Abstract
Effects of extracellular anions were studied in electrophysiological experiments on freshly isolated rat ventricular myocytes. Under current-clamp, action potential duration (APD) was prolonged by reducing the extracellular Cl− concentration and shortened by replacement of extracellular Cl− with I−. Under voltage-clamp, membrane potential steps or ramps evoked an anionic background current (IAB) carried by either Cl−, Br−, I− or NO3−. Activation of IAB was Ca2+- and cyclic AMP-independent, and was unaffected by cell shrinkage. IAB was insensitive to stilbene and fenamate anion transport blockers at concentrations that inhibit Ca2+-, cyclic AMP- and swelling-activated Cl− currents in ventricular cells of other mammals. These results suggest that IAB may be carried by a novel class of Cl− channel. Correlation of anion substitution experiments on membrane current and action potentials revealed that IAB could play a major role in controlling rat ventricular APD. These findings have important implications for those studying cardiac Cl− channels as potential targets for novel antiarrythmic agents.
Keywords: Ventricular myocyte, electrophysiology, action potential, Cl− current, anion channel, DIDS, niflumic acid, arrhythmia
Introduction
Cl− currents have been described in atrial and ventricular myocytes isolated from the hearts of many mammalian species and have been implicated in both cardiac physiology and pathophysiology (see reviews by: Ackerman & Clapham, 1993; Faivre & Bril, 1997; Hiraoka et al., 1998; Sorota, 1999). In cardiac myocytes, three main classes of Cl− current have been described: (i) a calcium-activated Cl− current activated following elevation of the intracellular Ca2+ concentration ([Ca2+]i) (Zygmunt & Gibbons, 1992; Laflamme & Becker, 1996); (ii) a swelling-activated current, activated following hypotonic shock (Du & Sorota, 1997; Vandenberg et al., 1997); and (iii) a cyclic AMP-activated current activated following β-adrenergic receptor stimulation (Harvey & Hume, 1989; Bahninsky et al., 1989; Levesque & Hume, 1995). The channel which carries the cyclic AMP-activated current is the product of the cystic fibrosis gene known as the cystic fibrosis transmembrane conductance regulator (CFTR; see Sorota, 1999). Activation of Cl− currents can potentially markedly affect generation and propagation of cardiac action potentials since the Cl− equilibrium potential (ECl) in intact ventricular cells is approximately −50 mV. Thus, at potentials hyperpolarized to ECl, inward Cl− current would induce depolarization and at potentials depolarized to ECl outward Cl− current would induce repolarization. It is this feature of cardiac Cl− channels that makes their blockade a potential approach for therapy of cardiac arrhythmia.
It is important to note that Cl− channels are generally permeable to other halides and other anions including amino acids such as aspartic acid and taurine in addition to Cl− (Hall et al., 1996), and therefore may be regarded as anion channels. We have used this feature of Cl− channel physiology to examine the effects of anions on the action potential in rat ventricular myocytes. Our results show that the duration of the rat ventricular action potential is acutely sensitive to extracellular anions. In addition, we have obtained evidence supporting the existence of a novel anionic background current (IAB) which is not dependent upon the presence of intracellular Ca2+, cyclic AMP or cell-swelling.
