Abstract
SDZ-RAD, 40-O-(2-hydroxyethyl)-rapamycin, is a novel macrolide immunosuppressant. Because of its synergistic interaction, SDZ-RAD is under clinical investigation as immunosuppressant in combination with cyclosporine after organ transplantation. Neurotoxicity is a critical side-effect of cyclosporine.
We studied the effect of SDZ-RAD and its combination with cyclosporine on high-energy phosphates, phosphocreatine (PCr) and nucleoside triphosphates (NTP), in brain slices using 31P-magnetic resonance spectroscopy (MRS).
Cyclosporine significantly reduced high-energy phosphates after 2 h in a dose-dependent manner (100 μg l−1: 93±3% of control (NTP), 91±3% (PCr); 500 μg l−1: 84±2% (NTP), 73±2 (PCr); 5000 μg l−1: 68±3% (NTP), 55±5% (PCr); n=6; P<0.02).
In contrast, after perfusion for 2 h, SDZ-RAD (500 μg l−1 and 5000 μg l−1) significantly increased high-energy phosphate concentrations in the brain slices (P<0.02). Even at the lowest concentration, SDZ-RAD protected brain energy metabolism against cyclosporine toxicity: 100 μg l−1 SDZ-RAD+5000 μg l−1 cyclosporine: 86±3% (NTP), 83±7% (PCr), n = 3, P<0.03 compared to cyclosporine alone. 5As evaluated using an algorithm based on Loewe isobolograms, the effects of SDZ-RAD/ cyclosporine combinations on brain energy reduction were antagonistic. Both drugs were found in mitochondria using h.p.l.c-MS analysis.
We conclude that cyclosporine inhibits mitochondrial high-energy phosphate metabolism, which can be antagonized by SDZ-RAD.
Keywords: SDZ-RAD; cyclosporine; neurotoxicity; interaction; antagonism, 31P n.m.r.; high-energy phosphate metabolism; rat brain slices
Introduction
The macrolide immunosuppressant SDZ-RAD is the 40-O-(2-hydroxyethyl) derivative of rapamycin. Like rapamycin (INN: sirolimus), the SDZ-RAD/FK-binding protein (FKBP) complex binds in T-lymphocytes to mTOR, the mammalian target of rapamycin. The result is inhibition of the interleukin-2-stimulated phosphorylation activation of p70-kd S6 protein kinase, and blockade of cell cycle progression at the G1-S-interface (Bohler et al., 1998; Schuler et al., 1997). SDZ-RAD synergistically interacts with cyclosporine (Schuurman et al., 1997). Although SDZ-RAD alone prolonged graft survival in a life-supporting kidney transplant animal model, it was shown that addition of cyclosporine significantly improved graft and animal survival. SDZ-RAD in combination with cyclosporine is presently in phase II-III clinical trials as an immunosuppressant after organ transplantation (Schuurman et al., 1998). In addition, SDZ-RAD prevented transplant arteriosclerosis (Cole et al., 1998), inhibited the development of bronchial lesions in a porcine heterotopic bronchial allograft model (Salminen et al., 1998) and was successfully used to treat acute rejection (Hausen et al., 1999).
Cyclosporine (INN; ciclosporin) is the primary agent of most immunosuppressive protocols after organ transplantation and it is also used in the therapy of autoimmune diseases (Kahan, 1989). However, cyclosporine therapy is limited by its side-effects with the most important being: nephrotoxicity, hypertension, hyperlipidemia and neurotoxicity (Kahan, 1989). Neurotoxicity of cyclosporine includes tremor, severe headache, epileptic seizures, paresthesia, cortical blindness, and stroke (Adams et al., 1987; Atkinson et al., 1984; Gijtenbeek et al., 1999; Hauben, 1996). MRI studies of patients treated with cyclosporine have found white matter disorders and gray matter hypoxic damage to be the most common manifestations of cyclosporine neurotoxicity (DeGroen et al., 1987; Goldstein et al., 1998; Hauben, 1996; Gijtenbeek et al., 1999).
In a recent in vitro study, we showed that cyclosporine concentrations at the lower limit of the therapeutic window in transplant patients (100 μg l−1) significantly decreased concentrations of the high-energy phosphates phosphocreatine (PCr) and nucleoside triphosphates (NTP) in rat brain slices, suggesting that energy phosphate metabolism is the metabolic pathway most sensitive to cyclosporine (Serkova et al., 1999). In the same model, rapamycin also reduced the concentrations of high-energy phosphates, while the combination of rapamycin and cyclosporine synergistically inhibited brain energy metabolism (Serkova et al., 1999).
It was our goal to evaluate the effect of the novel macrolide immunosuppressant SDZ-RAD and SDZ-RAD in combination with cyclosporine on high-energy phosphate metabolism in perfused rat brain slices.
Methods
Materials
Cyclosporine and SDZ-RAD were kindly provided by Novartis Pharma AG (Basel, Switzerland). One g l−1 stock solutions of each drug were prepared in distilled water containing 2% (w v−1) methyl cellulose (Sigma Chemicals, St. Louis, MO, U.S.A.). Stock solutions were stored at −80°C until use. In the controls, brain slices were always perfused with medium containing the same amount of drug-free vehicle, methyl cellulose, as in the corresponding study samples. Methyl cellulose at the highest concentration had no effect on brain metabolism. The perfusion medium was standard Krebs BSS, Medium ‘D' (0.1 μm sterile filtered) containing (mM) NaCl 100, KH2PO4 0.1, KCl 6.1, glucose 10, NaHCO3 24, CaCl2 1.2 (anhydrous), MgSO4.7H2O 1.2, and HEPES 25 (pH 7.4). Seven-day-old Wistar rats (12 g weight) were obtained from Charles River, Inc. (Wilmington, MA, U.S.A.). All animal protocols were reviewed and approved by the University of California, San Francisco, Committee on Animal Research.
