Abstract
The influence of 17 β-oestradiol on pressurized isolated rat mesenteric and coronary small arteries was investigated.
17 β-oestradiol caused rapid (t1.0<5 mins) concentration-dependent relaxations of pre-contracted pressurized (50 mmHg) isolated rat mesenteric and coronary arteries. Similar responses were observed in both vessel types. Significant relaxations were only observed at concentrations exceeding 3 μM.
The vasodilatory responses in both types of artery were unaffected by 10 μM L-nitro arginine (L-NNA) alone or in the presence of 10 μM indomethacin, inhibitors of nitric oxide and prostaglandin synthesis respectively. They were also unaffected by the pre-contracting agent used i.e. high K+ or U46619 (a thromboxane analogue).
Neither the oestrogen receptor antagonist ICI 182,780 (10 μM) nor the protein synthesis inhibitor cycloheximide (100 μM) had any effect on the responses of mesenteric arteries to 17 β-oestradiol.
17 α-oestradiol had only a minor effect on mesenteric arterial diameter over a concentration range similar to the effective vasodilatory range for 17 β-oestradiol.
Membrane impermeant 17 β-oestradiol conjugated to bovine serum albumin (β-oestradiol-17hemisuccinate-BSA) (E-H-BSA) resulted in a vasodilatation of pressurized arteries.
Wortmannin, an inhibitor of myosin light chain kinase, near maximally relaxed pressurized mesenteric arteries although the time course for the response was significantly slower than that for 17 β-oestradiol.
These results taken together suggest that the acute effects of 17 β-oestradiol on isolated pressurized arterial tone may be due to effects directly on the vascular smooth muscle via non-genomic mechanisms that involve a stereospecific interaction at the plasma membrane.
Keywords: 17 β-oestradiol, vascular, tone
Introduction
It has been known for many years that pre-menopausal women have a significantly lower incidence of cardiovascular disorders than men of a similar age do. After the menopause, however, the incidence increases such that the chance of developing coronary heart disease is comparable to men (Barrett-Connor & Bush, 1991). Hormone replacement therapy reduces the incidence of cardiac disorders leading to the suggestion that oestrogens may have a cardioprotective effect. While oestrogens have been shown to have a number of beneficial effects on the cardiovascular lipid profile, affecting cholesterol metabolism and deposition (Bush et al., 1983) and inhibiting atherosclerotic plaque formation (Sarrel, 1990), these effects can account for only 25–50% of the observed reduction in deleterious coronary events. This suggests that some other protective mechanism(s) are involved (Bush et al., 1987). One such mechanism maybe a direct vasodilatory effect of oestrogens on coronary arteries.
In vivo studies have shown that oestrogen infusion increases blood flow in both coronary (Sudhir et al., 1995) and systemic circulations (Rosenfeld et al., 1976; Magness & Rosenfeld, 1976). More direct studies on isolated arterial preparations have also demonstrated rapid relaxant effects of 17 β-oestradiol (Jiang et al., 1991; Mugge et al., 1993; Otter & Austin, 1998) again supporting the idea that the cardioprotection offered by oestrogens may, in part, be due to a direct vasodilatory effect. One of the criticisms with this hypothesis, however, is the fact that this effect on contractility is only observed at high (i.e. pharmacological), concentrations questioning its physiological importance. In vitro, the direct effects of 17 β-oestradiol on vascular tone has previously been studied in arteries mounted as strip or ring preparations with changes in contractility being measured as changes in isometric force (for methodological details see Mulvany & Halpern, 1977). A relatively new method of studying vascular reactivity is by pressure myography. Vessels mounted on this system are pressurized, allowed to maintain their physiological shape and experience true transmural pressures, with responsiveness being measured as a change in vessel diameter. It is now apparent that the method by which vascular responsiveness is studied is extremely important and, indeed, increased sensitivity to a number of agonists have been demonstrated in pressurized arteries compared to strip/ring preparations (Dunn et al., 1994; Falloon et al., 1995). To date only one study has investigated the effects of oestrogens on pressurized small arteries (Kakucs et al., 1998). Although in this study on the saphenous artery high concentrations of 17 β-oestradiol (i.e. >1 μM) were again required to produce a vasodilation it should be noted that the sensitivity of this vessel to the oestrogen in isometric systems, to allow direct comparison, is unknown. This study will investigate the acute effects of oestrogens on the contractility of pressurized small coronary and mesenteric arteries. When mounted as ring preparations these arteries have previously been found to vasodilate only to high concentrations of 17 β-oestradiol (Jiang et al., 1991; Otter & Austin, 1998). As it is the cardioprotective effects of oestrogens that have received much interest the vasodilatory actions of oestrogens may have some selectivity towards the coronary circulation. The responsiveness of isolated coronary and systemic pressurized arteries has not previously been compared. In the present study, therefore, the effects of 17 β-oestradiol were also compared in isolated rat mesenteric and coronary pressurized arteries.
The mechanisms whereby 17 β-oestradiol has this vasodilatory effect on arteries is poorly understood. In vivo effects of oestrogens on endothelium-dependent mechanisms have been reported (Williams et al., 1990; Herrington et al., 1994; Reiss et al., 1994) and indeed estrogenic regulation of myogenic tone, for example, has been attributed to nitric oxide dependent mechanisms (Geary et al., 1998; Skarsgard et al., 1997). It appears, however, that these effects are associated with chronic oestrogen exposure (Andersen et al., 1999). Isolated tissue studies investigating the acute effects of oestrogens have demonstrated both endothelium-dependent (total or partial) (McNeill et al., 1996; Otter & Austin, 1998) and endothelium-independent mechanisms (Jiang et al., 1991; Mugge et al., 1993). It is unclear why these differences exist but may depend on factors such as type, size and location of vessel, species and experimental methodology. A further aim of the present study was, therefore, to investigate the involvement of endothelial factors in the vasodilatory response of 17 β-oestradiol on isolated pressurized arteries.
Classically, steroid hormones exert their actions via interaction with nuclear/cytosolic oestrogen receptors and subsequent alteration of protein synthesis (Davidson & Lippman, 1989). Rapid effects of oestrogens on a variety of other physiological processes, including calcium homeostasis, have recently suggested the existence of non-genomic effects via as yet unidentified pathways (Benten et al., 1998). It is unclear whether the acute vasodilatory effects of 17 β-oestradiol on isolated arteries are due to genomic or non-genomic mechanisms. This will, therefore, also be investigated.
Thus the aims of the present study were (1) to examine the responses of isolated pressurized rat mesenteric small arteries and pressurized coronary small arteries to 17 β-oestradiol (2) to investigate the role of endothelial factors in the responses and (3) to investigate the genomic-dependence of oestrogen action.
Methods
Vessel isolation and cannulation
Male Wistar Kyoto rats (250–300 g) were killed by stunning and exsanguination. The mesentery or the heart were removed and placed in ice-cold physiological salt solution (PSS) of composition (mM): NaCl 119, KCl 4.7, MgSO4.7H2O 1.2, NaHCO3 25, KH2PO4 1.17, K2EDTA 0.03, glucose 5.5, CaCl2.2H2O 1.6 at pH 7.4. A 4th order mesenteric artery or the septal coronary artery were dissected from one animal and placed in the chamber of a pressure myograph (Living Systems Instrumentation (LSI), Burlington, VT, U.S.A.) containing cold PSS. Each vessel was cannulated onto two glass micropipettes as described previously (Izzard et al., 1996) pressurized to 50 mmHg using a pressure servo-control unit (LSI) and checked to ensure the absence of leaks. Vessels were then perfused with PSS at 37°C, gassed with 95% air/5% CO2. Lumen diameters were continuously measured using a video image analyser (LSI).
