Abstract
Cumulative concentration-effect curves for the selective prostanoid TP receptor agonist U46619 and six isoprostanes were constructed in the human isolated umbilical artery.
All compounds except 8-iso-PGF3α produced concentration-dependent contractions. The contractile response to the isoprostanes increased with each cumulative addition up to a point, after which subsequent addition reduced the contraction below the previous level. This [downturn] in the concentration-effect curve did not occur with U46619.
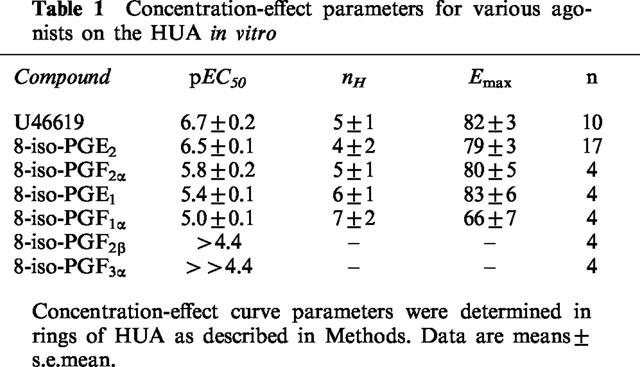
The potencies of the compounds tested were as follows (pEC50±s.e.mean): U46619, 6.7±0.2; 8-iso-PGE2, 6.5±0.1; 8-iso-PGF2α, 5.8±0.2; 8-iso-PGE1, 5.4±0.1; 8-iso-PGF1α, 5.0±0.1; 8-iso-PGF2β> 4.8; 8-iso-PGF3α>> 4.8 (n=4–17). Neither 8-iso-PGF2β nor 8-iso-PGF3α at 44 μM had a significant effect on cumulative concentration-effect curves to U46619.
The selective TP receptor antagonist GR32191 (0.1 μM) caused rightward shifts in the concentration-effect curves to all the active compounds. pA2 values for GR32191 against U46619, 8-iso-PGE2, 8-iso-PGF2α, 8-iso-PGE1 were 7.6±0.2, 9±1, 8.2±0.3 and 7.7±0.3, respectively (n=4).
Neither Nω-nitro-L-arginine methyl ester (100 μM) nor the selective DP receptor antagonist BW A868C (50 nM) affected the complex concentration-effect curve to 8-iso-PGE2 (n=3).
Stable contractions to U46619 (1–3 μM) were unaffected by anandamide at concentrations up to 60 μM.
Keywords: Human umbilical artery, contraction, isoprostanes, TP receptors, U46619
Introduction
The isoprostanes are stable arachidonic acid metabolites that appear in normal human plasma and urine at concentrations at least an order of magnitude greater than those of the prostaglandins (Morrow et al., 1990). Isoprostanes differ structurally from prostaglandins by the cis-orientation at the cyclopentane ring junction compared to the trans-orientation in the classical prostanoids (see Figure 1). Although originally thought to be formed exclusively by a cyclo-oxygenase-independent, free-radical catalyzed mechanism (Morrow et al., 1990), there is now mounting evidence to suggest that some isoprostanes can also be formed by a cyclo-oxygenase-dependent pathway (Jourdan et al., 1999; Montuschi et al., 1999). The isoprostane family is potentially very large. The F2-isoprostane family (isomers of prostaglandin (PG) F2α) alone is composed of four regioisomers each theoretically composed of eight racemic diastereomers (Morrow & Roberts, 1996). Not only F-isoprostanes, but also D- and E-ring isoprostanes are formed in vivo (Morrow et al., 1994).
Figure 1.

Chemical structures of 8-iso-PGE2, PGE2 and thromboxane A2.
Concentrations of isoprostanes, particularly the most widely studied 8-iso-PGF2α, are increased in a number of pathophysiological conditions including experimental diabetes (Wentzel et al., 1999), Alzheimer's disease (Montine et al., 1998), and anaphylaxis (Montuschi et al., 1999). But whether they contribute to these pathologies, as opposed to being mere markers for them, is largely unknown. In this regard, the pharmacology of the isoprostanes is poorly understood and knowledge is limited almost exclusively to 8-iso-PGF2α.
