Abstract
Transintestinal absorption of gamma-aminobutyric acid (GABA) via a pH-dependent mechanism is demonstrated in the model human intestinal epithelial cell line Caco-2.
Experiments with BCECF [2′,7′,-bis(2-carboxyethyl)-5(6)-carboxyfluorescein]-loaded Caco-2 cells demonstrate that GABA transport across the apical membrane is coupled to proton flow into the cell.
Short-circuit current (ISC) measurements using Caco-2 cell monolayers under voltage-clamped conditions demonstrate that pH-dependent GABA transport is a rheogenic process even in the absence of extracellular Na+, consistent with H+/GABA symport.
A range of GABA analogues were tested for their abilities to: (a) inhibit pH-dependent [3H]GABA uptake across the apical membrane; (b) stimulate H+ flow across the apical surface of BCECF-loaded Caco-2 cell monolayers; (c) increase inward ISC across voltage-clamped Caco-2 cell monolayers.
Nipecotic acid, isonipecotic acid, D,L-β-aminobutyric acid, and 3-amino-1-propanesulphonic acid each caused a marked acidification of intracellular pH and an increase in ISC when superfused at the apical surface of Caco-2 cell monolayers. In contrast L-α-amino-n-butyric acid failed to induce proton flow or ISC. The ability of these compounds to induce proton or current flow across the apical surface of this intestinal epithelium was closely related to the relative inhibitory effects on [3H]GABA uptake.
These observations demonstrate H+/GABA symport and suggest that this transport mechanism may be accessible as a route for oral absorption of therapeutically-useful GABA analogues.
Keywords: Proton-coupled transport, GABA, amino acid, human intestine, epithelium, Caco-2 cells, cell culture, intracellular pH, short-circuit current
Introduction
GABA is the main inhibitory neurotransmitter in the brain (MacDonald, 1997; Richens, 1992). Although no particular epilepsy has been shown to be caused by a change in GABAergic transmission, decreased GABA-mediated inhibition causes partial epilepsy (MacDonald, 1997). Thus reduced levels of brain GABA may result in epileptic conditions by a mechanism involving synaptic disinhibition (McNamara, 1999). Alternative pharmacological strategies to allow modulation of GABAergic activity could involve delivery of GABA receptor agonists or inhibition of mechanisms involved in rapid uptake of GABA into presynaptic terminals and surrounding glial cells (Krogsgaard-Larsen et al., 1987). Blockade of both neuronal and glial cell GABA uptake mechanisms would enhance the inhibitory effects of synaptically-released GABA whereas selective blockade of the glial cell GABA uptake mechanism would increase the amount of GABA returning to the neurone and would thus increase the effective GABA concentration in the nerve terminal (Krogsgaard-Larsen et al., 1987). Molecular cloning studies have identified four distinct GABA transporters (GAT-1, GAT-2, GAT-3 and GAT-4) in mouse brain (Liu et al., 1993). A number of GABA-related compounds (including nipecotic acid and a range of related cyclic amino acids) are potent inhibitors of neuronal and/or glial GABA transporters (Krogsgaard-Larsen et al., 1987; Borden et al., 1994). Thus pharmacological inhibition of GABA uptake via these mechanisms (the GAT transporters) provides an opportunity to increase GABAergic transmission.
The initial barrier to drug absorption is the intestinal epithelial cell wall. The treatment of CNS disorders such as epilepsy, therefore, is dependent upon the development of effective oral formulations of pharmaceutical agents (e.g. GABA receptor agonists and re-uptake inhibitors) (Richens, 1992). To be effective, novel orally-delivered anti-convulsant agents have to be transported across two cellular barriers (the intestine and blood–brain). Permeation across the blood–brain barrier has received most attention due to the relative impermeability of GABA and some related uptake inhibitors (Krogsgaard-Larsen et al., 1987). Relatively little is known about intestinal GABA transport although a number of amino acid transport mechanisms may be common to both cellular barriers (Smith & Stoll, 1999).
Intestinal absorption of GABA is likely to involve a transcellular pathway mediated via carrier proteins normally involved in ‘nutrient' absorption. Studies using rat intestine suggest that GABA shares a transporter with β-alanine (Nacher et al., 1994a) which has transport characteristics similar to the imino carrier (Munck et al., 1994). In addition baclofen (a GABAB receptor agonist used in the management of spasticity) is believed to share this intestinal transporter with GABA and β-alanine (Nacher et al., 1994b). Our previous studies have identified a novel H+/amino acid transporter at the apical membrane of human intestinal Caco-2 cells that has a similar substrate specificity to the rat imino carrier (Munck et al., 1994; Thwaites et al., 1995b; Thwaites & Stevens, 1999).
This study has two aims: firstly, to identify which nutrient carrier is involved in GABA transport in human intestinal tissues; and secondly, to determine whether selective GABA uptake inhibitors and receptor antagonists/agonists are transported by this nutrient carrier.
Methods
Materials
4-amino-n-[2,3-3H]butyric acid (GABA, specific activity 92 Ci.mmol−1) was from Amersham. β-[3-3H]alanine (specific activity 93 Ci.mmol−1), D-[1-14C]mannitol (specific activity 52 mCi.mmol−1) and D-[1-3H(N)]mannitol (specific activity 20 Ci.mmol−1) were obtained from NEN. [β-14C]β-alanine (specific activity 46 Ci.mmol−1) was from Sigma. Unlabelled amino acids, amino acid analogues, cell culture media and supplements were from Sigma. Cell culture plasticware was from Costar. Isonipecotic acid was obtained from Research Biochemicals International (RBI). Cycloleucine and 1-aminocyclopropane carboxylic acid were from Tocris. All other chemicals were from Merck and were of the highest quality available.
Cell culture
Caco-2 cells (passage number 103-119) were cultured as described previously (Thwaites et al., 1993a). Experiments were performed 13–26 days after seeding and 18–24 h after feeding.
