Abstract
Prostanoid receptors mediating inhibition of anti-IgE induced histamine release from rat peritoneal mast cells have been characterized pharmacologically. PGD2 and the specific DP receptor agonists BW 245C and ZK 118182 were the most potent inhibitors with half-maximal concentrations of 0.26, 0.06 and 0.02 μM respectively. The maximum inhibition attainable was 60–65% with 10−5 M BW 245C and ZK 118182.
Among several EP receptor agonists investigated, only PGE2 and the EP2/EP3 receptor agonist misoprostol induced significant inhibition (46.8±4.7% at 10−4 M and 18.7±6.8% at 10−5 M respectively). The IP receptor agonists cicaprost and iloprost were both less potent than the DP agonists in inhibiting histamine release (45.2±3.3% and 35.1±2.5% inhibition respectively at 10−5 M), whereas PGF2α and the TP receptor agonist U-46619 were only marginally effective.
The EP4/TP receptor antagonist AH 23848 failed to affect the inhibitory actions of PGD2 or PGE2 even at 10−5 M, whereas the DP/EP1/EP2 receptor antagonist AH 6809 slightly enhanced the effect of PGD2 at 10−6 M.
At concentrations of 3×10−6 to 10−5 M, the putative DP receptor antagonist ZK 138357 dose-dependently suppressed the inhibitory activities of the DP agonists, PGE2 and cicaprost. The antagonism of ZK 138357 against the DP receptor agonists appeared to be competitive with pA2 values of around six.
In conclusion, these data support our earlier proposal that an inhibitory DP receptor is the predominant prostanoid receptor in rat peritoneal mast cell. The properties of this receptor in relation to putative DP receptor subtypes reported in the literature are discussed.
Keywords: BW 245C, cicaprost, DP receptor, mast cells, prostaglandin, histamine release
Introduction
Prostanoids, including prostaglandins and thromboxanes, are synthesized via the cyclo-oxygenase pathway from arachidonic acid released from phospholipids in the plasma membrane by phospholipase A2 in response to a wide range of extracellular stimuli. Once synthesized, the prostanoids are quickly released and act as local hormones which modulate cellular functions in various physiological and pathological processes. Prostaglandins of the E and I subclasses make important contributions to the signs and symptoms of inflammatory diseases such as rheumatoid arthritis and asthma (Coleman et al., 1994b; Goodwin, 1991; Giles, 1990). The proinflammatory actions of prostanoids are mainly due to their direct dilating effects on the microvasculature and their synergism with other proinflammatory mediators to augment vascular permeability and sensitize pain receptors (Williams, 1979). However, a number of potential mechanisms for anti-inflammatory effects of prostaglandin, in particular PGEs have been identified, including the suppression of interleukin-1 production (Goodwin, 1991; Giles, 1990; Chouaib et al., 1985) and inhibition of neutrophil activation, superoxide release and leukotriene production (Fantone et al., 1983).
Although functional studies have demonstrated that various prostanoids can modulate mast cell function (Hogaboam et al., 1993; Peachell et al., 1988; Peters et al., 1982; 1992; Wescott & Kaliner, 1981) and previous radioligand binding studies have identified specific binding sites for prostaglandins E2, I2 and D2 in mouse mastocytoma P-815 cells (Hashimoto et al., 1990; Negishi, et al., 1991a,1991b; Oka et al., 1994; Yoshimura et al., 1989) and human basophils (Virgolin et al., 1992), definitive pharmacological characterization of prostanoid receptors on mast cells isolated in situ has not been reported. Five main types of prostanoid receptors, coded DP, EP, FP, IP and TP, have now been identified and their pharmacology has been extensively reviewed by Coleman et al. (1994b). Our preliminary studies (Chan & Lau, 1998) demonstrated that prostanoids in general suppressed histamine release from rat peritoneal mast cells with DP receptor agonists being the most potent. In the present study, we have attempted to characterize in more detail the inhibitory prostanoid receptor(s) in rat peritoneal mast cells by comparing the effects of various prostanoid agonists and antagonists.
Methods
Isolation/incubation medium
Full HEPES-buffered tyrode (FHB) at pH 7.4 containing (in mM): NaCl 137, KCl 2.7, CaCl2 1, NaH2PO4 0.4, N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES), 10, glucose 5.6 and MgCl2 1.2 was used throughout the study.
Purification of rat peritoneal mast cells
Male Sprague-Dawley rats weighing 200–300 g were actively immunized by an intraperitoneal injection of 0.5 ml phosphate-buffered saline containing ovalbumin (0.5 mg), Al(OH)3 (120 mg) and Bordetella pertussis (0.8 I.U.). Three to four weeks later, the sensitized animals were first anaesthetized with ether and then killed by decapitation. Mixed peritoneal cells were collected from each rat by peritoneal lavage with 20 ml of FHB supplemented with 1 mg ml−1 of bovine serum albumin (BSA-FHB). The cells were washed twice in ice-cold BSA-FHB by centrifugation (190×g, 4°C, 5 min), resuspended in 1 ml of ice-cold FHB, and then mixed with 4 ml of isotonic Percoll solution (SG=1.017) in calcium-free FHB. Mast cells were purified by centrifugation (190×g, 4°C, 25 min) through the continuous density gradient generated by the Percoll and were pelleted at the bottom of the tube. Residual Percoll was eliminated by two washes in BSA-FHB and a final wash in FHB. The purified mast cells were finally resuspended in the required amount of FHB.
Cell incubation and histamine assay
Four hundred μl of purified mast cells, which had been equilibrated in FHB at 37°C for 10 min, were added to 50 μl of FHB with or without a prostanoid. Fifty μl of anti-rat-IgE was added either at the same time as the cells or after the cells had been incubated with the prostanoid for 5 min. When the effects of prostanoid receptor antagonists were investigated, mast cells were first incubated with ibuprofen (10−5 M) for 10 min. Ibuprofen-treated cells were then mixed with the antagonist, agonist and anti-IgE at the same time except in experiments which evaluated the effects of preincubation. The cells were further incubated at 37°C for 10 min after the addition of anti-IgE and the reaction was terminated by the addition of ice-cold FHB. The supernatant and cell pellet were then separated by centrifugation (190×g, 4°C, 5 min). The cell pellet was resuspended in buffer and heated to 80°C for 15 min to liberate the residual histamine. The histamine contents of the supernatant and the cell pellet were determined spectrofluorometrically using a Hitachi F-4010 fluorescence spectrophotometer by the method of Shore et al. (1959).
