Abstract
Using subtype-selective 5-HT1 receptor agonists and/or the 5-HT1 receptor antagonist GR127935, we characterized in vitro the 5-HT receptor that mediates the contraction of human and bovine cerebral arteries. Further, we investigated which sumatriptan-sensitive receptors are present in human coronary artery by reverse-transcriptase polymerase chain reaction (RT–PCR).
Agonists with affinity at the 5-HT1B receptor, such as sumatriptan, alniditan and/or IS-159, elicited dose-dependent contraction in both human and bovine cerebral arteries. They behaved as full agonists at the sumatriptan-sensitive 5-HT1 receptors in both species. In contrast, PNU-109291 and LY344864, selective agonists at 5-HT1D and 5-HT1F receptors, respectively, were devoid of any significant vasocontractile activity in cerebral arteries, or did not affect the sumatriptan-induced vasocontraction. The rank order of agonist potency was similar in both species and could be summarized as 5-HT=alniditan>sumatriptan=IS-159>>>PNU-109291=LY344864.
In bovine cerebral arteries, the 5-HT1 receptor antagonist GR127935 dose-dependently inhibited the vasoconstrictions elicited by both 5-HT and sumatriptan, with respective pA2 values of 8.0 and 8.6.
RT–PCR studies in human coronary arteries showed a strong signal for the 5-HT1B receptor while message for the 5-HT1F receptor was weak and less frequently detected. Expression of 5-HT1D receptor mRNA was not detected in any sample.
The present results demonstrate that the triptan-induced contraction in brain vessels is mediated exclusively by the 5-HT1B receptor, which is also present in a majority of human coronary arteries. These results suggest that selective 5-HT1D and 5-HT1F receptor agonists might represent new antimigraine drugs devoid of cerebro- and cardiovascular effects.
Keywords: Migraine, serotonin receptors, vasoconstriction, meningeal arteries, coronary artery
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is a neurotransmitter which exerts a wide spectrum of modulatory effects in the peripheral and central nervous systems. It has been implicated in various disorders including the pathogenesis of migraine (Martin, 1997). 5-HT interacts with multiple receptors and numerous studies have emphasized the role of 5-HT1 receptors in the acute treatment of migraine headache (for reviews, see Martin, 1997; Schoenen, 1997; Goadsby, 1998). In this respect, the 5-HT1 receptor agonist sumatriptan (Peroutka & McCarthy, 1989; Humphrey & Feniuk, 1991; Adham et al., 1993) has proven to be a highly effective antimigraine compound (Ferrari, 1993; Gross et al., 1993; Tansey et al., 1993). Two mechanisms have been proposed to explain its clinical efficacy, namely a vasoconstriction of meningeal blood vessels and an inhibition of the pro-inflammatory response that results from the release of substance P and calcitonin gene-related peptide (CGRP) from activated trigeminovascular afferents (Humphrey & Feniuk, 1991; Moskowitz, 1992; Buzzi et al., 1995). However, it is still unclear whether or not the vascular and neuronal sites of action are both necessary for clinical efficacy.
Recent findings of distinct populations of 5-HT1 receptors in the trigeminovascular system and cerebral blood vessels (Hamel et al., 1993a,1993b; Rebeck et al., 1995; Bouchelet et al., 1996b; Longmore et al., 1997; Bonaventure et al., 1998) may offer an opportunity to highlight which of these neuronal or vascular effects, if any, confers anti-migraine properties. Indeed, pharmacological (Hamel & Bouchard, 1991), moleccular (Hamel et al., 1993b; Bouchelet et al., 1996b) and immunocytochemical investigations (Longmore et al., 1997) suggest that a 5-HT1B receptor is involved in the sumatriptan-induced cerebral vaosoconstriction, while 5-HT1D (Rebeck et al., 1995; Bouchelet et al., 1996b; Longmore et al., 1997; Bonaventure et al., 1998) and/or 5-HT1F (Bouchelet et al., 1996b; Johnson et al., 1997; Phebus et al., 1997) receptors would mediate the pre-synaptic inhibition of the trigeminovascular inflammatory response. Although this represents the predominant pattern of receptor distribution, expression of 5-HT1D and 5-HT1F receptor mRNAs has also been detected, respectively, in a subset and in a majority of human brain vessels (Bouchelet et al., 1996b). Such findings suggest that these receptors could possibly participate in the 5-HT1 receptor-mediated vasocontractile response to sumatriptan in a subpopulation of human cerebral vessels. In addition, sumatriptan and many of its new derivatives have been shown in vitro to induce contraction of human coronary arteries (Kaumann et al., 1994; Ferro et al., 1995; Maassen VanDenBrink et al., 1998), an undesirable side-effect of this therapy which has limited its use in individuals at risk for cardiovascular problems (Otterwanger et al., 1997; Dahlöf & Mathew, 1998). Hence, it appears of primary importance to clearly identify the 5-HT receptor(s) responsible for the vasomotor properties of sumatriptan and to understand their distribution not only in human brain vessels but also in coronary arteries. The present study was thus undertaken to assess the vasocontractile effect of selective 5-HT1D, 5-HT1B/1D and 5-HT1F receptor agonists in human and bovine brain vessels, two species in which sumatriptan has been shown to mediate vasoconstriction through a similar 5-HT1 receptor, suggested to correspond primarily to the 5-HT1B receptor subtype (Hamel et al., 1993a,1993b; Kaumann et al., 1993). Further, the expression of mRNAs for the three sumatriptan-sensitive 5-HT1 receptor was studied in human coronary arteries. Altogether, the results clearly indicate that 5-HT1B receptors are the only mediators of the vasocontractile response to sumatriptan and non-selective 5-HT1 receptor agonists in human and bovine brain vessels, and that this receptor is also expressed in a majority of human coronary arteries. Part of these results have appeared in an abstract form (Chauveau et al., 1994; Bouchelet et al., 1996a).
