & Moore, 1998b). Animals were housed for 1 week prior to the experiment. Animals were weighed, marked for identification purposes and allowed to habituate to the thermostatically controlled test room (ambient temperature, 22°C) for at least 1 h prior to commencement of the experiment. ‘Baseline' measurements of hindpaw mechanical nociceptive threshold were obtained as described below. Thereafter, hindpaw inflammation was produced by intraplantar injection of carrageenan (2% w v−1, 150 μl) into either hindpaw chosen at random. Nitroaspirin or aspirin (25–200 mg kg−1, p.o.) or an appropriate volume (5 ml kg−1, p.o.) of vehicle (0.5% w v−1 CMC) were administered 2.5 h after carrageenan injection and the mechanical nociceptive threshold determined 30 min thereafter.
The threshold response to a noxious mechanical stimulus was determined using an Ugo Basile algesiometer as described previously (Laird et al., 1996; Handy & Moore, 1998b). Briefly, each hindpaw was positioned in turn under a conical probe surface (tip radius approx. 1 mm) and gradually increasing pressure applied to the hindpaw surface until the animal vocalized at which point the measurement was terminated. Mechanical nociceptive threshold for both the injected and contralateral (i.e. non-injected) hindpaw were determined. Results (mean±s.e.mean, n=6) are shown as the difference in nociceptive threshold (arbitrary units) before (‘pre-') and 3 h after (‘post-') intraplantar injection of carrageenan. Statistical comparisons between groups was determined as the difference in ‘post-pre' values.
Measurement of formalin-induced hindpaw licking behaviour in the mouse
The formalin-induced hindpaw licking assay was performed as described previously (Moore et al., 1991; Morgan et al., 1992). Experiments were conducted between 12 : 00 and 17 : 00 h in a purpose-built, thermostatically-controlled (22°C) laboratory and in subdued lighting conditions. On the day of the experiment, animals were weighed, marked for identification purposes and transported to the laboratory 60 min prior to the start of the experiment in order to habituate to the environment. Throughout the pre-trial period, animals were allowed food and water ad libitum.
Mice were treated orally (time, 0 min) with nitroaspirin, aspirin (both 50–300 mg kg−1) or vehicle (0.5% w v−1 CMC; 1 ml kg−1) followed 15 min thereafter by intraplantar injection of formalin (10 μl, 5% v v−1 in saline) into the right hindpaw and immediate transfer to transparent plexiglass observation chambers (dimensions: 20×20×12 cm). The duration of time spent licking the injected hindpaw was monitored and recorded over the periods, 0–5 min (‘early' phase of licking) and 15–30 min (‘late' phase of licking) after formalin administration. Mirrors positioned behind the chambers enabled observation of the right hindpaw when it was obscured from direct view. Results are shown as time spent licking the injected hindpaw in s and are mean±s.e.mean (n=3–20). At the end of the experiment animals were killed by cervical dislocation and exsanguination.
Acetic acid-induced abdominal constriction assay
The acetic acid-evoked abdominal constriction test was employed as described previously (Moore et al., 1991; Morgan et al., 1992). Mice were injected i.p. with freshly prepared acetic acid (2% w v−1 in saline; pH 2.7; 10 ml kg−1) and transferred immediately to individual observation cages. The number of abdominal constrictions was monitored over the following 30 min. The abdominal constrictions (‘writhes') elicited by mice consisted of contractions of the abdominal muscles which progressed posteriorly and usually ended with simultaneous flexor extension of both hind limbs with arching of the back. Abdominal constrictions of two to three mice were counted at the same time using hand tally counters.
In these experiments, mice were injected with nitroaspirin, aspirin (both 2.5–100 mg kg−1, p.o.) or vehicle (0.5% w v−1 CMC; 10 ml kg−1, p.o.) 15 min prior to acetic acid administration followed 15 min thereafter by an i.p. injection of acetic acid and evaluation of abdominal constrictions as described above. In some experiments, nitroaspirin or aspirin (50 mg kg−1, p.o.) were administered at timed intervals (15–360 min) prior to injection of acetic acid. Results show number of abdominal constrictions per 30 min test period and are mean±s.e.mean (n=6). At the end of the observation period animals were killed by cervical dislocation and exsanguination.
Tail withdrawal (‘flick') assay
The tail withdrawal assay was performed essentially as described originally by D'Amour and Smith (1941). For these experiments, animals were restrained in a perspex tube and a radiant heat source applied to the tail (lower surface). In preliminary experiments, the radiant heat was adjusted to attain a mean baseline tail withdrawal latency of 2.5–3.5 s in control animals. In order to avoid tissue damage exposure to the noxious stimulus was automatically terminated at 10 s. Baseline latencies were measured on two consecutive occasions before drug administration and the mean value recorded. Thereafter, mice received nitroaspirin, aspirin (both 2.5–100 mg kg−1, p.o.) or vehicle (0.5% w v−1 CMC; 10 ml kg−1, p.o.). Tail withdrawal latencies (mean of two consecutive measurements) were monitored 15 min thereafter. Results (in s) show mean±s.e.mean (n=10). At the end of the experiment animals were killed by cervical dislocation and exsanguination.
Statistics
The statistical significance of differences between groups was determined by ANOVA followed by post hoc Dunnett's test. A probability (P) value of 0.05 or less was taken to indicate statistical significance.