Methods
Isolation and whole-cell voltage-clamp of ventricular myocytes from rat hearts was performed as described by Spencer & Berlin (1995). Action potentials were evoked by 20 ms stimulation pulses of 40% suprathreshold after switching to current-clamp using an Axopatch 200A amplifier (Axon Instruments). Myocytes were superfused at room temperature (20–25°C) with HEPES-buffered Tyrode's solution containing (in mM): NaCl 145, KCl 4, MgCl2 1, CaCl2 2, D-glucose 10, HEPES (neutralized to pH 7.4 with NaOH) 10 (solution A). During action potential experiments, NaCl in the Tyrode's solution was replaced by Na aspartate or Na iodide (NaI). For isolation of anionic currents, a standard test solution was used with following composition (in mM): N-methyl D-glucamine (neutralized to pH 7.4 using HCl) 135, MgCl2 2.5, D-glucose 10, TEA Cl 5, BaCl2 3, CdCl2 0.5, HEPES (pH 7.4, with CsOH) 5 (solution B). In ionic substitution experiments, the NMDG Cl in solution B was replaced by NMDG aspartate, I, Br or NO3. In some experiments an alternative test-solution (solution C) was used, based on solution B but containing (mM): BaCl2 0.5, nifedipine 0.01 and NMDG Cl (aspartate, I or NO3) 145. Nifedipine, diisothiocyanostilbene-2,2′-disulphonic acid (DIDS), niflumic acid, 9-anthracene carboxylic acid (9-AC), isobutylmethyl xanthine (IBMX), and forskolin (FSK) were added to these solutions from 50 mM stock solutions in dimethyl sulfoxide. In voltage-clamp experiments, cells were dialysed with a Cs-based ‘intracellular' solution which had the following composition (mM): Cs glutamate 75, CsCl 20, taurine 10, MgCl2 0.5, Cs EGTA 0.05, MgATP 10, tris phosphocreatine 5, tris GTP 0.1, pyruvic acid 5, PIPES (pH 7.1 with CsCO3) 30 (solution D). A similar intracellular medium with all Cs+ replaced by K+ and corrected to pH 7.1 with KOH was used in current clamp experiments (solution E). Specialist chemicals were purchased from Sigma Chemical Company, Poole, U.K.
In electrophysiological experiments, the reference Ag/AgCl electrode was immersed in a solution of 3 M KCl continuous with an agar bridge (4% agar in 3 M KCl) to minimise junction potential changes. Voltage-clamped myocytes were subjected to membrane potential steps from a holding potential of −80 mV at 0.33 Hz and ‘saw-toothed' ramps from −50 mV. During ramps, cells were depolarized at a rate of 0.32 V s−1 from −90 to +70 mV and back to −90 mV. Membrane currents and potentials were recorded on digital audio tape and subsequently digitized, signal-averaged over ten stimulations and filtered at frequencies appropriate to the Nyquist criterion (Stanley et al., 1984). Cell capacitance, for current normalization, was calculated by direct integration of the current transients evoked by 20 mV hyperpolarizing voltage steps of 5 ms duration, applied at 20 Hz. Mean measurements are presented with their respective standard errors, and statistical significance was assessed using the Student t-distribution. When stated in the text, ‘significance' refers to the 95% level of confidence (P<0.05).
Results
Shown in Figure 1a is a typical rat ventricular action potential recorded from a cell dialysed with solution E under current-clamp. The mean control action potential duration measured following 90% repolarization (APD90) was 66±11 ms (n=13). Reduction of the extracellular Cl− concentration ([Cl−]e) from 155 to 35 mM in Solution A caused slight hyperpolarization of resting membrane potential from −73±3 mV to −76±3 mV (n=15) and significantly prolonged APD90 by 13±4 ms (18±3%, n=9). Upon replacement of 94% of the extracellular chloride in solution A with iodide, resting membrane potential underwent a slight depolarization to −70±3 mV (n=9). This was coupled to a significant shortening in APD90 of 11±2 ms (24±3%, n=7) compared to control (see Figure 1a). These changes in APD persisted after normalization, as shown in Figure 1b, indicating that they were independent of the effects on resting potential. In order to determine the cause of these changes in APD, voltage-clamp experiments were performed under conditions that only allowed anion movement (using Solutions B & D). Shown in Figure 1c are the effects of applying 800 ms membrane potential steps in the presence of a range of extracellular chloride concentrations. The current-voltage (I–V) curves shown in Figure 1c, together with those from other experiments, allowed the determination of reversal potentials (Erev) for chloride. The experimentally derived values for Erev were −38±2 mV (n=12), −19±2 mV (n=7) and −17±1 mV (n=6) for 152, 97 and 37 mM chloride, respectively; with the corresponding theoretical reversal potentials being −52, −40 and −17 mV, respectively. Although the reversal potentials for 152 and 97 mM extracellular Cl− deviated from the theoretical, the current was acutely sensitive to changes in the concentration of the anion suggesting it was indeed carried through an anion channel. To verify this notion, 90% of the extracellular Cl− in solution B was substituted with I−, Br− or NO3−. Figure 1d shows current density I–V curves from these experiments. The outward current density was greater in the presence of I− and NO3− than Br− (which was similar to Cl−). The anion-dependent shifts in Erev in these experiments were used in the modified Goldman-Hodgkin-Katz equation (see Du & Sorota, 1997) to obtain relative permeabilities for these anions through the putative anion channel. Permeabilities of 1.8±0.3 for NO3− (n=4), 1.5±0.3 for I− (n=5), and 0.7±0.1 for Br−(n=3) were obtained, resulting in the following permeability sequence: NO3−⩾I−>Cl−,⩾Br−. To further explore the effects of NO3− and I−, voltage-clamped myocytes held at −50 mV in solution C were subjected to continuous trains of membrane potential ramps. For clarity, Figure 2a shows difference currents calculated by subtacting the current recorded during negative-going ramps in the presence of the relatively impermeant anion, aspartate−. The permeabilities of both NO3− and I−, relative to Cl−, were, coincidentally, found to be 1.5±0.1 (n=6) similar to the results from voltage-step experiments (above). Currents generated by voltage ramps in the presence of I− (solution C) or Cl− (solution B) were insensitive to 50–100 μM DIDS (n=7; e.g. Figure 2b), 50–100 μM niflumic acid (n=8; e.g. Figure 2c) or 50 μM 9-AC (data not shown), concentrations of blockers known to have marked inhibitory effects on cardiac Cl− channels (see Sorota, 1999). These results suggest that the current does not fall into a recognized class of cardiac anion currents. In order to confirm this notion, the effects of (i) extracellular application of 400 μM IBMX, 10.0 μM FSK and 0.1 μM isoproterenol (contained in solution B) were examined to test for CFTR involvement; (ii) extracellular tonicity was increased to verify whether cell shrinkage affected current amplitude; and (iii) 1 mM EGTA was added to the pipette solution to determine the requirement for intracellular Ca2+. Figure 2d shows that ramp currents obtained in the presence of Solution B were virtually unaffected by the elevation of the intracellular concentration of cyclic AMP (n=11) sufficient to double the amplitude of the L-type Ca2+ current in the same cells (not shown). Cell shrinkage induced by a 20% increase in the extracellular tonicity using NMDG aspartate was without effect (n=10), as was the addition of 1 mM EGTA to the intracellular solution (not shown), even though cell contractions were abolished. These observations all strongly suggest that the conductance demonstrated here did not fall into any of the three main classes of cardiac Cl− channel.
Figure 1.
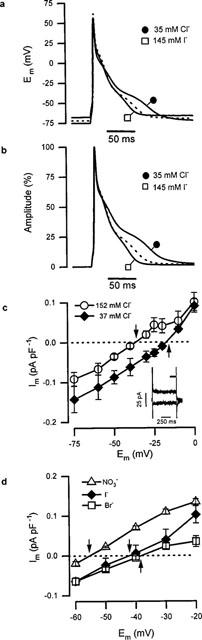
Electrophysiological recordings in the presence of different extracellular anions. (a) APs recorded during superfusion with solution A containing either 155 mM Cl− (dotted), 35 mM Cl− plus 120 mM aspartate− (•), or 10 mM Cl− plus 145 mM I− (□). (b) The same APs as shown in part (a) normalized relative to their maximum to eliminate variation in RMP; lines and symbols as in (a). (c) Mean current density current-voltage (I–V) plots at different [Cl−]e (in solution B). ○, [Cl−]e=152 mM (n=7), ⧫, [Cl−]e=37 mM (n=3). Inset: currents generated at test potentials of −100 and −25 mV in the presence of 152 mM Cl− (bar indicates current averaging period). (d) Mean I–V curves obtained in solution B containing 135 mM [Br−]e (□, n=3), [NO3−]e (▵, n=4) and [I−]e (⧫; n=3). The arrows shown in panels (c) and (d) indicate the reversal potential for the currents. In this, and the subsequent figure, error bars represent s.e.mean.
Figure 2.