Experimental protocol
Following an equilibration period of approximately 30 min each vessel was subjected to a [run-up] procedure consisting of three separate 2 min exposures to 60 mM KCl (isosmotically substituted for NaCl). The effect of oestrogens on vascular tone was investigated by stimulating vessels with 60 mM KCl or 10 μM U46619 (9,11-dideoxy-11α, 9α-epoxy methano-prostaglandin) (a thromboxane analogue) until a stable contraction was obtained. All drugs were added to the superfusate. Each vessel was subjected to increasing concentrations of 17 β-oestradiol from 0.01–30 μM and any relaxation at each concentration was allowed to reach a steady state. Solubility limitations prevented examination of concentrations in excess of 30 μM. Vessels were then washed with PSS and tone allowed to return to baseline. To determine the reversibility of the response a small number of vessels, pre-contracted with 60 mM KCl, were exposed to a single concentration (30 μM) of 17 β-oestradiol. Following relaxation the vessels were washed in 60 mM KCl (in the absence of 17 β-oestradiol) and tension allowed to rise.
Investigation of the role of the endothelium in responses to 17 β-oestradiol
The integrity of the endothelium was tested in each vessel by observing the relaxation response to 10 μM carbachol following pre-contraction with either 60 mM KCl or 10 μM U46619. Vessels which showed a relaxation of <15% were considered to possess a damaged endothelium and were discarded from the study. To determine the contribution of nitric oxide (NO) and prostaglandins to the carbachol response, experiments were repeated in the presence of 10 μM Nw-nitro-L-arginine (L-NNA), an inhibitor of NO synthase and in the additional presence of 10 μM indomethacin, an inhibitor of prostaglandin synthesis.
To investigate the involvement of NO and prostaglandins in the response to 17 β-oestradiol, concentration-response curves were constructed in the presence of 10 μM L-NNA alone or with 10 μM indomethacin. Vessels were incubated with the inhibitor for 30–40 min prior to experimentation. Responses to 17 β-oestradiol were directly compared to those obtained in the same tissues in the absence of the inhibitors.
Investigation of the effect of the oestrogen receptor antagonist ICI 182,780 on responses to 17 β-oestradiol
Following control concentration-response curves to 17 β-oestradiol in mesenteric arteries, vessels were incubated with 10 μM ICI 182,780 for 30–40 min then contracted with 60 mM KCl or 10 μM U46619. The concentration-response curve to 17 β-oestradiol was then repeated in the continued presence of ICI 182,780.
The effects of cycloheximide on responses to 17 β-oestradiol
To investigate the effects of protein synthesis inhibition on responses, mesenteric arteries were incubated for 1 h with 100 μM cycloheximide. At this concentration protein synthesis has been shown to be maximally inhibited (Waring, 1990). Vessels were constricted by depolarization and concentration-response curves to 17 β-oestradiol were examined. Responses were compared to those previously obtained on the same tissue in the absence of cycloheximide.
Responses to 17 α-oestradiol
Mesenteric arteries were contracted with 60 mM KCl and concentration-response curves to either 17 β-oestradiol or 17 α-oestradiol (0.01–30 μM) were constructed. Responses to both compounds were examined and directly compared in each tissue.
Responses to conjugated oestradiol
To investigate whether the acute vasodilatory effects to 17 β-oestradiol were mediated via intracellular interactions or via interactions/binding of 17 β-oestradiol at the plasma membrane the effects of the membrane impermeant 17 β-oestradiol conjugate β-oestradiol 17-hemisuccinate-BSA (E-H-BSA) on arterial diameter were studied and compared to those due to addition of 17 β-oestradiol. Control experiments with the membrane permeant conjugate β-oestradiol 17-hemisuccinate (E-H) and BSA were also carried out. To allow direct comparisons with the effects observed to 17 β-oestradiol, concentrations of E-H-BSA and E-H added were adjusted and expressed as concentrations of 17 β-oestradiol (i.e. 30 and 100 μM) within the conjugates. The concentration of BSA alone was also matched to that within E-H-BSA.
Responses to wortmannin
In order to investigate a different non-receptor-mediated method of relaxation pressurized rat mesenteric small arteries vessels were contracted with 60 mM KCl and 1 μM wortmannin (a membrane-permeant inhibitor of myosin light chain kinase) was added. The relaxation was allowed to reach a steady state and compared to the relaxation observed to 30 μM 17 β-oestradiol in the same tissue.
Drugs and chemicals
All drugs and chemicals, with the exception of ICI 182,780 were obtained from Sigma. ICI 182,780 was obtained from Tocris Cookson (Langford, Bristol, U.K.). Stock solutions of 17 β-oestradiol and 17 α-oestradiol were made by dissolving first in 100% ethanol and diluting in PSS (1 in 2000) to give a concentration of 100 μM. 10 mM stock solutions of indomethacin and ICI 182,780 were also made by dissolving in 100% ethanol. A 10 mM stock solution of U46619 was made by dissolving in a mixture of 100% ethanol and 1 mg kg−1 sodium carbonate (1 : 2). 10 mM stock solutions of E-H and E-H-BSA were made by dissolving in 95% ethanol and phosphate buffer solution respectively. Bovine serum albumin (BSA) was dissolved in KCl (60 mM). A 1 mM stock solution of wortmannin was made by dissolving in dimethylsulphoxide (DMSO). Carbachol, L-NNA and cycloheximide were dissolved in PSS.
Analysis of data
All results are expressed as mean±s.e.mean with n representing number of animals. All responses were normalized as a percentage of the change in diameter observed to 60 mM KCl or 10 μM U46619. Differences between groups were compared by analysis of variance and Student's t-test (paired or unpaired). Previous studies revealed no significant difference in time effect between first and second curves to 17 β-oestradiol and solvent only controls revealed no effect on vascular tone. All curves constructed in the presence of inhibitors were therefore compared to first curves constructed on the same tissue. It should be noted that, as full concentration-response curves to oestrogens could not always be obtained, EC50 values could not be accurately determined and statistical significance was therefore determined at each individual concentration.
Results
Resting parameters
In this study coronary arteries from the rat, pressurized to 50 mmHg, had a significantly larger mean diameter (280±6 μm, n=11) compared to mesenteric arteries (254±5 μm, n=40, P<0.05). 60 mM KCl or 10 μM U46619 induced near maximal, maintained lumenal narrowing in all tissues studied. The magnitude of the change in diameter in response to 60 mM KCl was 168±7 μm (n=24) for mesenteric and 168±11 μm (n=6) for coronary arteries and for U46619 was 149±5 μm (n=24) and 125±7 μm (n=5) for mesenteric and coronary arteries respectively.
Effects of 17 β-oestradiol on mesenteric and coronary arterial diameter
17 β-oestradiol had no effect on arterial diameter in unstimulated pressurized vessels. The oestrogen, however, caused a concentration-dependent relaxation of pressurized rat mesenteric and coronary arteries pre-contracted with either 60 mM KCl or 10 μM U46619. Figure 1 shows the effect of increasing concentrations of 17 β-oestradiol on a KCl-pre-contracted mesenteric artery and demonstrates rapid onset of the response with the maximum relaxation to each concentration occurring within 5 min. Significant relaxations were only observed at concentrations >3 μM. To examine the reversibility of the vasodilatory response, a small number of experiments (n=3) were carried out using a single concentration (30 μM) of 17 β-oestradiol. As is the full concentration response curves, 30 μM 17 β-oestradiol caused a rapid relaxation of depolarized tissues which was complete within 5 min. Upon washout with fresh KCl solution tension rose rapidly to original pre-contracted levels within 5 min, i.e. the vasodilation was rapidly reversible.
Figure 1.