Transport experiments
For transport experiments, the cell monolayers (grown on 24.5 mm diameter Transwell filters) were extensively washed in 4×500 ml of modified Krebs buffer [of composition, all mmol/l: NaCl, 137; KCl, 5.4; CaCl2, 2.8; MgSO4, 1.0; NaH2PO4, 0.3; KH2PO4, 0.3; HEPES, 10; glucose, 10 (pH to 7.4 at 37°C with Tris base)], or Na+-free Krebs as appropriate (identical in composition to above except choline chloride replaced NaCl and NaH2PO4 was omitted), placed in 6-well plates, each well containing 2 ml of prewarmed (37°C) Krebs buffer (pH 7.4, Na+-containing or Na+-free as appropriate). Aliquots (2 ml) of prewarmed Krebs [pH 5.5 (obtained by replacing 10 mM HEPES with 10 mM MES) or 7.4, Na+-containing or Na+-free as appropriate)] were then placed in the upper filter cup (apical solution). Radiolabelled substrates were used at tracer concentrations (0.5 μCi.ml−1) with unlabelled substrates added to give a final concentration of 100 μM. At the end of the 60 min incubation period samples of both apical and basal solutions were removed and cell monolayers were washed in 4×500 ml volumes of ice-cold Na+-containing or Na+-free Krebs buffer as appropriate (pH 7.4) to remove any loosely-associated radiolabel, and removed from the insert. Transepithelial radiolabelled GABA transport and accumulation were determined by scintillation counting and are expressed as pmol.cm−2.h−1 and as μM or a cell/medium (C/M) ratio, respectively.
Uptake experiments
For uptake experiments, the cell monolayers (grown on 12 mm diameter filters) were extensively washed in 4×500 ml of modified Na+-free Krebs buffer, placed in 12-well plates, each well containing 1 ml of prewarmed (37°C) modified Na+-free Krebs buffer (pH 7.4). Aliquots (0.5 ml) of prewarmed Na+-free Krebs (pH 7.4) were then placed in the upper filter cup (apical solution). The experimental composition of the buffers in the apical and basal chambers were identical except when stated otherwise. After preincubation, the apical solution was discarded and replaced with 500 μl of the appropriate prewarmed (37°C) uptake solution and uptake determined for 1–5 min. For uptake measurements, apical pH was varied between pH 5–8 by varying the relative concentration of HEPES and MES (total concentration 10 mM). Radiolabelled substrates were used at tracer concentrations (0.5–1.0 μCi.ml−1) with unlabelled substrates added to give a final concentration (unless stated) of 5–100 μM. In all cases pH was measured after addition of cold substrate and readjusted if required. At the end of the incubation period cell monolayers were washed in 4×500 ml volumes of ice-cold Na+-free Krebs buffer (pH 7.4) to remove any loosely-associated radiolabel, and removed from the insert. Cell monolayer-associated radiolabel was determined by scintillation counting and uptake is expressed as pmol.cm−2.min−1 or μM or as a cell/medium (C/M) ratio. Preliminary experiments determined that the uptake was linear up to 5 min (data not shown). Therefore, all further experiments were performed between 1 and 5 min.
Intracellular pH measurements
Intracellular pH (pHi) measurements were made as described previously (Thwaites et al., 1993a). Intracellular BCECF fluorescence was converted to pHi by comparison with values from an intracellular calibration curve using nigericin (10 μM) and high K+ solutions (Thomas et al., 1979). Results are expressed as pHi, ΔpHi or ΔpHi.min−1 [mean±s.e.mean (n)]. The rate of change of intracellular pH (ΔpHi.min−1), due to a change in the composition of the superfusate, was calculated by linear regression (Photon Counter System 4.7, Newcastle Photometric Systems) by comparison of the linear portions of the trace over 30–50 s (15–25 data points) periods before and after the change in composition. The specific change in ΔpHi.min−1, due to addition of a particular substrate, can be obtained by subtraction of the ΔpHi.min−1 in the presence of apical acidity alone from the total ΔpHi.min−1 determined upon addition of a particular substrate to the superfusate.
ISC measurements
Measurements of shortcircuit current (Isc) were made essentially as described previously (Thwaites et al., 1993a).
Calculation
Calculation of the percentage of the various ionic species of GABA as a function of pH was performed using the Henderson-Hasselbach equation (Wenzel et al., 1996) using the pKa and pKb values reported by Cohn & Edsall (1943).
Statistics
Results are expressed as mean±s.e.mean. Statistical comparison of mean values were made using one-way analysis of variance (ANOVA) using a corrected Bonferroni P value for the number of comparisons made.
Results
Transport experiments
Although net absorption of amino acids across the intestine is generally Na+-linked, figure 1a shows that in the absence of a transepithelial pH gradient (both apical and basolateral pH 7.4) net transport of GABA across Caco-2 cell monolayers is small and varies from net secretion (Jnet=−256±25 pmol. cm−2.h−1, n=4) to net absorption (151±30 pmol.cm−2.h−1, n=4) in the presence or absence of extracellular Na+, respectively (Figure 1a). Under these experimental conditions the cellular accumulation of GABA is also low, but does show Na+-dependency particularly at the basolateral membrane where the uptake is reduced from 204±5 (n=4) to 39±2 (n=4) μM when Na+ is removed from the extracellular solutions (Figure 1b). However, when the apical surface is acidified (apical pH 5.5, basolateral pH 7.4) net absorption of GABA across these human intestinal epithelial cell monolayers is stimulated in both the presence (854±69 pmol.cm−2.h−1, n=5) and absence (2201±164 pmol.cm−2.h−1, n=5) of Na+ (Figure 1a). In each case this large increase in Jnet is due to a pH-stimulated increase in Ja-b. Similarly at apical pH 5.5 accumulation across the apical membrane is also marked in both the presence (489±28 μM, n=5; cell/medium (C/M) ratio 4.9) and absence (627±23 μM, n=5; C/M ratio 6.3) of extracellular Na+ (Figure 1b). These observations suggest that the apical membrane of Caco-2 cell monolayers contains a Na+-independent, pH-dependent transport mechanism capable of transporting and accumulating GABA into the cell. Under all experimental conditions the transport (Figure 1a) and uptake (Figure 1b) of mannitol were low in comparison to GABA. For example, in the absence of extracellular Na+ (apical pH 5.5, basolateral pH 7.4) the Ja-b of GABA was 33 times greater than mannitol (conventionally used as an inert marker of paracellular flux).
Figure 1.