Drugs
The following prostanoid analogues are gifts from the sources indicated. Cicaprost, iloprost, sulprostone, ZK 118182 ((5Z, 13E) -(9R, 11R, 15S) -9-chloro-15-cyclohexyl-15-hydroxy-16, 17, 18, 19, 20-pentanor-3-oxa-5, 13-prostadienoic acid) and ZK 138357 ((5Z)-7-{( 2RS,4S,5S )-2-(2-chlorophenyl) -5-[(1E)-(3RS)-3-hydroxy -3- cyclohexyl -1- propenyl ] -1, 3-dioxolan-4-yl} -5-heptanoic acid) were from Schering AG, Germany. Butaprost was from Bayer, U.K. AH 23848 ([(±) 1α (Z), 2β, 5α,] -7 -[5 - [ [ (1, 1′ - biphenyl) - 4-yl] methoxy] - 2 - (4-morpholinyl)-3-oxocyclopentyl] -4-heptenoic acid), AH 6809 (6-isopropoxy-9-oxoxanthene-2-carboxylic acid), BW 245C (5-(6-carboxyhexyl)- 1 - (3 - cyclohexyl - 3 - hydroxypropyl) hydantoin) were from Glaxo-Wellcome, U.K. Misoprostol, SC-46275 (methyl 7-[ 2- [ 6-(1-cyclopenten-1-yl) -4R-hydroxy-4-methyl-1E, 5E-hexadienyl] -3- hydroxy -5- oxo-1R , 1-cyclopentyl]-4Z-heptenoate) were from Searle Research and Development, Skokie, U.S.A. PGD2, 13,14-dihydro-15-keto PGD2, PGE2, 9α,11β-PGF2, PGF2α and U-46619 were purchased from Cayman Chemicals. Anti-rat IgE was purchased from ICN Biomedicals. All other reagents were purchased from Sigma Chemicals, St. Louis, MO, U.S.A.
Dissolution and storage of drugs
With the exception of ZK 118182, which was dissolved in distilled water at concentration of 0.1 mM, all stock solutions of prostanoids were prepared at 10 mM or 100 mM (PGE2) in ethanol and were freshly diluted to desired working concentration in buffer upon use. Since ethanol at concentrations 40.1% significantly suppresses both spontaneous and anti-IgE induced histamine release, the maximum concentrations of prostanoids tested were limited to 10−5 or 10−4 M (PGE2).
Data analysis
In each experiment, the spontaneous histamine release in buffer alone was subtracted from all measurements. The percentage inhibition of anti-IgE induced histamine release (% Inhibition) was calculated from {(c - a)/c} × 100%, where c is the control anti-IgE induced histamine release in buffer and a is the anti-IgE induced histamine release in the presence of a prostanoid. All data are mean±standard error of mean (s.e.mean) for n independent observations and statistical analyses were performed using the Student's t-test. Concentrationinhibition curves were analysed by least squares nonlinear iterative regression with the ‘Prism' curve fitting programme (GraphPad software, San Diego, CA, U.S.A.) using a four-parameter logistic equation of the form:
where Y is the observed % inhibition, Ymax and Ymin are the maximum and minimum % inhibition, X is the logarithmic value of the drug concentration. EC50 is the concentration that gives a response halfway between Ymax and Ymin. In general, Ymin was fixed at 0 while the remaining parameters were determined from each independently fitted concentration-inhibition curve to generate average parameter estimates for each group of curves. These average estimates were used for the fitting of the final curves illustrated in the figures.
For the computation of the antagonist affinity of ZK 138357 against the DP agonists, the control concentration-inhibition curve for the effect of the prostanoid agonist alone was first fitted with Ymin fixed at 0 to produce estimates for Ymax, EC50 and Hill slope in the absence of ZK 138357. Assuming that ZK138357 inhibited the effects of prostanoid agonists competitively, all the concentration-inhibition curves in the presence of increasing concentrations of ZK 138357 from the same experiment should share the same Ymax as the control curve and were fitted accordingly to obtain estimates for the corresponding EC50 and Hill slope parameters. The mean values of Ymax, EC50 and Hill slope were then used to construct the final curves in Figure 6. For the calculation of pA2 values of ZK 138357 at single concentrations in an experiment, EC50 values estimated for the control and ZK 138357 affected curves from the same experiment were used in the equation:
where EC50A and EC50B are the agonist EC50 values in the presence of the antagonist and in buffer alone respectively, and [ZK 138357] is the molar concentration of ZK 138357. Results from all the experiments were then used to calculate the mean values listed in Table 2. Since maximum inhibition was not achieved with cicaprost and PGE2, best fitted concentration-inhibition curves generated by the Prism programme using the averaged data were illustrated in Figure 7.
Figure 6.
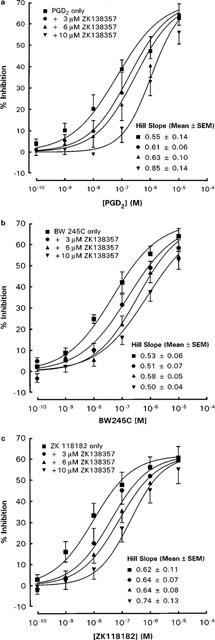
Effects of ZK 138357 on the inhibitory actions of (a) PGD2, (b) BW 245C and (c) ZK 118182 on anti-IgE induced histamine release from rat peritoneal mast cells. Mast cells were exposed simultaneously to ZK 138357, anti-IgE and the DP agonist. Spontaneous histamine release was 9.3±1.0, 10.8±0.8 and 9.1±1.0% whereas anti-IgE induced histamine release was 26.7±3.3, 26.4±3.3 and 28.5±4.0% for the PGD2 (n=6), BW 245C (n=6) and ZK 118182 (n=7) experiments respectively. Results are given as means±s.e.mean for the number of experiments indicated.
Table 2.
Calculated pA2 values for ZK 138357 against DP receptor agonists, PGE2 and cicaprost
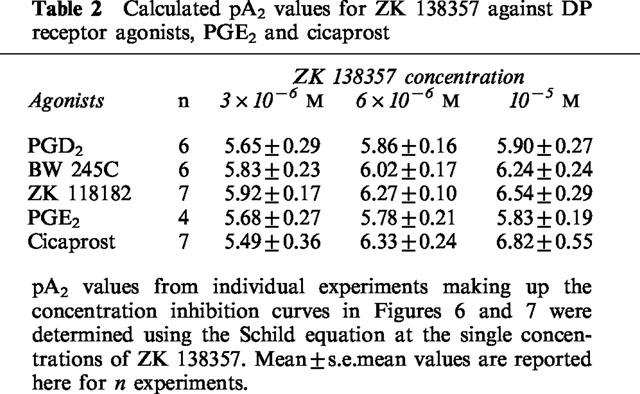
Figure 7.