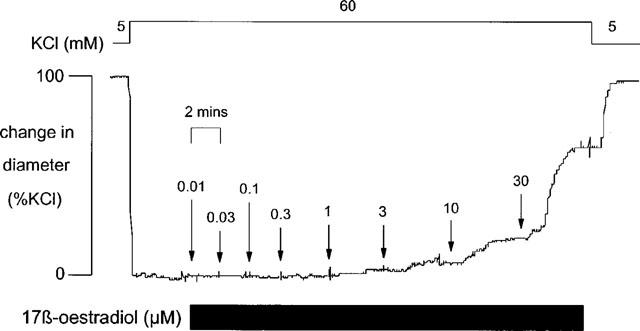
The response of rat isolated pressurized mesenteric small artery pre-contracted with 60 mM KCl to increasing concentrations of 17 β-oestradiol (0.01–30 μM).
The magnitude of the vasodilatory responses to 17 β-oestradiol were similar in both coronary (n=6, n=5) and mesenteric (n=8, n=12) arteries and were not significantly different in vessels contracted with either KCl or U46619 respectively (Figures 2 and 3).
Figure 2.
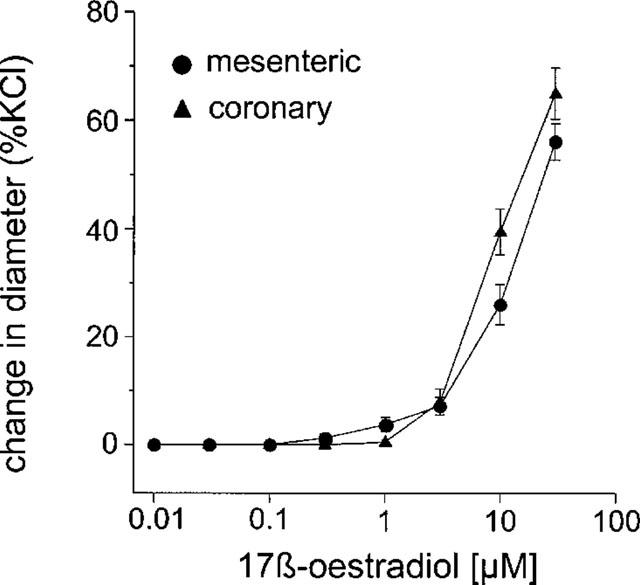
The effect of increasing concentrations of 17 β-oestradiol (0.01–30 μM) of rat isolated mesenteric and coronary small arteries pre-contracted with 60 mM KCl. The change in diameter is expressed as a percentage of the change in diameter of each tissue observed to 60 mM KCl.
Figure 3.
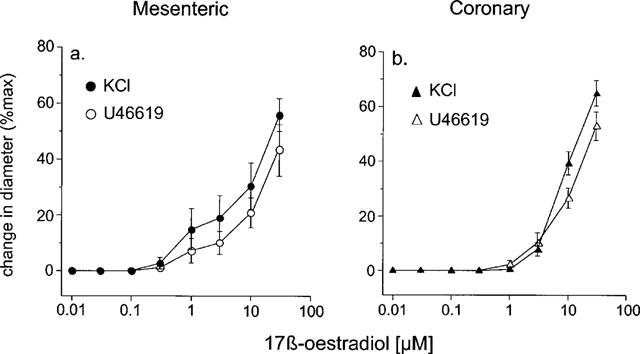
The effect of increasing concentrations of 17 β-oestradiol (0.01–30 μM) of (a) mesenteric and (b) coronary rat small arteries contracted with either 60 mM KCl or 10 μM U46619. The change in diameter is expressed as a percentage of the change in diameter of each tissue observed to 60 mM KCl or 10 μM U46619.
Investigation of the role of the endothelium
All tissues studied had a functionally intact endothelium as demonstrated by responses to the endothelium-dependent vasodilator carbachol. In depolarized mesenteric arteries 10 μM carbachol produced a relaxation of 43.9±2.14% (n=28). This was significantly reduced to only 2.39±1.06% (n=24) in the presence of L-NNA alone (P<0.001) and in the additional presence of indomethacin to 9.35±0.74% (n=3) (P<0.001). In mesenteric arteries constricted with U46619 (10 μM) carbachol induced a vasodilatation of 94.45±2.78% (n=3). This was significantly (P<0.001) greater than that observed in depolarized tissues and was not blocked by L-NNA alone or in the presence of indomethacin where responses were 100±0% (n=3) and 93.54±3.29% (n=3) respectively.
In coronary arteries similar responses were obtained to those observed in mesenteric arteries. In depolarized arteries carbachol produced a relaxation of 37.54±2.85% (n=11), a response which was completely blocked by L-NNA alone (n=10) and in the presence of indomethacin (4.54±4.54%, n=3, P<0.01). In vessels contracted with U46619 carbachol resulted in a vasodilatation of 98.85±1.15% (n=3) which was significantly greater than that observed in depolarized arteries (P<0.001). This response was unaffected by L-NNA alone (98.81±1.19%, n=3) and with indomethacin (95.65±6.15%, n=3).
Incubation with L-NNA had no significant effect on the contractions of either mesenteric or coronary arteries to KCl or to U46619. For mesenteric arteries changes in diameter were 154±13 μm (n=8) and 158±7 μm (n=8) for KCl and 156±9 μm (n=12) and 139±7 μm (n=12) for U46619 and, for coronary arteries, 192±12 μm (n=6) and 168±11 μm (n=6) for KCl and 126±11 μm (n=5) and 125±7 μm (n=5) for U46619 in the presence and absence of the inhibitor respectively.
L-NNA had no effect on the vasodilatory responses observed to 17 β-oestradiol in both mesenteric (n=8 for KCl, n=12 for U46619) and coronary (n=6 for KCl, n=5 for U46619) arteries. Again the responses obtained were independent of the pre-constricting agent used and the vessel type studied (Figure 4).
Figure 4.
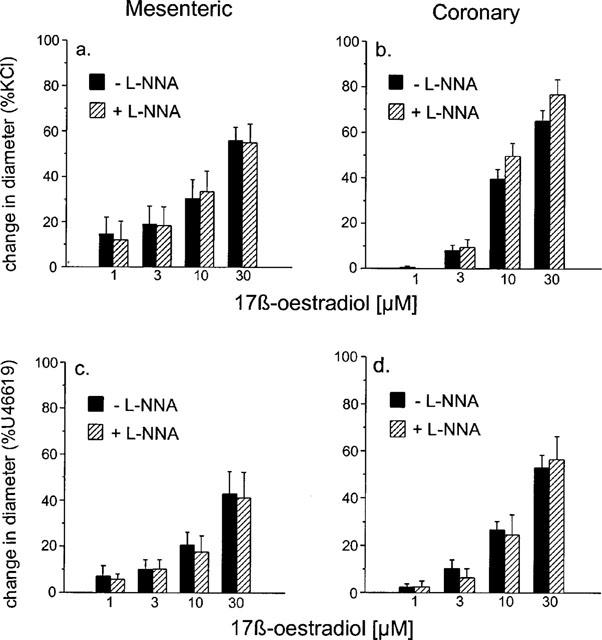
The effect of 10 μM L-NNA on responses to 17 β-oestradiol of (a) mesenteric and (b) coronary small arteries isolated from the rat pre-contracted with 60 mM KCl and (c) mesenteric and (d) coronary small arteries isolated from the rat pre-contracted with 10 μM U46619. The change in diameter is expressed as a percentage of the change in diameter of each tissue observed to 60 mM KCl (a,b) or 10 μM U46619 (c,d).
The effects of L-NNA and indomethacin on the responses to 17 β-oestradiol were examined in mesenteric arteries. Incubation with both inhibitors again did not significantly alter the magnitude of the contractile response to either KCl or U46619, reductions in lumenal diameter being, in the presence and absence of the inhibitors; 182±11 μm (n=6) and 178±7 μm (n=6) for KCl and 151±10 μm (n=6) and 154±7 μm (n=6) for U46619 respectively.