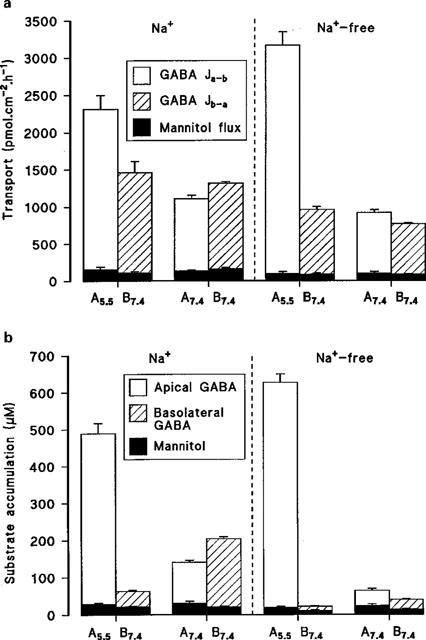
(a) Transepithelial transport of GABA (100 μM) across Caco-2 cell monolayers in the apical-to-basal (Ja-b) and basal-to-apical (Jb-a) directions (apical pH 5.5 or 7.4, basolateral pH 7.4) in the presence or absence of extracellular Na+. Mannitol transport under identical experimental conditions is also indicated. Results are expressed as pmol.cm−2.h−1 (mean±s.e.mean, n=4-5). (b) Accumulation of GABA (100 μM) across the apical or basolateral membrane (apical pH 5.5 or 7.4, basolateral pH 7.4) in the presence or absence of extracellular Na+. Mannitol uptake under identical experimental conditions is also indicated. Results are expressed as μM (mean±s.e.mean, n=4–5).
The Na+-independent, pH-dependent apical uptake of GABA was determined in comparison with the inert non-transported marker mannitol (Figure 2). In Na+ free conditions, over the apical pH range 5.0–8.0 (basolateral pH 7.4) GABA uptake was pH dependent being maximal between pH 5.0–5.6 and minimal between pH 7.7–8.0 (confirming previous observations with a range of dipolar amino acids; Thwaites & Stevens, 1999). In contrast the small mannitol uptake was not effected by apical pH. At pH 5.3 GABA uptake was 29.7 times greater than mannitol but only 1.5 times greater at pH 8.0 (see Figure 2). The acid-stimulated Na+-independent uptake of GABA showed saturation (Figure 3). The best fit curve was a Michaelis-Menten fit (1 site binding plus a linear component, r2=0.998) of the data which gives an apparent Km of 1.95±0.78 mM and Vmax for the saturable component of 2127±219 pmol.cm−2.min−1 (n=10) (linear component=181±19 pmol.cm−2.min−1/mM).
Figure 2.
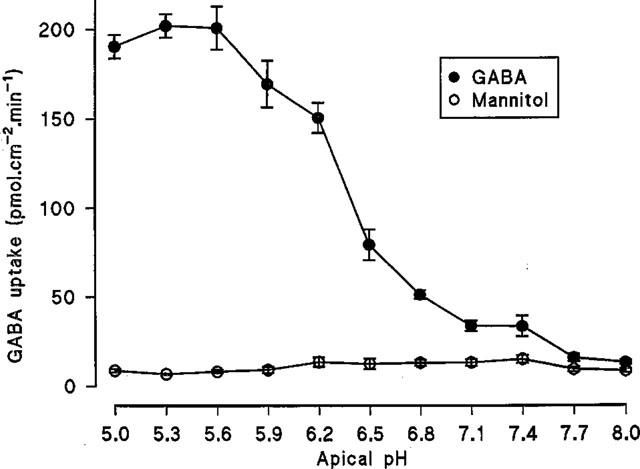
Na+-independent, pH-dependent uptake of GABA (100 μM) across the apical membrane of Caco-2 cell monolayers. Uptake was measured over 60s in Na+-free conditions over the apical pH range 5.0–8.0 (basolateral pH 7.4). Mannitol (100 μM) uptake was determined under identical experimental conditions. Results are expressed as pmol.cm−2.min−1 (mean±s.e.mean, n=5–6).
Figure 3.
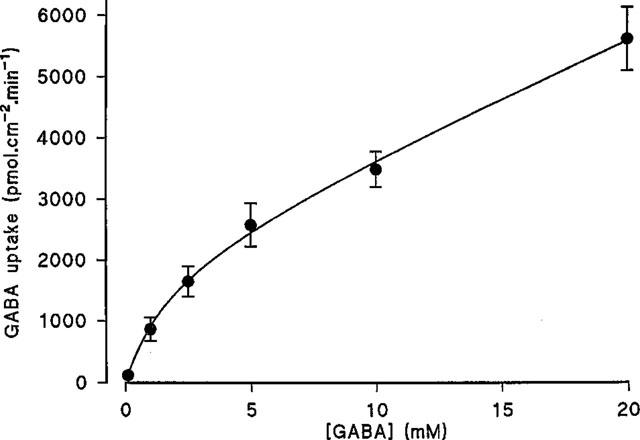
Concentration dependent uptake of GABA across the apical membrane of Caco-2 cell monolayers. Uptake was determined for 60s at apical pH 5.5 (basolateral pH 7.4) in the absence of extracellular Na+. Results are expressed as pmol.cm−2.min−1 (mean±s.e.mean, n=9–10). The curve was fitted using FigP.
Over the pH range used in this study GABA (pKa 4.23, pKb 10.43) is predominately present as a zwitterion (>85.5%) (Figure 4). The pH-dependent profile of GABA uptake observed in Figure 2 does not appear to be related to the relative abundance of the cationic form of GABA (see Table 1). In Na+-free conditions, GABA uptake was measured at a constant total substrate concentration of 10 μM at apical pH 5.0, 5.5, 6.0, 6.5 and 7.0. An increase in GABA uptake across the apical membrane of Caco-2 cell monolayers was observed as apical pH was reduced from 7.0 to 5.0 (Table 1). To discount the possibility that the increase in GABA uptake (Figures 1 and 2) was due simply to an increase in the concentration and, therefore, availability of the cationic form of GABA as pH was lowered, a separate series of experiments were performed. In these experiments the concentration (1.45 μM) of the cationic form of GABA was kept constant, as extracellular pH varied, by variation in the total concentration of GABA (Table 1). A similar dependence on pH was observed showing that H+-symport is likely rather than transport of the GABA cation.
Figure 4.