Methods
Tissue preparation
Vasomotor reactivity was measured in isolated segments of a temporal ramification of the middle cerebral artery obtained from calf or human (surgical biopsies of epileptic patients of either sex, obtained with permission from the Institutional research ethics committee) brain. Vessels were carefully isolated from the pia-arachnoid membrane, cleaned of blood and surrounding tissue under a dissecting microscope. They were then cut in 2–3 mm long segments and kept in an ice-cold Krebs-Ringer buffer solution (pH 7.4) (in mM): NaCl 118, KCl 4.5, MgSO4-7H2O 1.0, KH2PO4 1.0, NaHCO3 25, CaCl2-2H2O 2.5 and glucose 6.0. Coronary arteries used for the identification of 5-HT1 receptor mRNA expression were obtained with approval from the research ethics committee from human subjects of either sex who died from diseases not related to cardiovascular complications (n=6–12; post-mortem delay of 13.3±1.4 h). Distal portions of the coronary arteries were dissected from the hearts, cleaned of surrounding fat and adherent tissues, and were kept frozen at −80°C until use. They were then powdered with a pestle in liquid nitrogen and homogenized in TRIzol reagent (Gibco-BRL, Gaitherburg, MD, U.S.A.) with a polytron and by passage through a 18½½ needle gauge. Total RNA was extracted according to Chomczynski (1993), treated with RQ1-DNAase (Promega, Madison, WI, U.S.A.), precipitated with ethanol and used in reverse transcriptase-polymerase chain reaction (RT–PCR) experiments, taking the human trigeminal ganglion as control tissue (for details, see Bouchelet et al., 1996b).
Functional assays
The vasocontractile responses to 5-HT and 5-HT1 receptor agonists were determined in intact human and bovine brain vessels as routinely performed in our laboratory (Hamel & Bouchard, 1991; Hamel et al., 1993a,1993b). In brief, vessel segments were mounted between two L-shaped metal prongs in temperature-controlled (37°C) tissue baths (volume of 5 ml) containing the Krebs-Ringer solution (see above) bubbled with a mixture of 95% O2 and 5% CO2, and replaced every 15 min. Changes in muscle tension were measured by a force displacement transducer (Grass FT 103 D) and recorded on a Grass Polygraph Model 7E. The vessels were allowed to stabilize (45 min, 0.4 g for human and 0.4–0.6 g for bovine) and then the maximal contractile capacity was evaluated with a Krebs solution supplemented with K+ (124 mM). The vessels were washed and allowed to recover for an additional 30–45 min period.
Agonists
Log-concentration response curves were generated by cumulative addition (1 nM–10 μM) of either 5-HT, sumatriptan or selective 5-HT1B/1D, 5-HT1D or 5-HT1F receptor agonists. The order of the compounds was randomized from one experiment to another. For comparison, the maximal contractile response (EAmax) and relative potency (pD2 values or −log EC50, Van den Brink, 1977) were determined for each agonist, taking 5-HT as the reference compound. In a different series of experiments in bovine cerebral arteries, dose-response curves to sumatriptan were generated in the absence (control) and presence (plus a 30 min pre-incubation) of 10−6 M of the selective 5-HT1D receptor agonist (see below). In this case, the dose-response curves to sumatriptan in the presence of the agonist were expressed as a per cent of the control curves obtained for sumatriptan in the same vascular segments. The 5-HT1 receptor agonists used were: the non-selective 5-HT1B/1D/1F receptor agonist, sumatriptan (Peroutka & McCarthy, 1989; Humphrey & Feniuk, 1991; Adham et al., 1993; GlaxoWellcome, Greenford, U.K.), the 5-HT1B/1D receptor agonists alniditan (Leysen et al., 1996; Janssen Research Foundation, Beerse, Belgium) and/or serotonin-O-carboxymethylglycyltyrosinamide (IS-159, Boulenguez et al., 1991; Chauveau et al., 1994; Immunotech, Marseille, France), the selective 5-HT1D receptor agonist (S)-(−)-1[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]-N-methylisochroman-6-carboxamide (PNU-109291, Ennis et al., 1998; Pharmacia and Upjohn, Kalamazoo, MI, U.S.A.) and the selective 5-HT1F receptor agonist (R)-(+)-N-(3-dimethylamino-1,2,3,4,-tetrahydro-9H-carbazol-6-yl)-4-fluorobenzamide (LY344864, Phebus et al., 1997; Eli Lilly, Indianapolis, IN, U.S.A.). All compounds were graciously provided by the above respective companies. They were first diluted in water (10−2 or 10−3 M), with following dilutions being made directly in buffer.
Antagonist
The participation of 5-HT1 receptors in the vasocontractile response to 5-HT in bovine cerebral arteries was further assessed with the 5-HT1 receptor antagonist (N-[methoxy-3- (4-methyl-1-piperazinyl) phenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)[1,1-biphenyl]-4-carboxamide hydrochloride (GR127935, a gift from GlaxoWellcome, Greenford, U.K.) (Skingle et al., 1996). Dose-response curves to 5-HT and sumatriptan (1 nM to 10–100 μM) were performed in the absence and then in the presence (30 min pre-incubation) of different concentrations (1 or 10 nM to 0.1 or 1 μM) of GR127935, with the vessels being exposed only once (on rare occasions twice) to the antagonist. The potency of GR127935 was expressed as a pA2 value, and was calculated according to Van den Brink (1977) (for more details, see Hamel & Bouchard, 1991, Hamel et al., 1993b). The nature of the antagonism was further determined by Schild plot analysis (Arunlakshana & Schild, 1959) and the pA2 obtained from the Schild analysis compared to that calculated as described above. The pA2 values obtained from both methods were averaged and data are presented as overall pA2 values for GR127935 against either 5-HT or sumatriptan.
RT–PCR experiments
Following synthesis of cDNAs from the total RNA using random primers and avian myeloblastosis virus reverse transcriptase (RT) (for details, Bouchelet et al., 1996b), cDNA was heat denaturated (95°C, 3–5 min) and then subjected (2–4 μl) to PCR amplification with Taq polymerase using selective primers for the three sumatriptan-sensitive 5-HT1 receptors, namely the 5-HT1B, 5-HT1D and 5-HT1F receptors, (for more details see Bouchelet et al., 1996b). The timing for amplification was 85°C 3 min, 56°C 1 min, 72°C 5 min followed by 39 cycles of amplification (94°C 40 s, 55°C 40 s, 72°C 40 s) and a final elongation 5 min at 72°C. The PCR products (fragment size of 340 bp, 595 bp and 482 bp for the 5-HT1D, 5-HT1B and 5-HT1F receptors, respectively) were analysed by gel electrophoresis. Control reactions lacking reverse transcriptase were used to monitor for DNA contamination (−RT lane in Figure 5).
Figure 5.
