Abstract
The present study examined the effect of dopamine on rat jejunal electrolyte transport (rheogenic transport and Na+, K+-ATPase activity) in adult (60-day old) and young (20-day old) animals.
In young rats, dopamine, in the presence of phentolamine, produced an increase in jejunal Isc, this being completely abolished by SKF 83566, and not changed by S-sulpiride. SKF 38393, but not quinerolane, also increased Isc; this effect was abolished by SKF 83566 and ouabain, but not by furosemide. In adult rats, dopamine in the presence of phentolamine (0.2 μM) decreased Isc.
Na+, K+-ATPase activity in isolated jejunal epithelial cells from adult rats was 2.4 fold that in young rats. In the presence of phentolamine, both dopamine and SKF 38393, but not quinerolane, significantly decreased jejunal Na+, K+-ATPase activity in young animals but not in adult animals.
Binding [3H]-Sch 23390 to membranes of jejunal mucosa revealed the presence of a single class of receptors in both young and adult rats, with similar KD and Bmax values.
GTPγS and cholera toxin inhibited jejunal Na+, K+-ATPase activity in young, but not in adult rats. Co-incubation of pertussis toxin with dopamine was found to potentiate the inhibitory effects of dopamine upon the enzyme in both young and adult rats.
Regulation of Na+, K+-ATPase activity by cholera toxin-sensitive G proteins is absent in adult animals, and such difference may explain the failure of dopamine to inhibit intestinal Na+, K+-ATPase activity in adult rats.
Keywords: G-proteins; dopamine; D1 receptors; jejunum; electrolyte transport; Na+, K+-ATPase
Introduction
The intestinal tract has been shown to be of crucial importance in the regulation of body fluid and electrolyte homeostasis with particular relevance in the control of changes in body fluid and electrolyte intake (Binder, 1983). Previous studies on neurohumoral control of intestinal transport showed that catecholamines stimulate electrolyte absorption. In the rat, dopamine was also found to stimulate ileal and colonic fluid absorption via dopamine and α2-adrenoceptors (Donowitz et al., 1983). More recently, it has been suggested that the α2-adrenoceptors involved in the pro-absorptive or antisecretory actions in the rat intestinal epithelium are of the α2D or α2A-like subtype (Liu & Coupar, 1997). In contrast with this pro-absorptive or antisecretory profile of dopamine, other studies showed that endogenous dopamine reduces jejunal sodium transport in young rats submitted to a high salt diet (Finkel et al., 1994), this being accomplished by a decrease in Na+, K+-ATPase activity (Vieira-Coelho et al., 1998).
The current view of the intestinal dopaminergic system is that of a local non-neuronal system constituted by epithelial cells of intestinal mucosa, rich in aromatic L-amino acid decarboxylase (AADC) activity and using circulating or luminal 3,4-dihydroxyphenylalanine (L-DOPA) as a source for dopamine (Vieira-Coelho et al., 1997). Dopamine is particularly abundant in the mucosal cell layer (Eaker et al., 1988; Esplugues et al., 1985). Studies on the formation of dopamine from exogenous L-DOPA along the rat digestive tract showed that the highest AADC activity is located in the jejunum (Vieira-Coelho & Soares-da-Silva, 1993). Because the dopamine produced in this area is in close proximity to epithelial cells which contain receptors for the amine, it has been hypothesized that intestinal dopamine may act as a paracrine or autocrine substance (Vieira-Coelho et al., 1997). A high salt diet has been found to constitute an important stimulus for the production of dopamine in rat jejunal epithelial cells and this is accompanied, in 20-day old animals, by a decrease in sodium intestinal absorption (Finkel et al., 1994). The relative importance of this system in controlling sodium absorption assumes particular relevance in view of the findings that 40-day old rats submitted to a high salt diet have a fault in intestinal dopamine production during salt loading, in contrast to that occurring in 20-day old animals. The lack of changes in the jejunal function in response to high-salt diet coincides with the period in which the renal function has reached maturation (Robillard et al., 1992), suggesting the occurrence of complementary functions between the intestine and the kidney during development.
The present study was aimed to characterize the effect of dopamine on rat jejunal electrolyte transport (rheogenic transport and Na+, K+-ATPase activity) in young (20-day old) and adult (60-day old) rats and to determine the type of dopamine receptor involved. It is reported that, in the presence of α-adrenoceptor blockade, dopamine produces a decrease in young rat jejunal electrolyte absorption. This effect, most probably due to inhibition of Na+, K+-ATPase, is a D1 receptor-mediated event, being absent in adult rats. The results presented here also indicate that intestinal Na+, K+-ATPase activity in young rats is regulated by both cholera toxin (CTX) and pertussis toxin (PTX) sensitive G proteins, but regulation of the enzyme by CTX-sensitive G proteins is absent in adult animals. Such difference may explain the failure of dopamine to inhibit intestinal Na+, K+-ATPase activity in adult rats.
Methods
Tissue preparation
Adults (60-day old) male Wistar rats were fed a standard rat chow (Letica I.P.M R20) and received tap water ad libitum, whereas young (18–20-day old) rats were kept with the female progenitor for breast-feeding. Rats were killed by decapitation under ether anaesthesia and two segments of jejunum located 10–15 cm distal from the pyloric sphincter were removed. Each segment (2 cm long) of jejunum was cut longitudinally along the mesenteric border, washed free of luminal contents and the tissue pinned mucosal side down on a dental wax block. The serosa and muscularis were stripped away by dissection to obtain the epithelial sheets, as previously described (Nellans et al., 1974).
Electrophysiological studies
Epithelial sheets were mounted in Ussing chambers (window area 0.28 cm2) equipped with water-jacketed gas lifts, bathed on both sides with 10 ml of Krebs-Hensleit solution, gassed with 95% O2 and 5% CO2 and maintained at 37°C. D-Glucose (10 mM) was added to the serosal-side reservoir and an equimolar amount of mannitol to the mucosal-side reservoir. The Krebs-Hensleit solution contained (in mM): NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, CaCl2 2.5, MgSO4 1.2; pH was adjusted to 7.4 after gassing with 5% CO2 and 95% O2. Tissues were continuously voltage clamped to zero potential differences by application of external current, with compensation for fluid resistance, by means of an automatic voltage current clamp (DVC 1000, World Precision Instruments, Sarasota, Florida, U.S.A.). Transepithelial resistance (Ω cm2) was determined by altering the membrane potential stepwise (±5 mV) and applying the Ohmic relationship. Changes in short circuit current (μA cm−2) were continuously measured as an index of electrogenic ion transfer. The voltage/current clamp unit was connected to a PC via a BIOPAC MP1000 data acquisition system (BIOPAC Systems, Inc., Goleta, CA, U.S.A.). Data analysis was performed using AcqKnowledge 2.0 software (BIOPAC Systems, Inc., Goleta, CA, U.S.A.).