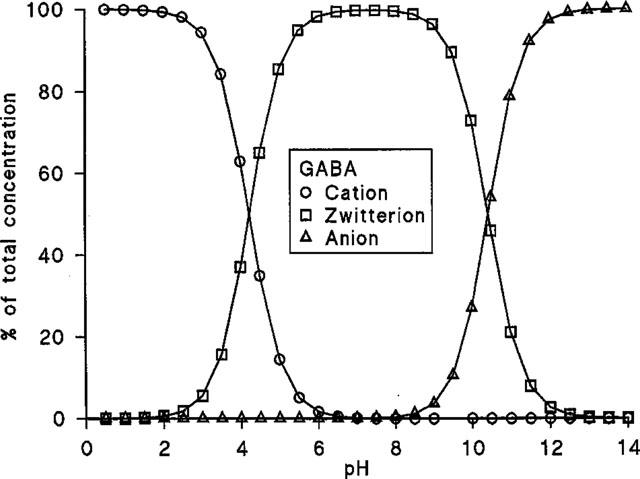
The proportion of GABA (as a per cent of the total concentration) in the cationic, zwitterionic and anionic forms.
Table 1.
GABA uptake
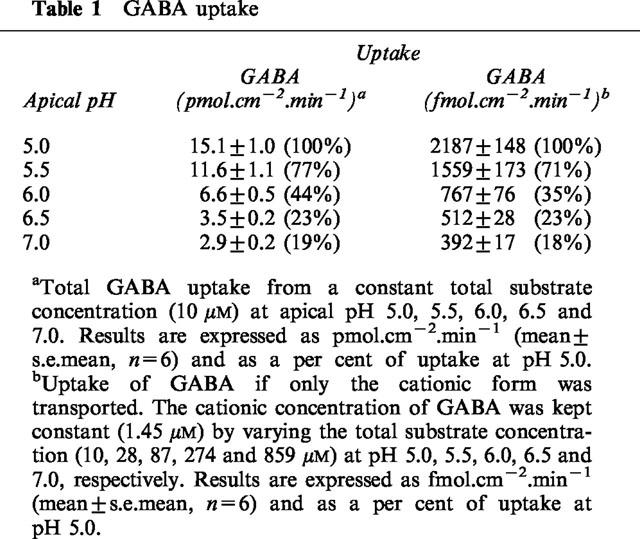
The pH dependent (apical pH 5.5, basolateral pH 7.4), Na+-independent uptake of [3H]GABA (5 or 100 μM) across the apical membrane of Caco-2 cell monolayers was determined in the presence and absence of a number of competing ‘cold' substrates (all 5 mM) (Figure 5a). GABA, β-alanine, nipecotic acid, isonipecotic acid and D,L-β-aminobutyric acid all significantly inhibited (P<0.001) [3H]GABA uptake. D-Cycloserine also significantly (P<0.001) reduced GABA uptake to 48.1±6.5 % (n=12) compared to control. 3-Amino-1-propanesulphonic acid also inhibited uptake but the difference between control was less significant (P<0.05). In contrast, D-α-amino-n-butyric acid and L-α-amino-n-butyric acid failed to reduce [3H]GABA uptake (P>0.05). There was no significant difference in the ability of GABA, β-alanine, D-cycloserine, nipecotic acid, isonipecotic acid or D,L-β-aminobutyric acid to inhibit [3H]GABA uptake (P>0.05).
Figure 5.
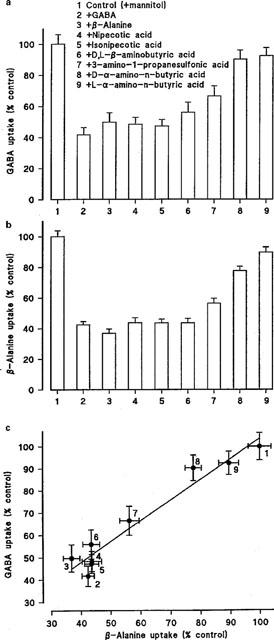
(a) pH-dependent (apical pH 5.5, basolateral pH 7.4), Na+-independent, [3H]GABA (5–100 μM) uptake (2–5 min) across the apical membrane of Caco-2 cell monolayers in the presence of a variety of cold substrates (all 5 mM). Results are expressed as per cent control (mean±s.e.mean, n=12). (b) pH-dependent (apical pH 5.5, basolateral pH 7.4), Na+-independent, [3H or 14C]β-alanine (5–100 μM) uptake (2–5 min) across the apical membrane of Caco-2 cell monolayers in the presence of a variety of cold substrates (all 5 mM). Results are expressed as per cent control (mean±s.e.mean, n=16–17). (c) Relationship between the abilities of various compounds [see (a) and (b)] to inhibit pH-dependent, Na+-independent, GABA and β-alanine uptake across the apical membrane of Caco-2 cells.
A similar pattern of inhibition was observed when the experiments were repeated using radiolabelled [3H or 14C]β-alanine (5–100 μM) as the transported substrate (compare Figure 5a,b). Figure 5c shows identity in the magnitude of inhibition by this range of substrates when either β-alanine or GABA uptake was measured. In conclusion the ability of these substrates to inhibit uptake of either GABA or β-alanine is in the order of GABA=β-alanine=D-cycloserine=nipecotic acid=isonipecotic acid=D,D-β-aminobutyric acid>3-amino-1-propanesulphonic acid>D-α-amino-n-butyric acid>L-α-amino-n-butyric acid.
Intracellular pH experiments
To confirm H+/GABA symport intracellular pH was monitored in Caco-2 cell monolayers loaded with the pH-sensitive dye BCECF during extracellular exposure to GABA (Figure 6). At the apical surface, exposure to 20 mM GABA at pH 7.4 (basolateral pH 7.4) led to a small but inconsistent acidification which was reversible when GABA was removed from the apical chamber. On reduction of apical pH to 5.5 an intracellular acidification was observed. Once the rate of this acidification due to the presence of apical pH 5.5 alone slowed (ΔpHi.min−1 0.024±0.008, n=5) GABA was added to the superfusate at pH 5.5 which led to an acceleration (P<0.001) of the initial rate of acidification (ΔpHi.min−1 0.215±0.032, n=5) compared to the change with apical acidity alone. In contrast addition of 20 mM GABA to the basolateral superfusate (apical pH 7.4) at basolateral pH 5.5 (ΔpHi.min−1 0.051±0.010, n=4) failed to increase (P>0.05) the rate of acidification seen with basolateral acidity alone (ΔpHi.min−1 0.053±0.017, n=4). This substrate-induced intracellular acidification at the apical surface is Na+-independent since a similar pHi change can be detected in the absence of extracellular Na+ (Figure 7). Note that the recovery after this solute-induced acid load is dependent on the presence of Na+ at the apical surface.