Responses to 17 β-oestradiol in the presence of L-NNA and indomethacin were not significantly different from those in the absence of the inhibitors (n=6 for KCl and n=6 for U46619 respectively) (Figure 5).
Figure 5.
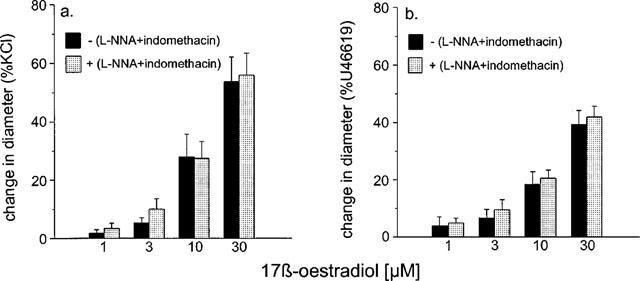
The effect of 10 μM L-NNA plus 10 μM indomethacin on the responses to 17 β-oestradiol of rat isolated mesenteric small arteries pre-contracted with (a) 60 mM KCl and (b) 10 μM U46619 to 17 β-oestradiol. The change in diameter is expressed as a percentage of the change in diameter of each tissue observed to 60 mM KCl (a) or 10 μM U44619 (b).
Effects of ICI 182,780 on responses to 17 β-oestradiol
Incubation with 10 μM ICI 182,780 had no effect on the magnitude of the change in diameter of isolated pressurized mesenteric arteries to 60 mM KCl (137±17 μm (n=6) and 181±23 μm (n=6)) and to 10 μM U46619 (109±20 μm (n=6) and 162±14 μm (n=6)) in the presence and absence of the antagonist respectively.
Incubation with ICI 182,780 had no effect on the magnitude of the relaxations observed to 17 β-oestradiol in arteries contracted with either KCl (n=6) or U46619 (n=6) (Figure 6).
Figure 6.
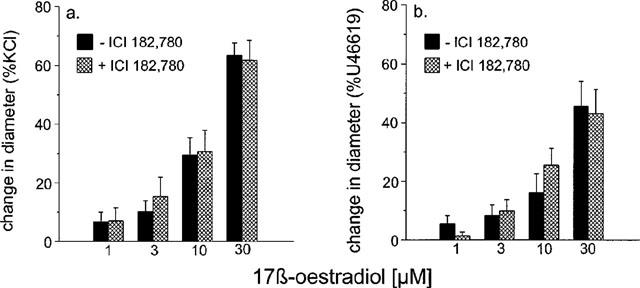
The effect of 10 μM ICI 182,780 on the responses to 17 β-oestradiol of rat isolated mesenteric small arteries pre-contracted with (a) 60 mM KCl and (b) 10 μM U46619. The change in diameter is expressed as a percentage of the change in diameter of each tissue observed to 60 mM KCl (a) or 10 μM U44619 (b).
Effects of cycloheximide
Incubation of tissues with 100 μM cycloheximide for 1 h had no effect on the resting lumenal diameter of pressurized arteries nor on the vasodilatory responses observed to increasing concentrations of 17 β-oestradiol on the same tissues (Figure 7). Cycloheximide also had no effect on the time course of the responses to oestradiol.
Figure 7.
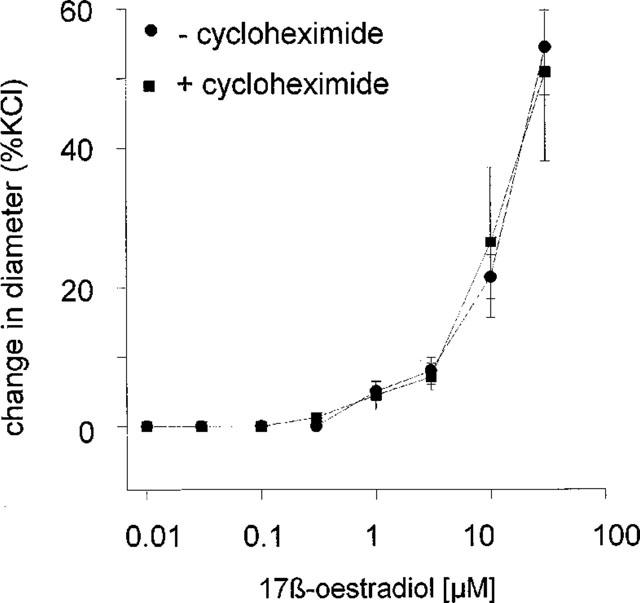
The effect of 100 μM cycloheximide on responses of isolated rat mesenteric artery to 17 β-oestradiol (0.01–30 μM). The change in diameter is expressed as a percentage change in diameter of each tissue to 60 mM KCl.
Effects of 17 α-oestradiol
17 α-oestradiol produced little relaxation of pressurized mesenteric arteries pre-contracted with 60 mM KCl (n=4). Although a small vasodilatory effect of 17 α-oestradiol was observed at high concentrations this was significantly smaller than that observed to 17 β-oestradiol at similar concentrations. Responses were 3.85±2.22% and 27.92±6.33% for 10 μM 17 α- and β-oestradiol and 11.85±6.96% and 54.49±5.30% for 30 μM 17 α-oestradiol and 17 β-oestradiol respectively (n=4) (P<0.005). (Figure 8).
Figure 8.
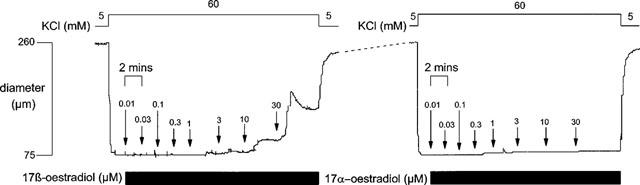
A comparison of the effect of 17 β-oestradiol (0.01–30 μM) and 17 α-oestradiol (0.01–30 μM) on rat isolated mesenteric small arteries pre-contracted with 60 mM KCl.
Effects of conjugated oestradiol
E-H-BSA produced no effect on vascular tone at concentrations that would result in 30 μM concentration of 17 β-oestradiol. At higher concentrations, however, a significant vasodilatory effect was observed. The magnitude of the vasodilatation of E-H-BSA was similar to that observed to E-H for similar concentrations of oestradiol (100 μM) being 46.03±6.04% KCl in response to E-H-BSA and 49.83±8.73% KCl for E-H. These responses were not significantly different from the responses observed to 30 μM 17 β-oestradiol alone on the same tissues (Table 1). BSA alone, at concentrations similar to those present in the E-H-BSA conjugate, had no effect on arterial tone.
Table 1.
A comparison of the effects of 17β-oestradiol, β-oestradiol-17-hemisuccinate and β-oestradiol-17-hemisuccinate-BSA on pressurized isolated rat mesenteric arteries
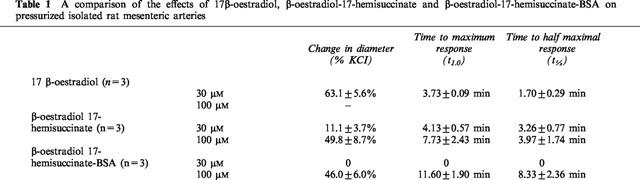
Effects of wortmannin
One μM wortmannin produced a gradual relaxation in pressurized mesenteric arteries pre-contracted with 60 mM KCl. The maximum relaxation was 85.6±3.3% (n=4) of the change in diameter observed to KCl which was significantly greater than the relaxation observed to 30 μM 17 β-oestradiol (53.4±2.9%, n=4, P<0.001) in the same tissues. The response to wortmannin was significantly slower compared to that due to 17 β-oestradiol (t1.0=35.4±3.7 and 4.8±0.3 mins respectively and t½5=14.8±2.8 and 1.3±0.5 mins respectively, both P<0.005) (n=4) (Figure 9).