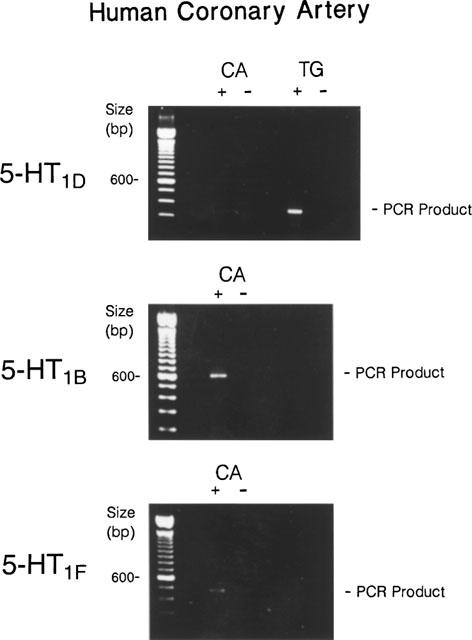
Identification of sumatriptan-sensitive 5-HT1 receptors in human coronary artery (CA) by RT–PCR. Representative agarose gel electrophoresis of PCR products showing the absence of 5-HT1D receptors in human coronary artery despite consistent amplification in the human trigeminal ganglion (TG). High intensity PCR products were obtained for 5-HT1B receptors in 60% of human coronary arteries while a weak signal was obtained for the 5-HT1F receptor in 40% of cases. Samples without reverse transcriptase (−) were included to monitor for possible contamination.
Statistical analysis
All results are mean±s.e.mean. Statistical significance was assumed when P<0.05, as determined by ANOVA followed by a Newman-Keuls comparison test, or Student t-test in the case of sumatriptan-induced contraction with and without PNU-109291.
Results
Vasomotor responses in cerebral arteries
In human cerebral arteries at basal tone, 5-HT, sumatriptan, alniditan and IS-159 consistently elicited a strong and dose-dependent vasoconstriction (Figure 1). The 5-HT1 receptor agonists sumatriptan, alniditan and IS159 behaved as full agonists, inducing maximal contractions which were not significantly different from that elicited by 5-HT (Table 1). However, while alniditan was as potent as 5-HT in inducing contraction of human cerebral arteries, sumatriptan and IS-159 both exhibited significantly lower pD2 values (Table 1). In contrast, the selective 5-HT1D and 5-HT1F receptor agonists PNU-109291 and LY344864, respectively, were both devoid of any significant vasocontractile activity in human brain vessels (Figure 1, Table 1).
Figure 1.
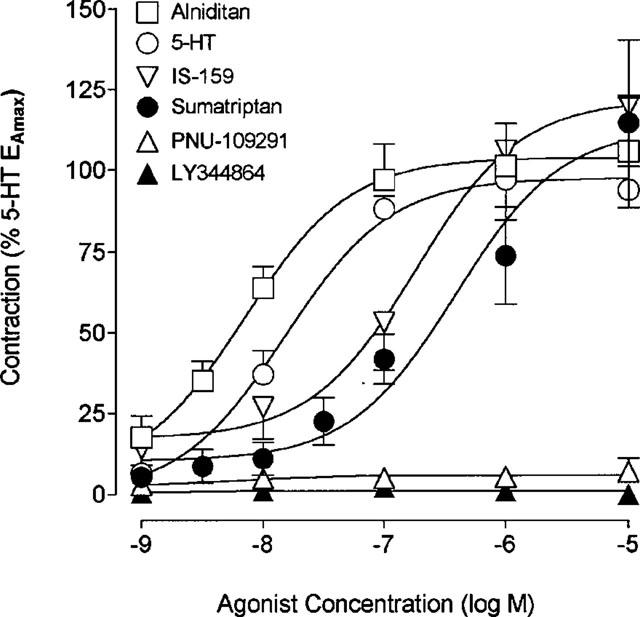
Concentration-response curves for 5-HT, sumatriptan, alniditan, IS-159, PNU-109291 and LY344864 in human cerebral arteries under resting tension. The EAmax for each agonist is expressed as a percentage of 5-HTEAmax measured in the same vascular segments. Complete information on potency, maximal response and number of vascular segments is given in Table 1. Vertical bars show s.e.mean of n=5–8.
Table 1.
Potencies of 5-HT1 receptor agonists in inducing contraction of human cerebral arteries
5-HT, sumatriptan and alniditan also elicited potent contraction of bovine cerebral arteries at basal tone (Figure 2). In this species, alniditan and sumatriptan both induced a significantly weaker maximal contraction than that mediated by 5-HT (55±3% and 36±5%, respectively of 5-HT EAmax, P<0.001, Table 2). As in human brain vessels, alniditan was as potent as 5-HT and sumatriptan exhibited a significantly lower pD2 values than these two agonists (Table 2). The selective 5-HT1D and 5-HT1F receptor agonists PNU-109291 and LY344864, respectively, did not elicit any significant contraction of bovine cerebral arteries (Figure 2, Table 2). In vessels incubated with 10−6 M PNU-109291, sumatriptan induced concentration-dependent contractions with a similar potency and a slightly lower but not significantly different maximal response (EAmax=88±8%, n=7) than those obtained in the same vessels tested before application of the selective 5-HT1D receptor agonist. The rank order of agonists potency in human and bovine cerebral arteries was thus comparable and could be summarized as: 5-HT=alniditan>sumatriptan=IS-159>>> PNU-109291=LY344864.
Figure 2.
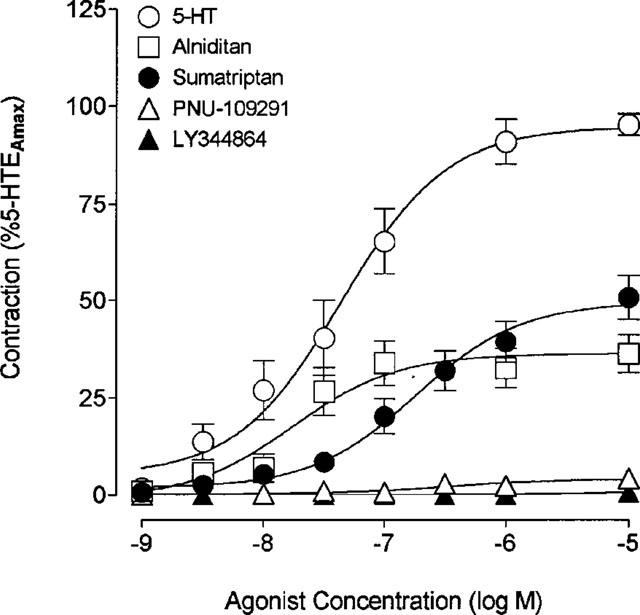
Concentration-response curves for 5-HT, sumatriptan, alniditan, PNU-109291 and LY344864 in bovine cerebral arteries under resting tension. The EAmax for each agonist is expressed as a percentage of 5-HT EAmax obtained in the same vascular segments. See Table 2 for detailed information on individual potency, maximal response and number of vascular segments. Vertical bars indicate s.e.mean of n=10–12.