Figure 6.
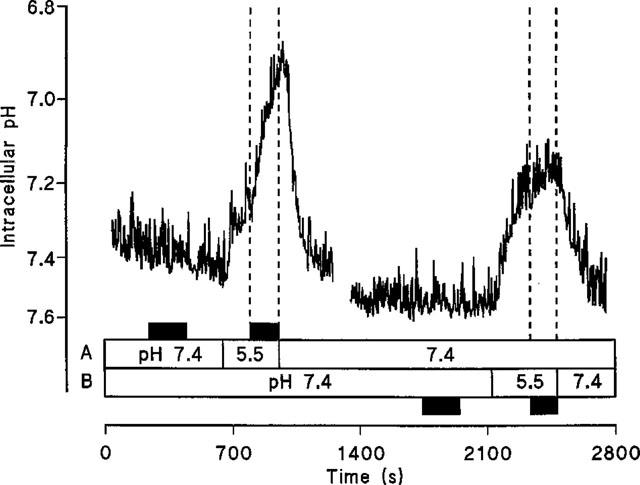
Intracellular pH measured in BCECF-loaded Caco-2 cell monolayers. The effect on pHi of exposure to 20 mM GABA (at pH 7.4 and pH 5.5) at either the apical (A) or basolateral (B) membranes of Caco-2 cell monolayers in the presence of extracellular Na+. Addition of GABA is indicated by the filled boxes. A representative trace of five separate experiments.
Figure 7.
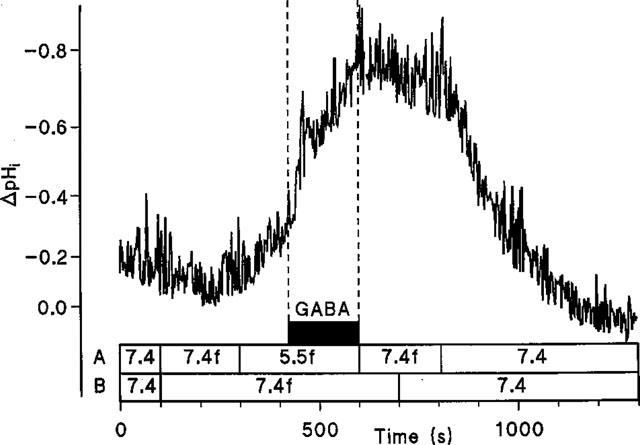
Intracellular pH measured in BCECF-loaded Caco-2 cell monolayers. The effect of GABA (20 mM) superfused across the apical membrane of Caco-2 cell monolayers in the absence of extracellular Na+. Lines A and B denote apical and basolateral solutions, respectively. The letter f denotes Na+-free solutions. Addition of GABA is indicated by the filled box. A representative trace of four separate experiments.
The substrates tested as potential inhibitors of GABA uptake (Figure 5) were also examined for their ability to induce H+-flow across the apical surface of Caco-2 cell monolayers (Figure 8). The compounds were all tested at an extracellular concentration of 20 mM (apical pH 5.5, basolateral pH 7.4). Note that the compounds to be tested were split into two groups because of experimental limitations. In each experiment the four test substrates were added in a random order. In each individual experiment the four substrates were flanked by additions of pH 5.5 alone to check the viability of the experiment with time. Within an experiment the rate of acidification due to pH 5.5 alone was obtained by averaging the rates at the beginning and end of each experiment. In the first series of experiments GABA [Figure 8a (ii); ΔpHi.min−1 0.428±0.068, n=5] significantly increased (P<0.01) the rate of acidification above the levels seen with pH 5.5 alone (Figure 8a (i) and (vi); ΔpHi.min−1 0.150±0.017, n=5). Nipecotic acid also significantly increased (Figure 8a (iii); ΔpHi.min−1 0.358±0.037, n=5) the rate of acidification compared to pH 5.5 alone (P<0.05). In contrast, neither D-α-amino-n-butyric acid (Figure 8a (iv); ΔpHi.min−1 0.235±0.018, n=5) nor L-α-amino-n-butyric acid (Figure 8a (v); ΔpHi.min−1 0.206±0.019, n=5) increased the rate of acidification compared to pH 5.5 alone (P>0.05). In the second series of experiments GABA again significantly increased (P<0.001) the rate of acidification (Figure 8b (iv); ΔpHi.min−1 0.466±0.042, n=5) compared to pH 5.5 alone (Figure 8b (i) and (vi); ΔpHi.min−1 0.118±0.004, n=5). Compared to pH 5.5 alone, D,L-β-aminobutyric acid (Figure 8b (ii); ΔpHi.min−1 0.457±0.017, n=5; P<0.001), isonipecotic acid (Figure 8b (v); ΔpHi.min−1 0.365±0.018, n=5; P<0.01), and 3-amino-1-propanesulphonic acid (Figure 8b (iii); ΔpHi.min−1 0.338±0.049, n=5; P<0.05) all significantly increased the rate of acidification. Thus the ability of the substrates to elicit a change in pHi is identical to their ability to inhibit GABA uptake. The ability of β-alanine (Thwaites et al., 1993a), nipecotic acid, isonipecotic acid, D,L-β-aminobutyric acid, and 3-amino-1-propanesulphonic acid to induce H+-inflow suggests that their inhibitory action is due to their ability to act as competitive transported substrates.
Figure 8.