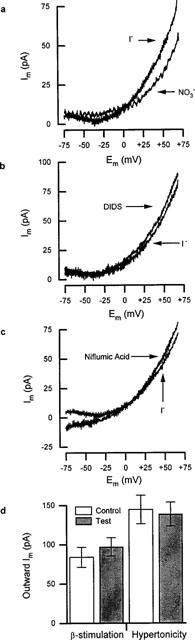
Voltage-clamp experiments using ramp protocols in the presence of different anions. (a) Anion-sensitive difference currents (Im) obtained from hyperpolarizing ramps during superfusion with solution C. The current recorded in 145 mM aspartate− was subtracted from the current observed in either 145 mM I− or NO3−. (b) Iodide-sensitive difference currents as in (a) before and after 5 min superfusion with 50 μM DIDS in the solution C. (c) difference currents as in (a) before and after 5 min superfusion with 50 μM niflumic acid in solution C. (d) histograms showing outward current amplitude at +60 mV before (control) and after increasing intracellular cyclic AMP levels (test; β-stimulation), and also before (control) and after cell shrinkage (test; hypertonicity) cell shrinkage (right). Details given in main text.
Discussion
Our results demonstrate the existence of, what appears to be, a novel anionic background current (IAB) in ventricular myocytes isolated from the rat heart: a finding with important implications for those interested in the potential value of Cl− channels as therapeutic targets. The channel underlying IAB appears to be permeable to a range of anions, but its activation is not dependent upon intracellular Ca2+, cyclic AMP or cell-swelling. Alteration of the anionic conditions, which affected the magnitude of IAB had profound effects on action potential duration, suggesting that IAB plays a role in controlling the profile of the rat ventricular action potential.
It appears that the permeability sequence for IAB (ie. NO3−⩾I−>Cl−⩾Br−) is similar to that of swelling-activated Cl− channels (Sorota, 1997), but, for halides, is the opposite to that of CFTR (Ackermann & Clapham, 1993; Sorota, 1999). However, upon altering the chloride gradient, the reversal potential of IAB deviated slightly from Nernstian theory suggesting that the biophysical characteristics of the channels carrying IAB deviate from ideality. This is perhaps not surprising however, in view of the apparently wide range of anions that support IAB.
Stilbenes and fenamates are extremely promiscuous blockers, affecting a wide range of anion transporters and channels. DIDS and niflumic acid at the concentrations used in this study (50–100 μM) abolish swelling-activated and Ca2+-activated ICl in cardiac cells (see Du & Sorota, 1997; Zygmunt & Gibbons, 1992). CFTR channels are also at-least partially sensitive to niflumic acid (Sorota, 1999). Yet these molecules and 9-AC were ineffective against IAB. Furthermore, the experiments designed to examine the role of [Ca2+]i, cyclic AMP and cell volume revealed no large changes in the magnitude of IAB. Taken together, these results provide strong evidence that IAB could be carried by a novel class of Cl− channel. However, the possibility that IAB channels represent an atypical variant of one of the three main classes of cardiac Cl− channel cannot be excluded.
Action potentials were observed to be prolonged by a reduction of [Cl−]e and shortened when I− was the predominant extracellular anion. When [Cl−]e was reduced from 155 to 35 mM, the observed Erev for Cl− shifted by +21 mV, readily explaining the prolongation of APD. Such a change in Cl− gradient would result in inward IAB flow during repolarization. This increase in inward Cl− current at potentials negative to ECl would counteract in part, some of the outward currents flowing, thereby prolonging APD. APD shortening after replacing extracellular Cl− by I− can be readily explained by the increase in outward IAB carried by I−. Superfusion with low [Cl−]e and extracellular I− caused hyperpolarization and depolarization of the RMP, respectively. Since an increased inward Cl− current at low [Cl−]e would be expected to depolarize the cell, these effects on RMP may be mediated independently of IAB. Nevertheless, upon normalizing the action potential (Figure 1b), it was shown that the effects of anions on APD were independent of those on RMP.
In conclusion, our results are consistent with the existence of a novel background anionic current in rat ventricular muscle (IAB) which may play a major role in controlling APD. These findings have important implications for those studying cardiac Cl− channels as potential targets for novel antiarrythmic agents. It remains to be determined whether IAB is present in human ventricular muscle, although preliminary evidence suggests that a background Cl− current may be present in human atria (Berul et al., 1997). Thus, IAB may represent a novel therapeutic target for anti-arrhythmic agents. In any event, in light of this data, caution should be exercised when interpreting the effects of anions or potential anti-arrhythmic agents on rat cardiac tissue.