Materials
Nitroaspirin (NCX-4016; 2-acetoxy-benzoate 2-(2-nitroxymethyl) phenyl ester) was obtained from NiCOX Ltd. (Sophia-Antipolis, France). Aspirin and carrageenan were purchased from Sigma Ltd. Aspirin and nitroaspirin were suspended in 0.5% w v−1 carboxymethylcellulose (CMC). Carrageenan was suspended in saline. In all cases a reasonable suspension was achieved after warming (45°C) and with constant sonication and agitation.
Results
Anti-inflammatory activity of nitroaspirin
Intraplantar injection of carrageenan evoked a time-dependent increase in hindpaw volume (indicative of oedema formation) comprising of a relatively rapid ‘early' phase (up to 180 min) followed by a more sustained plateau or ‘late' phase (180–360 min) (Figures 1 and 2). Pre-treatment of animals with nitroaspirin or aspirin administered either i.p. (Figure 1a) or p.o. (Figure 2a) resulted in a dose-related inhibition of carrageenan-evoked hindpaw oedema. The relative potency of nitroaspirin and aspirin varied depending upon the route of administration and the ‘phase' of the oedema response. When administered i.p the anti-inflammatory effect of nitroaspirin was considerably greater than that of aspirin in the ‘late' phase of the oedema response (measured at 180 min) with calculated ED50 values of 21.3 mg kg−1 and >100 mg kg−1 (equivalent to 64.3 μmol kg−1 and >555 μmol kg−1), respectively (Figure 1). In contrast, nitroaspirin and aspirin exhibited broadly similar anti-inflammatory activity in the ‘late' phase when administered p.o. (see Figure 2). Thus, at the highest dose used (100 mg kg−1, 360 min), orally administered nitroaspirin (aspirin in parenthesis) inhibited oedema formation by 46.9±1.6% (47.2±3.8%, both n=6, P<0.05). Interestingly, both nitroaspirin and aspirin administered i.p. (but not p.o.) also reduced the ‘early' phase of carrageenan-evoked hindpaw oedema. Once again nitroaspirin (ED50: 33.3 mg kg−1 equivalent to 99 μmol kg−1) was considerably more potent than aspirin (only 39% inhibition at 100 mg/kg−1) when measured 120 min after carrageenan injection.
Figure 1.
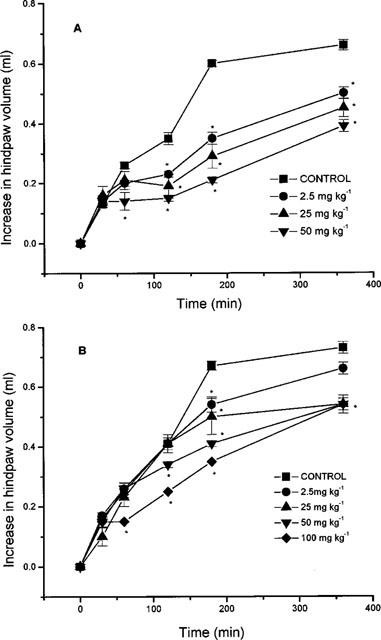
Effect of nitroaspirin (A) and aspirin (B) administered i.p. on carrageenan-induced hindpaw oedema formation in the rat. Results show increase in hindpaw volume (ml) and are mean±s.e.mean, n=6, *P<0.05 (ANOVA plus post-hoc Dunnett's test). Where no error bars are shown error lies within the dimensions of the symbol.
Figure 2.
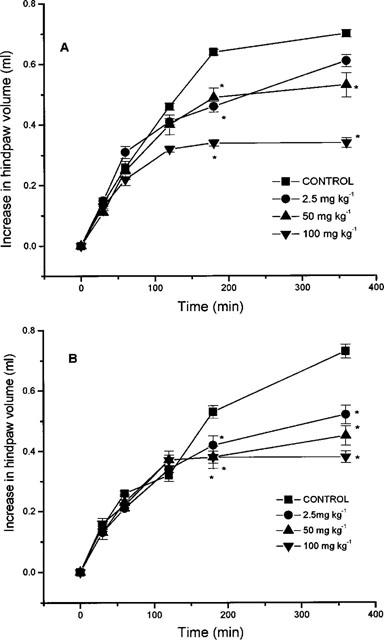
Effect of nitroaspirin (A) and aspirin (B) administered p.o. on carrageenan-induced hindpaw oedema formation in the rat. Results show increase in hindpaw volume (ml) and are mean±s.e.mean, n=6, *P<0.05 (ANOVA plus post-hoc Dunnett's test).
Anti-nociceptive activity of nitroaspirin
Carrageenan-induced hindpaw hyperalgesia (mechanical stimulation)
Intraplantar administration of carrageenan (2% w v−1, 150 μl) caused significant hindpaw hyperalgesia as evidenced by a reduction in the threshold for noxious mechanical stimulation from 9.92±1.0 to 3.38±0.37 units, n=6, P<0.05. In control experiments, intraplantar injection of saline (150 μl) did not alter the noxious mechanical stimulation threshold (data not shown).