Table 2.
Potencies of 5-HT1 receptor agonists in inducing contraction of bovine cerebral arteries

The contractions elicited by 5-HT and sumatriptan in bovine vessels were antagonized by GR127935, which induced a rightward shift in the dose-response curve to both agonists, together with a small but significant decrease in maximal responses for 5-HT (28%, P<0.001 at 10−6 M) and sumatriptan (21%, P<0.05) at the highest antagonist concentration (Figures 3 and 4). The mean pA2 value calculated for all GR127935 concentrations against 5-HT was 8.06±0.18. Schild plot analysis (r=0.99) of the inhibition yielded a pA2 value evaluated at the intercept of 7.89 (Figure 3B). An overall pA2 value of 7.98±0.12 (s.d.) was thus obtained for GR127935 against 5-HT. When sumatriptan was used as the agonist, GR127935 was a slightly more potent antagonist in inhibiting the contractile response with a calculated pA2 value of 8.50±0.39 (Figure 4A). In the Schild plot analysis (r=0.96), the pA2 value at intercept was 8.73 (Figure 4B), for an overall pA2 value of 8.61±0.12 (s.d.).
Figure 3.
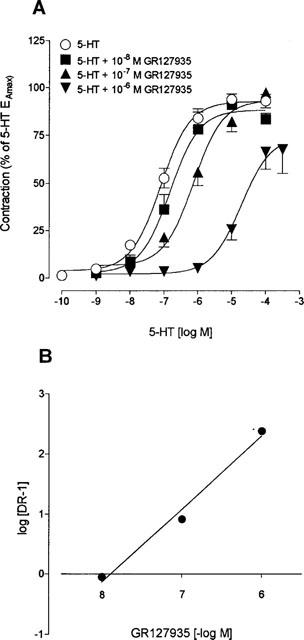
(A) Concentration-response curves for 5-HT in bovine cerebral arteries in the absence (control) and in presence of various concentrations (10−8–10−6 M) of GR127935. Values are expressed as percentage of the 5-HT EAmax measured in the same arterial segments and are means±s.e.means of n=20, 7, 11 and 9, respectively. (B) Schild plot analysis of the effect of GR127935 on 5-HT-induced contraction of bovine cerebral arteries (r=0.99, slope of 1.20±0.02).
Figure 4.
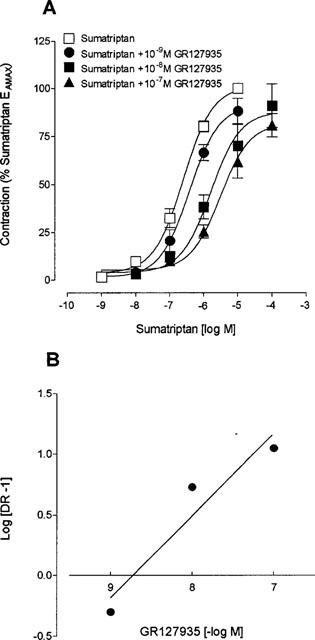
(A) Concentration-response curve for sumatriptan in bovine arteries in the absence (control) and in the presence of various concentrations (10−9–10−7 M) of GR127935. Values are expressed as percentage of the sumatriptan EAmax measured in the same arterial segments and are means±s.e.means of n=18, 5, 7, and 6, respectively. (B) Schild plot analysis of the effect of GR127935 on sumatriptan-induced contraction of bovine cerebral arteries (r=0.96, slope of 0.68±0.20).
5-HT1 receptor mRNAs expression in human coronary arteries
Upon gel electrophoresis, PCR products of the expected size for the 5-HT1D receptor were never detected in human coronary arteries despite successful amplification in control tissue (Figure 5). In contrast, the 5-HT1B receptor message was strongly expressed in a majority (>60%) of human coronary arteries and messages for the 5-HT1F receptor were detected in about 40% of human coronary arteries. The intensity of the latter PCR products, however, was systematically very faint on the ethidium bromide-stained agarose gels (Figure 5).
Discussion
The present data with selective 5-HT1D, 5-HT1B/1D and 5-HT1F receptor agonists allow to conclude that the 5-HT1B receptor is the exclusive mediator of the cerebral constriction elicited by sumatriptan and pharmacologically related compounds in both bovine and human brain arteries at basal tone. Furthermore, the results indicate that this receptor subtype is expressed in a majority of human coronary arteries, in which it is possibly responsible for the reported arterial vasoconstriction elicited by sumatriptan and other non-selective 5-HT1 receptor agonists (Kaumann et al., 1994; Ferro et al., 1995; Maassen VanDenBrink et al., 1998). Altogether these results suggest that selective 5-HT1D and/or 5-HT1F receptor agonists, if proven clinically effective, could represent a new generation of antimigraine compounds with a neuronal site of action and an increased cardiovascular safety.
The 5-HT1 receptor agonist sumatriptan was found to elicit a contraction of both human and bovine cerebral arteries, with a potency similar to that reported in previous studies in human (Parsons, 1991; Hamel et al., 1993b), bovine (Hamel et al., 1993b) and dog (Beattie & Connor, 1995) cerebral arteries. In bovine cerebral arteries, the maximal response elicited by sumatriptan and other 5-HT1 receptor agonists was predictably less than that of 5-HT, but was also slightly smaller than that reported previously by us for sumatriptan in the same preparation (Hamel et al., 1993b), probably a consequence of the seasonal variations in contractile 5-HT receptors (Vinall et al., 1991). The difference between 5-HT and sumatriptan maximal response, however, is most likely due to the participation of 5-HT2A receptors in the 5-HT-mediated vasoconstriction in this species (Frenken & Kaumann, 1984; De Wever et al., 1990; Foy et al., 1992), a population of receptors which is either not present or not functional in human brain vessels (Hamel & Bouchard, 1991; Kaumann et al., 1993). The vasocontractile response to 5-HT in bovine cerebral arteries was inhibited by the 5-HT1 receptor antagonist GR127935 with an overall potency (pA2≈8) which was slightly less than that observed when sumatriptan was used as the agonist (pA2≈8.6). The fact that, in bovine vessels, 5-HT also interacts with 5-HT2A receptors for which GR127935 has a lower affinity (pKi of 7.4) as compared to 5-HT1B/1D receptors (Skingle et al., 1996), most likely account for this weaker potency of GR127935 against 5-HT. Interestingly, GR127935 potency against sumatriptan in bovine vessels was identical to that observed for this antagonist against sumatriptan in ovine main branch (pKb=8.5) and second branch (pKb=8.7) middle cerebral arteries (Teng et al., 1998), and compared very well with that reported at rodent 5-HT1B receptors (pKi=8.5) (Skingle et al., 1996). However, it was slightly less than expected from a previous study in the dog basilar artery where it behaved as a very potent insurmountable antagonist (Skingle et al., 1996). These apparent discrepancies in potency and type of antagonism in dog cerebrovascular tissues may be partly related to species differences but could also be due to the fact that, in the present study, most vessels were exposed only once to this highly lipophilic antagonist in order to avoid tachyphylaxis, a phenomenon which would artificially increase the antagonist potency. The cerebrovascular potency of GR127935 in bovine vessels is thus fully compatible with an interaction with functional 5-HT1B/1D receptors, and not with 5-HT1F receptors for which it exhibits much lower affinity (pKi of 7.1, personal communication Dr H Connor).