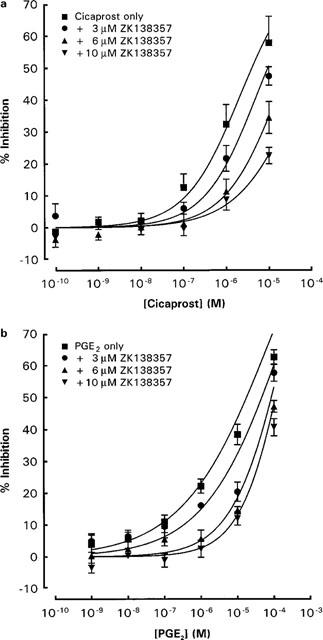
Effects of ZK 138357 on the inhibitory actions of (a) PGE2 and (b) cicaprost on anti-IgE induced histamine release from rat peritoneal mast cells. Mast cells were exposed simultaneously to ZK 138357, anti-IgE and the prostanoid agonist. Spontaneous histamine release was 12.2±0.9 and 11.2±0.9% whereas anti-IgE induced histamine release was 29.0±2.3 and 30.4±2.6% for the PGE2 (n=4) and cicaprost (n=7) experiments respectively. Results are given as means±s.e.mean for the number of experiments indicated.
Results
Establishing conditions for immunological release of histamine
Not more than 15% of total cellular histamine was released spontaneously by rat peritoneal mast cells incubated in buffer alone and none of the prostanoid analogues tested affected this spontaneous release. The anti-IgE challenge to the cells was adjusted so that histamine release was approximately 30% of total cellular histamine above the spontaneous level. Since the inhibitory effects of prostanoids on anti-IgE induced histamine release were not affected by pretreating the cells with ibuprofen (as illustrated in Figure 1 for the DP agonists), mast cells were not preincubated with ibuprofen in experiments screening for agonist activities in order to reduce the total cell incubation period. Furthermore, we had previously reported that endogenously produced prostanoids did not affect spontaneous and anti-IgE induced histamine release from rat peritoneal mast cells (Lau & Stenton, 1998). However, in experiments studying the effects of prostanoid receptor antagonists, potential interaction between endogenously synthesized prostanoids and the antagonists was eliminated by preincubating the mast cells with 10−5 M of ibuprofen for 10 min.
Figure 1.
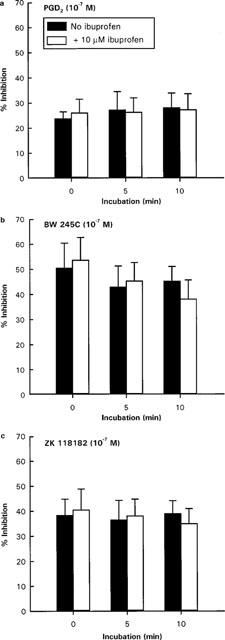
Effects of ibuprofen on (a) PGD2, (b) BW 245C and (c) ZK 118182 induced inhibition of anti-IgE stimulated histamine release from rat peritoneal mast cells. Cells were preincubated with ibuprofen (10−5 M) for 0, 5 or 10 min prior to the addition of DP agonist and anti-IgE. Spontaneous histamine release was 11–15% and anti-IgE induced histamine release was 29–36% above spontaneous level. Results are mean±s.e.mean for n=4.
Effects of DP receptor agonists and PGD2 metabolites
PGD2 and the two potent selective DP receptor agonists BW 245C and ZK 118182 caused concentration-dependent (10−9–10−5 M) inhibition of anti-IgE induced histamine release from rat peritoneal mast cells with similar levels of inhibition (56.5±3.7, 65.9±4.8 and 60.6±3.7% respectively) at the maximum concentration (10−5 M) tested (Figure 2a). When the concentrations which produced 30% inhibition (IC30) were compared (Table 1), ZK 118182 and BW 245C were significantly more potent than PGD2. Two metabolites of PGD2, 9α,11β-PGF2 and 13,14-dihydro-15-keto PGD2, were significantly less potent than PGD2 with maximum inhibitions of 42.0±6.8 and 13.4±5.8% respectively at 10−5 M. The inhibitory effects of PGD2 were similar when it was added with anti-IgE and when it was added 5 min before anti-IgE challenge (Figure 2a); similar results were obtained for the other prostanoids (data not shown). Hence all subsequent studies were performed without prior preincubation of cells with prostanoids to minimize degradation of the natural prostanoids.
Figure 2.
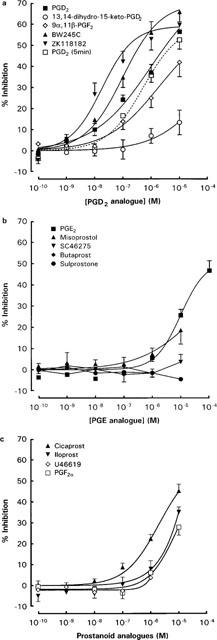
Concentration-inhibition curves for the effects of (a) PGD2 analogues (b) PGE2 analogues and (c) prostacyclin analogues, PGF2α and U-46619 on anti-IgE-induced histamine release from rat peritoneal mast cells. Spontaneous release of histamine ranged from 8.0 to 13.0%, whereas anti-IgE induced histamine release ranged from 24.0 to 35.8%. All data are means±s.e.mean for n experiments as indicated in Table 1.
Table 1.
Comparison of the inhibitory potencies of prostanoid analogues on anti-IgE induced histamine release from rat peritoneal mast cells
Effects of selective EP and IP agonists, PGF2α and U-46619
Among the various EP receptor agonists tested, only PGE2 and the EP2/EP3 receptor agonist, misoprostol caused significant inhibition of histamine release from anti-IgE activated rat peritoneal mast cells at concentrations higher than 10−7 M (Figure 2b). The EP1/EP3 agonist sulprostone and the selective EP3 agonist SC-46275, as well as the EP2 agonist butaprost, all had little effect even at 10−5 M. Dose-dependent inhibition of anti-IgE induced histamine release was also observed with the IP agonists cicaprost and iloprost at concentrations higher than 10−8 M (Figure 2c). Both PGF2α and the TP receptor agonist U-46619 induced minimal inhibition of anti-IgE induced histamine release from rat peritoneal mast cells at concentrations up to 10−6 M. 10−5 M of PGF2α produced 28.0±3.9% inhibition, whereas higher concentrations of U-46619 were not tested.