8-iso-PGF2α is a potent stimulant of vascular (Takahashi et al., 1992; Kang et al., 1993; Kromer & Tippins, 1996; Zhang et al., 1996; John & Valentin, 1997), airway (Kang et al., 1993; Kawikova et al., 1996), intestinal (Elmhurst et al., 1997) and uterine (Crankshaw, 1995) smooth muscles where its effects are sensitive to selective prostanoid TP receptor antagonists. Consequently, the effects of 8-iso-PGF2α are generally, though not exclusively, thought to be mediated by action at TP receptors (Kromer & Tippins, 1999). 8-iso-PGF2α is also a partial agonist at platelet TP receptors (Yin et al., 1994). 8-iso-PGE2 also has smooth muscle stimulant properties that are TP receptor antagonist-sensitive (Morrow et al., 1994; Fukunaga et al., 1993a) and this has led to speculation that spatial orientation of the side-chains is a more important determinant of isoprostane/receptor interactions than structural differences in the cyclopentane ring (Morrow & Roberts, 1996). Figure 1 shows the structure of 8-iso-PGE2 and compares it to both PGE2 and thromboxane A2, the endogenous ligand for TP receptors.
We recently demonstrated that the human umbilical artery (HUA) operationally expresses only one excitatory prostanoid receptor subtype, namely TP (Boersma et al., 1999). This preparation is therefore an ideal one in which to investigate operational aspects of the structure-activity relationships of isoprostanes with human TP receptors. In this study we have, therefore, compared the effects of a number of commercially available isoprostanes and the TP receptor agonist U46619 (Coleman et al., 1994) on the contractility of the HUA in vitro. Some of these data have been communicated to the British Pharmacological Society (Oliveira & Crankshaw, 1998; Oliveira et al., 1998).
Methods
Tissue collection and preparation
Sections of umbilical cords within 20 cm of the placenta were obtained from full-term vaginal or Caesarean births and collected in cold buffered physiological salt solution (BPSS). Tissues were stored at 4°C and used within 24 h. Human umbilical arteries were dissected free of Wharton's Jelly and cut into transverse rings 3–5 mm in length. Endothelial cells were mechanically removed and removal of the cells was confirmed by histology.
Isometric contractions
Rings were suspended on stainless-steel hooks and mounted in individual 5 ml jacketed muscle baths containing oxygenated (95% O2 and 5% CO2) physiological salt solution (PSS) at 37°C. One hook was anchored in the bath while the other was attached with silk thread to a FT-03 force displacement transducer writing to a 7D 8-channel polygraph (Grass Instruments). A resting tension of 30 mN was applied to each tissue ring. Individual rings were washed and allowed to equilibrate for 3 h under these conditions during which time spontaneous tone developed. Tissues were then challenged with 60 mM KCl. Once the maximum response to the KCl challenge was achieved, tissues were washed three times and allowed to equilibrate for 20 min to allow baseline to be reached again. The KCl challenge was performed a total of three times.
Agonist potency
Eighty minutes after the last KCl challenge had been washed out, concentration-effect experiments were performed by cumulative addition of agonists to produce approximately half log unit increases in the bath concentration per addition. When the response to the last agonist addition had reached a plateau, the PSS was washed from the bath and replaced with deionized water in order to induce a hypotonic shock. The contraction produced by the hypotonic shock was used to normalize all drug-induced responses (Boersma et al., 1999).