After a 30–45-min preincubation period, by which time the potential difference had stabilized, dopamine or SKF 38393, a selective dopamine D1 receptor selective agonist, was added to the serosal-side reservoir; ascorbic acid (1 mM) was present in the serosal bathing solution to reduce oxidation of dopamine. All agonist concentration-response curves were constructed in a cumulative manner; each new concentration was added as soon as the potential difference response to the prior concentration reached its nadir. Antagonists were added 20 min before construction of an agonist concentration-response curve. All drugs were added to the serosal side.
Isolation of jejunal epithelial cells
The method of cell isolation was similar to that previously described (Kimmich, 1990; Quaroni et al., 1979) with minor modifications. Briefly, the animals were killed by decapitation under anaesthesia and the abdominal cavity was immediately opened to excise a jejunal segment approximately 10 cm in length. The selected segment was placed on an ice cold glass plate and cut in smaller segments of approximately 1.5 cm in length, and rinsed free from blood and intestinal contents with saline (0.9% NaCl). The tissue fragments were everted with fine forceps and incubated for 45 min in 5 ml warm water (37°C) and gassed (95% O2 and 5% CO2) Hanks' solution with 0.06% collagenase type I (Sigma Chemical Co, St Louis, MO, U.S.A.). At the end of the incubation period the fragments were removed from the solution and the medium containing the detached cells was centrifuged (200×g, 4°C) for 4 min, and the cell pellet was re-suspended in Hanks' solution. Cell viability was estimated by the Trypan blue (0.04%; 1 min) exclusion method, and the percentage of viable cells (excluding the dye), determined by hemocytometer counting, was>90%.
Membrane preparations and D1 binding assay
Membranes from intestinal mucosa were obtained from adult and young male Wistar rats. After ether anaesthesia, a segment of jejunum (5–10 cm) was removed, opened longitudinally along the mesenteric border and rinsed free from the alimentary content with cold saline (0.9% NaCl) and the jejunal mucosa was removed with a scalpel. The mucosa thus obtained was homogenized in (mM): Tris-HCl 10, pH 7.4, containing sucrose 250, PMSF 1, EDTA 1, and 5 μg ml−1 each of leupeptin and pepstatine, with a Potter-Elvehjem teflon homogenizer, and centrifuged (20,000×g, 20 min, 4°C). Pellets were resuspended to a concentration of 2 mg protein ml−1 in (mM) Tris-HCl 10, pH 7.4 with MgCl2 5 and sucrose 250 and stored aliquoted at −80°C. Membranes were thawed at room temperature, centrifuged (20,000×g, 20 min, 4°C) and resuspended in binding buffer (mM): Tris-HCl 50, pH 7.4 with NaCl 120, KCl 5, CaCl2 2 and MgCl2 1. Saturation experiments were performed in four replicates in 96-well EIA/RIA plates (Costar) in a final volume of 0.2 ml binding buffer containing 0.1–10 nM [3H]-Sch 23390 and 100–200 μg membrane protein. Non-specific binding was determined in the presence of 10 μM of unlabelled Sch 23390 (Gil-Martín et al., 1997; Horiuchi et al., 1992). After 30 min incubation at 30°C in a shaking water bath, assays were terminated by vacuum filtration through glass fibre filtermats with the Brandel 96 cell Harvester (Brandel, Gaithsburg, U.S.A.). Filters were washed three times with 200 μl of cold 50 mM Tris-HCl pH 7.4, dried and impregnated with MeltiLex A (Wallac, Pharmacia) and DPM determined in a Microbeta counter (Wallac) with 20% efficiency.
Na+, K+-ATPase assay
Na+, K+-ATPase activity was measured by the method of Quigley & Gotterer (1969), with minor modifications. Briefly, epithelial cells were incubated with dopamine (1 μM) or SKF 38393 (10 nM), the selective D1 receptor agonist, for 15 min at 37°C. In some experiments, incubation was performed in the presence of D1 or D2 selective antagonists, respectively SKF 83566 (1 μM) and S-sulpiride (1 μM). In another set of experiments, cells were incubated in the presence of dibutyril cyclic AMP (2 mM), phorbol 12,13-dibutyrate (PDBu; 1 μM), phorbol 12-myristate 13-acetate (PMA; 1 μM), cyclic GMP (1 mM), guanosine 5′-o-(3-thiotriphosphate) (GTPγS; 50, 100 and 150 μM), guanosine 5′-o-(2-thiodiphosphate) (GDPβS; 50, 100 and 150 μM), cholera toxin (CTX; 0.1 μM) or pertussis toxin (PTX; 0.1 nM) for 30 min at 37°C. After the incubation period the cells were permeabilized by rapid freezing in dry ice-acetone and thawing. The reaction mixture contained (in mM): imidazole buffer 37.5, NaCl 75, KCl 5, sodium EDTA 1, MgCl2 5, 6 NaN3, tris(hydroxymethyl)aminomethane(tris) hydrochloride 75 and 100 μl cell suspension (100 μg protein). The reaction was initiated by the addition of 4 mM ATP. For determination of ouabain-insensitive ATPase, NaCl and KCl were omitted, and ouabain (1 mM; 100 μl) or vehicle (water; 100 μl) were added to the assay. After incubation at 37°C for 15 min, the reaction was terminated by the addition of 50 μl of ice-cold trichloroacetic acid. Samples were centrifuged (3000 r.p.m.), and liberated Pi in supernatant was measured by spectrophotometry at 740 nm. Na+, K+-ATPase activity is expressed as nanomoles Pi per milligram protein per minute and determined as the difference between total and ouabain-insensitive ATPase.
Assay of catecholamines
The assay of catecholamines and its metabolites in the jejunal mucosa (L-DOPA, noradrenaline, dopamine, 3,4-dihydroxyphenylacetic acid [DOPAC], 3-methoxytyramine [3-MT] and homovanillic acid [HVA]) was performed by h.p.l.c. with electrochemical detection, as previously described (Soares-da-Silva et al., 1995). In brief, aliquots of 1.5 ml of perchloric acid in which tissues had been kept were placed in 5 ml conical-based glass vials with 50 mg alumina and the pH of the samples adjusted to pH 8.6 by addition of Tris buffer. The adsorbed catecholamines were then eluted from the alumina with 200 μl of 0.2 M perchloric acid on Costar Spin-X microfilters; 50 μl of the eluate was injected into a h.p.l.c. (Gilson Medical Electronics, Villiers le Bel, France). For the quantification of 3-MT and HVA in tissue samples, 500 μl of perchloric acid in which tissues had been kept were filtered on Costar Spin-X microfilters and directly injected into the chromatograph. The lower limit of detection of L-DOPA, noradrenaline, dopamine, DOPAC, 3-MT and HVA ranged from 350 to 1000 fmol.