31P-MRS on perfused rat brain slices
Forty rat brain slices (350 μm thick) were prepared from the cortical region of a total of twelve Wistar rats as described previously (Espanol et al., 1992). Slices were transferred into a 20 mm diameter Wilmad NMR tube (Wilmad Glass Co., Buena, NJ, U.S.A.) and perfused with fresh medium in equilibrium with 95% O2/5% CO2 at 37°C. All 31P-MRS experiments were carried out using a Nalorac QUEST Model 4400 4.7 Tesla MRI animal scanner (Nalorac Inc., Martinez, CA, U.S.A.) at a frequency of 81 MHz for phosphorus nuclei. A one-pulse sequence with the following parameters was used: 45° top angle (27 μs), ±3125 Hz spectral width, 2K data point, 128 accumulations. The total acquisition time for each 31P spectrum was 10 min. Each brain slice preparation was used as its own control. Two hours after slice preparation, allowing for metabolic recovery, brain slices were perfused with cyclosporine or SDZ-RAD or with both drugs in combination. The study drugs were added to the perfusion medium at the following concentrations: 100, 500, and 5000 μg l−1. Brain slices were perfused at each drug concentration for 2 h. After perfusion with the study drugs, the brain slices were again perfused with drug-free medium. Viability of the brain slices and reversibility of the effects on high-energy phosphate metabolism were confirmed by a washout experiment after each exposure to the study drugs.
Chemical shifts were referenced to the phosphocreatine (PCr) peak at −2.33 p.p.m. 31P-MRS signal intensities of phosphorus metabolites were recorded and areas under the peak integrated using the MacFid software (Tecmag Inc., Bellair, TX, U.S.A.). Signals were normalized based on the intensity of the phosphomonoester (PME) signal. The following 31P-MRS signals were integrated and included in the data analysis: phosphomonoesters (PME), intracellular inorganic phosphate (Pi), phosphocreatine (PCr), nucleoside triphosphates (α-, β-, and γ-NTP), and the NAD+/NADH complex (see Figure 1A). Areas under the resonance peaks for all phosphorus metabolites were reproducible with less than 5% variation. Line widths of resonances did not change more than 2% over the course of each experiment. Both drugs and their combinations were found to have no effect on PME, and changes of other 31P-MRS signals are reported relative to the PME peak.
Figure 1.
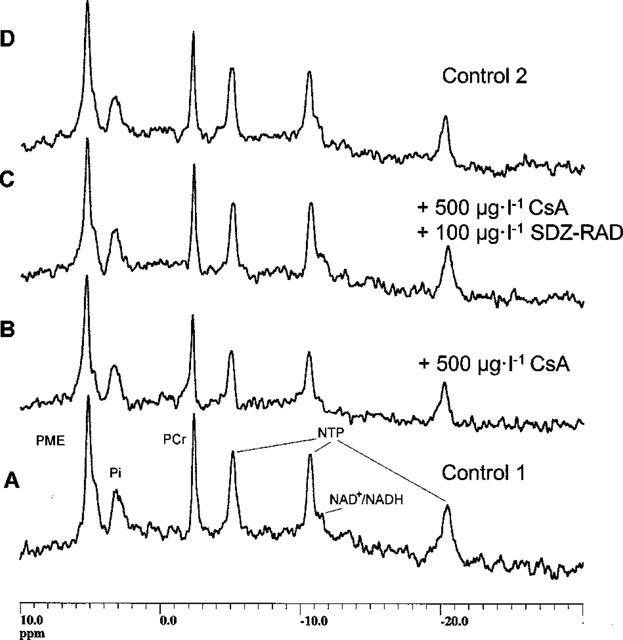
31P-MRS of perfused rat brain slices with and without immunosuppressive drugs. Four consecutive representative 31P-MRS spectra of a typical experiment with perfused rat brain slices are shown. Spectrum A: 120 min after perfusion with immunosuppressant-free medium (control 1). Spectrum B: 120 min after perfusion with 500 μg l−1 cyclosporine. Spectrum C: 120 min after perfusion with 500 μg l−1 cyclosporine and 100 μg l−1 SDZ-RAD. Spectrum D: 30 min after perfusion again with immunosuppressant-free medium (control 2). Abbreviations: NTP, nucleoside triphosphates; PCr, phosphocreatine; Pi, inorganic phosphate; PME, phosphomonoesters.
H.p.l.c.-MS analysis of cyclosporine and SDZ-RAD in rat brain slices
Because of the expected low tissue concentrations, h.p.l.c.-MS was used for quantification of cyclosporine and SDZ-RAD. After 31P-MRS, the slices were washed and homogenized with 1M KH2PO4 buffer pH 7.4 (2 ml). One ml of homogenate was removed and 28-, 40-diacetyl rapamycin and cyclosporin D were added as internal standards (Streit et al., 1996). The final concentration of the internal standards in the samples was 100 μg l−1. After addition of 2 ml methanol/0.2 mM ZnSO4 (80/20 v v−1) for protein precipitation, the samples were vortexed for 30 s and centrifuged at 1500 × g for 3 min. The supernatant was loaded on C18 extraction columns (Bond Elut LRC, Varian, Harbor City, CA, U.S.A.) by drawing the samples through columns using a vacuum system. Immunosuppressants and internal standards were eluted using 1.5 ml methylene chloride. Samples were evaporated to dryness under a stream of nitrogen. Residues were reconstituted in 120 μl acetonitrile/water (pH=3) 75/25 v v−1. Samples were transferred into micro h.p.l.c. vials and were analysed using a Hewlett-Packard (Palo Alto, CA, U.S.A.) h.p.l.c./electrospray-MS system. For single ion detection, the mass spectrometer was focused on the [M+Na]+ of SDZ-RAD (980 atomic mass units, amu), internal standard 28-,40-diacetyl rapamycin (1020 amu), cyclosporine (1224 amu), and the internal standard cyclosporin D (1238 amu). Concentrations of the immunosuppressants were calculated from an external standard curve and corrected on the basis of their internal standards.