Concentration-effect curves were constructed from the data obtained by fitting to a form of the logistic equation:
where E is the effect of the agonist, Emin is the effect in the absence of agonist, Emax is the maximum effect, C is the molar concentration of the agonist, nH is the slope parameter, and pEC50 is the negative log of the molar concentration of the agonist that produces a half-maximal response. Individual concentration-effect curves in which a reduced response was obtained at higher agonist concentrations were analysed by ignoring the points falling below and to the right of the peak response. In experiments where antagonists were used, they were added to the bath 60 min before the start of the concentration-effect experiment. Concentration-effect experiments were performed in antagonist-treated and matched control rings from the same donor. pEC50 values were calculated as described above. Single dose-ratio estimates of pA2 values were then determined according to the equation:
where EC50A is the agonist EC50 in the presence of antagonist, EC50C is the control agonist EC50 and [B] is the molar concentration of antagonist.
Effects of anandamide on stable contractions
Stable contractions to U46619 were obtained by adding either 1 or 3 μM of the agonist and allowing 30 min for the response to equilibrate. Thereafter, anandamide was added cumulatively as described for agonist potency experiments.
Drugs and chemicals
Anandamide (arachidonlyethanolamide), U46619 (9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α), 8-iso-PGE2, 8-iso-PGE1, 8-iso-PGF3α, 8-iso-PGF2α, 8-iso-PGF2β and 8-iso-PGF1α were obtained from Cayman Chemical (Ann Arbor, MI, U.S.A.). Indomethacin, 5-HT and Nω-nitro-L-arginine methyl ester (L-NAME) were obtained from Sigma (Oakville, ON, Canada). The following compounds were received as gifts: BW A868C (3-benzyl-5-(6-carboxyhexyl)-1-(2-cyclohexyl-2-hydroxyethylamino)hydantoin) from Wellcome (Beckenham, U.K.); GR32191 ([1R-[1α(Z),2β,3β,5α]]-(+)-7-[5-[[(1,1′-biphenyl)-4-yl]methoxy]-3-hydroxy-2-(1-piperidinyl)cyclopentyl]-4-heptenoic acid) from Glaxo-Wellcome (Stevenage, U.K.); All other chemicals were from BDH (Toronto, ON, Canada). Indomethacin was prepared as described by Curry et al. (1982). All other drugs were made as solutions in ethanol. Immediately before experiments, appropriate serial dilutions of drugs were made in double distilled water from concentrated stock solutions.
Solutions
The BPSS had the following composition (mM): N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid 5.0, NaCl 150, KCl 4.6, MgS04 1.2, CaCl2 2.5, glucose 11.1, and indomethacin 0.01, pH 7.4. The PSS was composed as follows (mM): KCl 4.6, MgSO4 1.16, NaH2PO4 1.16, CaCl2 2.5, NaCl 115.5, NaHCO3 21.9, glucose 11.1 and indomethacin 0.03.
Statistics
All data are expressed as means±s.e.mean. The concentration-effect parameters for U46619 in the absence and in the presence of isoprostanes were compared using a paired Student's t-test, values of P<0.05 were considered significant.
Results
U46619 caused concentration-dependent contraction of HUA. Contractions were slow to develop and tonic in nature at low agonist concentrations, with relatively slow phasic contractions often superimposed on the tonic background at higher concentrations. All the isoprostanes tested, except 8-iso-PGF3α at concentrations up to 44 μM, produced measurable contractions. Responses to the isoprostanes were similar in nature to those to U46619 except that the response to each cumulative addition of isoprostane increased up to a point, after which a subsequent addition reduced the contraction below the previous level. In some cases the response to the most effective concentration of isoprostane was tonic and well maintained, in other cases it was transient. Illustrative traces of the responses to U46619 and 8-iso-PGE2 are shown in Figure 2.
Figure 2.