Drugs
The compounds used were: adenosine 3′:5′-cyclic monophos-phate N6,2′-o-dibutyril (dibutyril cyclic AMP), cholera toxin, dopamine hydrochloride, forskolin, furosemide, guanosine 3′:5′-cyclic monophosphate sodium salt (cyclic GMP), guanosine 5′-o-(3-thiotriphosphate) (GTPγS), guanosine 5′-o-(2-thiodiphosphate) (GDPβS), ouabain, phentolamine hydrochloride, phorbol 12,13-dibutyrate (PDBu) and phorbol 12-myristate 13-acetate (PMA) obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.). Quinerolane, Sch 23390, SKF 38393, SKF 83566 and (S)-sulpiride were obtained from Research Biochemicals International (RBI, Natick, MA, U.S.A.). [3H]-Sch 23390 ([N-methyl-3H]R[+]-7-chloro-2,3.4,5-tetrahydro - 3 - methyl - 1 - phenyl - 1H - 3benzazepine-8-ol, specific activity 2600–3200 GBq mmol−1 was purchased from New England Nuclear (Boston, MA, U.S.A).
Analysis of data
Results are given as arithmetic means±s.e.mean. EC50 values and [3H]-Sch 23390 saturation parameters, Bmax and KD, were calculated using GraphPad Prism software package (Motulsky et al., 1994). Statistical analysis were performed by one-way analysis of variance (ANOVA) followed by Newman-Keuls test for multiple comparisons or Student's t-test. A P value less than 0.05 was assumed to denote a significant difference.
Results
Electrophysiological studies
Jejunal preparations from 20- and 60-day old rats exhibited a mean basal Isc value (in μA cm−2) of 28.7±2.9 and 23.1±2.4 (n=40), respectively. In 60-day old rats, dopamine (0.1–100 μM) when applied alone from the basal side produced a concentration dependent decrease in Isc with a EC50 value of 1.0 (0.4, 2.6) μM. In 20-day old rats, under the some experimental conditions, dopamine (0.1–100 μM) also produced a concentration dependent decrease in Isc with a EC50 value of 7.0 (3.3, 14.8) μM (Figure 1). However, in the presence of phentolamine (0.2 μM) a non-selective α-adrenoceptor antagonist, the effect of dopamine in young animals was a biphasic effect (Figure 1A). At low concentrations (0.1–3.0 μM), the effect of dopamine was a concentration dependent increase in Isc (maximal effect, 33.6±0.4% increase) with a EC50 value of 0.1 (0.07, 2.5) μM. At high concentrations (10–100 μM), the effect of dopamine was a concentration dependent decrease in Isc with a EC50 value of 35.5 (14.9, 84.5) μM (Figure 1A). By contrast, in adult animals the effect of dopamine (0.1–100 μM) in the presence of phentolamine (0.2 μM) was still a concentration dependent decrease in Isc and no increase was observed. In fact, in adult animals the addition of phentolamine (0.2 μM) produced a rightward shift of the dopamine concentration-dependent curve with a significant increase in EC50 values up to 12.9 (5.4, 30.7) μM (Figure 1B). In adult animals the effect of dopamine was completely abolished by 1 μM phentolamine (data not shown).
Figure 1.
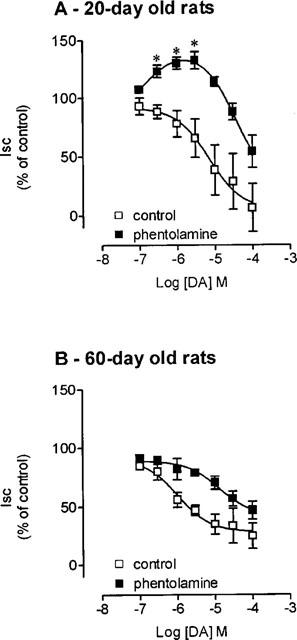
Concentration-response curves to dopamine (DA) in the absence (□) and the presence (▪) of phentolamine 0.2 μM on Isc in voltage-clamped rat jejunal epithelial sheets from (A) 20-day old and (B) 60-day old rats. Symbols represent means of four to five experiments per group; vertical lines show s.e.mean. *Significantly different from baseline values (P<0.05; Newman-Keuls test).
The increase in Isc produced by low concentrations (0.1–3.0 μM) of dopamine in 20-day old rats was completely abolished by the selective D1 antagonist, SKF 83566 (1 μM), but not by the selective D2 antagonist S-sulpiride (1 μM) (Figure 2A). The selective D1 agonist SKF 38393 (0.1–10 nM) applied from the basal side was also found to produce a concentration dependent increase in Isc (maximal effect, 30±6% increase) with a EC50 value of 0.31 (0.23, 0.43) nM, this being abolished by SKF 83566 (1 μM). By contrast, jejunal Isc was not significantly affected by quinerolane (0.1–100 nM), the selective D2 agonist, alone or in combination with S-sulpiride (data not shown). The increase in Isc produced by SKF 38393 (0.1–10 nM) was completely abolished by ouabain (1 mM) a Na+,K+-ATPase pump inhibitor, whereas furosemide (1 mM), a blocker of the Na+-K+-2Cl− transporter, failed to alter the response to D1 receptor stimulation (Figure 2).
Figure 2.
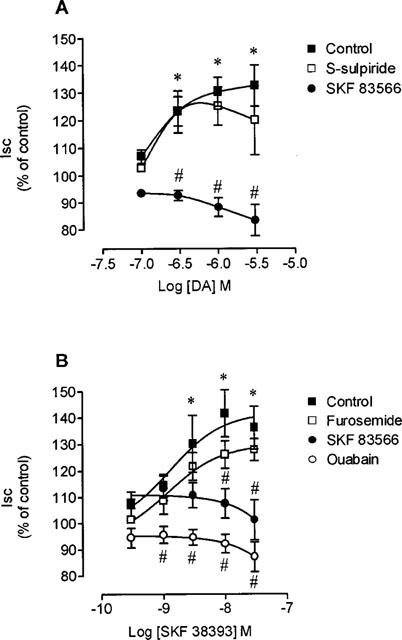
Concentration-response curves to (A) dopamine (DA) and (B) SKF 38393 on Isc in voltage-clamped jejunal epithelial sheets from 20-day old rats, in the absence and in the presence of 1 μM SKF 83566, 1 mM ouabain or 1 mM furosemide. Symbols represent means of four to five experiments per group; vertical lines show s.e.mean. Significantly different from baseline values (*P<0.05; Newman-Keuls test) and corresponding control values (#P<0.05; Newman-Keuls test).