Pre-treatment of animals (150 min after intraplantar carrageenan injection; i.e. 30 min before test) with nitroaspirin (25–200 mg kg−1, p.o.) or aspirin (25–200 mg kg−1, p.o.) inhibited carrageenan-induced hyperalgesia in the injected hindpaw without affecting the nociceptive mechanical threshold in the contralateral (i.e. non-injected) hindpaw (Figure 3). The calculated ED50 values for nitroaspirin and aspirin as inhibitors of mechanical hyperalgesia were 121.6 and 141.2 mg kg−1 (equivalent to 365 and 784 μmol kg−1), respectively.
Figure 3.
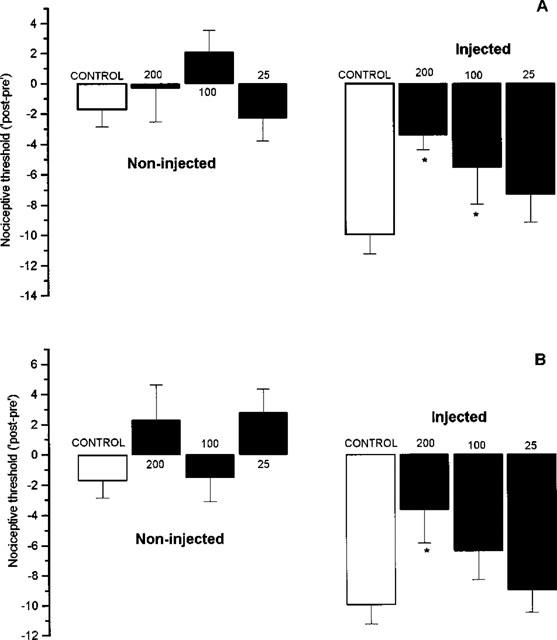
Effect of nitroaspirin (A) and aspirin (B) administered p.o. on carrageenan-induced mechanical hyperalgesia in the rat. Results show change (i.e. ‘post–pre') in nociceptive threshold (arbitrary units) to a noxious mechanical stimulus before and at 180 min after intraplantar injection of carrageenan. Drugs (mg kg−1) were administered 150 min after carrageenan injection. Results show mean±s.e.mean, n=6, *P<0.05 (ANOVA plus post-hoc Dunnett's test).
Formalin induced hindpaw licking behaviour
In control, (vehicle-injected) animals, intraplantar injection of formalin evoked a hindpaw licking response of 75.7±6.5 s (n=20) in the ‘early' phase (0–5 min) and 139.9±15.6 s (n=20) in the ‘late' (15–30 min) phase.
In contrast to the dose-related ability of both nitroaspirin and aspirin to reduce carrageenan-evoked hindpaw mechanical hyperalgesia in the rat, pretreatment of mice with nitroaspirin or aspirin (50 or 100 mg kg−1, p.o.) failed to affect either phase of the formalin-induced nociceptive licking behaviour (Figure 4). Higher doses of each drug produced more variable results with significant inhibition of ‘late' phase licking behaviour apparent at both 200 and 300 mg kg−1 of nitroaspirin. Aspirin (200 mg kg−1 but not 300 mg kg−1, p.o.) was also anti-nociceptive in the ‘late' phase.
Figure 4.
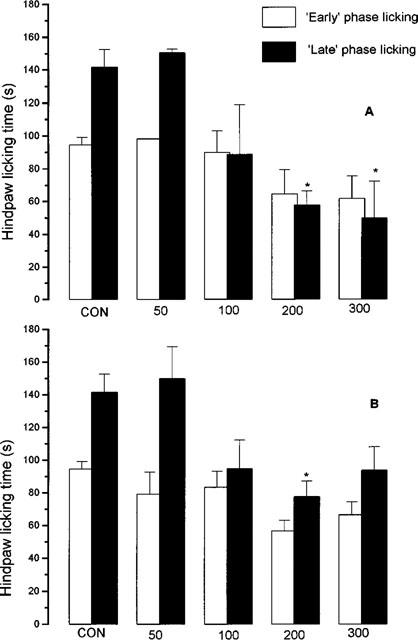
Effect of nitroaspirin (A) and aspirin (B) on mouse formalin-induced hindpaw licking behaviour in the ‘early' (0–5 min after intraplantar formalin injection) and ‘late' phases (15–30 min). Figures at the foot of each pair of columns indicate dose of drug administered p.o. (mg kg−1). Results shown mean±s.e.mean, n=3–20, *P<0.05 by ANOVA and post-hoc Dunnett's test.
Tail withdrawal assay
In these experiments, tail withdrawal latency following application of a topical heat source in control (vehicle-injected) animals was 2.4±0.15 s (n=20). Both nitroaspirin and aspirin (50 and 100 mg kg−1) exhibited anti-nociceptive activity as evidenced by a graded increase in withdrawal latency (Figure 5). Lower doses were without effect.
Figure 5.
