Abstract
The present study investigated the effect of curcumin on adriamycin (ADR) nephrosis in rats. The results indicate that ADR-induced kidney injury was remarkably prevented by treatment with curcumin. Treatment with curcumin markedly protected against ADR-induced proteinuria, albuminuria, hypoalbuminaemia and hyperlipidaemia. Similarly, curcumin inhibited ADR-induced increase in urinary excretion of N-acetyl-β-D-glucosaminidase (a marker of renal tubular injury), fibronectin and glycosaminoglycan and plasma cholesterol. Curcumin restored renal function in ADR rats, as judged by the increase in GFR. The data also demonstrated that curcumin protected against ADR-induced renal injury by suppressing oxidative stress and increasing kidney glutathione content and glutathione peroxidase activity. In like manner, curcumin abolished ADR-stimulated kidney microsomal and mitochondrial lipid peroxidation. These data suggest that administration of curcumin is a promising approach in the treatment of nephrosis caused by ADR.
Keywords: Adriamycin, curcumin, fibronectin, glycosaminoglycan, mitochondria
Introduction
The clinical use of adriamycin (ADR) is associated with nephrotic syndrome characterized by heavy proteinuria, albuminuria, hypoalbuminaemia and hyperlipidaemia (Bertani et al., 1982; Milner et al., 1991). Several lines of evidence suggest reactive oxygen species (ROS) as the principal mediator in the development of nephrosis caused by ADR (Mimnaugh et al., 1986; Milner et al., 1991). The hypothesis was proposed, that if ADR nephrotoxicity is related to free radical formation and lipid peroxidation then antioxidant therapy may protect ADR toxicity in kidney. Curcumin, the yellow curry pigment isolated from turmeric, represents a class of antiinflammatory (Srivastava & Srimal, 1985) antioxidants (Sharma, 1976) reported to be a potent inhibitor of ROS formation. Administration of curcumin has been reported to prevent renal lesions in streptozotocin diabetic rats (Suresh Babu & Srinivasan, 1998). Curcumin has been also shown to provide protection against oxidative stress in a renal cell line (Cohly et al., 1998). In addition, curcumin has been reported to reduce ischaemic renal injury (Shoskes, 1998). More recently, curcumin was shown to protect ADR cardiotoxicity in rats (Venkatesan, 1998). However, the protective effect of curcumin against ADR nephrosis has not been addressed to date. Therefore, the present study was undertaken to evaluate the protective effects of curcumin against ADR nephrotoxicity. Our results reveal here that curcumin suppressed ADR nephrotoxicity in rats.
Methods
Healthy male Wistar rats (275±10 g) were divided into four groups of six animals each: Group I, control (SA), was injected with 0.1 ml normal saline (0.9% NaCl). Group II received 200 mg kg−1 body weight of curcumin (CC) in 1% gum acacia, orally. Nephrosis (Group III, ADR) was induced by a single injection of ADR (7.5 mg kg−1 body weight, dissolved in 0.1 ml saline) through the tail vein. The ADR dosing regimen utilized in this study has been employed by other investigator (Bertani et al., 1982). Group IV (CC+ADR) received curcumin 7 days before ADR and daily thereafter throughout the study. All animals had free access to food and water ad libitum. Body weight, food intake and urine volume were recorded daily throughout the experimental period (30 days).
Collection of blood and urine
Thirty days after ADR, blood was collected in test tubes containing potassium-EDTA (1 mg ml−1) and plasma was separated by centrifugation at 2500×g for 15 min. Both control and treated groups were kept in individual metabolic cages for 24 h urine collection before sacrifice. Urine was centrifuged at 800×g for 10 min at 25°C to remove cells and particulate material and then dialyzed against phosphate-buffered saline (PBS), pH 7.4. Whole plasma and urine were stored at −70°C and thawed just before use.