The vasocontractile response obtained with alniditan and/or IS-159, two compounds not structurally related to sumatriptan and with low or no affinity at the 5-HT1F receptor subtype (respective pKi values of 6 and <5; Leysen et al.,1996; Hamel, 1996), further indicates that 5-HT1F receptors are not involved in the 5-HT1 receptor-mediated cerebral vasoconstriction in both man and bovine. The potent contraction elicited by the benzopyran derivative alniditan in human and bovine cerebral arteries also agrees with previous reports in the dog carotid artery (Van de Water et al., 1995) and the pig carotid arteriovenous anastomoses (De Vries et al., 1997), in which alniditan was found to be significantly more potent than sumatriptan. The high efficacy of alniditan in the present study fully agrees with its reported higher affinity at 5-HT1B/1D receptors than sumatriptan (Hamel et al., 1996; Leysen et al., 1996; Lesage et al., 1998). Moreover, alniditan is reportedly about ten times more potent than sumatriptan at the human 5-HT1B receptor and only twice as potent as sumatriptan at the human 5-HT1D receptor in mediating inhibition of adenylyl cyclase (Lesage et al., 1998). Based on this observation and the significantly greater potency for alniditan as compared to sumatriptan in eliciting cerebral vasoconstriction in the present study (at least 10 fold), we can suggest that these compounds act at a 5-HT1B receptor to elicit cerebral vasoconstriction.
This assumption is unequivocally supported by the lack of vasocontractile effect of the selective 5-HT1D and 5-HT1F receptor agonists PNU-109291 and LY344864, respectively. Indeed, PNU-109291 which has a 5000 times higher affinity at the 5-HT1D than the 5-HT1B receptor (Ki of 0.9 and 5775 nM, respectively; Ennis et al., 1998), did not elicit any significant contraction at concentrations up to 10−5 M. Further, the fact that 10−6 M PNU-109291, a concentration which is well in excess to its affinity at 5-HT1D receptors, failed to significantly affect the sumatriptan-induced vasocontractile response provided additional arguments for the absence of functional 5-HT1D receptors in cerebral vessels. Such a statement also agrees with recent studies which showed this compound to be devoid of any effect on carotid resistance in the cat (Ennis et al., 1998), and the sumatriptan-induced contraction of the porcine carotid arteriovenous anastomoses to be blocked by selective 5-HT1B, but not 5-HT1D receptor antagonists (DeVries et al., 1999). Similarly, a lack of vasomotor effect for the selective 5-HT1F receptor agonist LY344864 (Ki of 6 nM at 5-HT1F as compared to 549 and 575 nM at 5-HT1B and 5-HT1D receptors, respectively) (Phebus et al., 1997) has also been reported in peripheral blood vessels (Johnson et al., 1997; Phebus et al., 1997). Together with our present findings, these results suggest that the 5-HT1F receptor does not exert vasomotor effects in either brain or peripheral blood vessels. This statement is in line with our previous pharmacological correlation analyses (Hamel et al., 1993b) in human brain vessels that excluded the 5-HT1F receptor in the vasocontractile response to 5-HT and sumatriptan, this despite the presence of 5-HT1F mRNA associated with human brain vessels (Bouchelet et al., 1996b). However, a vascular localization for the receptor appears most unlikely. Indeed, smooth muscle cells from human pulmonary artery and aorta (Ullmer et al., 1995) and human brain microvessels (Cohen et al., 1999) were found not to express any, or very low levels of mRNA for either the 5-HT1D or the 5-HT1F receptor. Interestingly, in the human brain microcirculation, 5-HT1F receptors were expressed in astroglial cells which are closely associated with microvessels. It is thus possible that the 5-HT1D and 5-HT1F receptor messages detected in some human pial vessels (Bouchelet et al., 1996b) are localized in fibroblasts and cells of the pia-arachnoid membrane which may be closely attached to the vessel wall.
Expression of mRNA for 5-HT1 receptors in human coronary arteries indicated an absence of 5-HT1D, a predominance of 5-HT1B with a less frequent and overall weaker expression of 5-HT1F receptor subtype, an overall statement which is well compatible with the results from two recent studies (Ishida et al., 1999; Nilsson et al., 1999) which found barely detectable 5-HT1D receptors expression and no (Ishida et al., 1999) or relatively high (Nilsson et al., 1999) expression levels of 5-HT1F receptors.
In a certain proportion of human coronary arteries, however, we were unable to detect mRNA for either 5-HT1B or 5-HT1F receptor subtypes. Although we believe that this is not due to the selected oligonucleotide primers since they yielded highly reproducible results in human trigeminal ganglia (this study and Bouchelet et al., 1996b), we cannot exclude that the post mortem delay, RNAase activity and difference in the caliber of the arteries may play a role in this variability and small discrepancies between studies. In this regards, it is interesting to note that in a previous functional study (Ferro et al., 1995), about 50% of the human coronary artery segments were unable to constrict in response to either 5-HT or sumatriptan. As well, a large interpatient variability in coronary artery responses to 5-HT was previously reported by Kaumann et al. (1994). Taking this variability into account, the relatively low level of expression of the 5-HT1F receptor mRNA and the non-vascular cellular localization of this receptor in another isolated human cerebrovascular preparation (Cohen et al., 1999), the results of the present study strongly argues that the 5-HT1B is the most likely receptor to be activated by sumatriptan in human coronary arteries. Interestingly, many functional studies in human coronary arteries (Kaumann et al., 1994; Ferro et al., 1995; Maassen VanDenBrink et al., 1998) have previously attributed the sumatriptan-induced vasoconstriction to a pharmacologically defined 5-HT1D-like receptor, best characterized as 5-HT1B receptor (Kaumann et al., 1994). Together with a recent study in human temporal arteries (Verheggen et al., 1998), and the lack of association of 5-HT1F receptors with vascular cells of human brain (Cohen et al., 1999) and peripheral (Ullmer et al., 1995) vessels, our results in human coronary and cerebral arteries would support the statement that 5-HT1B receptors may be the general mediators of regional arterial vasoconstriction elicited by both 5-HT and sumatriptan in man (Verheggen et al., 1998).