Figure 9.
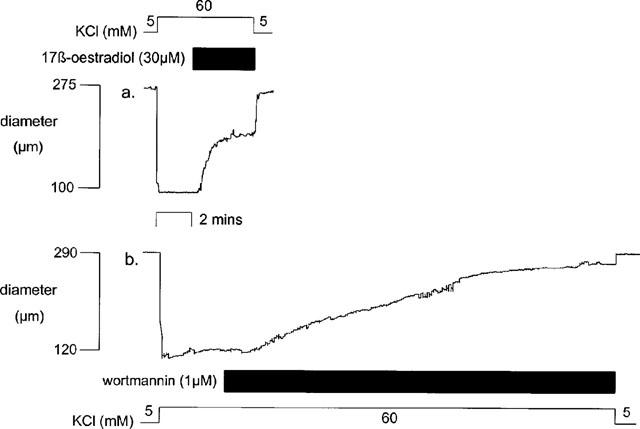
An example of the response of isolated pressurized rat mesenteric small arteries pre-contracted with 60 mM KCl to (a) 30 μM 17 β-oestradiol and (b) 1 μM wortmannin.
Discussion
The results of the present study demonstrate that 17 β-oestradiol causes a relaxation of isolated pressurized rat mesenteric and coronary vessels pre-contracted with either KCl or U46619. The magnitudes of the relaxations were independent of the pre-contracting agent used and the type of vessel. The vasodilatory responses were unaffected by L-NNA and indomethacin and were thus presumed independent of nitric oxide and prostaglandins. The relaxation observed to 17 β-oestradiol was unaffected by the oestrogen receptor antagonist ICI 182,780 and to cycloheximide, an inhibitor of protein synthesis. 17 α-oestradiol, a stereoisomer of 17 β-oestradiol, had only a small effect on arterial tone. 17 β-oestradiol rendered membrane-impermeant by conjugation to BSA had a vasodilatory effect on arteries.
A direct relaxant effect of pharmacological concentrations of 17 β-oestradiol has previously been demonstrated in a number of isolated vascular strip or ring preparations (Jiang et al., 1991; Mugge et al., 1993; Otter & Austin, 1998). In the present study it was found that pressurized arteries responded to 17 β-oestradiol over a similar concentration range to previous studies in non-pressurized isolated vessels including those we have previously reported in mesenteric arteries (Otter & Austin, 1998). Thus, even vessels mounted on the physiologically more relevant pressure myograph (where an enhanced sensitivity to a number of agonists has been shown (Dunn et al., 1994; Falloon et al., 1995)), exhibited a vasodilator response to 17 β-oestradiol only at concentrations which are significantly higher than circulating plasma concentrations of both rat and man: range 0.02–1 nM and 1–10 nM respectively (Brown-Grant et al., 1970; Abraham et al., 1972). This is in agreement with findings on pressurized saphenous arteries (Kakus et al., 1998) suggesting that neither the type of vessel or the experimental methodology used greatly effects in vitro arterial sensitivity to 17 β-oestradiol. In view of this it is still unknown whether this direct vasodilatory effect of oestrogens is physiologically important under normal conditions. It may become important in situations of compromised blood flow due, for example, to occlusion given that small changes in lumenal diameter have profound effects on vascular resistance to blood flow. This may increase greatly the local concentration of oestrogen that would in turn dilate the arteries increasing blood flow and therefore lowering the plasma concentration of the oestrogen. A further consideration is of course the fact that the circulating plasma concentration of 17 β-oestradiol may not adequately reflect the concentration of the lipophilic oestrogen at the site of action where it may be significantly higher. As the results of the present study demonstrate similar responses to 17 β-oestradiol in coronary and mesenteric small arteries, it appears that if the cardioprotective effects of the hormone are related in any way to its vasodilatory effects on the vasculature, they are not specific to coronary arteries but are likely due to general actions on the vasculature as a whole.
The mechanisms by which oestrogens influence arterial tone are unclear. In vivo administration of 17 β-oestradiol has been shown to increase endothelium-dependent vasodilatation to acetylcholine (Gilligan et al., 1994; 1995) and/or attenuate abnormal acetylcholine-induced vasoconstriction (Herrington et al., 1994; Collins et al., 1995) via mechanisms involving nitric oxide (Van Buren et al., 1992). In addition, stimulation of flow-induced vasodilatation following incubation with 17 β-oestradiol also appears to be endothelium/nitric oxide-dependent (Cockell & Poston, 1997). In contrast, however, in our isolated pressurized preparations neither L-NNA or indomethacin had any effect on the acute vasodilatory responses to 17 β-oestradiol in either coronary or mesenteric arteries. Thus, the acute responses of isolated pressurized arteries to 17 β-oestradiol are independent of nitric oxide and prostaglandins. It is now recognized that the endothelium may also release a further factor, endothelium derived hyperpolarizing factor (EDHF) which may also act to reduce contractility. EDHF has been shown to be particularly important in small resistance arteries (Urakami-Harasawa et al., 1997). In the present study we investigated the involvement of EDHF by examining and comparing responses to 17 β-oestradiol on tissues that had been pre-contracted by depolarization (60 mM KCl) to those contracted by activation with U46619. We have found that the relaxations evoked by carbachol in depolarized tissues were blocked by L-NNA and indomethacin suggesting that they were entirely due to release of NO and prostaglandins. In tissues pre-contracted with U46619, however, the responses to carbachol were not blocked by the inhibitors suggesting that some other endothelial factor, namely EDHF, may be involved. This may be expected as the contraction due to U46619 has been shown to be associated with much smaller changes in membrane potential than KCl (Plane & Garland, 1996). Although EDHF may thus have an effect in U46619 contracted arteries the magnitude of the relaxations due to 17 β-oestradiol were similar in both types of arteries making it unlikely that EDHF is involved in this response. Endothelium-independent responses to 17 β-oestradiol have previously been demonstrated in arterial ring preparations (Jiang et al., 1991; Mugge et al., 1993) whilst others have shown a full or partial dependence on the endothelium (McNeill et al., 1996; Otter & Austin, 1998). The reason for these differences is unclear and factors such as size and vascular origin of tissue, species and experimental methodology may clearly contribute to the results obtained.
The classical genomic actions of oestrogens and other steroids involve receptor activation, gene transcription and protein synthesis and thus exert their actions fairly slowly (Lin & Shain, 1985; Landers & Spelsberg, 1992; Losordo et al., 1994; Bayard et al., 1995). The acute vasodilatory responses to 17 β-oestradiol observed in the present study are, however, rapid with responses being complete within 5 min. This observation in itself may suggest that these acute effects may be mediated by non-genomic mechanisms and, in support of this, it was found that cycloheximide, an inhibitor of protein synthesis, had no effect on the responses. Clearly there are a number of possibilities which must be considered as to the mechanism of this acute oestrogenic effect. Firstly it may be possible that 17 β-oestradiol interacts with the classical oestrogen receptor to produce non-genomic effects. Secondly 17 β-oestradiol may be acting via another as yet unidentified receptor or stereo-specific binding site, and thirdly, the observed response may be due to non-specific effects of 17 β-oestradiol.