[3H]-Sch 23390 binding assay
To explore differences in the density of dopamine receptors that might explain the absence of D1 receptor-mediated responses in adult animals, radioligand-binding experiments with [3H]-Sch 23390 (0.1–10 nM) were performed in membranes prepared from young and adult rat intestinal mucosa. Non-linear analysis of saturation experiments with [3H]-Sch 23390 (Figure 3) revealed the presence of a single class of receptors in both young and adult rats, with no significant differences in KD values (young=3.4 nM [0.3; 6.5]; adult=7.5 nM [3.8; 11.2]). The maximal density of D1 binding sites (in fmol mg protein−1) in adult animals (539.3±64.9) was even greater than in young rats (315.8±49.3). The kinetic parameters of the D1 binding site reported here for adult rats differ from those described in the literature (Marmon et al., 1993). KD values are in the same range of magnitude, but Bmax values were found to be markedly higher in the present study (539.3 vs 1.37 fmoles mg−1 protein). The most likely explanation for this apparent discrepancy is concerned with the fact that these authors used the entire intestinal wall, whereas we used the intestinal mucosa only. This may suggest that D1 receptors would be preferentially located in the mucosal cells and not homogeneously distributed across the intestinal wall, which is in agreement with data obtained in autoradiography studies showing that D1 binding sites are localised primarily in the mucosal cells in the base of the crypts.
Figure 3.

Saturation curves of [3H]-Sch 23390 specific binding to rat jejunal mucosal membranes obtained from 20-(•) and 60-(○) day old rats. The inset shows the respective representative Scatchard plot. Non-specific binding was determined in the presence of unlabelled 10 μM SCH 23390. Each symbol represents the mean of four experiments performed in quadruplicate; vertical lines show s.e.mean.
Na+,K+-ATPase activity
Because the effects of dopamine upon Isc in young rats were sensitive to ouabain and antiabsorptive in nature (furosemide insensitive increases in Isc), it was decided to evaluate Na+,K+-ATPase activity in isolated jejunal epithelial cells. Na+,K+-ATPase activity (in nmol Pi mg protein−1 min−1) in adult rats (123±3) was found to be 2.4 fold higher than in young rats (52±4) (Figure 4). Both dopamine (1 μM; with added 0.2 μM phentolamine) and SKF 38393 (10 nM), but not quinerolane (10 nM), significantly (P<0.05) decreased jejunal Na+,K+-ATPase activity in young animals (Figure 4). By contrast, in adult animals dopamine (1 μM; with added 0.2 μM phentolamine), SKF 38393 (10 nM) and quinerolane (10 nM) failed to inhibit jejunal Na+,K+-ATPase (Figure 4). In agreement with the results from electrophysiological data, the inhibitory effect of dopamine (1 μM) in jejunal Na+,K+-ATPase activity from young animals was completely abolished by SKF 83566 (1 μM), but not by S-sulpiride (1 μM) (Figure 4).
Figure 4.
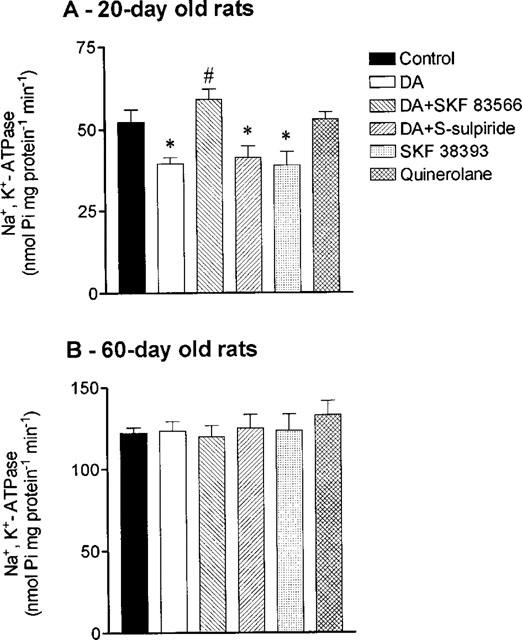
NA+, K+-ATPase activity in isolated jejunal epithelial cells from (A) 20- and (B) 60-day old rats in the absence (control) and the presence of 1 μM dopamine (DA) alone, 1 μM DA plus 10 nM SKF 83566, 1 μM DA plus 1 μM S-sulpiride, 10 nM SKF 38393 and 10 nM quinerolane. Columns represent means of four to five experiments per group; vertical lines show s.e.mean. Significantly different from corresponding control values (*P<0.05; Newman-Keuls test) and values for dopamine alone (#P<0.05; Newman-Keuls test).
Second messenger pathways thought to be involved in D1 receptor-mediated events include stimulation of protein kinase A (PKA) or C (PKC) pathways (Jose et al., 1992). To examine the hypothesis whether failure to respond to dopamine with inhibition of Na+,K+-ATPase activity was related or not to lack of responsiveness to stimulation of PKA or PKC, it was decided to evaluate the effect of stimuli leading activation of these pathways. As shown in Figure 5, both dibutyril cyclic AMP and forskolin were effective in reducing basal jejunal Na+,K+-ATPase activity. Similarly, PKC activation with PDBu and PMA was accompanied with inhibition of Na+,K+-ATPase activity. By contrast, cyclic GMP failed to alter Na+,K+-ATPase activity. Altogether, these results suggest that Na+,K+-ATPase in jejunal epithelial cells from adult rats respond with inhibition to stimuli usually involved in D1 receptor-mediated events.
Figure 5.
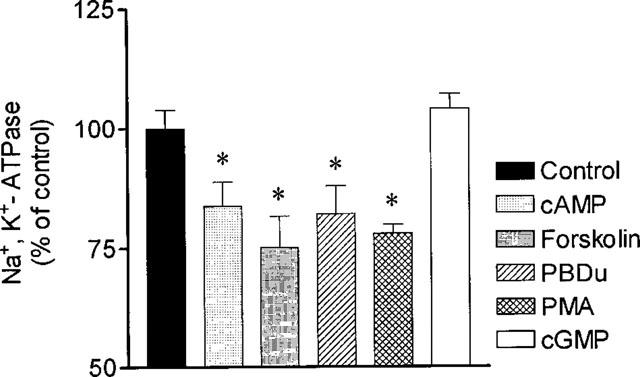
Effects of dibutyryl-cyclic AMP (2 mM), forskolin (10 μM), PDBu (1 μM), PMA (1 μM) and cyclic GMP (1 mM) upon jejunal NA+, K+-ATPase activity in 60-day old rats. Columns represent means of four experiments per group; vertical lines show s.e.mean. *Significantly different from corresponding control values (P<0.05; Newman-Keuls test).