Histology
Integrity and viability of the brain slices during perfusion and MRS analysis were verified by histology. Three slices were taken from the NMR tube immediately after the end of each 31P-MRS experiment. Slices were transferred to a tube containing 10% formalin in phosphate buffered 0.9% saline solution pH 7 (Sigma Chemicals, St. Louis, MO, U.S.A.). After 24 h at room temperature, tissues were washed, dehydrated, and embedded in paraffin. Adjacent 10 μm thick sections were cut in the plane of the slices, stained with cresyl violet (Nissl Stain), and examined with phase contrast light microscopy (Zeiss Axioskop Model 20, Thornwood, NY, U.S.A.). In representative fields of 100 adjacent cells, corresponding to areas of 104 μm2, shrunken and dead brain cells were counted and photographed.
Isolation of mitochondria
After 2 h perfusion with the study drugs or their combination, the concentrations of SDZ-RAD and/or cyclosporine in rat brain mitochondria were determined using h.p.l.c.-MS analysis. Mitochondrial from brain slices were prepared by a modified procedure as described previously (O'Gorman et al., 1996; Kristal & Dubinski, 1997). Briefly, brain slices were transferred into a centrifuge tube containing 10 ml ice-cold 250 mM sucrose, 2 mM HEPES and 1 mM EGTA (pH 7.4). After homogenization, the brain suspension was centrifuged at 900 × g in a Beckman J2-21 centrifuge for 10 min to remove cellular debris and nuclei. The supernatant was decanted into a centrifuge tube and re-centrifuged under the same conditions. The resulting supernatant was transferred into another centrifuge tube and centrifuged at 10 000 × g for 10 min to obtain an olive-green mitochondrial pellet. The mitochondrial pellet was further purified by 35 min centrifugation at 15 000 × g on a 20%- Percoll gradient. Percoll was removed from the mitochondria fraction by washing twice with the extraction medium and centrifuged again under the same conditions. The supernatant was removed, and the pellet was reconstituted in 1 ml of 1M KH2PO4 buffer pH 7.4. Mitochondria were extracted and prepared for h.p.l.c.-MS analysis as described above.
Analysis of the combined cyclosporine/SDZ-RAD effect on brain high-energy metabolism
The combined effects of cyclosporine and SDZ-RAD on brain energy metabolism were analysed using the algebraic algorithm described by Berenbaum (1978). This method is the mathematical surrogate of the geometric description of drug interactions by Loewe isobolograms. Synergism/antagonism indices were calculated using the following equation relating the effect of the doses of the drugs A, B,...X to the corresponding equieffective doses Ae, Be,...Xe when the drugs are used alone:
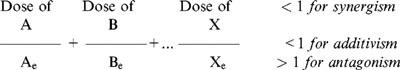 |
This analysis was based on a checkerboard matrix combining the following cyclosporine and SDZ-RAD concentrations: 0, 100, 500 and 5000 μg l−1, added to perfused rat brain slices. Concentrations of the high-energy phosphate PCr and NTP were measured in perfused rat brain slices by 31P-MRS as described above.
Data analysis
All results are given as means±standard deviation (s.d.). The results were compared either using unpaired Student's t-test (procedure T-Test, SAS, Version 6.12, SAS Institute, Cary, NC, U.S.A.) or analysis of variance in combination with Duncan grouping (procedure GLM, SAS).
Results
Effects of cyclosporine and SDZ-RAD on brain energy metabolism
Changes in brain energy metabolism were calculated from 31P-MRS experiments with perfused rat brain slices. Figure 1 shows four consecutive, representative 31P-MRS spectra from a typical 31P-MRS experiment for: (i) perfusion with medium containing drug-free vehicle (initial control, bottom, Figure 1A); (ii) after 2 h of perfusion with 500 μg ml−1 cyclosporine (Figure 1B); (iii) after 2 h of perfusion with a combination of 500 μg l−1 cyclosporine and 100 μg l−1 SDZ-RAD (Figure 1C), and (iv) after 30 min perfusion with medium containing drug-free vehicle (final control, top, Figure 1D). Two hours after perfusion with 500 μg l−1 cyclosporine alone (see Figures 2), normalized signals of the high-energy phosphates PCr and NTP were significantly reduced, i.e., [PCr/PME]: 73±2% of control (n=6, P<0.0001, Figure 2A); [NTP/PME]: 84±2% of control (P<0.003, Figure 2B). In addition, cyclosporine at this concentration also caused reduction of the NAD+/NADH signal, i.e., [NAD+-NADH/PME]: 69±9% of control (n=6, P<0.002, Figure 2C). The inhibitory effect of cyclosporine on high-energy phosphate metabolism and the NAD+/NADH signal was concentration dependent (P<0.001, analysis of variance, Figure 2).
Figure 2.
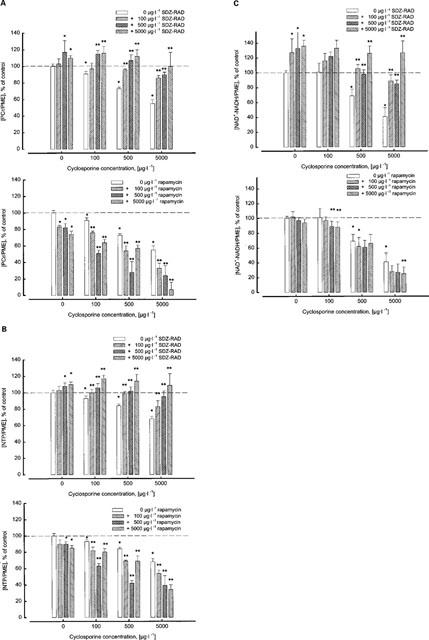
Dose-dependent effect of cyclosporine and SDZ-RAD alone and in combination on high-energy phosphate metabolism in rat brain slices and comparison with rapamycin. (A) Effect of cyclosporine and SDZ-RAD (top) or rapamycin (bottom) on [PCr/PME] ratio in perfused rat brain slices calculated from 31P-MRS after 2 h of perfusion with the study drugs. (B) Effect of CsA and SDZ-RAD (top) or rapamycin (bottom) on [NTP/PME] ratio after 2 h of perfusion with the study drugs. (C) Effect of CsA and SDZ-RAD (top) or rapamycin (bottom) on [NAD+-NADH/PME] ratio after 2 h of perfusion with the study drugs. Data for rapamycin in (A) and (B) was taken from Serkova et al. (1999). The n.m.r. experiments with rapamycin in (C) were carried out as described previously (Serkova et al., 1999). Significance levels: *Data are significantly different from untreated controls (n=3, P<0.05). **Data are significantly different from cyclosporine alone (n=3, P<0.05). Abbreviations: NTP, nucleoside triphosphates; PCr, phosphocreatine; PME, phosphomonoesters.