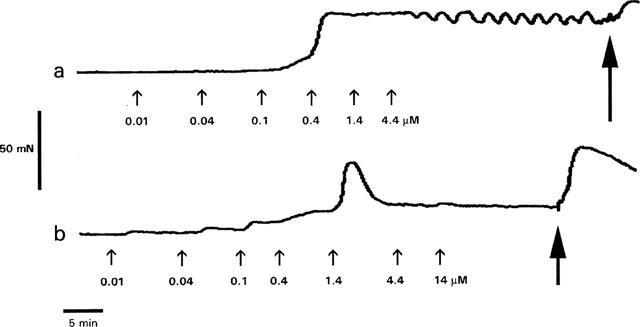
Sample traces showing the effect of cumulative addition of (a) U46619 and (b) 8-iso-PGE2 on tension development by rings of human umbilical artery. Agonists were added to the baths at the points indicated by the small arrows to give the cumulative concentrations shown. The large arrows indicate the points at which the bath contents were replaced with deionized water in order to induce a hypotonic shock. The peak response to hypotonic shock was used to normalize the drug-induced responses.
Responses to all active compounds were sensitive to the selective TP receptor antagonist GR32191 (Lumley et al., 1989) at 0.1 μM. GR32191 alone produced no measurable effect. Mean concentration-effect curves for the active compounds obtained from matched rings in the presence and absence of GR32191 are given in Figure 3. Concentration-effect parameters obtained from all control experiments with the compounds tested are given in Table 1. The pA2 values for GR32191 against U46619, 8-iso-PGE2, 8-iso-PGF2α and 8-iso-PGE1 were 7.6±0.2, 9±1, 8.2±0.3 and 7.7±0.3, respectively (n=4). pA2 values against 8-iso-PGF1α, 8-iso-PGF2β and 8-iso-PGF3α could not be calculated because of the low potency of the agonists.
Figure 3.
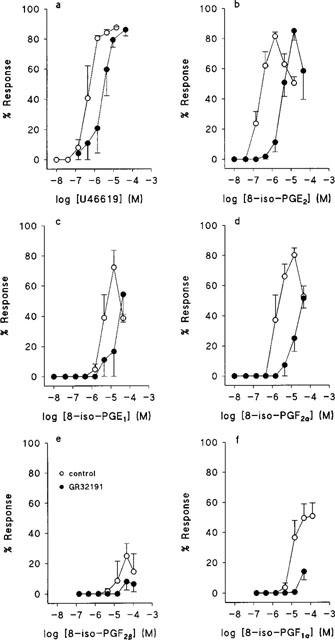
Effects of U46619 and some isoprostanes on tension development by rings of human umbilical artery. Mean concentration-effect curves obtained in the absence and presence of GR32191, 0.1 μM. (a) U46619, n=4; (b) 8-iso-PGE2, n=17; (c) 8-iso-PGE1, n=4; (d) 8-iso-PGF2α, n=4; (e) 8-iso-PGF2β, n=4; (f) 8-iso-PGF1α, n=4. Values are means±s.e.mean.
Table 1.
Concentration-effect parameters for various agonists on the HUA in vitro
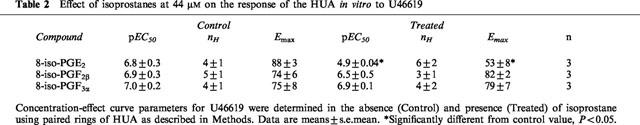
We tested some isoprostanes for antagonist activity. Neither of the two least potent compounds, 8-iso-PGF2β (effective at 4 to 44 μM with reduced maximum response, Figure 3), nor 8-iso-PGF3α (ineffective at concentrations up to 44 μM) at 44 μM had a significant effect on cumulative concentration-effect curves to U46619. However, 8-iso-PGE2, at the same concentration, significantly reduced both the pEC50 and the maximum response to U46619. Data from these experiments are summarized in Table 2. By contrast, 8-iso-PGE2 did not reduce the pEC50 for 5-HT. In these experiments pEC50, nH, and Emax values were 7.1±0.5, 3±1, and 68±15 in control and 7.5±0.4, 12±10, and 67±3 in isoprostane-treated rings, respectively (n=3).
Table 2.
Effect of isoprostanes at 44 μM on the response of the HUA in vitro to U46619
When cumulative concentration-effect experiments to 8-iso-PGE2 were performed in matched rings in either the presence or the absence of 100 μM L-NAME, the resulting concentration-effect curves were superimposable. Similar results were obtained when the selective DP receptor antagonist, BW A868C (Giles et al., 1989), at 50 nM was substituted for L-NAME. Data from these experiments are shown in Figure 4.