These findings are important as they imply that antimigraine drugs with affinity at the 5-HT1B receptor would be endowed with intrinsic potential cardiovascular activity (see Maassen VanDenBrink et al., 1998), a side-effect that has been seriously considered in susceptible patients (Dahlöf & Mathew, 1998).Whether or not a contractile effect at the level of brain vessels is necessary for a drug to be effective in migraine treatment still remains to be demonstrated. However, it is clear that effective antimigraine compounds such as sumatriptan, alniditan (Goldstein et al., 1995) and IS-159 (Hamel et al., 1996) share a common characteristic of high affinity at the 5-HT1B/1D receptors. As both 5-HT1D and 5-HT1F receptor agonists were found to be devoid of cerebrovascular activity (this study) while being active inhibitors of the trigeminovascular-mediated neurogenic inflammation response and/or c-fos expression in trigeminal nucleus caudalis in animal models (Cutrer et al., 1999; Ennis et al., 1998; Phebus et al., 1997), their clinical efficacy in migraine treatment, if proven, might offer new means to selectively target the putative neuronal locus of action of sumatriptan and derivatives. Clinical studies with subtype-selective compounds are mandatory and likely to provide new insights into the pathophysiology of migraine headache.
In conclusion, our results indicate that the 5-HT1B receptor is the exclusive mediator of the constriction elicited by 5-HT1 receptor agonists such as sumatriptan in human and bovine cerebral vessels, and they further show that this receptor is present in human coronary arteries. These findings raise the interesting possibility that new antimigraine drugs targeting selectively the neuronal 5-HT1D and/or 5-HT1F receptors may provide a safer cardiovascular profile. Furthermore, would the clinical efficacy of such compounds be demonstrated, the present findings would suggest that the vascular site of action of the triptans and other 5-HT1 receptor agonists is not required for clinical efficacy.
Acknowledgments
The authors are indebted to the patients and the Brain Bank of the Douglas Hospital Research Centre for the tissues used in this study. They also thank Dr M.J. Moreno for expert advice and Ms L. Michel for secretarial assistance. This study was supported by grants from the Medical Research Council of Canada (grant MA-9967) and the Heart and Stroke Foundation of Québec.
Abbreviations
- CGRP
calcitonin gene-related peptide
- GR127935
(N-[methoxy-3-(4-methyl-1-piperazinyl)phenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)[1,1-biphenyl]-4-carboxamide hydrochloride
- 5-HT
5-hydroxytryptamine, serotonin
- IS-159
serotonin-O-carboxymethylglycyltyrosinamide
- LY344864
(R)-(+)-N-(3-dimethylamino-1,2,3,4-tetrahydro-9H-carbazol-6-yl)-4-fluorobenzamide
- PNU-109291
(S)-(-)-1[2-[4-(4-methoxyphenyl)-1-piperazinyl]ethyl]-N-methylisochroman-6-carboxamide
- RT
reverse transcriptase
- RT–PCR
reverse transcriptase-polymerase chain reaction
References
- ADHAM N., KAO H.-T., SCHECHTER L.E., BARD J.A., OLSEN M., URQUHART D., DURKIN M., HARTIG P.R., WEINSHANK R.L., BRANCHEK T.A. Cloning of another human serotonin receptor (5-HT1F): A fifth 5-HT1 receptor coupled to the inhibition of adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 1993;90:408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEATTIE D.T., CONNOR H.E. The pre- and postjunctional activity of CP-122,288, a conformationally restricted analogue of sumatriptan. Eur. J. Pharmacol. 1995;276:271–276. doi: 10.1016/0014-2999(95)00080-5. [DOI] [PubMed] [Google Scholar]
- BONAVENTURE P., VOORN P., LUYTEN W.H., LEYSEN J.E. 5-HT1B and 5-HT1D receptor mRNA differential co-localization with peptide mRNA in the guinea pig trigeminal ganglion. Neuroreport. 1998;9:641–645. doi: 10.1097/00001756-199803090-00015. [DOI] [PubMed] [Google Scholar]
- BOUCHELET I., CASE B., HAMEL E. Expression of mRNA for serotonin (5-HT) receptors in human coronary arteries. Soc. Neurosci. Abst. 1996a;22 Part 2:1578. [Google Scholar]
- BOUCHELET I., COHEN Z., CASE B., SÉGUÉLA P., HAMEL E. Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral vessels. Mol. Pharmacol. 1996b;50:219–223. [PubMed] [Google Scholar]
- BOULENGUEZ P., CHAUVEAU J., SEGU L., MOREL A., DELAAGE M., LANOIR J. Pharmacological characterization of serotonin-O-carboxymethyl-glycyl-tyrosinamide, a new selective indolic ligand for 5-hydroxytryptamine (5-HT)1B and 5-HT1D binding sites. J. Pharmacol Exp. Ther. 1991;259:1360–1365. [PubMed] [Google Scholar]
- BUZZI M.G., BONAMINI M., MOSKOWITZ M.A. Neurogenic model of migraine. Cephalalgia. 1995;15:277–280. doi: 10.1046/j.1468-2982.1995.1504277.x. [DOI] [PubMed] [Google Scholar]
- CHAUVEAU J., VILLEMURE J.-G., DELAAGE M., HAMEL E. New 5-HT derivatives as agonists at ‘5-HT1D' receptors in human and cat cerebral arteries. 3rd. IUPHAR satellite meeting on serotonin. 1994.