In contrast, SDZ-RAD at concentrations as high as 5000 μg l−1 did not cause a reduction of high-energy phosphate concentrations. Compared with the controls, the signal intensities of PCr and NTP in the presence of 100 μg l−1 SDZ-RAD were unchanged after 2 h perfusion (Figure 2). At higher concentrations (500 μg l−1 and 5000 μg l−1), SDZ-RAD significantly increased high-energy phosphate metabolite concentrations, i.e. [PCr/PME]: 117±14% of control at 500 μg l−1 (n=3, P<0.02, Figure 2A) and 110±3% of control at 5000 μg l−1 (n=3, P<0.0006); [NTP/PME]: 108±4% of control at 500 μg l−1 (n=3, P<0.004, Figure 2B) and 110±3% of control at 5000 μg l−1 (n=3, P<0.0006). Addition of SDZ-RAD to the perfusion medium also led to a significant increase of the NAD+/NADH signal (Figure 2C), i.e. [NAD+-NADH/PME]: 127±19% of control at 100 μg l−1 (n=6, P<0.006), 133±22% of control at 500 μg l−1 (n=3, P<0.005), and 136±8% of control at 5000 μg l−1 (n=3, P<0.0001).
When combined with 500 μg l−1 cyclosporine, 100 μg l−1 SDZ-RAD abolished the cyclosporine-induced reduction of high-energy metabolism in perfused rat brain slices (Figures 1 and 2). This change was significant as compared to the measurements when SDZ-RAD was absent. This SDZ-RAD protective effect against cyclosporine-induced reduction of brain energy metabolism was observed even when the highest cyclosporine dose and the lowest SDZ-RAD dose were combined: 5000 μg l−1 cyclosporine plus 100 μg l−1 SDZ-RAD, i.e. [PCr/PME]: 86±3% of control (n=3, P<0.001 compared with cyclosporine effects in the absence of SDZ-RAD, Figure 2A); [NTP/PME]: 83±7% of control (n=3, P<0.03, Figure 2B); [NAD+-NADH/PME]: 89±8% (n=3, P<0.001, Figure 2C). Analysis of whether or not the interaction of SDZ-RAD and cyclosporine was of a synergistic, additive, or antagonistic nature yielded indices for the combined effects of cyclosporine and SDZ-RAD for phosphocreatine as well as NTP which were clearly greater than 1 for all concentration combinations tested (Table 1). The indices indicated that SDZ-RAD antagonized the cyclosporine-induced reduction of high-energy phosphates in rat brain slices.
Table 1.
Synergism/antagonism indices of cyclosporine and SDZ-RAD calculated from 31P-MRS spectra of perfused rat brain slices

Stability of the brain slices during perfusion
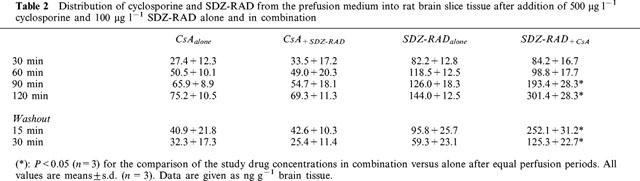
The intracellular pH as calculated from the 31P-MRS chemical shift of intracellular inorganic phosphate was 7.19±0.03 and unchanged in all perfusion experiments. No MRS-detectable, statistically significant changes were observed in either the intracellular inorganic phosphate (PI) or phosphomonoester (PME) signal intensity during treatment with cyclosporine, or with SDZ-RAD, or with both combined. Histological analyses showed no morphological changes and tissue degradation or cell death even at the highest concentrations of SDZ-RAD or cyclosporine after a perfusion period of 2 h. Viability of the brain slices and reversibility of the effects on high-energy phosphate metabolism were confirmed in all experiments by washout data collected after exposure to the study drugs. After 30 min of washout, NMR signal intensities returned to normal and were not significantly different from those during the initial control perfusion period. By this time, more than 50% of the study drugs were washed out of the tissues (Table 2).
Table 2.
Distribution of cyclosporine and SDZ-RAD from the prefusion medium into rat brain slice tissue after addition of 500 μg l−1 cyclosporine and 100 μg l−1 SDZ-RAD alone and in combination
SDZ-RAD and cyclosporine distribution in tissue and mitochondria of perfused rat brain slices
SDZ-RAD and cyclosporine distributions in brain slices and mitochondria were determined as a function of time using h.p.l.c./electrospray-MS.
Brain tissue concentrations
Perfusion with 500 μg l−1 cyclosporine for 30 min resulted in cyclosporine tissue concentrations of 27.4±12.3 ng g−1 tissue (n=3, Table 2), After 2 h of perfusion with 500 μg l−1 cyclosporine, the tissue concentration of cyclosporine increased to 75.2±10.5 ng g−1. Addition of 100 μg l−1 SDZ-RAD to the perfusion medium produced SDZ-RAD tissue concentrations of 82.2±12.8 ng g−1 after 30 min and 144.0±12.5 ng g−1 after 2 h (n=3, Table 2). The combination of SDZ-RAD and cyclosporine significantly increased the brain tissue concentrations of SDZ-RAD but did not affect those of cyclosporine (Table 2).