The genomic effects may be inhibited by oestrogen receptor antagonists such as ICI 182,780 (Wakeling & Bowler, 1992). In the present study, however, it was found that ICI 182,780 had no effect on the acute vasodilatory actions of 17 β-oestradiol. Similar effects have also been demonstrated in vivo in canine coronary arteries (Sudhir et al., 1995). These results suggest, therefore, that the acute vasodilatory effects of 17 β-oestradiol are mediated via non-genomic effects that are independent of the classical oestrogen receptor. The effects may therefore be mediated via interactions at another as yet unidentified binding site/receptor or may be due to non-specific effects for example changes in membrane fluidity. In the present study, however, it was found that 17 α-oestradiol induced only minor changes in arterial diameter implying that the vasodilatory response is stereospecific. This clearly suggests the involvement of a specific binding site or receptor. This binding site could be intracellular or on the plasma membrane.
To investigate the cellular point of interaction of 17 β-oestradiol the effect of 17 β-oestradiol rendered membrane-impermeant by conjugation to BSA (E-H-BSA) was investigated. This membrane impermeant oestrogen also reduced tone in pressurized arteries suggesting that these acute effects of oestrogens are mediated via interactions at the plasma membrane. It is recognized that higher concentrations of conjugated 17 β-oestradiol were required to elicit a response than unconjugated oestrogen. However, similar effects were seen with the membrane permeable conjugate E-H to the membrane impermeant E-H-BSA and conjugation reduces the affinity of 17 β-oestradiol binding at the classical oestrogen receptors (De Goeij et al., 1986). Membrane binding of oestrogens have previously been demonstrated in a number of different cell types although specific binding sites and/or receptors have not yet been characterized (Nenci et al., 1981; Bression et al., 1986; Pietras & Szego, 1977). Functional evidence for the existence of binding sites on the plasma membrane has been obtained using conjugated membrane impermeant oestrogens in a variety of cell types including neurons (Mermelstein et al., 1996; Gu & Moss, 1998), splenic T cells (Benten et al., 1998) and pancreatic β cells (Nadal et al., 1998) where oestrogens have been reported to have a variety of non-genomic effects including those on Ca2+ homeostasis (Le Mellay et al., 1997; Nadal et al., 1998; Beyer & Raab, 1998; Benten et al., 1998) and various ion channels (Nadal et al., 1998; Mermelstein et al., 1996; Gu & Moss, 1998). Additionally, morphological analysis of cells labelled with anti-oestrogenic antibodies, or fluorescently-tagged BSA conjugated to oestradiol, supports a membranous binding site for 17 β-oestradiol (Pappas et al., 1995; Benten et al., 1998). It is possible that the reported localization of oestradiol-BSA-FITC conjugates in other cell types is due to BSA binding to arachidonic acid in membrane as has been proposed for smooth muscle (Beck et al., 1998). Intracellular liberation of arachidonic acid is likely to have complex effects on contractility (Gong et al., 1992; van der Zee et al., 1995) but could possibly result in inhibition of trans-sarcolemmal Ca2+ influx (Nagano et al., 1995) and thereby illicit vasodilatation. However, in the present study, BSA alone did not alter vascular pressure responsiveness at concentrations equivalent to those present when conjugated to oestradiol. These results, taken together with those of the present study, suggest that, in addition to their well-documented genomic effects, oestrogens may also have non-genomic effects that appear to be mediated predominantly via stereospecific interactions at the plasma membrane.
Although the existence of the classical oestrogen receptor (ERα) in vascular tissues has been known for many years it has recently been shown that oestrogens may also influence gene expression through a newly described oestrogen receptor (ERβ), both subtypes showing a similar binding affinity for oestrogens (Pace et al., 1997). It is unclear which receptors mediate the non-genomic effects of oestrogens such as those of the present study. Although ERβ has been found in vascular smooth muscle (Register & Adams, 1998; Makela et al., 1999) it has been localized, using immunohistochemistry, to the cell nuclei of non-vascular tissues (Saunders et al., 1997) suggesting that it may not be mediating the membranous effects seen in the present study. In contrast ERα have been detected on the plasma membrane (Norfleet et al., 1999). In ovary cells, however, transfection of cDNA for ERα and ERβ resulted in expression of both receptor subtypes and, while the majority were found in the nucleus a small number (<3%) of both receptors were located in the membrane (Razandi et al., 1999). Thus, while it is clear that both receptor subtypes may be expressed in the cell membrane it is clear that the relative amounts of the receptors may vary greatly with cell type which itself may influence cellular distribution (Wilson & McPhaul, 1996). In the absence of any data in vascular smooth muscle itself therefore it is impossible to determine whether it is ERα, ERβ or another as yet unidentified ER or specific binding site which is involved in the responses we observed. This is further complicated by a lack of data concerning the specificity of ICI 182,780. Whilst the compound has been shown to inhibit the genomic effects of oestrogens, which could presumably be due to activation of both receptor subtypes, in a wide range of tissues limited data suggests that it may exhibit subtype selective effects on receptor stability (Van Den Bemd et al., 1999). This emphasizes the need for the development of selective antagonists for the two identified ER subtypes.
Smooth muscle contractility is governed primarily by the calcium-calmodulin dependent phosphorylation of the regulatory myosin light chain (MLC20) by myosin light chain kinase (MLCK). Kitazawa et al. (1997) showed that 17 β-oestradiol reduced force and MLC20 phosphorylation in isolated rabbit femoral artery. Here, we have shown that (non-receptor-mediated) inhibition of MLCK with wortmannin does result in a dramatic relaxation of pre-contracted mesenteric arteries. The time-course of relaxation with MLCK inhibition, although similar to that previously reported in another vascular preparation (Takayama et al., 1996), was significantly slower than with 17 β-oestradiol. Although the relative lipophilicities of 17 β-oestradiol and wortmannin are unknown, this again supports the notion that the initial site of action of 17 β-oestradiol is on the smooth muscle plasma membrane consistent with reports that the vasodilatory action of oestrogens may be related to reductions of plasmalemmal Ca2+ influx and consequent decreases in myosin light chain phosphorylation (Nakajima et al., 1995; Kitizawa et al., 1997).
In conclusion, therefore, we have demonstrated that 17 β-oestradiol similarly relaxes pressurized pre-contracted rat coronary and mesenteric arteries although pharmacological concentrations are required for any effects to be observed. Summarily: (i) the rapid endothelial-independent vasodilatory action of acute 17 β-oestradiol administration; (ii) the stereospecificity of the effect; (iii) the significantly slower time-course of action of another pharmacological intervention acting downstream of receptor activation and impinging on the same mechanistic end-point; (iv) the ineffectiveness of classical oestrogen receptor inhibitors; and (v) the effectiveness of membrane-impermeant oestradiol; all point to the existence of a non-genomic smoooth muscle membranous vasodilatory action of pharmacological doses of oestradiol.
Acknowledgments
This work was supported by The Wellcome Trust.