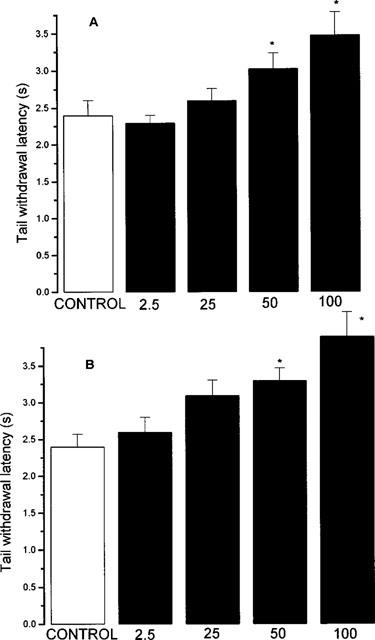
Effect of nitroaspirin (A) and aspirin (B) on tail withdrawal latency to a noxious thermal stimulus in the mouse. Results show tail withdrawal latency (s) 30 min after p.o. administration of drug. Figures at the base of each column indicate dose administered (mg kg−1, p.o.). Results show mean±s.e.mean, n=10, *P<0.05 (ANOVA plus post-hoc Dunnett's test).
Acetic acid induced abdominal constrictions
Intraperitoneal injection of acetic acid (2% w v−1, 0.1 ml 10 g−1) resulted in 49.6±1.9 abdominal constrictions (n=10) in the 30 min period thereafter. In the present experiments, oral administration of nitroaspirin or aspirin produced a dose-related inhibition of acetic acid induced abdominal constrictions (Figure 6). The calculated ED50 values for nitroaspirin and aspirin were 51.2 and 43.7 mg kg−1 (equivalent to 154.7 and 242.8 μmol kg−1). The time course of anti-nociceptive activity of nitroaspirin and aspirin was also investigated in this model. Pretreatment of animals with an approximate ED50 (i.e. 50 mg kg−1, p.o.) of nitroaspirin or aspirin either 15 min or 180 min prior to acetic acid injection revealed no difference in the resulting anti-nociceptive effect. In contrast, reduced anti-nociceptive activity was apparent in animals pretreated with either compound 360 min prior to acetic acid injection (Figure 7). Thus, these experiments suggest that the time course of anti-nociceptive activity of nitroaspirin and aspirin is similar in this model.
Figure 6.
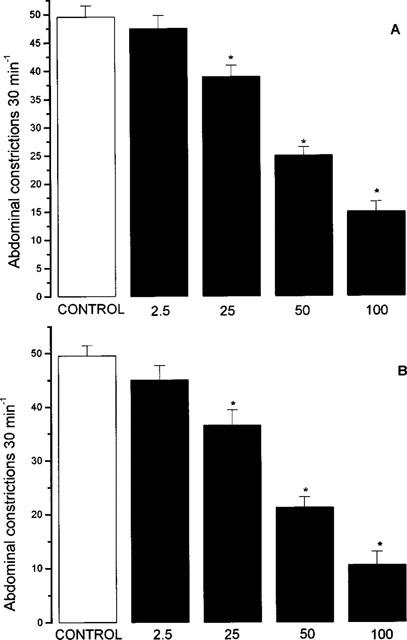
Effect of nitroaspirin (A) and aspirin (B) on acetic acid induced abdominal constrictions in the mouse. Results show number of abdominal constrictions over a 30 min period following i.p. acetic acid injection. Drugs were administered p.o. 15 min prior to acetic acid injection. Figures at the base of each column indicate dose administered (mg kg−1, p.o.). Results show mean±s.e. mean, n=10, *P<0.05 (ANOVA plus post-hoc Dunnett's test).
Figure 7.
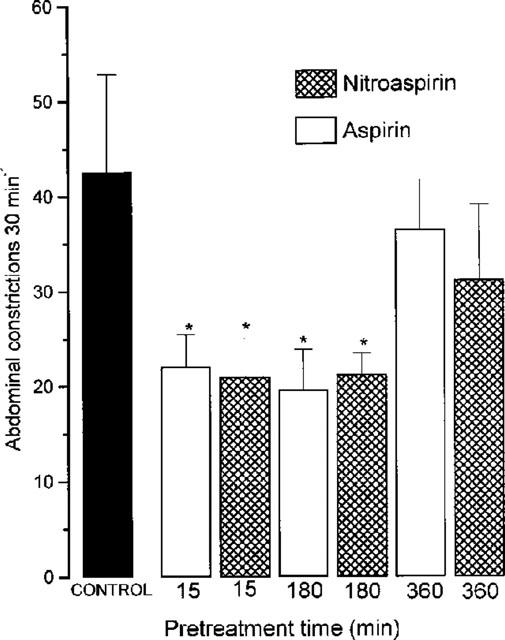
Effect of nitroaspirin and aspirin (both 50 mg kg−1, p.o.) on acetic acid induced abdominal constrictions in the mouse. Results show number of abdominal constrictions over a 30 min period following i.p. acetic acid injection. Drugs were administered p.o. either 15, 180 or 360 min prior to acetic acid injection. Results show mean±s.e.mean, n=6, *P<0.05 (ANOVA plus post-hoc Dunnett's test).
Discussion
Anti-oedema effect of nitroaspirin
The hindpaw oedema which follows intraplantar carrageenan injection involves a complex and time-dependent synthesis/release of a plethora of different inflammatory mediators. Previous research from this and other laboratories has suggested that the ‘early' phase response is triggered mainly by histamine, bradykinin, 5-hydroxytryptamine and nitric oxide (synthesized by the neuronal nitric oxide synthase isoform), whilst the ‘late' phase results primarily from the formation of pro-inflammatory prostanoids and nitric oxide (synthesized by the inducible nitric oxide synthase isoform) (DiRosa et al., 1971; Garcia Leme et al., 1973; Handy & Moore, 1998; Holsapple et al., 1980; Salvemini et al., 1996; Vinegar et al. (1969).