Concentrations in mitochondria
Both cyclosporine and SDZ-RAD were found in the mitochondria of rat brain slices. In mitochondria, the presence of SDZ-RAD significantly changed cyclosporine concentrations and vice versa. When brain slices were perfused with medium containing 500 μg l−1 cyclosporine for 2 h, the cyclosporine concentration in mitochondria was 80.5±12.4 ng g−1, when brain slices were perfused with 100 μg l−1 SDZ-RAD, the mitochondrial concentration was 27.2±11.7 ng g−1 (mean±s.d., n=5). In comparison with each drug alone, perfusion of brain slices with a combination of 100 μg l−1 SDZ-RAD and 500 μg l−1 cyclosporine significantly reduced the cyclosporine concentration in mitochondria by 25% (59.5±2.1 ng g−1, P<0.015), while the SDZ-RAD concentration increased by 74% (47.4±7.1 ng g−1, P<0.015).
Discussion
In contrast to cyclosporine and the macrolide immunosuppressants tacrolimus and rapamycin (Massicot et al., 1997; Serkova et al., 1996; 1997), SDZ-RAD did not inhibit high-energy phosphate metabolism in vitro. This is especially interesting, considering that SDZ-RAD and rapamycin are structurally related. In addition, SDZ-RAD significantly antagonized cyclosporine-induced reduction of brain high-energy phosphates even when the molar cyclosporine/SDZ-RAD ratio was 50 : 1.
In previous studies (Serkova et al., 1996; 1997) we demonstrated that cyclosporine, tacrolimus, and rapamycin affect high-energy phosphate metabolism, the intracellular concentrations of osmoregulators (such as aspartate, taurine and hypotaurine), the intracellular concentrations of the neurotransmitter glutamate, membrane turn-over, and tricarboxylic acid (TCA) cycle. High-energy phosphate metabolism was the metabolic pathway most sensitive to cyclosporine and rapamycin, and it was significantly reduced at perfusate concentrations as low as 100 μg l−1 (Serkova et al., 1999). Cyclosporine perfusate concentrations of 100 μg l−1 corresponded to the lower limit of the blood concentration range targeted in transplant patients (Oellerich et al., 1995). Various previous studies (Gabe et al., 1998; Henke et al., 1992; Massicot et al., 1997; Riuz-Cabello et al., 1994; Uemoto et al., 1989) demonstrated that cyclosporine specifically inhibits mitochondrial energy production and decreases ATP level in target organs of its toxicity such as kidney, liver and intestine in vitro as well as in vivo. Impairment of energy metabolism in the brain results in excitotoxic neuronal death in various neurodegenerative illnesses (Beal, 1992). Therefore, in this study, we focused on the effects of SDZ-RAD on brain high-energy phosphate metabolism alone and in combination with cyclosporine. One advantage of using 31P-MRS is its non-invasive character. Thus, in our study, brain slice preparations could be used as their own controls. A previous report established that quantification of high-energy phosphates by 31P-MRS and by a reference h.p.l.c. assay led to identical results (Auffermann et al., 1988).
In the past, different mechanisms have been proposed to explain the effect of cyclosporine on mitochondrial NTP concentrations. Cyclosporine increases free radical formation (Serino et al., 1993; Wang & Salahudeen, 1994) leading among other effects to reduction of oxidative phosphorylation and to decreased mitochondrial ATP-concentrations (Packer, 1998). The immunophilin FKBP-12, the major intra-cellular binding protein of rapamycin and SDZ-RAD (Schuler et al., 1997), is an important component in the regulation of ryanodine and inositol 1,4,5,-triphosphate receptors, both major intracellular calcium channels (Salducci et al., 1992; Schreiber, 1991; Shou et al., 1998; Snyder et al., 1998). It has been hypothesized that inhibition of FKBP-12 by macrolide immunosuppressants results in calcium overload of mitochondria which inhibits oxidative phosphorylation (Salducci et al., 1992). This hypothesis is not supported by the results of our study. Both rapamycin as well as SDZ-RAD bind and inhibit FKBP-12 (Schuler et al., 1997). Rapamycin (Serkova et al., 1997; 1999), but not SDZ-RAD, affected high-energy phosphate metabolism indicating that neither FKBP-12 nor the FKBP-12/calcineurin complex are significantly involved.
Although our study did not produce exact identification of the molecular mechanisms underlying the antagonistic interaction between SDZ-RAD and cyclosporine, our results suggest a direct stimulation of mitochondrial oxidative phosphorylation by SDZ-RAD. At perfusate concentrations of 500 and 5000 μg l−1, SDZ-RAD significantly increased high-energy phosphate concentrations. This trend was even detectable at concentrations as low as 100 μg l−1. SDZ-RAD increased mitochondrial NTP-production rather than increase glycolysis. The latter would have increased lactate concentrations. Because intracellular pH remained unchanged when the brain slices were perfused with SDZ-RAD, it is highly unlikely that significant increases occurred in lactate concentrations. In addition to high-energy phosphates, SDZ-RAD increased NAD+/NADH concentrations, a marker of mitochondrial oxidative phosphorylation (Erecinska & Wilson, 1982).
Intracellular radical formation by cyclosporine (Wolf et al., 1997) has mainly been attributed to its interaction with cytochrome P450 enzymes (Ahmed et al., 1993; Serino et al., 1994). We have recently confirmed that SDZ-RAD is a substrate for cytochrome P450 3A (own unpublished data) just as cyclosporine is (Kronbach et al., 1988). Thus a competitive interaction might be a potential mechanism for the antagonistic interaction. However, this seems unlikely for two reasons. Brain microsomes contain only 0.5–2% of the cytochrome P450 concentration of liver microsomes (Hedlund et al., 1998). In addition, cyclosporine and SDZ-RAD bind to cytochrome P450 3A enzymes with similar KM values. Here we demonstrated that addition of 100 μg l−1 SDZ-RAD to 5000 μg l−1 cyclosporine, resulting into a molar SDZ-RAD/cyclosporine ratio of approximately 1 : 50, increased high-energy phosphate concentrations by 56%, a value that would not correlate with the expected extent of a potential cytochrome P450 3A interaction.