Effects of prostanoid antagonists
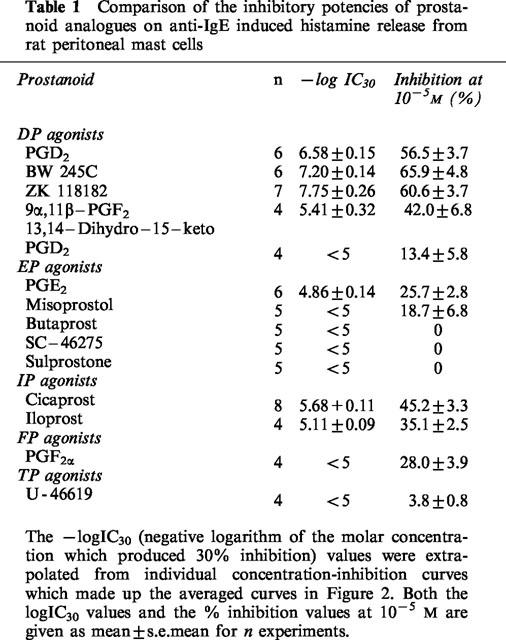
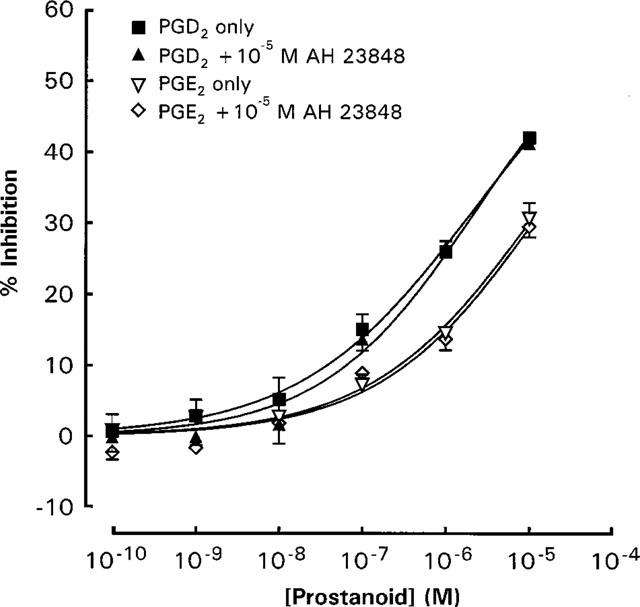
The EP4 antagonist AH 23848 at 10−5 M was without effect on the inhibitory activity of PGE2 and PGD2 (Figure 3). The DP/EP1/EP2 receptor antagonist AH 6809 at 10−7 M did not affect the inhibitory effect of PGD2, whereas at 10−6 M the PGD2 log concentration-response curve was shifted to the left by about one log unit (Figure 4). Increasing the preincubation period for 10−7 M of AH 6809 to 10 min before the addition of PGD2 and anti-IgE did not significantly alter the effect of AH 6809 (Figure 5).
Figure 3.
Effects of 10−5 M AH 23848 on the inhibitory actions of PGD2 and PGE2 on anti-IgE induced histamine release from rat peritoneal mast cells. Ibuprofen (10−5 M) -treated mast cells were exposed simultaneously to anti-IgE, the prostaglandin and AH 23848. Spontaneous histamine release was 12.2±0.9 and 11.2±0.9%, whereas anti-IgE induced histamine release were 29.0±2.3 and 30.4±2.6% for the PGD2 and PGE2 experiments respectively. Results are given as means±s.e.mean for n=4.
Figure 4.
Effects of AH 6809 on the inhibitory actions of PGD2 on anti-IgE induced histamine release from rat peritoneal mast cells. Ibuprofen (10−5 M) treated mast cells were exposed to anti-IgE and PGD2 together with AH 6809. Spontaneous release of histamine was 13.9±1.1% whereas anti-IgE induced histamine release was 31.9±3.3%. Results are given as means±s.e.mean for n=4.
Figure 5.
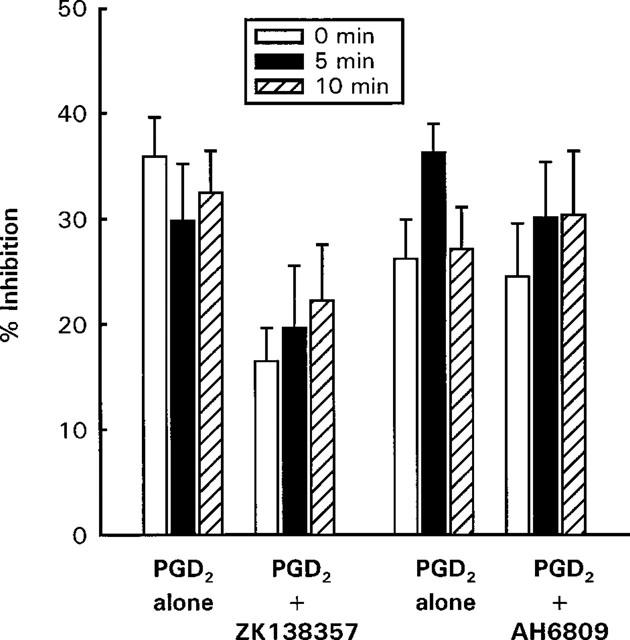
Effects of preincubation with ZK 138357 (10−5 M) and AH 6809 (10−7 M) on the inhibitory actions of PGD2 (10−6 M) on anti-IgE induced histamine release from rat peritoneal mast cells. Ibuprofen (10−5 M) treated mast cells were exposed to anti-IgE and PGD2 after 0, 5 or 10 min preincubation with the DP antagonists. For the ZK 138357 experiments, spontaneous histamine release was 9.8±1.0% (0 min), 11.5±1.1% (5 min) and 11.2±1.1% (10 min), whereas anti-IgE induced histamine release was 33.3±4.6% (0 min), 31.4±2.7% (5 min) and 31.6±3.1% (10 min). The corresponding values for the AH 6809 experiments were 13.9±1.1, 13.3±0.8, 13.9±1.5, 31.9±3.3, 31.7±3.0 and 31.8±4.4%. Results are given as means±s.e.mean for n=4.
The putative DP receptor antagonist ZK 138357, at concentrations of 3×10−6, 6×10−6 and 10−5 M caused progressive rightward shifts of dose-inhibition curves for PGD2, ZK 118182, BW 245C, PGE2 and cicaprost (Figures 6 and 7). As with AH 6809, increasing the preincubation period with cells did not significantly alter the antagonist potency of ZK 138357 against PGD2 (Figure 5). Student t-test analysis comparing the Hill slopes of the dose-inhibition curves of the three DP agonists in the presence of increasing concentrations of ZK 138357 with that of the corresponding control curve showed no significant difference and there was little evidence of suppression of the maximum response. This profile is consistent with competitive antagonism. Use of the Schild equation gave comparable pA2 values of about six for ZK 138357 versus the three DP agonists (Table 2). Full dose-inhibition curves in the presence of ZK 138357 were not obtained for PGE2 and cicaprost due to their lower potencies. However, the available portions of the inhibition curves appear to be parallel to the corresponding control curves (Figure 7), and the pA2 values calculated from extrapolated EC50 values are similar to those obtained for the DP agonists (Table 2).