Figure 4.
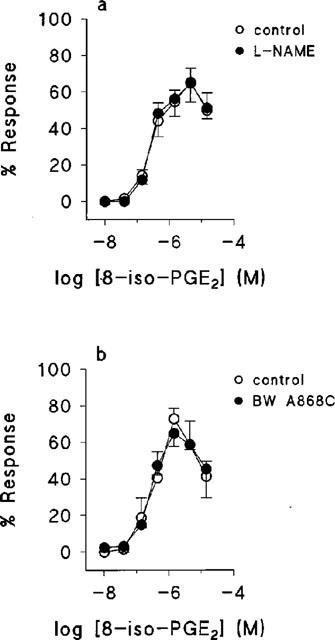
The effect of L-NAME and BW A868C on the response of human umbilical artery to 8-iso-PGE2. Mean concentration-effect curves obtained in the absence and presence of (a) 100 μM L-NAME, (b) 50 nM BWA 868C. In both cases n=3. Values are means ±s.e.mean.
Stable contractions of HUA to 1 or 3 μM U46619 were not affected in any way by cumulative addition of the endogenous cannabimimetic eicosanoid, anandamide (Felder & Glass, 1998), up to 60 μM.
Discussion
Under the conditions of our experiments, the HUA operationally expresses only one subtype of excitatory prostanoid receptor, TP (Boersma et al., 1999). All the active isoprostanes we tested were sensitive to the selective TP receptor antagonist GR32191, and it is therefore most likely that the effects we saw were mediated by human TP receptors. The slopes of the concentration effect-curves were very steep (Figure 3) compatible with our previous observations on HUA under these conditions (Boersma et al., 1999). The relatively large variability in slope parameters (Tables 1 and 2) may be attributed in part to inaccuracies introduced by the necessity to ignore the downturn phase in the analysis of excitatory concentration-effect curves. However, considerable variability in the slope parameter was also observed for agonists that did not produce a downturn (Boersma et al., 1999) suggesting significant inter-preparation variation. The reason for this variation is unclear to us.
The potency order shown in Table 1 suggests several conclusions regarding the structural requirements for isoprostane/receptor activation. Firstly, the superior potency of 8-iso-PGE2 over 8-iso-PGF2α and of 8-iso-PGE1 over 8-iso-PGF1α demonstrates that E-ring compounds are more potent than F-ring compounds. Secondly, the superior potency of 8-iso-PGE2 over 8-iso-PGE1 and of 8-iso-PGF2α over 8-iso-PGF1α demonstrates that doubly unsaturated compounds are more potent than singly unsaturated compounds. Thirdly, the much greater potency of 8-iso-PGF2α over 8-iso-PGF2β shows the importance of the α-configuration. 8-iso-PGF1α appears to be a partial agonist since it elicited a lower maximum response than U46619 (Figure 2, Table 1) and did not demonstrate any inhibitory effects. The extremely low potencies of 8-iso-PGF2β and 8-iso-PGF3α are likely to be attributable to a loss of affinity, since we were unable to demonstrate any antagonist activity for these compounds as opposed to that shown by 8-iso-PGE2 (Table 2).
GR32191 has a pKb of 8.1 against U46619 in HUA (Boersma et al., 1999) and a pA2 of 8.2 against U46619 in human pulmonary artery (Lumley et al., 1989), evidence supports action at TP receptors in both cases. The pA2 values that we obtained for GR32191 against isoprostanes in the present study are compatible with action at TP receptors. The large standard error on the pA2 estimate against 8-iso-PGE2 results from one tissue that was almost completely blocked by GR32191. Isoprostanes have been suggested to act at distinct isoprostane receptors (Fukunaga et al., 1993b) but the present results do not support the existence of distinct isoprostane receptors in HUA. However, isoprostanes do have actions that are not TP receptor antagonist-sensitive (Elmhurst et al., 1997; Jourdan et al., 1997), the structure-activity requirements for these actions remain unknown.