Other potential interactions may affect distribution into brain tissue and mitochondria. In a previous study (Serkova et al., 1999), we showed that combined perfusion with rapamycin and cyclosporine increased cyclosporine tissue concentrations in rat brain slices and this contributed to the synergistic interaction between rapamycin and cyclosporine. However, rapamycin was not detected in mitochondria of rat brain slices (own unpublished data). In contrast, SDZ-RAD was able to enter mitochondria. This can be expected to be part of the explanation why rapamycin and SDZ-RAD exhibit different effects on brain energy metabolism. Despite their structural similarity, sirolimus and SDZ-RAD are substrates of different ATP-binding cassette transporters (Crowe & Lemaire, 1998). This is major reason for the significantly better oral bioavailability of SDZ-RAD in comparison with rapamycin due to less countertransport by intestinal ATP-binding cassette transporters and, thus, better membrane permeability (Crowe et al., 1999). ATP-binding cassette transporters are also located in the mitochondrial membrane (Csere et al., 1998; Leighton & Schatz, 1995). Although their role in limiting access of drugs into the mitochondria has not yet been studied, different affinities of rapamycin and SDZ-RAD to ATP-binding cassette transporters constitute a potential mechanism, which may explain the differences in intra-cellular distribution. As demonstrated in our study, cyclosporine increased SDZ-RAD concentrations in mitochondria while SDZ-RAD reduced cyclosporine concentrations. Our results suggest that SDZ-RAD antagonized cyclosporine-induced reduction of high-energy phosphates by stimulation of mitochondrial oxidative phosphorylation and by reduction of cyclosporine distribution into mitochondria.
It has been hypothesized that reduction of high-energy phosphate concentrations plays a significant role in cyclosporine nephrotoxicity (Henke et al., 1992; Riuz-Cabello et al., 1994). If so, SDZ-RAD, as demonstrated here in brain tissue, might antagonize cyclosporine-induced inhibition of oxidative phosphorylation in other organs as well. In vivo studies are ongoing to determine if our results correlate with measures of toxicity and to establish whether or not co-administration of SDZ-RAD and cyclosporine is a clinically significant path to reducing cyclosporine toxicity in transplant patients.
Acknowledgments
The authors thank Dr Pak H. Chan (Stanford University) for the helpful discussions. This study was supported by NIH grant GM26691 (L.Z. Benet), the Alexander-von Humboldt Foundation grant V-3-FLF-1052812 (N. Serkova), the Deutsche Forschungsgemeinschaft grants CH95/6-2 (U. Christians) and Ha 1967/2-1 (B. Hausen), the Hedco Foundation (R.E. Morris) and the Marian and Ralph Falk Trust Foundation (R.E. Morris).
Abbreviations
- amu
atomic mass units
- MS
mass spectrometry
- NTP
nucleoside triphosphates
- PCr
phosphocreatine
- Pi
inorganic phosphate
- PME
phosphomonoesters
References
- ADAMS D.H., GUNSON B., PONSFORD S., BOON A., HONIGSBERG L., WILLIAMS A., BUCKELS J., ELIAS E., MCMASTER P. Neurological complications following liver transplantation. Lancet. 1987;i:949–952. doi: 10.1016/s0140-6736(87)90294-7. [DOI] [PubMed] [Google Scholar]
- AHMED S.S., STROBEL H.W., NAPOLI K.L., GREVEL J. Adenochrome reaction implicates oxygen radicals in metabolism of cyclosporine A and FK506 in rat and human liver microsomes. J. Pharmacol. Exp. Ther. 1993;265:1047–1054. [PubMed] [Google Scholar]
- ATKINSON K., BIGGS J., DARVENIZA P., BOLAND J., CONCANNON A., DODDS A. Cyclosporineassociated centralnervoussystem toxicity after allogenic bonemarrow transplantation. N. Engl. J. Med. 1984;310:527. doi: 10.1056/NEJM198402233100815. [DOI] [PubMed] [Google Scholar]
- AUFFERMANN W., WU S., PARMLEY W.W., HIGGINS C.B., SIEVERS R., WIKMAN-COFFELT R. Reversibility of acute alcohol cardiac depression: 31P-NMR in hamsters. FASEB J. 1988;2:256–263. doi: 10.1096/fasebj.2.3.3350237. [DOI] [PubMed] [Google Scholar]
- BEAL M.F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses. Ann. Neurol. 1992;31:119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- BERENBAUM M.C. A method for testing for synergy with any number of agents. J. Infect. Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- BOHLER T., WAISER J., BUDDE K., LICHTER S., JAUHO A., FRITSCHE L., KORN A., NEUMAYER H.H. The in vivo effect of rapamycin derivative SDZ-RAD on lymphocyte proliferation. Transplant. Proc. 1998;30:2195–2197. doi: 10.1016/s0041-1345(98)00588-0. [DOI] [PubMed] [Google Scholar]
- COLE O.J., SHEHATA M., RIGG K.M. Effect of SDZ-RAD on transplant arteriosclerosis in the rat aortic model. Transplant. Proc. 1998;30:2200–2203. doi: 10.1016/s0041-1345(98)00590-9. [DOI] [PubMed] [Google Scholar]