In order to examine the specificity of ZK 138357, the effect of the antagonist against inhibition of immunologically induced histamine release by the two well documented mast cell stabilizers, disodium cromoglycate (DSCG) and theophylline was studied. As illustrated in Figure 8, 10−5 M of ZK 138357 did not affect the inhibitory effects of DSCG and theophylline.
Figure 8.
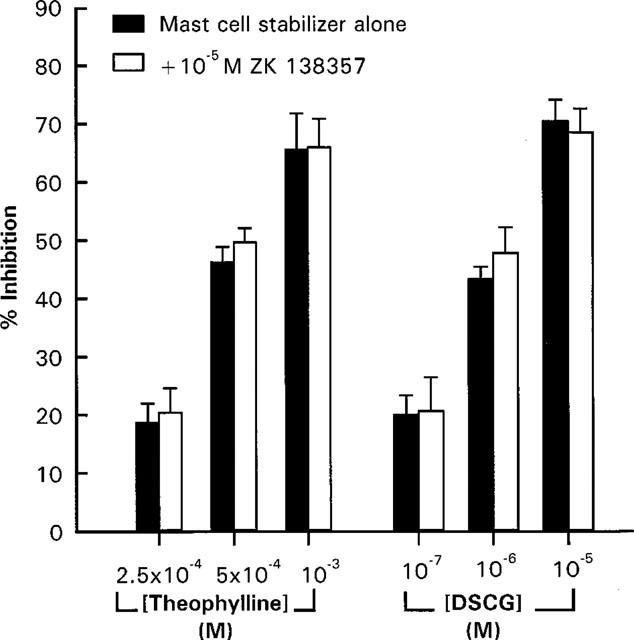
Effects of ZK 138357 on the inhibitory actions of theophylline and disodium cromoglycate (DSCG) on anti-IgE induced histamine release from rat peritoneal mast cells. Mast cells were exposed simultaneously to ZK 138357, anti-IgE and the mast cell inhibitor. Spontaneous histamine release was 9.9±0.2% whereas anti-IgE induced histamine release was 36.3±1.6%. Results are given as means±s.e.mean for n=5.
Discussion
Our studies on the rat peritoneal mast cell have shown that prostanoid agonists covering the five prostanoid receptor types could inhibit histamine release, and no evidence for excitatory actions was obtained. Thus, none of the prostanoids increased the release of histamine in the resting state; this included PGE2, PGF2α and the TXA2 mimetic U-46619, all of which are known to activate excitatory prostanoid receptors (EP1, EP3, FP and TP) in other systems (see Coleman, 1998; Coleman et al., 1994b). These findings agree with earlier reports on rat and human mast cells (Albro et al., 1972; Peters et al.,1982; Takei & Endo,1994; Wescott & Kaliner, 1981). In BNu-2cl3 cultured mouse mast cells however, there is the potential for excitatory actions through both EP3 receptors and novel IP receptors linked to phosphoinositide metabolism via pertussis toxin-sensitive G proteins (Oka et al., 1993). The absence of excitatory EP receptors in rat peritoneal mast cells is further confirmed by our observation that the EP1/EP3 agonist, sulprostone (Bunce et al., 1991) and the highly potent and selective EP3 agonist SC-46275 (Savage et al., 1993) did not alter spontaneous or immunologically-induced histamine release.
The ranking of inhibitory potencies on the rat peritoneal mast cell indicates the presence of a DP-receptor (Table 1). First, PGD2 is active at concentrations as low as 10 nM and is considerably more potent than PGF2α and U-46619. Secondly, two purportedly selective DP-receptor agonists were at least five times more potent than PGD2. BW 245C is an older agent previously shown to be slightly more potent than PGD2 in activating adenylate cyclase in rat peritoneal mast cells (Narumiya & Toda, 1985). ZK 118182 is a newer prostanoid which is more potent then PGD2 in raising cyclic AMP levels in human platelets (Darius et al., 1994) and suppressing superoxide production in human neutrophils (Pons et al., 1994). Thirdly, the DP-receptor antagonist ZK 138357 (Lydford et al., 1996) blocks the action of PGD2, BW 245C and ZK 118182 to similar extents. The preferred DP antagonist in this type of study would be BW A868C because of its high affinity (pA2=8.5–9.3) and specificity (Giles et al., 1989). Unfortunately this compound is not available for general use, and instead we have been obliged to use ZK 138357, which has some chemical resemblance to BW A868C but is a somewhat less potent DP blocker. ZK 138357 appeared to act specifically in our experiments, with the highest concentration used (10−5 M) having no effect on inhibition of histamine release due to either the anti-allergic compound disodium cromoglycate or the phosphodiesterase inhibitor theophylline. K.H. Thierauch and colleagues have reported that ZK 138357 has a high affinity for the DP receptor on human platelets (pA2=7.7), but has no affinity for other prostanoid receptors (unpublished abstracts of 9th International Conference in Prostaglandins and Related Compounds, Florence, Italy, 1994).
Another DP antagonist AH 6809 (pA2=5.0; Keery & Lumley, 1988) was tested in our system in the knowledge that it also blocks EP1-receptors (pA2=7.1; Coleman et al., 1985; Lawrence et al., 1992) and EP2-receptors (pA2=6.5; Regan et al., 1994). In our experiments, it had no effect on PGD2-induced inhibition at 10−7 M and enhanced the inhibition at 10−6 M. The latter effect may be due to its ability to inhibit cyclic-AMP-phosphodiesterase, as found in rat lung mast cells (Keery & Lumley, 1988). Thus it was impossible to determine whether AH 6809 has significant DP blocking activity in the present study.
Since the concentration-inhibition curves of the prostanoid receptor agonists in general have Hill slopes of less than one, the observed inhibitory effects might be mediated by more than one receptor. Hence the question remains as to whether inhibitory EP or IP receptors are present on the rat peritoneal mast cell. Activation of EP2 and/or EP4 receptors (linked to adenylate cyclase) could account for the inhibitory activity of PGE2, and in this context, binding sites for PGE1 and PGE2 have been identified in mouse mastocytoma P815 cells (Hashimoto et al., 1990; Nishigaki et al., 1995). Furthermore, both prostaglandin D2 and BW245C were reported to produce a BW868C resistant phase of agonism in rabbit jugular vein which might be mediated through EP2 receptors (Giles et al., 1989). However, we favour the alternative explanation that PGE2 is a low potency DP agonist, based on two pieces of evidence. Firstly, ZK 138357 blocked the action of PGD2 and PGE2 to similar extent. Secondly, the selective EP2 agonist butaprost did not inhibit histamine release, and the EP4 antagonist AH 23848 (Coleman et al., 1994a) failed to affect the inhibitory activities of either PGD2 or PGE2. The relatively flat agonist concentration-inhibition curves may be due to the fact that we are measuring an indirect physiological consequence of receptor activation instead of measuring the immediate biochemical change induced by receptor activation. Actually, when we monitored the effects of PGD2 on intracellular cyclic AMP level, a concentration-response curve with a Hill slope of near unity was observed (data not shown).