There are several possible explanations for the downturn in the concentration-effect curves to the isoprostanes at high agonist concentrations (Figure 2). In rat isolated pulmonary artery 8-iso-PGF2α causes constriction by a TP receptor-mediated pathway, and dilatation by a non-TP receptor mechanism that involves the release of NO (Jourdan et al., 1997). If such a mechanism were operative in HUA, a complex concentration-effect curve to isoprostanes would likely result. However, NO is unlikely to contribute to the effects we observed since we had mechanically removed the endothelium, the primary source of NO in blood vessels (Pollock et al., 1991). This notion is supported by the failure of the NO synthase inhibitor, L-NAME, to alter the concentration-effect curve to 8-iso-PGE2 in any way (Figure 3a).
Alternatively, isoprostanes might simultaneously activate mutually opposing prostanoid receptor populations. The role of DP receptors in opposing the constrictor effects of 8-iso-PGE2 was ruled out by the failure of BW A868C to alter the concentration-effect curve to 8-iso-PGE2 in any way (Figure 3b). Less direct evidence also argues against the involvement of EP2, EP4 or IP receptors. The inability of PGE2 or cicaprost to reverse a U46619-induced contraction (Boersma et al., 1999) suggest that none of these receptors is operationally expressed at a sufficient level to mediate the downturn in response to the isoprostanes. The arachidonic acid metabolite, anandamide relaxes vascular smooth muscle by a number of putative mechanisms, including stimulation of cannabinoid receptors (Mombouli et al., 1999). The failure of anandamide to reverse U46619-induced contractions provides indirect evidence that isoprostanes do not relax the HUA at high concentrations through release of anandamide or mimicry of its actions.
A third possible explanation for the downturn in the concentration-effect curves to isoprostanes is receptor desensitization at high agonist concentrations. Two observations are consistent with this hypothesis. Firstly, the downturn was shifted by GR32191 (Figure 2), suggesting it was a TP receptor-mediated event. This argument is dependent upon the well-recognized selectivity of GR32191 (Lumley et al., 1989; Coleman et al., 1994), however, if the downturn is mediated by a heretofore-uncharacterized receptor, insensitivity to GR32191 cannot be assumed. Secondly, the partial agonist 8-iso-PGF1α did not produce a downturn. If receptor desensitization were the explanation, we would have expected U46619 to produce a downturn also, which it did not (Figure 2). The discrepancy is unlikely to result from isoprostanes and U46619 acting at different receptors since 8-iso-PGE2 antagonized U46619, but not 5-HT. Differential effects of the agonists could be mediated by the same receptor if there is agonist-specific dual coupling of TP receptors to both excitatory and inhibitory pathways, however, we have no current evidence to support such a mechanism.
In conclusion, we have demonstrated that a number of isoprostanes cause constriction of the HUA, most likely by activation of TP receptors. Structural determinants of the potency of these compounds in this system reside in both the cyclopentane ring and the side chains. Free plasma concentrations of isoprostanes during oxidative stress associated with smoking (Morrow et al., 1995) or cystic fibrosis (Collins et al., 1999) are between 0.2 and 0.9 nM. Concentrations at the site of production are likely to be considerably higher and capable of TP receptor activation. Our data suggest that the isoprostanes should be considered to be important pharmacological agents, not mere markers for oxidative stress.
Acknowledgments
We thank the Labour & Delivery staff at Chedoke McMaster Hospital for helping us to collect umbilical cords. We are grateful to all those who provided drugs used in this study. The Medical Research Council of Canada supported this work.