- CROWE B., BRÜLISAUER A., DÜRR L., GUNTZ P., LEMAIRE M. Absorption and intestinal metabolism of SDZ-RAD and rapamycin in rats. Drug Metab. Dispos. 1999;27:627–632. [PubMed] [Google Scholar]
- CROWE A., LEMAIRE M. In vitro and in situ absorption of SDZ-RAD using a human intestinal cell line (Caco-2) and a single pass perfusion model in rats: Comparison with rapamycin. Pharm. Res. 1998;15:1666–1672. doi: 10.1023/a:1011940108365. [DOI] [PubMed] [Google Scholar]
- CSERE P., LILL R., KISPAL G. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett. 1998;441:266–270. doi: 10.1016/s0014-5793(98)01560-9. [DOI] [PubMed] [Google Scholar]
- DE GROEN P.C., AKSAMIT A.J., RAKELA J., FORBES G.S., KROM R.A.F. Central nervous system toxicity after liver transplantation. N. Engl. J. Med. 1987;317:861–866. doi: 10.1056/NEJM198710013171404. [DOI] [PubMed] [Google Scholar]
- ERECINSKA A., WILSON D.F. Regulation of cellular energy metabolism. J. Membr. Biol. 1982;70:1–14. doi: 10.1007/BF01871584. [DOI] [PubMed] [Google Scholar]
- ESPANOL M.T., LITT L., YANG G.Y., CHANG L.H., CHAN P.H., JAMES T.L., WEINSTEIN P.R. Tolerance of low intracellular pH during hypercapnia by rat cortical slices: A 31P/1H NMR study. J. Neurochem. 1992;59:1820–1828. doi: 10.1111/j.1471-4159.1992.tb11015.x. [DOI] [PubMed] [Google Scholar]
- GABE S.M., BJARNASON I., TOLOU-GHAMARI Z., TREDGER J.M., JOHNSON P.G., BARCLAY G.R., WILLIAMS R., SILK D.B.A. The effect of tacrolimus (FK506) on intestinal barrier function and cellular energy production in humans. Gastroenterology. 1998;115:67–74. doi: 10.1016/s0016-5085(98)70366-x. [DOI] [PubMed] [Google Scholar]
- GIJTENBEEK J.M., VAN DEN BENT M.J., VECHT C.J. Cyclosporine neurotoxicity: a review. J. Neurol. 1999;246:339–346. doi: 10.1007/s004150050360. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L.S., HAUG M.T., PERL J., PERL M.K., MAURER J.R., ARROLIGA A.C., MEHTA A.C., KIRBY T., HIGGINS B., STILLWELL P.S. Central nervous system complications after lung transplantation. J. Heart Lung Transplant. 1998;17:185–191. [PubMed] [Google Scholar]
- HAUBEN M. Cyclosporine neurotoxicity. Pharmacotherapy. 1996;16:576–583. [PubMed] [Google Scholar]
- HAUSEN B., BOEKE K., BERRY G.J., SEGARRA I.T., CHRISTIANS U., MORRIS R.E. Suppression of acute rejection in allogeneic rat lung transplantation: a study of the efficacy of rapamycin derivative (SDZ RAD) used alone and in combination with a microemulsion formulation of cyclosporine. J. Heart Lung Transplant. 1999;18:150–159. doi: 10.1016/s1053-2498(98)00020-5. [DOI] [PubMed] [Google Scholar]
- HEDLUND E., GUSTAFSSON J.-A., WARNER M. Cytochrome P450 in the brain: 2B or not 2B. Trends Pharmacol. Sci. 1998;19:82–85. doi: 10.1016/s0165-6147(97)01165-6. [DOI] [PubMed] [Google Scholar]
- HENKE W., NICKEL E., JUNG K. Cyclosporine A inhibits ATP net uptake of rat kidney mitochondria. Biochem. Pharmacol. 1992;43:1021–1024. doi: 10.1016/0006-2952(92)90608-l. [DOI] [PubMed] [Google Scholar]
- KAHAN B.D. Cyclosporine. N. Engl. J. Med. 1989;321:1727–1737. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- KRISTAL B.S., DUBINSKY J.M. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and -independent pathways. J. Neurochem. 1997;69:524–538. doi: 10.1046/j.1471-4159.1997.69020524.x. [DOI] [PubMed] [Google Scholar]
- KRONBACH T., FISCHER V., MEYER U.A. Cyclosporine metabolism in human liver: identification of a cytochrome P450IIIA gene family as the major cyclosporine-metabolizing enzyme explains interaction of cyclosporine with other drugs. Clin. Pharmacol. Ther. 1988;43:630–635. doi: 10.1038/clpt.1988.87. [DOI] [PubMed] [Google Scholar]
- LEIGHTON J., SCHATZ G. An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J. 1995;14:188–195. doi: 10.1002/j.1460-2075.1995.tb06989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSICOT F., MARTIN C., DITERTRE-CATELLA H, ELLOUK-ACHARD S., PHAM-HUY C., THEVENIN M., RUCAY P., WARNET J.-M., CLAUDE J.-R. Modulation of energy status and cytotoxicity induced by FK506 and cyclosporin A in a renal epithelial cell line. Arch. Toxicol. 1997;71:529–531. doi: 10.1007/s002040050423. [DOI] [PubMed] [Google Scholar]
- O'GORMAN E., BEUTNER G., WALLIMANN T., BRDICZKA D. Differential effects of creatine depletion on the regulation of enzyme activities and on creatine-stimulated mitochondrial respiration in skeletal muscle, heart, and brain. Biochim. Biophys. Acta. 1996;1276:161–170. doi: 10.1016/0005-2728(96)00074-6. [DOI] [PubMed] [Google Scholar]
- OELLERICH M., ARMSTRONG V.W., KAHAN B., SHAW L., HOLT D.W., YATSCOFF R., LINDHOLM A., HALLORAN P., GALLICANO K., WONIGEIT K., SCHUTZ E., SCHRAN H., ANNESLEY T. Lake Louise consensus conference on cyclosporin monitoring in organ transplantation: Report of the consensus panel. Ther. Drug Monit. 1995;17:642–654. doi: 10.1097/00007691-199512000-00017. [DOI] [PubMed] [Google Scholar]