Analytical methods
Urinary protein, creatinine and albumin, and plasma albumin were measured according to standard methods. Urine was also analysed for N-acetyl-β-D-glucosaminidase (NAG) (Moore & Morris, 1982), fibronectin (FN) (Rennard et al., 1980) and glycosaminoglycan (GAG) (uronic acid content) (Benjelloun et al., 1993). The glomerular filtration rate (GFR) was determined by insulin clearance according to standard methods. Plasma cholesterol was measured (Parekh & Jung, 1970). Excised kidneys were analysed for lipid peroxides (Ohkawa et al., 1979), glutathione (Moron et al., 1979), glutathione peroxidase (Rotruck et al., 1973) and total lipids (Folsch et al., 1957).
In vitro lipid peroxidation
In a separate study, microsomal and mitochondrial lipid peroxidation were assayed essentially following the method of Mimnaugh et al. (1986). A total of 48 rats were used to isolate microsomes and mitochondria. Kidneys from four rats were pooled to get sufficient amount of microsomal or mitochondrial fractions for these experiments and they were repeated six times. Both the microsomal and mitochondrial studies were done immediately. Kidney microsomes or mitochondria (1 mg protein ml−1) were incubated in the absence or presence of 100 μM ADR in KCl-Tris-HCl buffer. Curcumin (1, 10 or 100 μM) was added directly to the incubation mixtures. The reaction was initiated using an NADPH-generating system (microsomes) or NADH (mitochondria). The reaction was terminated after 60 min by adding ice-cold trichloroacetic acid, and lipid peroxidation products were estimated (Mimnaugh et al., 1986).
Statistical analysis
Results are mean±s.d. of six rats. One way analysis of variance (ANOVA) with post hoc Bonferroni was used to find statistical significance.
Results
Intravenous ADR caused a decrease in body weight gain, however, curcumin treatment significantly (P<0.001) attenuated ADR-induced decrease in body weight. As shown in Table 1, ADR rats had elevated urine protein, albumin and NAG levels that were markedly decreased by curcumin. ADR rats exhibited an increase in urinary FN and GAG excretion. Interestingly, ADR rats treated with curcumin had urinary FN and GAG levels that were similar to control groups. ADR-induced reduction in GFR, measured as an index of renal function, was significantly (P<0.001) suppressed by curcumin. ADR-induced increase in plasma cholesterol, and a decrease in albumin levels were remarkably attenuated by curcumin. In kidneys exposed to ADR there was a significant increase in lipid peroxides and total lipids while the same was attenuated by curcumin. Similarly, ADR-induced decrease in glutathione content and glutathione peroxidase activity of kidney tissue was suppressed by curcumin. Treatment of microsomes or mitochondria with ADR resulted in a significant (P<0.001) increase of lipid peroxidation (Figure 1), as measured by the formation of thiobarbituric acid reactive substances. Interestingly, curcumin inhibited ADR-induced microsomal and mitochondrial lipid peroxidation in a dose-dependent manner.
Table 1.
Beneficial effect of curcumin on ADR nephrosis
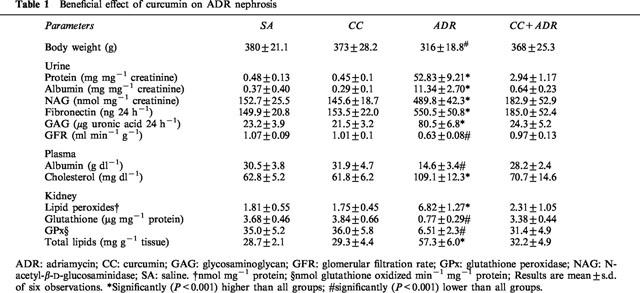
Figure 1.
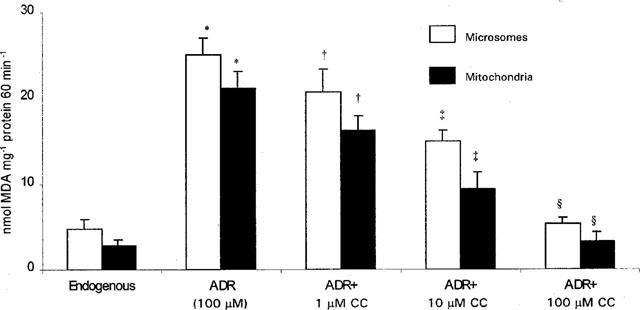
Dose-dependent protective effect of curcumin against ADR-induced microsomal and mitochondrial lipid peroxidation. Microsomes or mitochondria were incubated with NADPH-generating system or NADH, respectively in the absence or presence of 100 μM ADR and lipid peroxidation was measured by the thiobarbituric acid method. Curcumin (1, 10 or 100 μM) was added to incubation mixtures before adding NADPH-generating system or NADH. Data are mean±s.d. of six observations. *Significantly (P<0.001) higher than all groups; †significantly (P<0.05) lower than ADR; ‡significantly (P<0.01) lower than ADR; ≈rcub;significantly (P<0.001) lower than ADR, but not significant compared to controls.