Abbreviations
- BPSS
buffered physiological salt solution
- HUA
human umbilical artery
- L-NAME
Nω-nitro-L-arginine methyl ester
- PG
prostaglandin
- PSS
physiological salt solution
References
- BOERSMA J.I., JANZEN K.M., OLIVEIRA L., CRANKSHAW D.J. Characterization of excitatory prostanoid receptors in the human umbilical arteryin vitro. Br.J.Pharmacol. 1999;128:1505–1512. doi: 10.1038/sj.bjp.0702965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- COLLINS C.E., QUAGGIOTTO P., WOOD L., O'LOUGHLIN E.V., HENRY R.L., GARG M.L. Elevated plasma levels of F2α isoprostane in cystic fibrosis. Lipids. 1999;34:551–556. doi: 10.1007/s11745-999-0397-1. [DOI] [PubMed] [Google Scholar]
- CURRY S.H., BROWN E.A., KUCK H., CASSIN S. Preparation and stability of indomethacin solutions. Can. J. Physiol. Pharmacol. 1981;60:988–992. doi: 10.1139/y82-139. [DOI] [PubMed] [Google Scholar]
- CRANKSHAW D. Effects of the isoprostane, 8-epi-prostaglandin F2α, on the contractility of the human myometrium in vitro. Eur. J. Pharmacol. 1995;285:151–158. doi: 10.1016/0014-2999(95)00398-5. [DOI] [PubMed] [Google Scholar]
- ELMHURST J.L., BETTI P.-A., RANGACHARI P.K. Intestinal effects of isoprostanes: Evidence for the involvement of prostanoid EP and TP receptors. J. Pharmacol. Exp. Ther. 1997;283:1198–1205. [PubMed] [Google Scholar]
- FELDER C.C., GLASS M. Cannabinoid receptors and their endogenous agonists. Annu. Rev. Pharmacol. Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- FUKUNAGA M., MAKITA N., ROBERTS L.J., II, MORROW J.D., TAKAHASHI K., BADR K.F. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am. J. Physiol. 1993b;264:C1619–C1624. doi: 10.1152/ajpcell.1993.264.6.C1619. [DOI] [PubMed] [Google Scholar]
- FUKUNAGA M., TAKAHASHI K., BADR K.F. Vascular smooth muscle actions and receptor interactions of 8-iso-prostaglandin E2, an E2-isoprostane. Biochem. Biophys. Res. Commun. 1993a;195:507–515. doi: 10.1006/bbrc.1993.2075. [DOI] [PubMed] [Google Scholar]
- GILES H., LEFF P., BOLOFO M.L., KELLY M.G., ROBERTSON A.D. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br. J. Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHN G.W., VALENTIN J.-P. Analysis of the pulmonary hypertensive effects of the isoprostane derivative, 8-iso-PGF2α, in the rat. Br. J. Pharmacol. 1997;122:899–905. doi: 10.1038/sj.bjp.0701441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOURDAN K.B., EVANS T.W., CURZEN N.P., MITCHELL J.P. Evidence for a dilator function of 8-iso prostaglandin F2α in rat pulmonary artery. Br. J. Pharmacol. 1997;120:1280–1285. doi: 10.1038/sj.bjp.0701052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOURDAN K.B., EVANS T.W., GOLDSTRAW P., MITCHELL J.A. Isoprostanes and PGE2 production in human isolated pulmonary artery smooth muscle cells: concomitant and differential release. Faseb. J. 1999;13:1025–1030. doi: 10.1096/fasebj.13.9.1025. [DOI] [PubMed] [Google Scholar]
- KANG K.H., MORROW J.D., ROBERTS L.J., II, NEWMAN J.H., BANERJEE M. Airway and vascular effects of 8-epi-prostaglandin F2α in isolated perfused rat lung. J. Appl. Physiol. 1993;74:460–465. doi: 10.1152/jappl.1993.74.1.460. [DOI] [PubMed] [Google Scholar]
- KAWIKOVA I., BARNES P.J., TAKAHASHI T., TADJKARIMI S., YACOUB M.H., BELVISI M.G. 8-epi-PGF2α, a novel noncyclooxygenase-derived prostaglandin, constricts airways in vitro. Am. J. Respir. Crit. Care. Med. 1996;153:590–596. doi: 10.1164/ajrccm.153.2.8564103. [DOI] [PubMed] [Google Scholar]