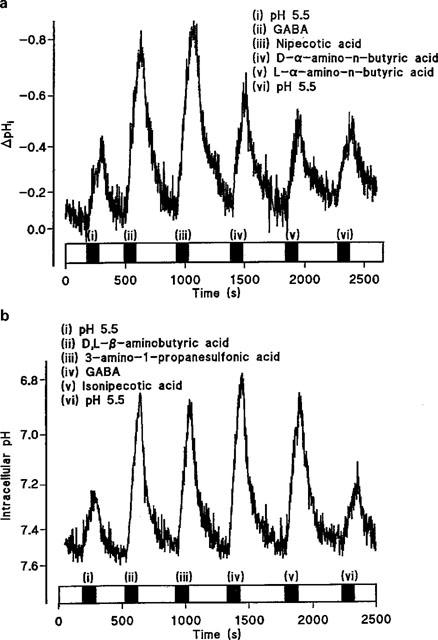
Intracellular pH measured in BCECF-loaded Caco-2 cell monolayers. (a) The effect on intracellular pH of sequential addition of (i) pH 5.5 alone; (ii) GABA; (iii) nipecotic acid; (iv) D-α-amino-n-butyric acid; (v) L-α-amino-n-butyric acid; (vi) pH 5.5 alone. All substrates are at 20 mM at apical pH 5.5 (basolateral pH 7.4). A representative trace of five separate experiments. (b) The effect on intracellular pH of sequential addition of (i) pH 5.5 alone; (ii) D,L-β-aminobutyric acid; (iii) 3-amino-1-propanesulphonic acid; (iv) GABA; (v) Isonipecotic acid; (vi) pH 5.5 alone. All substrates are at 20 mM at apical pH 5.5 (basolateral pH 7.4). The additions of substrates are indicated by the filled bars; open bars indicate superfusion of the pH 7.4 solution at the apical surface. A representative trace of five separate experiments.
ISC measurements
The rheogenic nature of this transport process was measured by the ability of the substrates to induce inward ISC in voltage-clamped Caco-2 cell monolayers (Figure 9). In Na+-free conditions, addition of GABA (20 mM) to the apical surface of voltage-clamped Caco-2 cell monolayers was associated with an increase in inward ISC above the levels noted with pH 5.5 alone (Figure 9a). Nipecotic acid, isonipecotic acid, D,L-β-aminobutyric acid, and 3-amino-1-propanesulphonic acid also stimulated inward ISC to a similar level as GABA whereas the current observed in the presence of D-α-aminobutyric acid (P<0.05) and L-α-aminobutyric acid (P<0.01) was significantly reduced compared to GABA (Figure 9b). Since H+/GABA symport is rheogenic the ΔISC measured is an independent assessment of substrate-induced H+-flow. These data, therefore, confirm that nipecotic acid, isonipecotic acid, D,L-β-aminobutyric acid and 3-amino-1-propanesulphonic acid act as competitive transported substrates for the H+/GABA symporter in human intestinal cells. Finally, the NMDA receptor partial agonist 1-aminocyclopropanecarboxylic acid (which acts at the glycine site) stimulated ISC in a similar fashion to GABA (61±5 (n=4) % of the GABA response) whereas the structurally-related antagonist cycloleucine (1-aminocyclopentanecarboxylic acid) only induced a small ISC (13±4 (n=4) %, P<0.01) compared to GABA.
Figure 9.
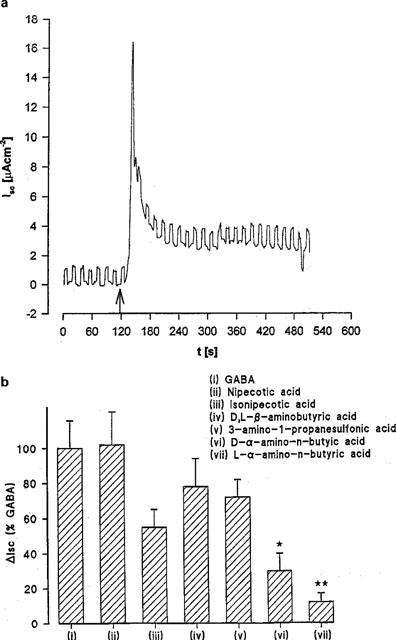
The rheogenic nature of GABA transport in short-circuited Caco-2 cell monolayers. Epithelial monolayers of Caco-2 cells were continually short-circuited in Na+-free media and the short-circuit current (ISC) response measured. (a) Time dependence of inward ISC after incubation in apical media of pH 5.5 (basolateral pH 7.4) and exposure to 20 mM GABA (see arrow). Square-wave pulse is excursion of the voltage-clamp to +1 mV as a measure of epithelial conductance. Representative trace of ten individual experiments. (b) Maximal ΔISC in the presence of GABA and GABA analogues (all 20 mM) in Na+-free media at apical pH 5.5 (basolateral pH 7.4). Results are expressed as a percentage of the response to GABA (mean±s.e.mean, n=6–10; *, P<0.05 versus GABA; **, P<0.01 versus GABA).
Discussion
This study provides evidence to suggest that GABA can undergo rapid transepithelial transport across the intestinal wall. Accumulation across the apical membrane and generation of overall net transfer (net absorption) result from the transport activity of a pH-dependent and Na+-independent H+/GABA symporter. The competition experiments, and measurements of pHi and ISC suggest that a number of GABA analogues (nipecotic acid, isonipecotic acid, D,L-β-aminobutyric acid and 3-amino-1-propanesulphonic acid) are transported substrates for this carrier. Although direct measurements of transepithelial transport of each of these substrates is required to identify whether-or-not the substrates can exit the basolateral membrane previous experiments with β-alanine, L-alanine, α-methylaminoisobutyric acid, proline and glycine (Thwaites et al., 1993a,1993b, 1994, 1995b,1995c) suggest that transepithelial transport will occur. Therefore, the oral route of absorption mediated via ‘nutrient' carrier proteins could be a potential route of delivery for some GABA uptake inhibitors and receptor agonists/antagonists. Clearly further work at the basolateral membrane is required.
A number of studies of amino acid transport using rabbit renal BBMV (Jessen et al. 1989, 1991; Rajendran et al. 1987) or confluent monolayers of the human intestinal epithelial cell line Caco-2 (grown on permeable filters) (Thwaites et al. 1993a,1993b, 1994, 1995c), suggest that a H+-coupled amino acid carrier(s) is present at the apical surface of both mammalian intestinal and renal epithelia. This novel H+/amino acid transport mechanism also transports the orally-active antibiotic (and modulator of the NMDA receptor) D-cycloserine (Ranaldi et al., 1994; Thwaites et al., 1995a). In vivo the driving force (the H+-electrochemical gradient) for such a transporter exists at the apical surface of the mammalian small intestinal epithelium in the form of the acid microclimate (McEwan et al., 1988; Rawlings et al., 1987).
The pH-sensitivity of GABA uptake across the apical surface of Caco-2 cell monolayers is demonstrated in Figures 1 and 2. That the transporter is a H+/GABA symporter is supported not only by the demonstrations of GABA-induced H+-flow and current flow (both in Na+-free conditions) and by the fact that over the pH range used in this study GABA is predominantly in the form of the zwitterion (Figure 4) but also by the results in Table 1. Table 1 shows clearly that the uptake is independent of the concentration of the cationic form of GABA since a similar pattern of pH-dependent uptake is observed when the GABA cation concentration was either varied or kept constant.