At this stage it was considered important to evaluate the involvement of G proteins in the regulation of Na+,K+-ATPase in epithelial intestinal cells from young and adult rats. To demonstrate that G proteins were involved in the regulation of intestinal Na+,K+-ATPase, isolated epithelial cells were incubated in the presence of GTPγS (15, 50 and 150 μM), a nonhydrolyzable analogue of GTP, that irreversibly activates G proteins (Gurich & Beach, 1994). This resulted in a concentration dependent inhibition of intestinal Na+,K+-ATPase activity in young animals (Figure 6A), with no significant effect in adult animals (Figure 6B). The compound GDPβS, an analogue of GTP that does not activate G proteins (Gurich & Beach, 1994), had no affect on intestinal Na+,K+-ATPase in both young and adult animals (Figure 6). These results demonstrate that non-specific activation of G proteins can regulate intestinal Na+,K+-ATPase in young, but not adult rats.
Figure 6.
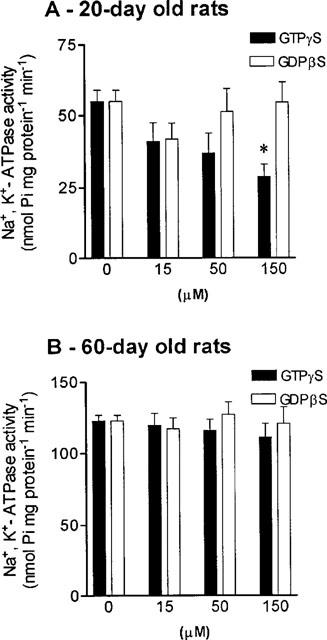
Effects of guanosine 5′-O-(3-thiotriphosphate) (GTPγS) and guanosine 5′-o-(2-thiodiphosphate) (GDPβS), on intestinal Na+, K+-ATPase activity from (A) 20- and (B) 60-day old rats. Columns represent means of four to five experiments per group; vertical lines show s.e.mean. *Significantly different from corresponding control values (P<0.05; Newman-Keuls test).
Since GTPγS activates all classes of G proteins, the effects of cholera toxin and pertussis toxin that ribosylates the α-subunit of the Gs and Gi/o classes of G proteins, respectively, were also evaluated. Cholera toxin 0.1 μM inhibited Na+,K+-ATPase activity by 23.6±4.4% in young rats, but had no effect in adult rats (Figure 7). In both young and adult animals, pretreatment of intestinal cells with pertussis toxin 0.1 nM failed to affect Na+,K+-ATPase activity. However, co-incubation of PTX with dopamine was found to potentiate the inhibitory effects of dopamine (1 μM) upon Na+,K+-ATPase in young rats (40.4±5.8% reduction). In adult animals, although dopamine 1 μM alone failed to inhibit intestinal sodium pump activity, the addition of PTX (0.1 nM) plus dopamine (1 μM) resulted in a significant (P<0.05) inhibition of Na+,K+-ATPase activity (15.2±3.9% reduction).
Figure 7.
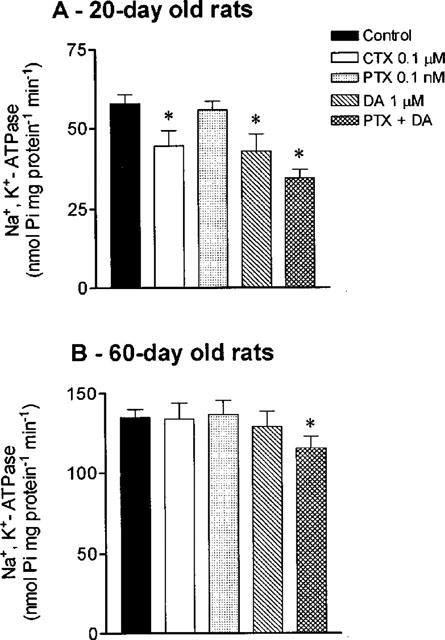
Effects of cholera toxin (CTX; 0.1 μM), pertussis toxin (PTX; 0.1 nM), dopamine (DA; 1 μM), PTX (0.1 nM) plus DA (1 μM) on intestinal Na+, K+-ATPase activity in (A) 20- and (B) 60-day old rats. Columns represent means of four to five experiments per group; vertical lines show s.e.mean. *Significantly different from corresponding control values (P<0.05; Newman-Keuls test).
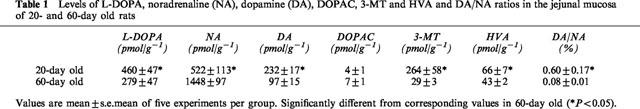
Table 1, indicates the levels of L-DOPA, noradrenaline, dopamine and amine metabolites in the jejunal mucosa of young and adult rats. As can be observed in the table, levels of dopamine were more than twice in young rats compared to the adult animals, whereas levels of noradrenaline were less than half in the former. This resulted in that dopamine/noradrenaline tissue ratios in young animals were 7.5 fold those in adult animals.
Table 1.
Levels of L-DOPA, noradrenaline (NA), dopamine (DA), DOPAC, 3-MT and HVA and DA/NA ratios in the jejunal mucosa of 20- and 60-day old rats
Discussion
The results presented here clearly show that the effects of dopamine on jejunal electrolyte (rheogenic) transport and on Na+,K+-ATPase activity in young rats differs markedly from those observed in adult animals. In young animals the effects of dopamine are mediated through the activation of D1 receptors and these occur predominantly at low concentrations of the amine, resulting in inhibition of Na+,K+-ATPase activity. Jejunal Na+,K+-ATPase activity in young rats is regulated by both CTX- and PTX-sensitive G proteins, but regulation of the enzyme by CTX-sensitive G proteins is absent in adult animals. Such difference may explain the failure of dopamine to inhibit intestinal Na+,K+-ATPase activity in adult rats. However, jejunal Na+,K+-ATPase in adult rats appears to be an effector protein of both PKA and PKC, as stimulation of PKA- and PKC-mediated pathways (usually involved in D1 receptor activation) produce marked inhibition of sodium pump activity.