At present, the best chance of pharmacologically identifying an IP-receptor lies with the prostacyclin analogue cicaprost, due to its high potency and specificity (Dong et al., 1986; Lawrence et al., 1992). For example, it inhibits human platelet aggregation over the 0.1–1 nM concentration range (Armstrong et al., 1989) and relaxes vascular smooth muscle between 1 and 10 nM (see Jones et al., 1997). On other prostanoid systems we have come to expect cicaprost to be at least 100 times less potent than the appropriate standard agonist (e.g. PGE2 on EP receptors). In the present study, cicaprost was a fairly weak agonist in absolute terms (−logIC30=5.68), but was only less potent than PGD2 by around 1 log unit. However, ZK 138357 blocked cicaprost with an affinity similar to that found with PGD2 as agonist, suggesting that cicaprost is indeed a DP agonist on rat peritoneal mast cells.
Although there is no formal inclusion of DP-receptor subtypes in the current prostanoid receptor classification scheme (Coleman, 1998), there is some evidence in the literature for their existence. The first indication came from the work of Jones (1976; 1978) on the vasoconstrictor actions of PGD2 in the sheep, rabbit, pig and dog. This receptor system differed from the classical (adenylate cyclase-linked) DP-receptor in the human platelet in that a ‘natural' 15(S)-15-hydroxy substituent in the ω-chain was not essential to high biological potency. PGD2, 15(R) PGD2, 13,14-dihydro-15-keto PGD2 and 15-O-methyl PGD2 were all potent vasoconstrictors, and considerably more potent than the corresponding PGF2α analogues. However, only PGD2 showed inhibitory activity on human platelets. It is of interest that Rangachari & Betti (1993) were able to provisionally detect both DP-receptor subtypes in a single preparation, the dog isolated colonic epithelium. Short-circuit current (a measure of active Cl− secretion) was usually increased by PGD2 and always by 9α,11β PGF2 (classical DP-receptor), whereas short-circuit current was decreased occasionally by PGD2 and always by 13,14-dihydro-15-keto PGD2 (non-classical DP-receptor). It is clear from our results on the rat peritoneal mast cell that 9α,11β PGF2 is a moderately potent agonist and 13,14-dihydro-15-keto PGD2 a very weak agonist, and this argues against the presence of the non-classical DP-receptor.
Narumiya & Toda (1985) have argued for the existence of three subtypes of DP-receptor. The first of these, in guinea-pig trachea and dog cerebral artery, was difficult to define as a DP-receptor, since the two most active excitatory agonists tested, PGD2 and its 17-phenyl-ω-trinor analogue, were not particularly potent (⩾100 nM required for contraction). Furthermore, both preparations are known to be highly sensitive to the contractile actions of TP agonists and a TP antagonist was not used in the study. In addition, the guinea-pig trachea contains an excitatory EP1 system, on which the corresponding 17-phenyl-ω-trinor derivative of PGE2 is a highly potent agonist (EC50∼2 nM) (Lawrence et al., 1992). Activation of the second putative DP-receptor inhibited the growth of the mouse L-1210 leukaemia cell line and 9-deoxy-Δ9,12 PGD2 was about three times more potent than PGD2 (Fukushima et al., 1982; Kikawa et al., 1984). The problem here is that micromolar concentrations of PGD analogues are required, and there is the possibility of a non-prostanoid receptor mechanism whereby an enone unit in or generated from the PGD molecule covalently interacts with a component of the growth regulatory system. The remaining receptor, which was present in the tissue under study here, rat peritoneal mast cells, did indeed appear to be a DP receptor. The available, although limited, biological data for PGD2, BW 245C and ZK 118182 acting on DP systems are shown in Table 3. The three human systems show reasonable agreement for agonist potencies, bearing in mind that the human platelet data are from a biochemical as opposed to a functional assay. On the rabbit and rat preparations, BW 245C appears to be somewhat less potent, but the differences are too small to conclude even tentatively the existence of DP receptor subtypes, and species variants would seem more likely.
Table 3.
Comparison of the potencies of PGD2, BW 245C and ZK 118182 in different isolated preparations
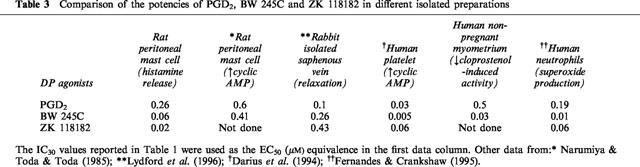
The pA2 value of around 6 for ZK 138357 estimated in the current study is intermediate between the two previously reported pA2 values of 7.25 in human PMN leukocytes and 5.09 in the rabbit isolated saphenous vein for blocking the actions of BW 245C (Lydford et al., 1996). Although these values were only estimated by using a single concentration of the antagonist and hence may represent extremes of estimation, the authors postulated that they may implicate the presence of DP receptor subtypes in the two systems. This is worthy of further investigation, although it is relevant to consider the work of Jones and co-workers on TP-receptors in smooth muscle and platelets (Tymkewycz et al., 1991). Very good agreement was obtained between agonist potencies across both species and preparations, but the affinities of a range of structurally different antagonists varied considerably and no firm conclusions could be drawn about TP-receptor subtypes.
In conclusion, the current study has positively demonstrated that a PGD2-specific receptor is the predominant prostanoid receptor in rat peritoneal mast cells. This receptor is potently activated by ZK 118182 and blocked, probably competitively, by the related compound ZK 138357. Since mast cell activation is an integral part of allergic reactions, development of highly specific agonists for the mast cell DP receptor may be useful in the management of allergic diseases such as asthma, providing that similar observations in human mast cells are obtained in future studies.
Acknowledgments
This work was supported by grants from the University Grant Committee of Hong Kong. The generous gifts of drugs by companies listed in the Methods section are gratefully acknowledged.