Acknowledgments
R.Z. Kozlowski is a British Heart Foundation Lecturer. This work was funded in part by the Medical Research Council of Great Britain.
Abbreviations
- APD
action potential duration
- CFTR
cystic fibrosis transmembrane conductance regulator
- RMP
resting membrane potential
- s.e.mean
standard error of mean
References
- ACKERMAN M.J., CLAPHAM D.E. Cardiac Cl− channels. Trends. Cardiovasc. Med. 1993;3:23–28. doi: 10.1016/1050-1738(93)90024-Z. [DOI] [PubMed] [Google Scholar]
- BAHINKSY A., NAIRN A.C., GREENGARD P., GADSBY D.C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989;340:718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- BERUL C.I., SWEETEN T., VETTER V.L., MORAD M. Lack of cystic fibrosis transmembrane regulator-type Cl− current in pediatric human atrial myocytes. Life Sci. 1997;60:189–197. doi: 10.1016/s0024-3205(96)00615-7. [DOI] [PubMed] [Google Scholar]
- DU X., SOROTA S. Cardiac swelling-induced Cl− current depolarizes canine atrial myocytes. Am. J. Phys. 1997;272:H1904–H1916. doi: 10.1152/ajpheart.1997.272.4.H1904. [DOI] [PubMed] [Google Scholar]
- FAIVRE J., BRIL A. The cardiac Cl− channels as molecular targets for antiarrhythmic therapy. Fr. Pharm. Rev. Comm. 1997;9:61–70. [Google Scholar]
- HALL J.A., KIRK J., POTTS J.R., RAE C., KIRK K. Anion channel blockers inhibit swelling-activated anion, cation and non-electrolyte transport in HeLa cells. Am. J. Phys. 1996;271:C579–C588. doi: 10.1152/ajpcell.1996.271.2.C579. [DOI] [PubMed] [Google Scholar]
- HARVEY R.D., HUME J.R. Autonomic regulation of a Cl− current in heart. Science. 1989;244:983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- HIRAOKA M., KAWANO S., HIRANO Y., FURUKAWA T. Role of cardiac chloride currents in changes in action potential characteristics and arrhythmias. Cardiovasc. Res. 1998;40:23–33. doi: 10.1016/s0008-6363(98)00173-4. [DOI] [PubMed] [Google Scholar]
- LAFLAMME M.A., BECKER P.L. Ca2+-induced current oscillations in rabbit ventricular myocytes. Circ. Res. 1996;78:707–716. doi: 10.1161/01.res.78.4.707. [DOI] [PubMed] [Google Scholar]
- LEVESQUE P.C., HUME J.R. ATPo but not cAMPi activates a Cl− conductance in mouse ventricular myocytes. Cardiovasc. Res. 1995;29:336–343. [PubMed] [Google Scholar]
- SOROTA S. Insights into the structure, distribution and function of the cardiac chloride channels. Cardiovasc. Res. 1999;42:361–376. doi: 10.1016/s0008-6363(99)00039-5. [DOI] [PubMed] [Google Scholar]
- SPENCER C.I., BERLIN J.R. Control of sarcoplasmic reticulum calcium release during calcium loading in isolated rat ventricular myocytes. J. Physiol. 1995;488:267–279. doi: 10.1113/jphysiol.1995.sp020965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANLEY W.D., DOUGHERTY G.R., DOUGHERTY R. Digital Signal Processing. Reston, Virginia, U.S.A.: Prentice-Hall; 1984. [Google Scholar]
- VANDENBERG J.I., BETT G.C., POWELL T. Contribution of a swelling-activated Cl− current to changes in the cardiac action potential. Am. J. Physiol. 1997;73:C541–C547. doi: 10.1152/ajpcell.1997.273.2.C541. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT A.C., GIBBONS W.R. Properties of the calcium-activated Cl− current in heart. J. Gen. Physiol. 1992;99:391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]