Discussion
The main objective of this study was to investigate the hypothesis that curcumin has a protective effect against the development of ADR renal injury. The data presented in this study reveal that curcumin is a potent inhibitor of ADR nephrosis. Experiments performed to examine the mechanism by which curcumin was exerting its protective effect on ADR nephrosis revealed that antioxidant, antiinflammatory, membrane stabilizing and hypolipidemic properties may constitute an important part of its therapeutic effects. First, curcumin treatment prevented ADR-induced decrease in body weight gain. This provides evidence that curcumin directly protects ADR general toxicity. Second, curcumin suppressed renal toxicity by blocking oxidative injury in the kidney because it prevented ADR-induced rise in lipid peroxides. Similarly, curcumin suppressed ADR-stimulated lipid peroxidation in kidney microsomes and mitochondria in a dose-dependent manner. Curcumin treatment also increased the glutathione content and glutathione peroxidase activity of kidney tissues in ADR rats. This increase in both the nonenzymatic and enzymatic antioxidants may play significant role in the mechanism of the protective effect of curcumin (Sharma, 1976). Curcumin has been shown to inhibit hydrogen peroxide induced oxidative injury in a renal cell line (Cohly et al., 1998). Curcumin is also protective against ADR cardiac injury (Venkatesan, 1998). These findings support that curcumin suppresses oxidative injury in a wide variety of tissues including heart and kidney. Third, the observation that curcumin treatment was accompanied by significant reduction in urinary excretion of GAG in ADR rats suggests that this treatment may influence the integrity of the glomerular basement membrane. Because the basement membrane is subject to damage in ADR nephrosis (Raats et al., 1997), curcumin treatment would retard the abnormal passage of high molecular weight macromolecules from the blood to the urinary space. In addition, curcumin might play a critical role in suppressing ROS mediated destruction of basement membrane and proteinuria (Raats et al., 1997). In this respect, it is interesting to note that ADR has been demonstrated to cause increased urinary excretion of GAG and loss of glomerular GAG in rats (Benjelloun et al., 1993). Fourth, the renoprotective effect of curcumin is also evident by a remarkable improvement of renal function, as judged by the restoration of GFR in ADR rats. It is also interesting to note that urinary FN levels (Soose et al., 1991) are modulated in curcumin-treated ADR rats. Fifth, the prevention of ADR-induced increases in the urinary levels of NAG supports the idea that curcumin exhibits potent antiinflammatory (Shoskes, 1998) and membrane stabilizing properties (Srivastava & Srimal, 1985) and thus prevents heavy proteinuria and exposure to proinflammatory cytokines that induce the damage of the tubular epithelial cells in ADR rats. Finally, the hypolipidaemic property of curcumin could also contribute to its beneficial effects on ADR renal injury. Excessive production and accumulation of lipids can have a devastating effect on renal structure and function (Hutchison, 1993). Our results showing decreased plasma and kidney lipids in curcumin-treated ADR rats are consistent with the hypolipidaemic action of curcumin. Recently, the hypolipidaemic property of curcumin in streptozotocin diabetic rats was reported (Suresh Babu & Srinivasan, 1997).
In summary, the present findings demonstrate that curcumin has multiple therapeutic activities that are beneficial in the kidney and thus curcumin is a promising candidate to block ADR nephrosis. Further studies are in progress.
Acknowledgments
We thank Mr V. Elango for his help in animal studies.