- CHOMCZYNSKI P. A reagent for the single-step simultaneous isolation RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–535. [PubMed] [Google Scholar]
- COHEN Z., BOUCHELET I., VILLEMURE J.-G., BALL R., STANIMIROVIĆ D.B., HAMEL E. Molecular and pharmacological characterization of functional serotonin receptors in human brain microcirculation and astrocytes. J. Cereb. Blood Flow Metab. 1999;19:908–917. doi: 10.1097/00004647-199908000-00010. [DOI] [PubMed] [Google Scholar]
- CUTRER F.M., YU X.J., AYATA G., MOSKOWITZ M.A., WAEBER C. Effects of PNU-109,291, a selective 5-HT1D receptor agonist, on electrically induced dural plasma extravasation and capsaicin-evoked c-fos immunoreactivity within the trigeminal nucleus caudalis. Neuropharmacololy. 1999;38:1043–1053. doi: 10.1016/s0028-3908(99)00032-5. [DOI] [PubMed] [Google Scholar]
- DAHLÖF C.G., MATHEW N. Cardiovascular safety of 5-HT1B/1D agonists–is there a cause for concern. Cephalalgia. 1998;18:539–545. doi: 10.1046/j.1468-2982.1998.1808539.x. [DOI] [PubMed] [Google Scholar]
- DE VRIES P., APAYDIN S., VILLALÓN C.M., HEILIGERS J.P.C., SAXENA P.R. Interactions of GR127935, a 5-HT1B/1D receptor ligand, with functional 5-HT receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1997;355:423–430. doi: 10.1007/pl00004964. [DOI] [PubMed] [Google Scholar]
- DE VRIES P., WILLEMS E.W., HEILIGERS J.P., VILLALON C.M., SAXENA P.R. Investigation of the role of 5-HT1B and 5-HT1D receptors in the sumatriptan-induced constriction on porcine carotid arteriovenous anastomoses. Br. J. Pharmacol. 1999;127:405–412. doi: 10.1038/sj.bjp.0702572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WEVER B., ROOMAN R.P., DE BRABANDER M. Serum and serotonin induce retraction of calf aortic smooth muscle (CASM) cells in vitro: inhibition by ketanserin, a 5-HT2 receptor antagonist. Exp. Cell Res. 1990;186:109–114. doi: 10.1016/0014-4827(90)90216-w. [DOI] [PubMed] [Google Scholar]
- ENNIS M.D., GHAZAL N.B., HOFFMAN R.L., SMITH M.W., SCHLACHTER S.K., LAWSON C.F., IM W.B., PREGENZER J.F., SVENSSON K.A., LEWIS R.A., HALL E.D., SUTTER D.M., HARRIS L.T., MCCALL R.B. Isochroman-6-carboxamides as highly selective 5-HT1D agonists: potential new treatment for migraine without cardiovascular side effects. J. Med. Chem. 1998;41:2180–2183. doi: 10.1021/jm980137o. [DOI] [PubMed] [Google Scholar]
- FERRARI M.D. Sumatriptan in the treatment of migraine. Neurology. 1993;43:S43–S47. [PubMed] [Google Scholar]
- FERRO A., LONGMORE J., HILL R.G., BROWN M.J. A comparison of the contractile effect of 5-hydroxytryptamine, sumatriptan and MK-462 on human coronary artery in vitro. Br. J. Pharmacol. 1995;40:245–251. doi: 10.1111/j.1365-2125.1995.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOY R.A., MYLES J.L., WILKERSON R.D. Characterization of 5-hydroxytryptamine receptors in bovine coronary arteries. J. Pharmacol. Exp. Ther. 1992;261:601–606. [PubMed] [Google Scholar]
- FRENKEN M., KAUMANN A.J. Interaction of ketanserin and its metabolite ketanserinol with 5-HT2 receptors in pulmonary and coronary arteries of calf. Naunyn Schmiedeberg's Arch. Pharmacol. 1984;326:334–339. doi: 10.1007/BF00501438. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J. Serotonin receptors and the acute attack of migraine. Clin. Neurosci. 1998;5:18–23. [PubMed] [Google Scholar]
- GOLDSTEIN J., SCHELLENS R., DIENER H.C., DAHLOF C., OLESEN J., SENARD J.M., STEINER T., SIMARD D., VINGERHOETS I.Alniditan, a novel non-indole derivative 5-HT1D-receptor agonist: a SC dose-finding trial 1995. 6th International Headache Research Seminar, Copenhagenp. 56
- GROSS M. The use of sumatriptan in the treatment of migraine. Br. J. Clin. Pract. 1993;47:205–207. [PubMed] [Google Scholar]
- HAMEL E. 5-HT1D receptors: pharmacology and therapeutic potential. Serotonin Research Alert. 1996;1:19–29. [Google Scholar]
- HAMEL E., BOUCHARD D. Contractile 5-HT1 receptors in human isolated pial arterioles: correlations with 5-HT1D binding sites. Br. J. Pharmacol. 1991;102:227–233. doi: 10.1111/j.1476-5381.1991.tb12158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMEL E., FAN E., LINVILLE D., TING V., VILLEMURE J.-G., CHIA L.-S. Expression of mRNA for the serotonin 5-hydroxytryptamine 1Dβ receptor subtype in human and bovine cerebral arteries. Mol. Pharmacol. 1993a;44:242–246. [PubMed] [Google Scholar]
- HAMEL E., GRÉGOIRE L., LAU B. 5-HT1 receptors mediating contraction in bovine cerebral arteries: a model for human cerebrovascular [5-HT1Dβ] receptors. Eur. J. Pharmacol. 1993b;242:75–82. doi: 10.1016/0014-2999(93)90012-7. [DOI] [PubMed] [Google Scholar]
- HUMPHREY P.P.A., FENIUK W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol. Sci. 1991;12:444–446. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- ISHIDA T., HIRATA K.I., SAKODA T., KAWASHIMA S., AKITA H., YOKOYAMA M. Identification of mRNA for 5-HT1 and 5-HT2 receptor subtypes in human coronary arteries. Cardiovasc. Res. 1999;41:267–274. doi: 10.1016/s0008-6363(98)00162-x. [DOI] [PubMed] [Google Scholar]
- JOHNSON K.W., SCHAUS J.M., DURKIN M.M., AUDIA J.E., KALDOR S.W., FLAUGH M.E., ADHAM N., ZGOMBICK J.M., COHEN M.L., BRANCHEK T.A., PHEBUS L.A. 5-HT1F receptor agonists inhibit neurogenic inflammation in guinea pigs. NeuroReport. 1997;8:2237–2240. doi: 10.1097/00001756-199707070-00029. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., FRENKEN M., POSIVAL H., BROWN A.M. Variable participation of 5-HT1-like receptors and 5-HT2 receptors in serotonin-induced contraction of human isolated coronary arteries:5-HT1-like receptors resemble cloned 5-HT1Dβ receptors. Circulation. 1994;90:1141–1153. doi: 10.1161/01.cir.90.3.1141. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., PARSONS A.A. and , BROWN A.M. Human arterial contractile receptors. Cardiovasc. Res. 1993;27:20094–20103. doi: 10.1093/cvr/27.12.2094. [DOI] [PubMed] [Google Scholar]