- KROMER B.M., TIPPINS J.R. Coronary artery constriction by the isoprostane 8-epi prostaglandin F2α. Br. J. Pharmacol. 1996;119:1276–1280. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROMER B.M., TIPPINS J.R. The vasoconstrictor effect of 8-epi prostaglandin F2α in the hypoxic rat heart. Br. J. Pharmacol. 1999;126:1171–1174. doi: 10.1038/sj.bjp.0702433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUMLEY P., WHITE B.P., HUMPHREY P.P.A. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br. J. Pharmacol. 1989;97:783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOMBOULI J.V., SCHAEFFER G., HOLZMANN S., KOSTNER G.M., GRAIER W.F. Anandamide-induced mobilization of cytosolic Ca2+ in endothelial cells. Br. J. Pharmacol. 1999;126:1593–1600. doi: 10.1038/sj.bjp.0702483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTINE T.J., MARKESBERY W.R., MORROW J.D., ROBERTS L.J., II Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer's disease. Ann. Neurol. 1998;44:410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- MONTUSCHI P., CURRO D., RAGAZZONI E., PREZIOSI P., CIABATTONI G. Anaphylaxis increases 8-iso-prostaglandin F2α release from guinea-pig lung in vitro. Eur. J. Pharmacol. 1999;365:59–64. doi: 10.1016/s0014-2999(98)00859-0. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., FREI B., LONGMIRE A.W., GAZIANO J.M., LYNCH S.M., SHYR Y., STRAUSS W.E., OATES J.A., ROBERTS L.J. Increase in circulating products of lipid peroxidation (F2- isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., HILL K.E., BURK R.F., NAMMOUR T.M., BADR K.F., ROBERTS L.J., II A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORROW J.D., MINTON T.A., MUKUNDAN C.R., CAMPBELL M.D., ZACKERT W.E., DANIEL V.C., BADR K.F., BLAIR I.A., ROBERTS L.J., II Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 1994;269:4317–4326. [PubMed] [Google Scholar]
- MORROW J.D., ROBERTS L.J., II The isoprostanes: current knowledge and directions for future research. Biochem. Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA L., CRANKSHAW D.J.Excitatory effects of isoprostanes in the human umbilical artery in vitro Br. J. Pharmacol. 1998125109P [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVEIRA L., STALLWOOD N., CRANKSHAW D.J.An investigation into the downturn in response to 8-isoprostaglandin E2 in human umbilical artery in vitro Br. J. Pharmacol. 1998125110P [Google Scholar]
- POLLOCK J.S., FORSTERMANN U., MITCHELL J.A., WARNER T.D., SCHMIDT H.H., NAKANE M., MURAD F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI K., NAMMOUR T.M., FUKUNAGA M., EBERT J., MORROW J.D., ROBERTS L.J., II, HOOVER R.L., BADR K.F. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2α, in the rat. J. Clin. Invest. 1992;90:136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENTZEL P., WELSH N., ERIKSSON U.J. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered prostaglandin E2 levels in rat embryos exposed to a diabetic environment. Diabetes. 1999;48:813–820. doi: 10.2337/diabetes.48.4.813. [DOI] [PubMed] [Google Scholar]
- YIN K., HALUSHKA P.V., YAN Y.-T., WONG P.Y.K. Antiaggregatory activity of 8-epi-prostaglandin F2α and other F- series prostanoids and their binding to thromboxane A2/prostaglandin H2 receptors in human platelets. J. Pharmacol. Exp. Ther. 1994;270:1192–1196. [PubMed] [Google Scholar]
- ZHANG R., OGLETREE M.L., MORELAND S. Characterization of the thromboxane A2/prostaglandin endoperoxide receptors in aorta. Eur. J. Pharmacol. 1996;317:91–96. doi: 10.1016/s0014-2999(96)00697-8. [DOI] [PubMed] [Google Scholar]