Abbreviations
- BSA
bovine serum albumin
- DMSO
diemthylsulphoxide
- E-H
β-oestradiol 17-hemisuccinate
- E-H-BSA, β-oestradiol 17-hemisuccinate-BSA; LSI
Living Systems Instrumentation
- L-NNA
Nw-nitro-L-arginine
- PSS
physiological salt solution
- NO
nitric oxide
- U46619
9,11-dideoxy-11α, 9α-epoxy methano-prostaglandin
References
- ABRAHAM G.E., ODELL W.D., SWERDLOFF R.S., HAPPER K. Simultaneous radioimmunoassay of plasma FSH, LH, progesterone, 17-hydroxyprogesterone, and estradiol-17β during the menstrual cycle. J. Clin. Endocr. 1972;34:312–318. doi: 10.1210/jcem-34-2-312. [DOI] [PubMed] [Google Scholar]
- ANDERSEN H.L., WEIS J.U., FJALLAND B., KORSGAARD N. Effect of acute and long-term treatment with 17 β-estradiol on the vasomotor responses in the rat aorta. Br. J. Pharmacol. 1999;126:159–168. doi: 10.1038/sj.bjp.0702289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRETT-CONNOR E., BUSH T.L. Estrogen and coronary heart disease in women. J. Am. Med. Assoc. 1991;265:1861–1867. [PubMed] [Google Scholar]
- BAYARD F., CLAMENS S., MEGGETTO F., BLAES N., DELSOL G., FAYE J.C. Estrogen synthesis, estrogen metabolism, and functional estrogen receptors in rat arterial smooth muscle cells in culture. Endocrinology. 1995;136:1523–1529. doi: 10.1210/endo.136.4.7895662. [DOI] [PubMed] [Google Scholar]
- BECK R., BERTOLINO S., ABBOT S.E., AARONSON P.I., SMIRNOV S.V. Modulation of arachidonic acid release and membrane fluidity by albumin in vascular smooth muscle and endothelial cells. Circ. Res. 1998;83:923–931. doi: 10.1161/01.res.83.9.923. [DOI] [PubMed] [Google Scholar]
- BENTEN W.P.M., LIEBERHERR M., GIESE G., WUNDERLICH F. Estradiol binding to cell surface raises cytosolic free calcium in T cells. FEBS Letts. 1998;422:349–353. doi: 10.1016/s0014-5793(98)00039-8. [DOI] [PubMed] [Google Scholar]
- BEYER C., RAAB H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. Eur. J. Neurosci. 1998;10:255–262. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- BRESSION D., MICHARD M., LE DAFNIET M., PAGESY P., PEILLON F. Evidence for a specific estradiol binding site on rat pituitary membranes. Endocrinology. 1986;119:1048–1051. doi: 10.1210/endo-119-3-1048. [DOI] [PubMed] [Google Scholar]
- BROWN-GRANT K., EXLEY D., NAFTOLIN F. Peripheral plasma oestradiol and lutenizing hormone concentrations during the oestrous cycle of the rat. J. Endocr. 1970;48:295–296. doi: 10.1677/joe.0.0480295. [DOI] [PubMed] [Google Scholar]
- BUSH T.L., BARRETT-CONNOR E., COWAN L.D., CRIQUI M.H., WALLACE R.B., SUCHINDDRAN C.M., TYROLER H.A., RIFKIND B.M. Cardiovascular mortality and noncontraceptive use of oestrogen in women: results from the Lipid Research Clinics Program follow-up study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- BUSH T.L., COWAN L.D., BARRETT-CONNOR E. Estrogen use and all-cause mortality: preliminary results from the Lipid Research Clinics Program Follow-up Study. JAMA. 1983;249:814–820. doi: 10.1001/jama.249.7.903. [DOI] [PubMed] [Google Scholar]
- COCKELL A.P., POSTON L. 17 β-oestradiol stimulates flow-induced vasodilatation in isolated small mesenteric arteries from prepubertal female rats. Am. J. Obstet. Gynecol. 1997;177:1432–1438. doi: 10.1016/s0002-9378(97)70087-5. [DOI] [PubMed] [Google Scholar]
- COLLINS P., ROSANO G.M.C., SARREL P.M., ULRICH L., ADAMOPOULOS S., BEALE C.M., MCNEILL J.G., POOLE-WILSON P.A. 17 β-estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995;92:24–30. doi: 10.1161/01.cir.92.1.24. [DOI] [PubMed] [Google Scholar]
- DAVIDSON N.E., LIPPMAN M.E. The role of estrogens in growth regulation of breast cancer. Crit. Rev. Oncol. 1989;1:89–111. [PubMed] [Google Scholar]
- DE GOEIJ A.F.P.M., VAN ZEELAND J.K., BEEK C.J., BOSMAN F.T. Steroid-bovine serum albumin conjugates: molecular characterisation and their interaction with androgen and estrogen receptors. J. Steroid Biochem. 1986;24:1017–1031. doi: 10.1016/0022-4731(86)90355-9. [DOI] [PubMed] [Google Scholar]
- DUNN W.R., WELLMAN G.C., BEVAN J.A. Enhanced resistance artery sensitivity to agonists under isobaric compared with isometric conditions. Am. J. Physiol. 1994;266:H147–H155. doi: 10.1152/ajpheart.1994.266.1.H147. [DOI] [PubMed] [Google Scholar]
- FALLOON B.J., STEPHENS N., TULIP J.R., HEAGERTY A.M. Comparison of small artery sensitivity and morphology in pressurised and wire-mounted preparations. Am. J. Physiol. 1995;268:H670–H678. doi: 10.1152/ajpheart.1995.268.2.H670. [DOI] [PubMed] [Google Scholar]
- GEARY G.G., KRAUSE D.N., DUCKLES S.P. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am. J. Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., BADAR D.M., PANZA J.A., QUYYUMI A.A., CANNON R.O. Effects of estrogen replacement therapy on peripheral vasomotor function in postmenopausal women. Am. J. Cardiol. 1995;75:264–268. doi: 10.1016/0002-9149(95)80033-o. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., QUYYUMI A.A., CANNON R.O. Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- GONG M.C., FUGLSANG A., ALESSI D., KOBAYASHI S., COHEN P., SOMLYO A.V., SOMLYO A.P. Arachidonic acid inhibits myosin light chain phosphatase and sensitises smooth muscle to calcium. J. Biol. Chem. 1992;267:21492–21498. [PubMed] [Google Scholar]
- GU Q., MOSS R.L. Novel mechanism for non-genomic action of 17 β-oestradiol on kainate-induced currents in isolated rat CA1 hippocampal neurones. J. Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRINGTON D.M., BRADEN G.A., WILLIAMS J.K., MORGAN T.M. Endothelial-dependent coronary vasomotor responsiveness in postmenopausal women with and without estrogen replacement therapy. Am. J. Cardiol. 1994;73:951–952. doi: 10.1016/0002-9149(94)90136-8. [DOI] [PubMed] [Google Scholar]
- IZZARD A.S., BUND S.J., HEAGERTY A.M. Myogenic tone in mesenteric arteries from spontaneously hypertensive rats. Am. J. Physiol. 1996;270:H1–H6. doi: 10.1152/ajpheart.1996.270.1.H1. [DOI] [PubMed] [Google Scholar]
- JIANG C., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Endothelium-dependent relaxation of rabbit coronary artery by 17 β-oestradiol in vivo. Br. J. Pharmacol. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKUS R., VARBIRO S., SZEKAS B., NADASY G.L., ACS N., MONOS E. Direct relaxing effect of estradiol-17beta and progesterone on rat saphenous artery. Microvasc. Res. 1998;56:139–143. doi: 10.1006/mvre.1998.2093. [DOI] [PubMed] [Google Scholar]
- KITAZAWA T., HAMADA E., KUAZAWA K., GAZNABI A.K.M. Non-genomic mechanism of 17 β-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J. Physiol. 1997;499:497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDERS J.P., SPELSBERG T.C. New concepts in steroid hormone action: transcription factors, protooncogenes, and the cascade model for steroid regulation of gene expression. Crit. Rev. Euk. Gene Exp. 1992;2:19–63. [PubMed] [Google Scholar]
- LE MELLAY V., GROSSE B., LIEBERHERR M. Phospholipase C β and membrane action of calcitriol and estradiol. J. Biol. Chem. 1997;272:11902–11907. doi: 10.1074/jbc.272.18.11902. [DOI] [PubMed] [Google Scholar]
- LIN A.L., SHAIN S.A. Estrogen mediated cytoplasmic and nuclear distribution of rat cardiovascular estrogen receptors. Arteriosclerosis. 1985;5:668–677. doi: 10.1161/01.atv.5.6.668. [DOI] [PubMed] [Google Scholar]