It has been known for many years that aspirin, and similar NSAID, reduce carrageenan-induced hindpaw oedema in the rat (Flower et al., 1972). Most reports suggest that NSAID preferentially inhibit the ‘late' phase response presumably by inhibiting the inducible COX-2 isoform which is believed to be responsible for the generation of pro-inflammatory prostanoids in the later stages of this and other inflammatory models (Vane & Botting, 1998). In the present experiments, both aspirin and nitroaspirin caused dose related inhibition of the ‘late' phase of oedema regardless of the route of administration (i.e., i.p. or p.o.) employed. These results, therefore, confirm a preliminary report in which single, equimolar doses of nitroaspirin and aspirin reduced carrageenan-induced hindpaw oedema in the rat (Takeuchi et al., 1998).
As nitroaspirin and aspirin exhibit similar COX inhibitory activity in vivo (Cuzzolin et al., 1996; Wallace et al., 1999a), it seems likely that the ‘late' phase inhibitory effect of both drugs depends upon inhibition of hindpaw prostanoid formation presumably by an effect on COX-2 activity, which is likely to be the predominant isoform at this stage in the response (Seibert et al., 1994; Vane et al., 1998). Interestingly, nitroaspirin or aspirin administered i.p. (but not p.o.) also reduced the ‘early' phase response. Although some reports suggest that COX-2 may be present as early as 60–90 min after induction of an inflammatory response (Seibert et al., 1994) the possibility that nitroaspirin (and aspirin) may, additionally, reduce hindpaw constitutive COX-1 activity to bring about an anti-oedema effect in the ‘early' phase should be considered. Certainly, aspirin reduces inflammation in a carrageenan-induced pleurisy model in the rat over a considerably longer time period than either NS-398 or nimesulide (relatively selective COX-2 inhibitors) (Gilroy et al., 1998), which suggests a pro-inflammatory role for prostanoids generated by both COX-1 and COX-2 at least in this model (Gilroy et al., 1998).
The relative potency of nitroaspirin vis-a-vis aspirin is clearly dependent both upon the time course of the carrageenan response (i.e. ‘early' c.f. ‘late' phase) and upon the route of drug administration. Thus, nitroaspirin (administered i.p.) is more potent than aspirin (by comparison of ED50 values; 8.6×on a mol for mol basis) than aspirin in the ‘late' phase. Similarly, nitroaspirin (administered i.p.) also exhibits greater anti-inflammatory activity than does aspirin in the ‘early' (time, 60 min) phase (e.g. nitroaspirin; 46% inhibition at 50 mg kg−1 equivalent to 151 μmol kg−1 c.f. aspirin; 40% inhibition at 100 mg kg−1 equivalent to 555 μmol kg−1). However, it should be noted that the improved anti-oedema potency of nitroaspirin (c.f. aspirin) is no longer apparent when drugs are administered orally. In this context, a NO-releasing derivative of mesalamine (5-aminosalicylic acid) has recently been reported to exhibit greater anti-inflammatory activity than the parent compound as an inhibitor of colitis in the rat (Wallace et al., 1999b).
Assuming that nitroaspirin acts simply as a ‘pro-drug' for anti-inflammatory aspirin it might be expected that the two compounds would exhibit similar potency in these experiments. This is not the case. The reason(s) underlying this discrepancy in potency are not clear and have not been directly addressed in the present study. However, we believe that some comment is appropriate. Perhaps the simplest explanation is that i.p. administered nitroaspirin is accumulated more readily or over a longer period in the carrageenan-inflamed hindpaw than is aspirin. Unfortunately, to the best of our knowledge, there have been no published reports which compare the accumulation and dispersal of nitroaspirin/aspirin (and their metabolites such as salicylate) at the site of inflammation. However, the observation that i.p. injected nitroaspirin in the rat results in a delay in the time required to achieve peak plasma salicylate concentration (6 h) when compared with aspirin (3 h; Tagliaro et al., 1996) suggests that differences in pharmacokinetic profile may be important in determining the relative anti-inflammatory potency of nitroaspirin vis-à-vis aspirin. Alternatively, nitroaspirin may exhibit additional pharmacological activity (c.f. aspirin) by virtue of what is presumed to be a slow release of nitric oxide (see del Soldato et al., 1999). In this context, nitroaspirin (but not aspirin) has recently been shown to inhibit caspase 1 activity and, thus, interleukin-1β formation (Fiorucci et al., 1999) and also reduces nuclear factor κB (NF-κB) activation (Minto et al., 1997) although it should be noted that an inhibitory effect of aspirin on NF-κB activation has also been reported (Yin et al., 1998). The NO-releasing derivative of mesalamine has also been demonstrated to reduce leucocyte adherence to the vascular endothelium (Wallace et al., 1999b). In each case, nitric oxide has been shown to be the active moiety within the nitroaspirin molecule. Whether nitroaspirin affects leucocyte function at the site of inflammation in vivo or whether other, as yet unidentified, COX-independent mechanisms contribute to the anti-oedema effect of this compound would appear to warrant further study.