The Km for GABA uptake, the dependence on medium pH and independence on Na+, clearly distinguish the intestinal H+/GABA transporter from the brain GAT transporters which show relatively high affinity for GABA (<80 μM) and Na+ and Cl−-dependence (Liu et al., 1993; Nelson, 1998). However, the broad similarity in transported substrates (GABA, β-alanine, taurine, betaine and nipecotic acid) suggest that the intestinal transporter could be related to the GAT family of neurotransmitter transporters (Nelson, 1998).
Our previous studies (Thwaites et al., 1995a,1995c; Thwaites & Stevens, 1999) demonstrate that as well as GABA, β-alanine, glycine, L-alanine, taurine, proline, MeAIB, hydroxyproline, AIB (α-aminoisobutyric acid), sarcosine, betaine and D-cycloserine are also substrates for this transport mechanism. The transporter prefers dipolar amino acids with small side chains including those with an extra methyl group on the α-carbon atom (Thwaites & Stevens, 1999). The nature of the amino group does not seem to be important since substrates containing N-methylation, the amino group on the β or γ-carbon atoms, or side-chains bonded to the nitrogen on the α-carbon are all transported (Thwaites & Stevens, 1999). The major restriction on transport seems to be the nature of the side-chain since amino acids containing branching on the β or γ-carbons, bulky aromatic side chains, hydroxyl groups, amino groups, sulphur-containing groups, cationic, or anionic groups are all excluded (Thwaites & Stevens, 1999). These observations are supported by the results in this study where the linear compounds GABA and 3-amino-1-propanesulphonic acid are transported. The methyl group on the β-carbon in D,L-β-aminobutyric acid does not reduce transport although an extension of the side chain on the α-carbon, as seen in L and D-α-amino-n-butyric acid, causes a reduction in transport. Thus these experiments utilising GABA and some related compounds are able to provide further information about the pharmacological requirements of the intestinal H+/amino acid transporter. A considerable amount of conformational flexibility is characteristic of GABA (Krogsgaard-Larsen et al. 1987). The heterocyclic groups of nipecotic acid and isonipecotic acid in which the NH groups are expressed, with different spatial arrangements with respect to the acid moiety, indicate that a minimal separation of these groups is required.
Notwithstanding this partial structure-activity relationship (SAR) for GABA and its analogues, the ability to deliver useful therapeutically-active agents for GABA via the oral route is of considerable importance. Two further problems which need to be overcome to deliver GABA and analogues to the brain successfully are to avoid first pass elimination (metabolism) by the liver and to cross the blood–brain barrier. The blood–brain barrier is considered to be relatively impermeable to GABA although a recent study suggests that GABA is transported via a saturable process into rat blood–brain barrier capillaries (Garcia et al., 1998). Moreover, after i.p. injection of large doses of [3H]GABA, concentrations of approximately 5 mM in plasma are detected, and although the labelled GABA represents only a small fraction of the total endogenous neurotransmitter (0.014%) in the brain, because of rapid uptake it represents approximately 7% of GABA in the nerve endings (Vignolo et al., 1992). Furthermore, D-cycloserine (which acts via NMDA receptors) affects long-term memory processing in rats after subcutaneous administration (Flood et al., 1992) and, therefore, must cross the blood–brain barrier in vivo. Thus efficient delivery of novel anti-convulsants across the gastrointestinal tract may lead to central actions despite poor blood–brain barrier permeability. To achieve adequate delivery via the oral route a greater understanding of the SAR characteristics of mediated transport at three sites (the small intestine, the blood–brain barrier and the GAT transporters) is required.
In conclusion, the characteristics of GABA transport across the apical membrane of this human intestinal epithelial cell line are consistent with H+/zwitterionic GABA cotransport. The substrate specificity of this transport mechanism is not unlike that of the GAfT transporter family (Liu et al., 1993; Nelson, 1998) and is similar to that described for the Na+-dependent (Cl−-independent) imino acid carrier in rat small intestine (Munck et al. 1994). Finally, the current study demonstrates that the H+/amino acid cotransporter could provide a potential route for oral absorption of GABA and some related compounds.
Acknowledgments
Charlotte Ward provided excellent technical assistance. This study was supported by the Wellcome Trust and the BBSRC.
Abbreviations
- AIB
α-aminoisobutyric acid
- BBMV
brush-border membrane vesicles
- BCECF
2′,7′,-bis(2-carboxyethyl)-5(6)-carboxyfluorescein
- GABA
gamma-aminobutyric acid
- Isc
short-circuit current
- MeAIB
α-methylaminoisobutyric acid
- pHi
intracellular pH
- SAR
structure-activity relationship
References
- BORDEN L.A., DHAR T.G.M., SMITH K.E., WEINSHANK R.L., BRANCHEK T.A., GLUCHOWSKI C. Tiagabine, SK-and-F 89976-A, CI-966 and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur. J. Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- COHN E.J., EDSALL J.T. Proteins, amino, acids and peptides as ions and dipolar ions. Reinhold Publishing Corporation, N.Y.; 1943. [Google Scholar]
- FLOOD J.F., MORLEY J.E., LANTHORN T.H. Effect on memory processing by D-cycloserine, an antagonist of the NMDA glycine receptor. Eur. J. Pharmacol. 1992;221:249–254. doi: 10.1016/0014-2999(92)90709-d. [DOI] [PubMed] [Google Scholar]
- GARCIA S.C., MORETTI M.B., GARAY M.V.R., BATLLE A. δ-Aminolevulinic acid transport through blood–brain barrier. Gen. Pharmacol. 1998;31:579–582. doi: 10.1016/s0306-3623(98)00038-x. [DOI] [PubMed] [Google Scholar]