- LESAGE A., WOUTERS R., VAN GOMPEL P., HEYLEN L., VANHOENACKER P., HAEGEMAN G., LUYTEN W.H.M.L., LEYSEN J.E. Agonistic properties of alniditan, sumatriptan and dihydroergotamine on human 5-HT1B and 5-HT1D receptors expressed in various mammalian cell lines. Br. J. Pharmacol. 1998;123:1655–1665. doi: 10.1038/sj.bjp.0701766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEYSEN J.E., GOMMEREN W., HEYLEN L., LUYTEN W.H., VAN DE WEYER I., VANHOENACKER P., HAEGEMAN G., SCHOTTE A., VAN GOMPEL P., WOUTERS R., LESAGE A.S. Alniditan, a new 5-hydroxytryptamine1D agonist and migraine-abortive agent: ligand-binding properties of human 5-hydroxytryptamine1Dα, human 5-hydroxytryptamine1Dβ, and calf 5-hydrytryptamine 1D receptors investigated with 3H 5-hydroxytryptamine and 3H alniditan. Mol. Pharmacol. 1996;50:1567–1580. [PubMed] [Google Scholar]
- LONGMORE J., SHAW D., SMITH D., HOPKINS R., MCALLISTER G., PICKARD J.D., SIRINATHSINGHJI D.J.S., BUTLER A.J. Differential distribution of 5-HT1D- and 5-HT1B-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia. 1997;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- MAASSENVANDENBRINK A., REEKERS M., BAX W.A., FERRARI M.D., SAXENA P.R. Coronary side-effect potential of current and prospective antimigraine drugs. Circulation. 1998;98:25–30. doi: 10.1161/01.cir.98.1.25. [DOI] [PubMed] [Google Scholar]
- MARTIN G.R.Serotonin receptor involvement in the pathogenesis and treatment of migraine Headache Blue Books of Practical Neurology 1997vol 17Butterworth-Heineman; 25–38.eds Silberstein S & Goadsby PJ. pp [Google Scholar]
- MOSKOWITZ M.A. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol. Sci. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- NILSSON T., LONGMORE J., SHAW D., PANTEV E., BARD J.A., BRANCHEK T., EDVINSSON L. Characterization of 5-HT receptors in human coronary arteries by molecular and pharmacological techniques. Eur. J. Pharmacol. 1999;372:49–56. doi: 10.1016/s0014-2999(99)00114-4. [DOI] [PubMed] [Google Scholar]
- OTTERWANGER J.P., WILSON J.H., STRICKER B.H. Drug-induced chest pain and myocardial infarction. Reports to a national centre and review of the literature. Eur. J. Clin. Pharmacol. 1997;53:105–110. doi: 10.1007/s002280050346. [DOI] [PubMed] [Google Scholar]
- PARSONS A.A.. 5-HT receptors in human and animal cerebrovasculature. Trends Pharmacol. Sci. 1991;12:310–315. doi: 10.1016/0165-6147(91)90583-e. [DOI] [PubMed] [Google Scholar]
- PEROUTKA S.J., MCCARTHY B.G. Sumatriptan (GR 43175) interacts selectively with 5-HT1B and 5-HT1D binding sites. Eur. J. Pharmacol. 1989;163:133–136. doi: 10.1016/0014-2999(89)90406-8. [DOI] [PubMed] [Google Scholar]
- PHEBUS L.A., JOHNSON K.W., ZGOMBICK J.M., GILBERT P.J., VAN BELLE K., MANCUSO V., NELSON D.L.G., CALLIGARO D.O., KIEFER A.D., JR, BRANCHEK T.A., FLAUGH M.E. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci. 1997;61:2117–2126. doi: 10.1016/s0024-3205(97)00885-0. [DOI] [PubMed] [Google Scholar]
- REBECK G.W., MAYNARD K.I., HYMAN B.T., MOSKOWITZ M.A. Selective 5-HT1Dα serotonin receptor gene expression in trigeminal ganglia: Implications for antimigraine drug development. Proc. Natl. Acad. Sci. U.S.A. 1995;91:3666–3669. doi: 10.1073/pnas.91.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOENEN J. Acute migraine therapy: the newer drugs. Curr. Op. Neurol. 1997;10:237–243. doi: 10.1097/00019052-199706000-00012. [DOI] [PubMed] [Google Scholar]
- SKINGLE M., BEATTIE D.T., SCOPES D.I.C., STARKEY S.J., CONNOR H.E., FENIUK W., HUMPHREY P.P.A., TYERS M.B. GR127935: a potent orally active 5-HT1D receptor antagonist. Behav. Br. Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- TANSEY M.J, , PILGRIM A.J., LLOYD K. Sumatriptan in the acute treatment of migraine. J. Neurol. Sci. 1993;114:109–116. doi: 10.1016/0022-510x(93)90057-6. [DOI] [PubMed] [Google Scholar]
- TENG G.Q., WILLIAMS J., ZHANG L., PURDY R., PEARCE W.J. Effects of maturation, artery size, and chronic hypoxia on 5-HT receptor type in ovine cranial arteries. Am. J. Physiol. 1998;275:R742–753. doi: 10.1152/ajpregu.1998.275.3.R742. [DOI] [PubMed] [Google Scholar]
- ULLMER C., SCHMUCK K., KALKMAN H.O., LÜBBERT H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- VAN DEN BRINK F.G.General theory of drug-receptor interactions. Drug-receptor interaction models. Calculation of drug parameters Kinetics of Drug Action 1977Springer-Verlag, Berlin; J.M. Van Rossum (ed). p. 169 [Google Scholar]
- VAN DE WATER A.D., AUBIOUL J., VAN GERVEN W., VAN AMMEL K., DE CLERCK F. Selective vasoconstriction by alniditan in the carotid vascular bed of anaesthetized dogs. Eur. J. Pharmacol. 1995;299:127–137. doi: 10.1016/0014-2999(95)00848-9. [DOI] [PubMed] [Google Scholar]
- VERHEGGEN R., HUNDESHAGEN A.G., BROWN A.M., SCHINDLER M., KAUMANN A.J. 5-HT1B receptor-mediated contractions in human temporal artery: evidence from selective antagonists and 5-HT receptor mRNA expression. Br. J. Pharmacol. 1998;124:1345–1354. doi: 10.1038/sj.bjp.0701929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINALL P.E., MICHELE J.J., SIMEONE F.A. Seasonal variations of serotonin-induced contractility in vitro in bovine middle cerebral artery. Blood Vessels. 1991;28:547–551. doi: 10.1159/000158902. [DOI] [PubMed] [Google Scholar]