Anti-nociceptive effect of nitroaspirin
Nitroaspirin and aspirin exhibited anti-nociceptive activity in all four animal models studied. However, both the absolute and the relative potency of the two compounds varies depending upon the experimental model used. Thus, both nitroaspirin and aspirin exhibited greater anti-nociceptive activity in animal models of ‘inflammatory' pain (e.g. carrageenan-induced hindpaw hyperalgesia; acetic acid induced abdominal constrictions) and were less effective in models of ‘acute' pain (e.g. tail flick test; responsiveness to mechanical noxious stimulation in the non-injected hindpaw) and in hyperalgesia states dependent more on ‘central' as opposed to ‘peripheral' sensitisation (e.g. late phase formalin-induced hindpaw licking) (see reviews by Willis, 1992; Woolf, 1993). Interestingly, the time course of the anti-nociceptive effect of nitroaspirin and aspirin (assessed as inhibition of acetic acid induced abdominal constrictions in the mouse) was very similar with no difference in activity detected when drugs were administered either 15 min or 180 min prior to acetic acid injection.
The profile of anti-nociceptive activity of aspirin identified in these experiments is in broad agreement with previous reports in the literature. Thus, published ED50 values (43.7 mg kg−1 in this study) for aspirin in the mouse abdominal constriction test have ranged from 24 mg kg−1 (Santos et al., 1995) through 31.6 mg kg−1 (Inoue et al., 1994) to 81.4 mg kg−1 (Satyanarana et al., 1995). In addition, similar doses of aspirin to those used in this study reduce the hyperalgesia associated with hindpaw injection in the rat of carrageenan (Calhoun et al., 1992) or other algogens such as yeast (Seegers et al., 1981) but have only marginal effect on the tail flick response to noxious thermal stimulus (Millan et al., 1995) and no effect on either the ‘early' or ‘late' phase hindpaw licking response to intraplantar formalin injection even at doses as high as 400 mg kg−1 (Rahman et al., 1994; Wheeler-Aceto & Cowan, 1991).
Since this is the first study to examine the anti-nociceptive effect of nitroaspirin in a range of behavioural tests similar comparisons with the results of other researchers are clearly not available. However, as was the case for the anti-oedema effect, nitroaspirin was also marginally more potent (mol for mol) than aspirin as an inhibitor both of carrageenan-induced hindpaw hyperalgesia in the rat (2.1 fold comparing ED50 values) and acetic acid-induced abdominal constrictions in the mouse (1.6 fold comparing ED50 values). It seems likely that inhibition of hindpaw COX-2 enzyme activity plays a major part in the anti-nociceptive activity of nitroaspirin and aspirin. Experiments using highly selective COX-2 inhibitors (e.g. rofecoxib, Ehrich et al., 1999) strongly suggest that the therapeutic benefit of NSAID in inflammatory pain results from inhibition of COX-2 activity although whether the COX-2 isoform involved is sited in the inflamed tissue or in the spinal cord (see Smith et al., 1998) or both remains unclear. In contrast, bearing in mind the brief (30 min) duration of the present experiments, the effect of nitroaspirin and aspirin on acetic acid induced abdominal constrictions is more likely to reflect COX-1 rather than COX-2 inhibition. Whether the albeit modestly augmented anti-nociceptive potency of nitroaspirin (c.f. aspirin) suggests additional COX-independent mechanisms for the nitro derivative requires more detailed analysis.
Conclusion
The present experiments represent the first systematic attempt to compare the anti-oedema and anti-nociceptive activity of aspirin and nitroaspirin. We conclude that these two compounds are qualitatively similar in their actions. However, on a mol for mol basis, nitroaspirin appears to be more potent than aspirin. The possibility that nitroaspirin exhibits additional anti-inflammatory actions over and above those of aspirin is intriguing and merits further investigation.
Abbreviations
- CMC
carboxymethylcellulose
- COX
cyclo-oxygenase
- NSAID
non-steroidal anti-inflammatory drug
- PGE2
Prostaglandin E2
- PGI2
Prostaglandin I2
References
- AL-SWAYEH O.A., CLIFFORD R.H., MOORE P.K. Nitroaspirin – a novel anti-inflammatory and anti-hyperalgesic agent. Mediat. Inflamm. 1999;8:S95. [Google Scholar]
- CALHOUN W., GILMAN S.C., DATKO L.J., COPENHAVER T.W., CARLSON R.P. Interaction studies of tilomisole, aspirin and naproxen in acute and chronic inflammation with assessment of gastrointestinal irritancy in the rat. Agents Actions. 1992;36:99–106. doi: 10.1007/BF01991236. [DOI] [PubMed] [Google Scholar]
- CUZZOLIN L., ADAMI A., DEGAN M., CRIVELLENTE F., BONAPACE S., MINUZ P., BENONI G. Effect of single and repeated doses of a new nitroderivative of acetylsalicylic acid on platelet TxA2 production in rats. Life Sci. 1996;58:207–208. doi: 10.1016/0024-3205(96)00038-0. [DOI] [PubMed] [Google Scholar]