In the absence of phentolamine, when α-adrenoceptors operate, the main effect of dopamine was a decrease in Isc in jejunal sheets from both young and adult rats. This was particularly evident at high concentrations of the amine and indicates that at this level of agonist concentration the predominant effects are those mediated by α-adrenoceptors and not through the activation of receptors specific for the amine, which is in agreement with data in the literature (Goldberg & Rajfer, 1985). Because levels of endogenous dopamine in the jejunal mucosa are relatively low, it is unlikely the main effects of dopamine would be promoted through activation of α-adrenoceptors. In adult rats, despite effective α-adrenoceptor blockade with phentolamine the effect of dopamine is still a decrease in Isc. This is in agreement with previous work performed in adult rats showing that the effects of dopamine on jejunal Isc are mediated by α2-adrenoceptors (Vieira-Coelho & Soares-da-Silva, 1998). On the other hand, in young rats, when phentolamine is present, the effect of dopamine is a biphasic one. At low concentrations (0.1–3.0 μM), dopamine increases Isc (EC50=0.1 μM), whereas at higher concentrations (10–100 μM) the effect of dopamine is an opposite one, resulting in a concentration-dependent decrease in Isc (EC50=35.5 μM). The finding that this increase in Isc by low concentrations of dopamine was completely abolished by selective D1, but not by selective D2, receptor blockade, strongly suggests that these effects are mediated through the activation of specific dopamine receptors of the D1 subtype. It is interesting, however, to observe that the rightward shift of the concentration-response curve for dopamine in the absence and the presence of phentolamine in adult rats (12 fold) is greater than that observed in young rats (8 fold). This may indicate that the sensitivity of the α-adrenoceptors to dopamine in adult rats is greater than that in young animals, which is in agreement with the marked difference in EC50 values for dopamine (in the absence of phentolamine) between young (7.0 (3.3, 14.8) μM) and adult (1.0 (0.4, 2.6) μM) rats. Thus, it might be concluded that in the jejunum of 20-day old rats the α-adrenergic response to dopamine are not easy to arouse. The reason for this to occur can not be attributed exclusively to the presence in young rats of an antagonistic dopaminergic component, but it is possible that this may contribute for the observed response to the amine. Another possible explanation may be that adult animals are endowed with a density of α-adrenoceptors greater than that in young rats. To our knowledge there is no indication in the literature that this is the case for the rat jejunum. However, the ontogeny of α-adrenoceptors has been quite well defined in other tissues and the overall view fits well the suggestion of a age dependent receptor density (Gitler et al., 1991; Guimarães et al., 1994; McMillian et al., 1983; Takayanagi et al., 1989). On the other hand, it is interesting to mention that noradrenaline levels in the jejunal wall of 20-day rats were markedly higher (28 fold) than in 60-day old rats, which is in agreement with previous findings (Finkel et al., 1994; Vieira-Coelho et al., 1998). In the present study, the subtype of α-adrenoceptor involved in this response of dopamine has not been examined.
Under our experimental conditions an increase in jejunal Isc can be due to a decrease in sodium absorption, a decrease in chloride secretion or both (Vieira-Coelho & Soares-da-Silva, 1998). The finding that only ouabain, but not furosemide prevented the increase in Isc produced by SKF 38393 strongly suggests that the effect of D1 receptor activation is a decrease in sodium movement across the jejunal epithelium from the mucosal to the serosal side, most probably as a result of Na+,K+-ATPase inhibition. This view is reinforced by the observation that both dopamine and the selective D1 agonist, SKF 38393, produced a significant inhibition of the Na+,K+-ATPase activity in isolated jejunal epithelial cells obtained from young rats. It is interesting to note that in adult rats dopamine is devoid of inhibitory effect upon Na+,K+-ATPase, which fits well the data obtained on rheogenic transport. This failure of dopamine to inhibit jejunal Na+K+-ATPase activity is most likely not related to the absence of receptors for the amine, since density of binding sites for [3H]-Sch 23390 was even slightly greater in adult than in young rats, with similar KD's. On the other hand, jejunal Na+,K+-ATPase was also found to respond with inhibition to stimuli that are known to be involved in transduction mechanisms set into motion during activation of D1 receptors, such as stimulation of PKA and PKC pathways (Bertorello & Aperia, 1990b; Hussain & Lokhandwala, 1998; Jose et al., 1992). In fact, the inhibitory effects of dopamine upon renal Na+,K+-ATPase activity is accompanied by activation of adenylate cyclase (Bertorello & Aperia, 1990a) and phospholipase C (Vyas et al., 1992).
Altogether, these results would suggest that jejunal cells from adult rats are endowed with the necessary machinery to respond to dopamine; the cells are endowed with a considerable number of receptors and jejunal Na+,K+-ATPase is an effector protein for PKA and PKC. Thus, this data suggests that abnormal regulation of Na+,K+-ATPase by dopamine may not arise from a defect in effector mechanisms. Rather, they suggest the defect occurs prior to the effector mechanism, most probably at the level of G proteins. In fact, GTPγS, a nonhydrolyzable analogue of GTP, that irreversibly activates G proteins, decreased in a concentration dependent manner jejunal Na+,K+-ATPase activity in young but not in adult rats. This view is reinforced by the fact that GDPβS, an analogue of GTP that does not activate G proteins, had no effect on intestinal Na+,K+-ATPase in both young and adult animals. Treatment of jejunal cells with CTX also inhibited Na+,K+-ATPase activity, and it would appear, therefore, that a G protein of the Gs class is involved in the inhibitory pathway. Because dopamine inhibits Na+,K+-ATPase activity, it is suggested the effects of dopamine upon Na+,K+-ATPase are mediated via Gs pathway. Failure of dopamine, GTPγS and CTX to inhibit jejunal Na+,K+-ATPase activity in adult rats is in agreement with the suggestion that the αs-subunit is subnormal. Because PTX blocks the activation of Gi/o pathways by agonists (Birnbaumer, 1990; Sunyer et al., 1989), and the inhibitory effect of dopamine was not blocked by PTX, it is suggested that inhibition of sodium pump by dopamine is not mediated by G proteins of the Gi/o class. Rather, the results presented here indicate that Gi/o proteins may mediate a stimulatory pathway that involves dopamine, since inhibition of jejunal Na+,K+-ATPase activity by dopamine is potentiated by PTX. These results, combined with the CTX results, suggest that dopamine has a dual stimulatory and inhibitory effect on Na+,K+-ATPase activity that is mediated by Gs and Gi/o class of G proteins, respectively. This is particularly evident in young rats, which respond to CTX and dopamine with inhibition of Na+,K+-ATPase activity, the latter effect being potentiated by PTX. To a less extent, this is also true for adult rats. One possible interpretation is that binding of dopamine to its receptor activates both Gs and Gi/o; stimulatory αi/o pathway is counterbalanced by inhibitory αs pathway. Net inhibition by dopamine is achieved via an inhibitory βγ pathway, which is subnormal in adult animals. Alternatively, it might be hypothesized that subnormal activation of βγ pathway and αs-subunit would result from defective dissociation of the α-subunit from the βγ-subunits. The findings presented here, namely those concerning the effects of dopamine, GTPγS, CTX and PTX alone and plus dopamine upon jejunal Na+,K+-ATPase activity, are quite similar to those observed at the kidney level in spontaneous hypertensive rats (SHR) and their corresponding normotensive controls, the Wistar-Kyoto (WKY) rats (Gurich & Beach, 1994). Dual activation of G proteins by one ligand/receptor interaction has been described in other systems (Eason et al., 1992; Gurich & Beach, 1994). At the kidney level, there is evidence that PTX-sensitive G proteins of Gi/o class also play a role in dopamine-mediated inhibition of Na+,K+-ATPase activity, most likely via stimulation of D2-like receptors (Bertorello & Aperia, 1989; 1990a). This is unlikely to be the case in the rat jejunum, given the fact that dopamine-mediated inhibition of Na+,K+-ATPase activity and increases in Isc were insensitive to S-sulpiride, a selective D2 receptor antagonist. Furthermore, the selective D2 receptor agonist quinerolane failed to alter Na+,K+-ATPase activity and Isc.