- LOSORDO D.W., KEARNEY M., KIM E.A., JEKANOWSKI J., ISNER J.M. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89:1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- MAGNESS R.R., ROSENFELD C.R. Local and systemic estradiol-17β: effects on uterine and systemic vasodilatation. Am. J. Physiol. 1976;256:E536–542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- MAKELA S., SAVOLAINEN H., AAVIK E., MYLLARNIEMI M., STRAUSS L., TASKINEN E., GUSTAFSSON J.A., HAYRY P. Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors alpha and beta. Proc. Nat. Acad. Sci. 1999;96:7077–7082. doi: 10.1073/pnas.96.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNEILL A.M., DUCKLES S.P., KRAUSE D.N. Relaxant effects of 17 beta-estradiol in the rat tail artery are greater in females than males. Eur. J. Pharmacol. 1996;308:305–309. doi: 10.1016/0014-2999(96)00374-3. [DOI] [PubMed] [Google Scholar]
- MERMELSTEIN P.G., BECKER J.B., SURMEIER D.J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUGGE A., RIEDEL M., BARTON M., KUHN M., LICHTLEN P.R. Endothelium independent relaxation of human coronary arteries by 17 β-oestradiol in vitro. Cardiovasc. Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NADAL A., ROVIRA J.M., LARIBI O., LEON-QUINTO T., ANDREU E., CRISTINA R., SORIA B. Rapid insulinotropic effect of 17 β-estradiol via a plasma membrane receptor. FASEB J. 1998;12:1341–1348. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- NAGANO N., IMAIZUMI Y., WATANABE M. Modulation of calcium channel currents by arachidonic acid in single smooth muscle cells from vas deferens of the guinea-pig. Br. J. Pharmacol. 1995;116:1887–1893. doi: 10.1111/j.1476-5381.1995.tb16678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAJIMA T., KITAZAWA T., HAMADA E., HAZAMA H., OMATA M., KURACHI Y. 17 β-oestradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur. J. Pharmacol. 1995;294:625–635. doi: 10.1016/0014-2999(95)00602-8. [DOI] [PubMed] [Google Scholar]
- NENCI I., MARCHETTI E., MARZOLA A., FABRIS G. Affinity chromatography visualises specific estrogen receptor binding sites on plasma membrane of breast cancer cells. J. Steroid Biochem. 1981;14:1139–1146. doi: 10.1016/0022-4731(81)90043-1. [DOI] [PubMed] [Google Scholar]
- NORFLEET A.M., THOMAS M.L., GAMETCHU B., WATSON C.S. Estrogen receptor-α detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology. 1999;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- OTTER D., AUSTIN C. Effects of 17 β-oestradiol on rat isolated coronary and mesenteric artery tone: Involvement of nitric oxide. J. Pharm. Pharmacol. 1998;50:531–538. doi: 10.1111/j.2042-7158.1998.tb06195.x. [DOI] [PubMed] [Google Scholar]
- PACE P., TAYLOR J., SUNTHARALINGAM S., COOMBES R.C., ALI S. Human estrogen receptor β binds DNA in a manner similar to and dimerizes with estrogen receptor α. J. Biol. Chem. 1997;41:25832–25838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- PAPPAS T.C., GAMETCHU B., WATSON C.S. Membrane estrogen receptors identified by multiple antibody labelling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- PIETRAS R.J., SZEGO C.M. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- PLANE F., GARLAND C.J. Influence of contractile agonists on the mechanism of endothelium-dependent relaxation of rat isolated mesenteric artery. Br. J. Pharmacol. 1996;119:191–193. doi: 10.1111/j.1476-5381.1996.tb15970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZANDI M., PEDRAM A., GREENE G.L., LEVIN E.R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in chinese hamster ovary cells. Molec. Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- REGISTER T.C., ADAMS M.R. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J. Steroid Biochem. 1998;64:187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- REIS S.E., GLOTH S.T., BLUMENTHAL R.S., RESAR J.R., ZACUR H.A., GERSTENBLITH G., BRINKER J.A. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- ROSENFELD C.R., MORRISS F.H., BARRAGLIA F.C., MAKOWSKI E.L., MESCHIA G. Effect of estradiol-17 β on blood flow to reproductive and nonreproductive tissue in pregnant ewes. Am. J. Obstet. Gynecol. 1976;124:618–629. doi: 10.1016/0002-9378(76)90064-8. [DOI] [PubMed] [Google Scholar]
- SARREL P.M. Ovarian hormones and the circulation. Maturitas. 1990;12:287–298. doi: 10.1016/0378-5122(90)90008-t. [DOI] [PubMed] [Google Scholar]
- SAUNDERS P.T.K., MAGUIRE S.M., GAUGHAN J., MILLAR M.R. Expression of oestrogen receptor beta (ERβ) in multiple rat tissues visualised by immunohistochemistry. J. Endocrinol. 1997;154:R13–R16. doi: 10.1677/joe.0.154r013. [DOI] [PubMed] [Google Scholar]
- SKARSGARD P., VAN BREEMEN C., LAHER I. Estrogen regulates myogenic tone in pressurised cerebral arteries by enhanced basal release of nitric oxide. Am. J. Physiol. 1997;273:H2248–H2256. doi: 10.1152/ajpheart.1997.273.5.H2248. [DOI] [PubMed] [Google Scholar]
- SUDHIR K., CHOU T.M., MULLEN W.L., HAUSMANN D., COLLINS P., YOCK P.G., CHATTERJEE K. Mechanisms of estrogen-induced vasodilatation: in vivo studies in canine coronary conductance and resistance arteries. J. Am. Coll. Cardiol. 1995;26:807–814. doi: 10.1016/0735-1097(95)00248-3. [DOI] [PubMed] [Google Scholar]
- TAKAYAMA M., OZAKI H., KARAKI H. Effects of a myosin light chain kinase inhibitor, wortmannin, on cytoplasmic Ca2+ levels, myosin light chain phosphorylation and force in vascular smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:120–127. doi: 10.1007/BF00178711. [DOI] [PubMed] [Google Scholar]
- URAKAMI-HARASAWA L., SHIMOKAWA H., NAKASHIMA M., EGASHIRA K., TAKESHITA A. Importance of endothelium-derived hyperpolarising factor in human arteries. J. Clin. Invest. 1997;100:2793–2799. doi: 10.1172/JCI119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BUREN G.A., YANG D., CLARK K.E. Estrogen-induced uterine vasodilatation is antagonised by l-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am. J. Obstet. Gynecol. 1992;167:828–833. doi: 10.1016/s0002-9378(11)91597-x. [DOI] [PubMed] [Google Scholar]
- VAN DEN BEMD G.J., KUIPER G.G., POLS H.A., VAN LEEUWEN J.P. Distinct effects on the conformation of estrogen receptor alpha and beta by both the antiestrogens ICI 164,384 and ICI 182,780 leading to opposite effects on receptor stability. Biochem. Biophys. Res. Commun. 1999;22:1–5. doi: 10.1006/bbrc.1999.0864. [DOI] [PubMed] [Google Scholar]
- VAN DER ZEE L., NELEMANS A., DEN HERTOG A. Arachidonic acid is functioning as a second messenger in activating the Ca2+ entry process on H1-histaminoceptor stimulation in DDT1 MF-2 cells. Biochem. J. 1995;305:859–864. doi: 10.1042/bj3050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKELING A.E., BOWLER J. ICI 182,780, a new antioestrogen with clinical potential. J. Steroid Biochem. Mol. Biol. 1992;43:173–177. doi: 10.1016/0960-0760(92)90204-v. [DOI] [PubMed] [Google Scholar]
- WARING P. DNA fragmentation induced by macrophages by gliotoxin does not require protein synthesis and is preceded by raised inositol triphosphate levels. J. Biol. Chem. 1990;265:14476–14480. [PubMed] [Google Scholar]
- WILLIAMS J.K., ADAMS M.R., KLOPFENSTEIN H.S. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]