- D'AMOUR F.E., SMITH D.L. A method for determining loss of pain sensation. J. Pharm. Exp. Ther. 1941;72:74–79. [Google Scholar]
- DAVIES N.M., ROSETH A.G., APPLEYARD C.B., MCKNIGHT W., DEL SOLDATO P., CALIGNANO A., CIRINO G., WALLACE J.L. NO-naproxen vs naproxen: ulcerogenic, analgesic and anti-inflammatory effects. Aliment. Pharmacol. Ther. 1997;11:69–79. doi: 10.1046/j.1365-2036.1997.115286000.x. [DOI] [PubMed] [Google Scholar]
- DELSOLDATO P., SORRENTINO R., PINTO A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol. Sci. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- DIROSA M., GIROUD J.P., WILLOUGHBY D.A. Studies of mediators of the acute inflammatory response in rats in different sites by carrageenan and turpentine. J. Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- EHRICH E.W., DALLOB A., DELEPELEIRE I., VAN HECKEN A., RIENDEAU D., YUAN W.Y., PORRAS A., WITTREICH J., SEIBOLD J.R., DESCHEPPER P., MEHLISCH D.R., GERTZ B.J. Characterisation of rofecoxib as a cyclooxygenase-2 isoform inhibitor and demonstration of analgesia in the dental pain model. Clin. Pharm Ther. 1999;65:336–347. doi: 10.1016/S0009-9236(99)70113-X. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., ANTONELLI E., SANTUCCI L., MORELLI O., MIGLIETTI M., FEDERICI B., MANNUCCI R., DEL SOLDATO P., MORELLI A. Gastrointestinal safety of nitric oxide derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- FLOWER R.J., GRYGLEWSKI R.J., HERBACZYNSKA-CEDRO K., VANE J.R. Effects of anti-inflammatory drugs on prostaglandin biosynthesis. Nature. 1972;238:104–106. doi: 10.1038/newbio238104a0. [DOI] [PubMed] [Google Scholar]
- FUNG H.B., KIRSCHENBAUM H.L. Selective cyclooxygenase-2 inhibitors for the treatment of arthritis. Clin. Ther. 1999;21:1131–1157. doi: 10.1016/S0149-2918(00)80018-1. [DOI] [PubMed] [Google Scholar]
- GARCIA LEME J., HAMAMURA L., LEITE M.P., ROCHA E SILVA M. Pharmacological analysis of the acute inflammatory response induced in the rat's paw by local injection of carrageenan and by heating. Br. J. Pharmacol. 1973;48:88–96. doi: 10.1111/j.1476-5381.1973.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILROY D.W., TOMLINSON A., WILLOUGHBY D.A. Differential effects of inhibitors of cyclooxygenase (cyclooxygenase 1 and cyclooxygenase 2) in acute inflammation. Eur. J. Pharmacol. 1998;355:211–217. doi: 10.1016/s0014-2999(98)00508-1. [DOI] [PubMed] [Google Scholar]
- HANDY R.L.C., MOORE P.K. A comparison of the effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS activity. Br. J. Pharmacol. 1998a;123:1083–1088. doi: 10.1038/sj.bjp.0701735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANDY R.L.C., MOORE P.K. Effect of selective inhibitors of neuronal nitric oxide synthase on carrageenan-induced mechanical and thermal hyperalgesia. Neuropharmacology. 1998b;279:37–43. doi: 10.1016/s0028-3908(97)00201-3. [DOI] [PubMed] [Google Scholar]
- HOLSAPPLE M.P., SCHNUR M., YIM G.K. Pharmacological modulation of edema mediated by prostaglandin, serotonin and histamine. Agents Actions. 1980;10:368–373. doi: 10.1007/BF01971442. [DOI] [PubMed] [Google Scholar]
- INOUE K., FUJISAWA H., MOTYONAGA A., INOUE Y., KYOI T., UEDA F., KIMURA K. Antiinflammatory effects of etodolac; comparison with other nonsteroidal antiinflammatory drugs. Biol. Pharm Bull. 1994;17:1577–1583. doi: 10.1248/bpb.17.1577. [DOI] [PubMed] [Google Scholar]
- LAIRD J.M.A., MASON G.S., WEBB J., HILL R.G., HARGREAVES R.J. Effects of a partial agonist and a full antagonist acting at the glycine site of the NMDA receptor on inflammation-induced mechanical hyperalgesia in rats. Br. J. Pharmacol. 1996;117:1487–1492. doi: 10.1111/j.1476-5381.1996.tb15311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLAN M.J., SEGUIN L., HONORE P., GIRARDON S., BERVOETS K. Pro- and antinociceptive actions of serotonin (5-HT1A) agonists and antagonists in rodents: relationship to algesiometric paradigm. Behavioural Brain Res. 1995;73:69–77. doi: 10.1016/0166-4328(96)00073-3. [DOI] [PubMed] [Google Scholar]
- MINTO M., DEL SOLDATO P., GHEZZI P., FRATELLI M. A novel acetylsalicylic acid nitroderivative inhibits NF-κB activation and is antiproliferative to L929 cells. Inflamm. Res. 1997;46:S3. [Google Scholar]
- MONCADA S., FERREIRA S.H., VANE J.R. Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature. 1973;246:217–219. doi: 10.1038/246217a0. [DOI] [PubMed] [Google Scholar]