Intestinal function is determined by a developmental process that has a great impact in maintaining electrolyte and water metabolism (Younoszai et al., 1978). At this stage of maturation, the possible occurrence of complementary functions between the intestine and the kidney may be essential to achieve adequate electrolyte and water homeostasis. In fact, intestinal Na+,K+-ATPase activity in young rats was lower than in adult rats, was sensitive to inhibition by dopamine (anti-absorptive) and less sensitive to the α2-adrenoceptor stimulation (pro-absorptive) in comparison with that occurring in animals with a mature renal function. All the data reported here in young rats demonstrate that the intestine at this age is less absorptive and more susceptive to secretion. This is in agreement with the clinical finding that the major disorder of water and electrolyte metabolism in children is dehydrating diarrhoea, that contributes to higher mortality rates at young ages (Meyers, 1994). Because lack of response to dopamine in adult rats at the intestinal level appears to result from subnormal regulation of G proteins (subnormal activation of αs-subunit, subnormal activation of βγ pathway or subnormal dissociation of the α-subunit from the βγ-subunits) is compatible with a healthy life, in contrast to that occurring at the kidney level in SHR, one should think more on evolutionary or adaptation processes rather than on defective mechanisms.
In conclusion, the data presented here strongly suggest that in young rats, dopamine produce a decrease in jejunal electrolyte absorption, in the presence of α-adrenoceptor blockade. This effect, most probably due to inhibition of Na+,K+-ATPase, is likely to be mediated by D1 receptors and is not present in adult rats. Jejunal Na+,K+ATPase activity in young rats is regulated by both CTX- and PTX-sensitive G proteins, but regulation of the enzyme by CTX-sensitive G proteins is absent in adult animals. Such difference may explain the failure of dopamine to inhibit intestinal Na+,K+-ATPase activity in adult rats.
Acknowledgments
Supported by grant SAU/29/95 from Fundação para a Ciência e a Tecnologia.
Abbreviations
- AADC
aromatic L-amino acid decarboxylase
- CTX
cholera toxin
- DOPAC
3,4-dihydroxyphenylacetic acid
- GDPβS
guanosine 5′-o-(2-thiodiphosphate)
- GTPγS
guanosine 5′-o-(3-thiotriphosphate)
- HVA
homovanillic acid
- L-DOPA
3,4-dihydroxyphenylalanine
- 3-MT
3-methoxytyramine
- PDBu
phorbol 12,13-dibutyrate
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PMSF
Phenylmethyfsulphonyl fluoride
- PTX
pertussis toxin
References
- BERTORELLO A., APERIA A. Regulation of Na(+)-K(+)-ATPase activity in kidney proximal tubules: involvement of GTP binding proteins. Am. J. Physiol. 1989;256:F57–F62. doi: 10.1152/ajprenal.1989.256.1.F57. [DOI] [PubMed] [Google Scholar]
- BERTORELLO A., APERIA A. Inhibition of proximal tubule Na(+)-K(+)-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am. J. Physiol. 1990a;259:F924–F928. doi: 10.1152/ajprenal.1990.259.6.F924. [DOI] [PubMed] [Google Scholar]
- BERTORELLO A., APERIA A. Short-term regulation of Na+,K(+)-ATPase activity by dopamine. Am. J. Hypertens. 1990b;3:51S–54S. doi: 10.1093/ajh/3.6.51s. [DOI] [PubMed] [Google Scholar]
- BINDER H.J.Absorption and secretion of water and electrolytes by small and large intestine Gastrointestinal Disease 1983Philadelphia: W.B. Saunders; 812–829.In Seisinger, O. & Fordtran, O. eds [Google Scholar]
- BIRNBAUMER L. G-proteins in signal transduction. Annu. Rev. Pharmacol. Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- DONOWITZ M., ELTA G., BATTISTI L., FOGEL R., LABEL-SCHWARTZ E. Effects of dopamine and bromocriptine on rat ileal and colonic transport. Gastroenterology. 1983;84:516–523. [PubMed] [Google Scholar]
- EAKER E.Y., BIXLER G.B., DUNN A.J., MORESHEAD W.V., MATHIAS J.R. Dopamine and norepinephrine in the gastrointestinal tract of mice and the effects of neurotoxins. J. Pharmacol. Exp. Ther. 1988;244:438–442. [PubMed] [Google Scholar]
- EASON M.G., KUROSE H., HOLT B.D., RAYMOND J.R., LIGGETT S.B. Simultaneous coupling of alpha2-adrenergic receptors to two G-proteins with opposing effects. J. Biol. Chem. 1992;267:15795–15801. [PubMed] [Google Scholar]
- ESPLUGUES J.V., CARAMONA M.M., MOURA D., SOARES-DA-SILVA P. Effects of chemical sympathectomy on dopamine and noradrenaline content of the dog gastrointestinal tract. J. Auton. Pharmacol. 1985;5:189–195. doi: 10.1111/j.1474-8673.1985.tb00119.x. [DOI] [PubMed] [Google Scholar]
- FINKEL Y., EKLOF A.C., GRANQUIST L., SOARES-DA-SILVA P., BERTORELLO A.M. Endogenous dopamine modulates jejunal sodium absorption during high-salt diet in young but not in adult rats. Gastroenterology. 1994;107:675–679. doi: 10.1016/0016-5085(94)90114-7. [DOI] [PubMed] [Google Scholar]
- GIL-MARTIN E., FERNANDEZ-BRIERA A., CALVO P. Effects of chronic ethanol treatment withdrawal on (3H)SCH23390 binding to rat strital membranes. Neuropharmacology. 1997;36:101–106. doi: 10.1016/s0028-3908(96)00159-1. [DOI] [PubMed] [Google Scholar]
- GITLER M.S., PICCIO M.M., ROBILLARD J.E., JOSE P.A. Characterization of renal alpha-adrenoceptor subtypes in sheep during development. Am. J. Physiol. 1991;260:R407–R412. doi: 10.1152/ajpregu.1991.260.2.R407. [DOI] [PubMed] [Google Scholar]
- GOLDBERG L.I., RAJFER S.I. Dopamine receptors: applications in clinical cardiology. Circulation. 1985;72:245–248. doi: 10.1161/01.cir.72.2.245. [DOI] [PubMed] [Google Scholar]