Abbreviations
- ADR
adriamycin
- CC
curcumin
- FN
fibronectin
- GAG
glycosaminoglycan
- GFR
glomerular filtration rate
- NADH
nicotinamide adenine dinucleotide, reduced form
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- NAG
N-acetyl-β-D-glucosaminidase
- ROS
reactive oxygen species
- SA
saline
References
- BENJELLOUN A.S., MERVILLE P., CAMBAR J., APARICIO M. Effects of low-protein diet on urinary glycosaminoglycan excretion in adriamycin-treated rats. Nephron. 1993;64:242–248. doi: 10.1159/000187321. [DOI] [PubMed] [Google Scholar]
- BERTANI T., POGGI A., POZZONI R., DELAINI F., SACCHI G., THOUA Y., MECCA G., REMUZZI G., DONATI M.B. Adriamycin induced nephrotic syndrome in rats: Sequence of pathologic events. Lab. Invest. 1982;46:16–23. [PubMed] [Google Scholar]
- COHLY H.H.P., TAYLOR A., ANGEL M.F., SALAHUDEEN A.K. Effect of turmeric, turmerin and curcumin on H2O2-induced renal epithelial (LLC-PK1) cell injury. Free Rad. Biol. Med. 1998;24:49–54. doi: 10.1016/s0891-5849(97)00140-8. [DOI] [PubMed] [Google Scholar]
- FOLSCH J., LEES M., STANLEY G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- HUTCHISON F.N. Proteinuria, hyperlipidemia and the kidney. Miner. Electrolyte Metab. 1993;19:127–136. [PubMed] [Google Scholar]
- MILNER L.S., WEI S.H., HOUSER M.T. Amelioration of glomerular injury in doxorubicin hydrochloride nephrosis by dimethylthiourea. J. Lab. Clin. Med. 1991;118:427–434. [PubMed] [Google Scholar]
- MIMNAUGH E.G., TRUSH M.A., GRAM T.E. A possible role for membrane lipid peroxidation in anthracycline nephrotoxicity. Biochem. Pharmacol. 1986;35:4327–4335. doi: 10.1016/0006-2952(86)90713-6. [DOI] [PubMed] [Google Scholar]
- MOORE J.C., MORRIS J.W. A simple automated colorimetric method for determination of N-acetyl-β-D-glucosaminidase. Ann. Clin. Biochem. 1982;19:157–159. doi: 10.1177/000456328201900305. [DOI] [PubMed] [Google Scholar]
- MORON M.S., DEPIERRE J.W., MANNERVIK B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;528:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- PAREKH A.C., JUNG D.H. Cholesterol determination with ferric acetate-uranium acetate and sulfuric acid-ferrous sulfate reagents. Anal. Chem. 1970;42:1423–1427. [Google Scholar]
- RAATS C.J.I., BAKKER M.A.H., VAN DEN BORN J., BERDEN J.H.M. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J. Biol. Chem. 1997;272:26734–26741. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- RENNARD S.I., BERG R., MARTIN G.R., FOIDART J.M., GEHRON ROBEY P. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal. Biochem. 1980;104:205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- ROTRUCK J.T., POPE A.L., GANTHER H.E., HAFEMAN D.G., HOEKSTRA W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- SHARMA O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- SHOSKES D.A. Effect of bioflavanoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation. 1998;66:147–152. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- SOOSE M., GWINNER W., GROTKAMP J., HANSEMANN W., STOLTE H. Altered renal fibronectin excretion in early adriamycin nephrosis of rats. J. Pharmacol. Exp. Ther. 1991;257:493–499. [PubMed] [Google Scholar]
- SRIVASTAVA R., SRIMAL R.C. Modification of certain inflammation induced biochemical changes by curcumin. Ind. J. Med. Res. 1985;8:215–223. [PubMed] [Google Scholar]
- SURESH BABU P., SRINIVASAN K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa), in streptozotocin induced diabetic rats. Mol. Cell. Biochem. 1997;166:169–175. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- SURESH BABU P., SRINIVASAN K. Amelioration of renal lesions associated with diabetes by dietary curcumin in streptozotocin diabetic rats. Mol. Cell. Biochem. 1998;181:87–96. doi: 10.1023/a:1006821828706. [DOI] [PubMed] [Google Scholar]
- VENKATESAN N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br. J. Pharmacol. 1998;124:425–427. doi: 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]