- MOORE P.K., OLUYOMI A.O., BABBEDGE R.C., WALLACE P., HART S.L. L-NG nitroarginine methyl ester exhibits antinociceptive activity in the mouse. Br. J. Pharmacol. 1991;102:198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C.V.J., BABBEDGE R.C., GAFFEN Z., WALLACE P., HART S.L., MOORE P.K. Synergistic anti-nociceptive effect of L-NG-nitro arginine methyl ester (L-NAME) and flurbiprofen in the mouse. Br. J. Pharmacol. 1992;106:493–497. doi: 10.1111/j.1476-5381.1992.tb14362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAHMAN A.F.M.M., TAKAHISHI M., KANETO H. Involvement of pain associated anxiety in the development of morphine tolerance in formalin-treated mice. Jap. J. Pharmacol. 1994;65:313–317. doi: 10.1254/jjp.65.313. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.-Q., WYATT P.A., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS A.R.S., NIERO R., FILHO Y.C., YUNES R.A., PIZZOLATTI M.G., DELLEMONACHE F., CALIXTO J.B. Antinociceptive properties of steroids isolated from Phyllanthus-Corcovadensis in mice. Planta Med. 1995;61:329–332. doi: 10.1055/s-2006-958093. [DOI] [PubMed] [Google Scholar]
- SATYANARANA K., RAO M.N.A. Synthesis of 4-[5-(substituted aryl) 4,5 dihydro 1H pyrazol-3-yl] 3 phenylsydnones as antinflammatory, antiarthritic and analgesic agents. Eur. J. Med. Chem. 1995;30:641–645. [Google Scholar]
- SEEGERS A.J., JAGER L.P., ZANDBERG P., VAN NOORDWIJK J. The anti-inflammatory, analgesic and antipyretic activities of non-narcotic analgesic drug mixtures in rats. Arch. Int. Pharmacodyn. Ther. 1981;251:237–254. [PubMed] [Google Scholar]
- SEIBERT K., ZHANG Y., LEAHY K., HAUSER S., MASFERRER J., PERKINS W., LEE L., ISAKSON P. Pharmacological and biochemical demonstration of the role of cyclooxygenase-2 in inflammation and pain. Proc. Natl. Acad. Sci., U.S.A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C.J., ZHANG Y., MASFERRER J.L., SEIBERT K., ISAKSON P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGLIARO F., CUZZOLIN L., ADAMI A., SCARCELLA D., CRIVELLENTE F., BENONI G. Pharmacokinetics of a new nitroderivative of acetylsalicyclic acid after a single dose in rats. Life Sci. 1996;60:101–106. doi: 10.1016/s0024-3205(96)00599-1. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI K., UKAWA H., KONAKA A., KITAMURA M., SUGAWA Y. Effect of nitric oxide-releasing aspirin derivative on gastric functional and ulcerogenic responses in rats: comparison with plain aspirin. J. Pharm. Exp. Ther. 1998;286:115–121. [PubMed] [Google Scholar]
- UKAWA H., YAMAKUNI H., KATO S., TAKEUCHI K. Effects of cycloxygenase-2 selective and nitric oxide releasing nonsteroidal anti-inflammatory drugs on gastric ulcerogenic and healing responses in experimental aninmals. Dig. Dis. Sci. 1997;43:2003–2011. doi: 10.1023/a:1018846912032. [DOI] [PubMed] [Google Scholar]
- VANE J.R., BAKHLE Y.S., BOTTING R.M. Cyclooxygenases 1 and 2. Ann. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- VANE J.R., BOTTING R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998;47:S78–S87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- VINEGAR R., SCHREIBER W., HUGO R. Biphasic development of carrageenin edema in rats. J. Pharmacol. Exp. Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- WALLACE J.L. Nonsteroidal antiinflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., WILSON T.L., DEL SOLDATO P., CIRINO G. Reduction of shock-induced gastric damage by a nitric oxide releasing aspirin derivative: role of neutrophils. Am. J. Physiol. 1997;273:G1246–G1251. doi: 10.1152/ajpgi.1997.273.6.G1246. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MUSCARA M.N., MCKNIGHT W., DICAY M., DEL SOLDATO P., CIRINC G. In vivo antithrombotic effects of a nitric oxide releasing aspirin derivative, NCX-4016. Thrombosis Res. 1999a;93:43–50. doi: 10.1016/s0049-3848(98)00134-0. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M., CIRINO G. Novel non-steroidal antiinflammatory derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994a;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M., CIRINO G. A diclofenac derivative without ulcerogenic properties. Eur. J. Pharmacol. 1994b;257:249–255. doi: 10.1016/0014-2999(94)90136-8. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., VERGNOLLE N., MUSCARA M.N., ASFAHA S., CHAPMAN K., MCKNIGHT W., DEL SOLDATO P., MORELLI I., FIORUCCI S. Enhanced anti-inflammatory effects of a nitric oxide releasing derivative of mesalamine in rats. Gastroenterology. 1999b;117:557–566. doi: 10.1016/s0016-5085(99)70448-8. [DOI] [PubMed] [Google Scholar]
- WHEELER-ACETO H., COWAN A. Standardisation of the rat paw formalin test for the evaluation of analgesics. Psychopharmacology. 1991;104:35–44. doi: 10.1007/BF02244551. [DOI] [PubMed] [Google Scholar]
- WILLIS W.D. Hyperalgesia and Allodynia. Raven Press, New York; 1992. [Google Scholar]
- WOOLF C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1993;308:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- YIN M.-J., YAMAMOTO Y., GAYNOR R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]