- GUIMARÃES S., MOURA D., PAIVA M.Q., VAZ-DA-SILVA M.J. Lack of pre- and postjunctional beta adrenoceptor-mediated effects in the canine saphenous vein at birth. J. Pharmacol. Exp. Ther. 1994;268:990–995. [PubMed] [Google Scholar]
- GURICH R.W., BEACH R.E. Abnormal regulation of renal proximal tubule Na+-K+-ATPase by G proteins in spontaneous hypertensive rats. Am. J. Physiol. 1994;267:F1069–F1075. doi: 10.1152/ajprenal.1994.267.6.F1069. [DOI] [PubMed] [Google Scholar]
- HORIUCHI A., ALBRECHT F.E., EISNER G.M., JOSE P.A., FELDER R.A. Renal dopamine receptors and pre- and post-cAMP-mediated Na+ transport defect in spontaneously hypertensive rats. Am. J. Physiol. 1992;263:F1105–F1111. doi: 10.1152/ajprenal.1992.263.6.F1105. [DOI] [PubMed] [Google Scholar]
- HUSSAIN T., LOKHANDWALA M.F. Renal dopamine receptor function in hypertension. Hypertension. 1998;32:187–197. doi: 10.1161/01.hyp.32.2.187. [DOI] [PubMed] [Google Scholar]
- JOSE P.A., RAYMOND J.R., BATES M.D., APERIA A., FELDER R.A., CAREY R.M. The renal dopamine receptors. J. Am. Soc. Nephrol. 1992;2:1265–1278. doi: 10.1681/ASN.V281265. [DOI] [PubMed] [Google Scholar]
- KIMMICH G.A. Isolation of intestinal epithelial cells and evaluation of transport functions. Meth. Enzymol. 1990;192:324–340. doi: 10.1016/0076-6879(90)92080-w. [DOI] [PubMed] [Google Scholar]
- LIU L., COUPAR I.M. Role of alpha2- adrenoceptors in the regulation of intestinal water transport. Br. J. Pharmacol. 1997;120:892–898. doi: 10.1038/sj.bjp.0700958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMON L.M., ALBRECHT F., CANESSA L.M., HOY G.R., JOSE P.A. Identification of dopamine 1A receptors in the rat small intestine. J. Surg. Res. 1993;54:616–620. doi: 10.1006/jsre.1993.1094. [DOI] [PubMed] [Google Scholar]
- MCMILLIAN M.K., SCHANBERG S.M., KUHN C.M. Ontogeny of rat hepatic adrenoceptors. J. Pharmacol. Exp. Ther. 1983;227:181–186. [PubMed] [Google Scholar]
- MEYERS A. Fluid and electrolyte therapy for children. Curr. Opin. Pediatr. 1994;6:303–309. doi: 10.1097/00008480-199406000-00012. [DOI] [PubMed] [Google Scholar]
- MOTULSKY H.J., SPANNARD P., NEUBIG R. GraphPad Prism (version 1.0) GraphPad Prism Software Inc.: San Diego, U.S.A; 1994. [Google Scholar]
- NELLANS H.N., FRIZZELL R.A., SCHULTZ S.G. Brush-border processes and transepithelial Na and Cl transport by rabbit ileum. Am. J. Physiol. 1974;226:1131–1141. doi: 10.1152/ajplegacy.1974.226.5.1131. [DOI] [PubMed] [Google Scholar]
- QUARONI A., WANDS R.L., TRESSTAD K.L., ISSELBACHER K.J. Epitheliod cell cultures from rat small intestine. J. Cell. Biol. 1979;80:248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIGLEY J.P., GOTTERER G.S. Distribution of Na,K-stimulated ATPase activity in rat intestinal mucosa. Biochim. Biophys. Acta. 1969;173:456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- ROBILLARD J., SEGAR J., SMITH F., GUILLERY E., JOSE P. Mechanisms regulating renal sodium excretion during development. Pediatr. Nephrol. 1992;6:205–213. doi: 10.1007/BF00866320. [DOI] [PubMed] [Google Scholar]
- SOARES-DA-SILVA P., PESTANA M., VIEIRA-COELHO M.A., FERNANDES M.H., ALBINO-TEIXEIRA A. Assessment of renal dopaminergic system activity in the nitric oxide-deprived hypertensive rat model. Br. J. Pharmacol. 1995;114:1403–1413. doi: 10.1111/j.1476-5381.1995.tb13362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNYER T., MONASTIRSKY B., CODINA J., BIRNBAUMER L. Studies on nucleotide and receptor regulation of Gi proteins: effects of pertussis toxin. Mol. Endocrinol. 1989;3:1115–1124. doi: 10.1210/mend-3-7-1115. [DOI] [PubMed] [Google Scholar]
- TAKAYANAGI I., MAEDA O., KOIKE K. Effect of aging on presynaptic alpha 2-adrenoceptor mechanisms in guinea pig ileum. Comp. Biochem. Physiol. C. 1989;92:419–423. doi: 10.1016/0742-8413(89)90077-7. [DOI] [PubMed] [Google Scholar]
- VIEIRA-COELHO M.A., SOARES-DA-SILVA P. Dopamine formation, from its immediate precursor 3,4-dihydroxyphenylalanine, along the rat digestive tract. Fund. Clin. Pharmacol. 1993;7:235–243. doi: 10.1111/j.1472-8206.1993.tb00237.x. [DOI] [PubMed] [Google Scholar]
- VIEIRA-COELHO M.A., SOARES-DA-SILVA P. Alpha2-adrenoceptors mediate the effect of dopamine on adult rat jejunal electrolyte transport. Eur. J. Pharmacol. 1998;356:59–65. doi: 10.1016/s0014-2999(98)00500-7. [DOI] [PubMed] [Google Scholar]
- VIEIRA-COELHO M.A., GOMES P., SERRÃO M.P., SOARES-DA-SILVA P. Renal and Intestinal Autocrine Monoaminergic Systems: dopamine versus 5-hydroxytryptamine. Clin. Exp. Hypertens. 1997;19:43–58. doi: 10.3109/10641969709080803. [DOI] [PubMed] [Google Scholar]
- VIEIRA-COELHO M.A., LUCAS TEIXEIRA V.A., FINKEL Y., SOARES-DA-SILVA P., BERTORELLO A.M. Dopamine dependent inhibition of jejunal Na+,K+-ATPase during high-salt diet in young but not in adult rats. Am. J. Physiol. 1998;275:1317–1323. doi: 10.1152/ajpgi.1998.275.6.G1317. [DOI] [PubMed] [Google Scholar]
- VYAS S.J., EICHBERG J., LOKHANDWALA M.F. Characterization of receptors involved in dopamine-induced activation of phospholipase-C in rat renal cortex. J. Pharmacol. Exp. Ther. 1992;260:134–139. [PubMed] [Google Scholar]
- YOUNOSZAI M.K., SAPARIO R.S., LAUGHLIN M. Maturation of jejunum and ileum in rats. J. Clin. Invest. 1978;68:271–280. doi: 10.1172/JCI109126. [DOI] [PMC free article] [PubMed] [Google Scholar]