Abbreviations
- BSA-FHB
full HEPES buffered tyrode supplemented with 1 mg/ml of bovine serum albumin
- DSCG
disodium cromoglycate
- FHB
full HEPES-buffered tyrode
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- IC30
the prostanoid concentration which produced 30% inhibition
- −logIC30
negative logarithm of the molar concentration which produced 30% inhibition
References
- ALBRO P., THOMAS R., FISHBEIN L. Prostaglandins: action on mast cells in vitro. Prostaglandins. 1972;1:133–144. doi: 10.1016/0090-6980(72)90076-7. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG R.A., LAWRENCE R.A., JONES R.L., WILSON N.H., COLLIER A. Functional and ligand binding studies suggest heterogeneity of platelet prostacyclin receptors. Br. J. Pharmacol. 1989;97:657–668. doi: 10.1111/j.1476-5381.1989.tb12001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUNCE K.T., CLAYTON N.M., COLEMAN R.A., COLLINGTON E.W., FINCH H., HUMPHRAY J.M., HUMPHREY P.P.A., REEVES J.J., SHELDRICK R.L.G., STABLES R. GR63799X–a novel prostanoid with selectivity for EP3 receptors. Adv. Prostaglandin Thromboxane Leukotriene Res. 1991;21:379–382. [PubMed] [Google Scholar]
- CHAN C.L., LAU H.Y.A. Effects of prostanoid receptor agonists on immunologically activated rat peritoneal mast cells. Inflamm. Res. 1998;47:S20–S21. doi: 10.1007/s000110050247. [DOI] [PubMed] [Google Scholar]
- CHOUAIB S., WELTE K., MERTELSMANN R., DUPONT B. Prostaglandin E2 acts at two distinct pathways of lymphocyte activation: inhibition of interleukin 2 production and downregulation of transferrin receptor expression. J. Immunol. 1985;135:1172–1179. [PubMed] [Google Scholar]
- COLEMAN R.A.Prostanoids receptors The IUPHAR Compendium of Receptor Characterization and Classification 1998Burlington Press: Cambridge; 229–244.In: Girdlestone D. (ed.) [Google Scholar]
- COLEMAN R.A., DENYER L.H., SHELDRICK R.L.The influence of protein binding on the potency of the prostanoid EP1-receptor blocking drug AH 6809 Br. J. Pharmacol. 198586p. 203 [Google Scholar]
- COLEMAN R.A., GRIX S.P., HEAD S.A., LOUTTIT J.B., MALLETT A., SHELDRICK R.L. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994a;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution and structure of the receptors and their subtypes. Pharmacol. Rev. 1994b;46:205–229. [PubMed] [Google Scholar]
- DARIUS H., MICHAEL-HEPP J., THIERAUCH K.H., FISCH A. Inhibition of human platelets and polymorphonuclear neutrophils by the potent and metabolically stable prostaglandin D2 analog ZK 118182. Eur. J. Pharmacol. 1994;258:207–213. doi: 10.1016/0014-2999(94)90482-0. [DOI] [PubMed] [Google Scholar]
- DONG Y.J., JONES R.L., WILSON N.H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable prostacyclin analogues. Br. J. Pharmacol. 1986;87:97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANTONE J.C., MARASCO W.A., ELGAS L.J., WARD P.A. Anti-inflammatory effects of prostaglandin E1: in vivo modulation of the formyl peptide chemotactic receptor on the rat neutrophil. J. Immunol. 1983;130:1495–1497. [PubMed] [Google Scholar]
- FERNANDES B., CRANKSHAW D. Functional characterization of the prostanoid DP receptor in human myometrium. Eur. J. Pharmacol. 1995;283:73–81. doi: 10.1016/0014-2999(95)00288-v. [DOI] [PubMed] [Google Scholar]
- FUKUSHIMA M., KATO T., OTA K., ARAI Y., NARUMIYA S., HAYAISHI O. 9-Deoxy-Δ9-prostaglandin D2, a prostaglandin D2 derivative with potent anti-neoplastic and weak smooth muscle-contracting activities. Biochem. Biophys. Res. Commun. 1982;109:626–633. doi: 10.1016/0006-291x(82)91986-6. [DOI] [PubMed] [Google Scholar]
- GILES H. More selective ligands at eicosanoid receptor subtypes improve prospects in inflammatory and cardiovascular research. Trends Pharmacol. Sci. 1990;11:301–305. doi: 10.1016/0165-6147(90)90224-v. [DOI] [PubMed] [Google Scholar]
- GILES H., LEFF P., BOLOFO M.L., KELLY M.G., ROBERTSON A.D. The classification of prostaglandin D2 DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br. J. Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN J.S. Are prostaglandins proinflammatory, anti-inflammatory, both or neither. J. Rheumatol. 1991;18 suppl. 28:26–29. [PubMed] [Google Scholar]
- HASHIMOTO H., NEGISHI M., ICHIKAWA A. Identification of a prostacyclin receptor coupled to the adenylate cyclase system via a stimulatory GTP-binding protein in mouse mastocytoma P-815 cells. Prostaglandins. 1990;40:491–505. doi: 10.1016/0090-6980(90)90111-8. [DOI] [PubMed] [Google Scholar]
- HOGABOAM C.M., BISSONNETTE E.T., CHIN B.C., BEFUS D., WALLACE J.L. Prostaglandins inhibit inflammatory mediator release from rat mast cells. Gastroenterology. 1993;104:122–129. doi: 10.1016/0016-5085(93)90843-2. [DOI] [PubMed] [Google Scholar]
- JONES R.L. Sites of action of prostaglandins on the cardiovascular system with particular reference to the sheep. Acta Biol. Med. Germ. 1976;35:1091–1096. [PubMed] [Google Scholar]
- JONES R.L. Definition of prostaglandin-sensitive arterial constrictor systems. Acta Biol. Med. Germ. 1978;37:837–844. [PubMed] [Google Scholar]
- JONES R.L., QIAN Y.M., WONG H.N.C., CHAN H.W., YIM P.C. Prostanoid action on the human pulmonary vascular system. Clin. Exp. Pharmacol. Physiol. 1997;24:969–972. doi: 10.1111/j.1440-1681.1997.tb02730.x. [DOI] [PubMed] [Google Scholar]
- KEERY R.J., LUMLEY P. AH 6809, a prostaglandin DP-receptor blocking drug on human platelets. Br. J. Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIKAWA Y., NARUMIYA S., FUKUSHIMA M., WAKATSUKA H., HAYAISHI O. 9-Deoxy-Δ9, Δ12-13,14-dihydro-prostaglandin D2, a metabolite of prostaglandin D2 formed in human plasma. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1317–1321. doi: 10.1073/pnas.81.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAU H.Y.A., STENTON G.R. Effects of non-steroidal anti-inflammatory drugs and cyclooxygenase-2 specific inhibitors on mediator release from rat peritoneal mast cells. Inflamm. Res. 1998;47:S22–S23. doi: 10.1007/s000110050248. [DOI] [PubMed] [Google Scholar]