- JESSEN H., JORGENSEN K.E., ROIGAARD-PETERSEN H., SHEIKH M.I. Demonstration of H+and Na+-coupled co-transport of β-alanine by luminal membrane vesicles of rabbit proximal tubule. J. Physiol. 1989;411:517–528. doi: 10.1113/jphysiol.1989.sp017587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESSEN H., VORUM H., JORGENSEN K.E., SHEIKH M.I. Na+-and H+gradient-dependent transport of α-aminoisobutyrate by luminal membrane vesicles from rabbit proximal tubule. J. Physiol. 1991;436:149–167. doi: 10.1113/jphysiol.1991.sp018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P., FALCH E., LARSSON O.M., SCHOUSBOE A. GABA uptake inhibitors: relevance to antiepileptic drug research. Epilepsy Res. 1987;1:77–93. doi: 10.1016/0920-1211(87)90012-x. [DOI] [PubMed] [Google Scholar]
- LIU Q.R., LOPEZ-CORCUERA B., MANDIYAN S., NELSON H., NELSON N. Molecular characterization of four pharmacologically distinct γ- aminobutyric acid transporters in mouse brain. J. Biol. Chem. 1993;268:2106–2112. [PubMed] [Google Scholar]
- MACDONALD R.L.Inhibitory synaptic transmission Epilepsy: A Comprehensive Textbook 1997Philadelphia: Lippincott-Raven Publishers; 265–275.In: Engel Jr. J. & Pedley, T.A. (eds) [Google Scholar]
- MCNAMARA J.O. Emerging insights into the genesis of epilepsy. Nature. 1999;399 Suppl:A15–A22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- MCEWAN G.T.A., DANIEL H., FETT C., BURGESS M.N., LUCAS M.L. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc. R. Soc. Lond. (Series B) 1988;234:219–237. doi: 10.1098/rspb.1988.0045. [DOI] [PubMed] [Google Scholar]
- MUNCK B.G., MUNCK L.K., RASMUSSEN S.N., POLACHE A. Specificity of the imino acid carrier in rat small intestine. Am. J. Physiol. 1994;266:R1154–R1161. doi: 10.1152/ajpregu.1994.266.4.R1154. [DOI] [PubMed] [Google Scholar]
- NACHER A., POLACHE A., MOLL-NAVARRO M.J., PLA-DELFINA J.M., MERINO M. Intestinal absorption pathway of γ-aminobutyric acid in rat small intestine. Biopharm. Drug Disposition. 1994a;15:359–371. doi: 10.1002/bdd.2510150503. [DOI] [PubMed] [Google Scholar]
- NACHER A., POLACHE A., MOLL-NAVARRO M.J., PLA-DELFINA J.M., MERINO M. Influence of γ-aminobutyric acid on baclofen intestinal absorption. Biopharm. Drug Disposition. 1994b;15:371–382. doi: 10.1002/bdd.2510150504. [DOI] [PubMed] [Google Scholar]
- NELSON N. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- RAJENDRAN V.M., BARRY J.A., KLEINMAN J.G., RAMASWAMY K. Proton-gradient dependent transport of glycine in rabbit renal brush-border membrane vesicles. J. Biol. Chem. 1987;262:14974–14977. [PubMed] [Google Scholar]
- RANALDI G., ISLAM K., SAMBUY Y. D-Cycloserine uses an active transport mechanism in the human intestinal cell line Caco-2. Antimicrobial Agents and Chemother. 1994;38:1239–1245. doi: 10.1128/aac.38.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAWLINGS J.M., LUCAS M.L., RUSSEL R.I. Measurement of jejunal surface pH in situ by plastic pH electrode in patients with coeliac disease. Scand. J. Gastro. 1987;22:377–384. doi: 10.3109/00365528709078608. [DOI] [PubMed] [Google Scholar]
- RICHENS A. New drugs for epilepsy: a rapidly changing scene. Acta Neurol. Scand. 1992;140 Suppl:65–70. doi: 10.1111/j.1600-0404.1992.tb04473.x. [DOI] [PubMed] [Google Scholar]
- SMITH Q.R., STOLL J.Molecular characterization of amino acid transporters at the blood-brain barrier Brain Barrier Systems 1999Alfred Benzon Symposium 45, Alfred Benzon Foundation, Copenhagen, Denmark; 303–317.In: Paulson, O.B., Knudsen, G.M. & Moos, T. (eds) [Google Scholar]
- THOMAS J.A., BUSHBAUM R.N., ZIMNIAK A., RACKER E. Intracellular pH measurements in Ehrlich ascites tumour cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2230–2238. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., ARMSTRONG G., HIRST B.H., SIMMONS N.L. D-Cycloserine transport in human intestinal epithelial (Caco-2) cells is mediated by a H+-coupled amino acid transporter. Br. J. Pharmacol. 1995a;115:761–766. doi: 10.1111/j.1476-5381.1995.tb14998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., BROWN C.D.A., HIRST B.H., SIMMONS N.L. Na+-independent, H+-coupled transepithelial β-alanine absorption by human intestinal Caco-2 cell monolayers. J. Biol. Chem. 1993a;268:18438–18441. [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., BROWN C.D.A., HIRST B.H., SIMMONS N.L. L-Alanine absorption in human intestinal cells driven by the proton electrochemical gradient. J. Membr. Biol. 1994;140:143–151. doi: 10.1007/BF00232902. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., COOK M.J., HIRST B.H., SIMMONS N.L. H+-coupled (Na+-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett. 1993b;333:78–82. doi: 10.1016/0014-5793(93)80378-8. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., HIRST B.H., SIMMONS N.L. H+- coupled α-methylaminoisobutyric acid transport in human intestinal Caco-2 cells. Biochim. Biophys. Acta. 1995b;1234:111–118. doi: 10.1016/0005-2736(94)00268-t. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., SIMMONS N.L. The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J. Membr. Biol. 1995c;145:245–256. doi: 10.1007/BF00232716. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., STEVENS B.C. H+-zwitterionic amino acid symport at the brush-border membrane of human intestinal epithelial (Caco-2) cells. Exp. Physiol. 1999;84:275–284. [PubMed] [Google Scholar]
- VIGNOLO L., CUPELLO A., MAINARDI P., RAPALLINO M.V., PATRONE A., LOEB C. Accumulation of labelled gamma-aminobutyric acid into rat brain and brain synaptosomes after i.p. injection. Neurochem. Res. 1992;17:193–199. doi: 10.1007/BF00966799. [DOI] [PubMed] [Google Scholar]
- WENZEL U., GEBERT I., WEINTRAUT H., WEBER W.M., CLAUSS W., DANIEL H. Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PepT1 and in human intestinal Caco-2 cells. J. Pharmacol. Exp. Ther. 1996;277:831–839. [PubMed] [Google Scholar]