- PACKER L. α-Lipoic acid: a metabolic antioxidant which regulates NF-κB signal transduction and protects against oxidative injury. Drug. Metab. Rev. 1998;30:245–275. doi: 10.3109/03602539808996311. [DOI] [PubMed] [Google Scholar]
- RUIZ-CABELLO J., BUSS W.C., COLLIER S.W., GLAZER R.I., COHEN J.S. Changes in ATP after cyclosporin A treatment in a renal epithelial cell line in the rat studied by 31P-NMR spectroscopy. Res. Commun. Mol. Pathol. Pharmacol. 1994;86:3–13. [PubMed] [Google Scholar]
- SALDUCCI M., CHAULET-MONGES A.M., BERLAND Y., DUSSOL B., ELSEN R., CREVAT A. The restoration of ATP synthesis may explain the protective effect of calcium antagonists against cyclosporine A nephrotoxicity. Life Sci. 1992;50:2053–2058. doi: 10.1016/0024-3205(92)90571-6. [DOI] [PubMed] [Google Scholar]
- SALMINEN U.S., ALHO H., TASKINEN E., MAASILTA P., IKONEN T., HARJULA A.L.J. Effects of rapamycin analogue SDZ RAD on obliterative lesions in a porcine heterotopic bronchial allograft model. Transplant. Proc. 1998;30:2204–2205. doi: 10.1016/s0041-1345(98)00591-0. [DOI] [PubMed] [Google Scholar]
- SCHREIBER S.L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- SCHULER W., SEDRANI R., COTTENS S., HÄBERLIN B., SCHULZ M., SCHUURMAN H.J., ZENKE G., ZERWES H.G., SCHREIER M.H. SDZ-RAD, a new rapamycin derivative. Pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- SCHUURMAN H.J., COTTENS S., FUCHS S., JOERGENSEN J., MEERLOO T., SEDRANI R., TANNER M., ZENKE G., SCHULER W. SDZ-RAD, a new rapamycin derivative: Synergism with cyclosporine. Transplantation. 1997;64:32–35. doi: 10.1097/00007890-199707150-00007. [DOI] [PubMed] [Google Scholar]
- SCHUURMAN H.J., SCHULER W., RINGERS J., JONKER M. The macrolide SDZ-RAD is efficacious in a nonhuman primate model of allotransplantation. Transplant. Proc. 1998;30:2198–2199. doi: 10.1016/s0041-1345(98)00589-2. [DOI] [PubMed] [Google Scholar]
- SERINO F., GREVEL J., NAPOLI K.L., KAHAN B.D., STROBEL H.W. Generation of oxygen free radicals during the metabolism of cyclosporine A: a cause-effect relationship with metabolism inhibition. Mol. Cell. Biochem. 1993;122:101–112. doi: 10.1007/BF01076094. [DOI] [PubMed] [Google Scholar]
- SERINO F., GREVEL J., NAPOLI K.L., KAHAN B.D., STROBEL H.W. Oxygen radical formation by the cytochrome P450 system as a cellular mechanism for cyclosporine toxicity. Transplant. Proc. 1994;26:2916–2917. [PubMed] [Google Scholar]
- SERKOVA N., BRAND A., CHRISTIANS U., LEIBFRITZ D. Evaluation of the effects of immunosuppressants on neuronal and glial cells in vitro by multinuclear magnetic resonance spectroscopy. Biochim. Biophys. Acta. 1996;1314:93–104. doi: 10.1016/s0167-4889(96)00081-x. [DOI] [PubMed] [Google Scholar]
- SERKOVA N., CHRISTIANS U., FLOEGEL U., PFEUFFER J., LEIBFRITZ D. Assessment of the mechanism of astrocyte swelling induced by the macrolide immunosuppressant sirolimus using multinuclear NMR spectroscopy. Chem. Res. Toxicol. 1997;10:1359–1363. doi: 10.1021/tx970071k. [DOI] [PubMed] [Google Scholar]
- SERKOVA N., LITT L., JAMES T.L., SADÉE W., LEIBFRITZ D., BENET L.Z., CHRISTIANS U. Evaluation of individual and combined neurotoxicity of immunosuppressants cyclosporine and sirolimus by in vitro multinuclear NMR. J. Pharmacol. Exp. Ther. 1999;289:800–808. [PubMed] [Google Scholar]
- SHOU W.N., AGHDASI B., ARMSTRONG D.L., GUO Q.X., BAO S.D., CHARNG M.J., MATHEWS L.M., SCHNEIDER M., HAMILTON S.L., MATZUK M.M. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- SNYDER S.H., LAI M.M., BURNETT P.E. Immunophilins in the nervous system. Neuron. 1998;21:283–294. doi: 10.1016/s0896-6273(00)80538-3. [DOI] [PubMed] [Google Scholar]
- STREIT F., CHRISTIANS U., SCHIEBEL H.M., NAPOLI K.L., ERNST L., LINCK A., KAHAN B.D., SEWING K.-F. Sensitive and specific quantification of sirolimus (rapamycin) and its metabolites in blood of kidney graft recipients by HPLC/electrospray-mass spectrometry. Clin. Chem. 1996;42:1417–1425. [PubMed] [Google Scholar]
- UEMOTO S., TANAKA K., ASONUMA K., OKAMURA R., KITAKADO Y., MATSUOKA S., OZAKI N., OZAWA K., HASHIDA T., INUI K., HORI R. Effect of cyclosporine on oxidative phosphorylation and adenylate energy of regenerating rat liver. Res. Exp. Med. 1989;189:313–320. doi: 10.1007/BF01855036. [DOI] [PubMed] [Google Scholar]
- WANG C., SALAHUDEEN A.K. Cyclosporine nephrotoxicity: Attenuation by an antioxidant-inhibitor of lipid peroxidation in vitro and in vivo. Transplantation. 1994;58:940–946. doi: 10.1097/00007890-199410270-00014. [DOI] [PubMed] [Google Scholar]
- WOLF A., TRENDELENBURG C.F., DIEZ-FERNANDEZ C.F., PRIETO S., HOUY E., TROMMER W.E., CORDIER A. Cyclosporine A-induced oxidative stress in rat hepatocytes. J. Pharmacol. Exp. Ther. 1997;280:1328–1334. [PubMed] [Google Scholar]