- LAWRENCE R.A., JONES R.L., WILSON N.H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br. J. Pharmacol. 1992;105:271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYDFORD S.J., LI S.W., MCKECHINE K.C.W. Comparison of prostanoid DP-receptors in the rabbit isolated saphenous vein and human neutrophil. Br. J. Pharmacol. 1996;117:190P. doi: 10.1111/j.1476-5381.1996.tb15148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARUMIYA S., TODA N. Different responsiveness of prostaglandin D2-sensitive systems to prostaglandin D2 and its analogues. Br. J. Pharmacol. 1985;85:367–375. doi: 10.1111/j.1476-5381.1985.tb08870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEGISHI M., HASHIMOTO H., ICHIKAWA A. Differential regulation of thrombin- or ATP-induced mobilization of intracellular Ca2+ by prostacyclin receptor in mouse mastocytoma cells. Biochem. Biophys. Res. Commun. 1991a;176:102–107. doi: 10.1016/0006-291x(91)90895-e. [DOI] [PubMed] [Google Scholar]
- NEGISHI M., HASHIMOTO H., YATSUNAMI K., KUROZUMI S., ICHIKAWA A. TEI-9063, a stable and highly specific prostacyclin analogue for the prostacyclin receptor in mastocytoma P-815 cells. Prostaglandins. 1991b;42:225–237. doi: 10.1016/0090-6980(91)90112-s. [DOI] [PubMed] [Google Scholar]
- NISHIGAKI N., NEGISHI M., HONDA A., SUGIMOTO Y., NAMBA T., NARUMIYA S., ICHIKAWA A. Identification of prostaglandin E receptor ‘EP2' cloned from mastocytoma cells as EP4 subtype. FEBS Lett. 1995;364:339–341. doi: 10.1016/0014-5793(95)00421-5. [DOI] [PubMed] [Google Scholar]
- OKA M., NEGISHI M., NISHIGAKI N., ICHIKAWA A. Two types of prostacyclin receptor coupling to stimulation of adenylate cyclase and phosphatidylinositol hydrolysis in a cultured mast cell line, BNu-2c13 cells. Cell. Signal. 1993;5:643–650. doi: 10.1016/0898-6568(93)90059-u. [DOI] [PubMed] [Google Scholar]
- OKA M., NEGISHI M., YAMAMOTO T., SATOH K., HIROHASHI T., ICHIKAWA A. Prostacyclin (PGI) receptor binding and cyclic AMP synthesis activities of PGI1 analogues, SM-10906 and its methyl ester, SM-10902, in mastocytoma P-815 cells. Biol. Pharm. Bull. 1994;17:74–77. doi: 10.1248/bpb.17.74. [DOI] [PubMed] [Google Scholar]
- PEACHELL P.T., MACGLASHAN D.W., LICHTENSTEIN L.M., SCHLEIMER R.P. Regulation of human basophil and lung mast cell function by cyclic adenosine monophosphate. J. Immunol. 1988;140:571–579. [PubMed] [Google Scholar]
- PETERS S.P., KAGEY-SOBOTKA A., MACGLASHAN D.W., LICHTENSTEIN L.M. Effect of prostaglandin D2 in modulating histamine release from human basophils. J. Immunol. 1992;228:400–406. [PubMed] [Google Scholar]
- PETERS S.P., SCHULMAN E.S., SCHLEIMER R.P., MACGLASHAN D.W., NEWBALL H.H., LICHTENSTEIN L.M. Dispersed human lung mast cells, pharmacologic aspects and comparison with human lung tissue fragments. Am. Rev. Respir. Dis. 1982;216:1034–1039. doi: 10.1164/arrd.1982.126.6.1034. [DOI] [PubMed] [Google Scholar]
- PONS F., WILLIAMS T.J., KIRK S.A., MCDONDALD F., ROSSI A.G. Pro-inflammatory and anti-inflammatory effects of the stable prostaglandin D2 analogue, ZK 118.182. Eur. J. Pharmacol. 1994;261:237–247. doi: 10.1016/0014-2999(94)90113-9. [DOI] [PubMed] [Google Scholar]
- RANGACHARI P.K., BETTI P.A. Biological activity of metabolites of PGD2 on canine proximal colon. Am. J. Physiol. 1993;264:G886–G894. doi: 10.1152/ajpgi.1993.264.5.G886. [DOI] [PubMed] [Google Scholar]
- REGAN J.W., BAILEY T.J., PEPPERL D.J., PIERCE K.L., BOGARDUS A.M., DONELLO J., FAIRBAIRN C.E., KEDZIE K.M., WOODWARD D.F., GIL D.W. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol. Pharmacol. 1994;46:213–220. [PubMed] [Google Scholar]
- SAVAGE M.A., MOUMMI C., KARABATSOS P.J., LANTHORN T.H. SC-46275: A potent and highly selective agonist at the EP3 receptor. Prostag. Leukotr. Ess. 1993;49:939–943. doi: 10.1016/0952-3278(93)90179-z. [DOI] [PubMed] [Google Scholar]
- SHORE P.A., BURKHALTER A., COHN V.H. A method for the fluorometric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 1959;127:182–186. [PubMed] [Google Scholar]
- TAKEI M., ENDO K. Histamine release and calcium concentration in rat mast cells are dependent on intracellular ATP: effects of prostaglandin D2. Prostag. Leukotr. Ess. 1994;50:357–362. doi: 10.1016/0952-3278(94)90247-x. [DOI] [PubMed] [Google Scholar]
- TYMKEWYCZ P.M., JONES R.L., WILSON N.H., MARR C.G. Heterogeneity of thromboxane A2 (TP-) receptors: evidence from antagonist but not agonist potency measurements. Br. J. Pharmacol. 1991;102:607–614. doi: 10.1111/j.1476-5381.1991.tb12220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRGOLIN I., LI S., SILLABER C., MAJDIC O., SINZINGER H., LECHNER K., BETTELHEIM P., VALENT P. Characterization of prostaglandin (PG)-binding sites expressed on human basophils. J. Biol. Chem. 1992;267:12700–12708. [PubMed] [Google Scholar]
- WESCOTT S., KALINER M. The effects of histamine and prostaglandin D2 on rat mast-cell cyclic AMP and mediator release. J. Allergy Clin. Immunol. 1981;68:383–391. doi: 10.1016/0091-6749(81)90137-8. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br. J. Pharmacol. 1979;65:517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMURA S., MIZUNO Y., KIMURA K., YATSUNAMI K., FUJISAWA J., TOMITA K., ICHIKAWA A. Prostaglandin D2 receptor of mastocytoma P-815 cells - possible regulation by phosphorylation and dephosphorylation. Biochim. Biophys. Acta. 1989;981:69–76. doi: 10.1016/0005-2736(89)90083-7. [DOI] [PubMed] [Google Scholar]


