Abstract
This study examined whether activation of group II metabotropic glutamate (mGlu) receptors in the substantia nigra pars reticulata (SNr) could reverse akinesia in a rodent model of Parkinson's disease (PD).
Male Sprague Dawley rats, stereotaxically cannulated above either the SNr or third ventricle, were rendered akinetic by injection of reserpine (5 mg kg−1 s.c.). Eighteen hours later, the rotational behaviour induced by unilateral injection of the group II mGlu receptor agonist, (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV), was examined.
Following intranigral injection, DCG-IV (0.125–0.75 nmol in 0.1 μl) produced a dose-dependent increase in net contraversive rotations (n=6–8 animals per dose), reaching a maximum of 395±51 rotations 60 min−1 after 0.75 nmol. The effects of DCG-IV (0.5 nmol) were inhibited by 63.0±9.0% following 30 min pre-treatment with the group II mGlu receptor antagonist, (2S)-α-ethylglutamic acid (EGLU; 100 nmol in 0.2 μl; n=6).
Following intraventricular injection, DCG-IV (0.125–1.5 nmol in 2 μl) produced a dose-dependent increase in bilateral locomotor activity (n=6–7 animals per dose), reaching a maximum of 180±21 locomotor units 30 min−1 after 0.5 nmol. Pre-treatment with EGLU (200 nmol in 2 μl) inhibited the effects of DCG-IV (0.5 nmol) by 68.2±12.3% (n=5).
These data show that activation of group II mGlu receptors in the SNr provides relief of akinesia in the reserpinized rat model of PD. The reversal seen following intraventricular administration supports the likely therapeutic benefit of systemically-active group II mGlu receptor agonists in PD.
Keywords: Akinesia, autoreceptor, basal ganglia, DCG-IV, metabotropic glutamate receptor, reserpine, substantia nigra, subthalamic nucleus, Parkinson's disease
Introduction
Current treatments for Parkinson's disease (PD) rely heavily on dopamine replacement therapies which, although effective in the early stages of treatment, are accompanied by debilitating dyskinesias and reduced efficacy with long-term use (Stocchi et al., 1997). For these reasons, alternative treatments that do not rely on dopamine replacement are being investigated.
The loss of striatal dopamine innervation in PD produces many downstream changes in activity of the basal ganglia components. In particular, the subthalamic nucleus (STN), which innervates the output regions of the basal ganglia, the substantia nigra pars reticulata (SNr) and the internal globus pallidus (GPi) or rodent homologue, the entopeduncular nucleus (EPN), becomes markedly overactive (Mitchell et al., 1989). Being glutamatergic in nature (Robledo & Feger, 1990; Brotchie & Crossman, 1992), overactivity of STN neurones results in increased glutamate-mediated excitation of the SNr and GPi/EPN which is ultimately believed to lead to inhibition of thalamocortical feedback and subsequent generation of the akinetic symptoms seen in PD (Parent et al., 1995).
Accordingly, previous studies have demonstrated that surgical inactivation of STN efferent transmission either through subthalamotomy (Bergman et al., 1990) or by high frequency stimulation-induced depolarizing blockade (Benazzouz et al., 1993) alleviates motor disturbances in animal models of PD. Moreover, direct injection of NMDA- and AMPA-type glutamate receptor antagonists into the SNr and GPi/EPN has been shown to alleviate Parkinsonian symptoms in these animals (see Starr et al., 1997 for review). However, neither of these approaches is ideal. On the one hand, surgical intervention is often irreversible and, especially following STN lesion, leads to the generation of dyskinesias through excessive inhibition of STN activity (Bergman et al., 1990). On the other hand, systemic administration of NMDA- and AMPA-type glutamate receptor antagonists is accompanied by unaccept-able side-effects such as psychostimulation, sedation or ataxia, that result from widespread blockade of these receptors (Starr et al., 1997).
An alternative approach to resolving this overactive glutamatergic transmission might be to reduce the level of glutamate release from the STN terminals in the SNr and GPi/EPN. Group II metabotropic glutamate (mGlu) receptors may provide an ideal means of achieving this. Group II mGlu receptors are negatively coupled through Gi/o to adenylate cyclase and voltage-operated calcium channels and their activation brings about membrane hyperpolarization (reviewed by Conn & Pin, 1997). This functional response, coupled with their presynaptic localization in brain regions such as the striatum (Testa et al., 1998) and hippocampus (Shigemoto et al., 1997) has led to the suggestion that group II mGlu receptors operate as autoreceptors controlling the release of glutamate in target areas.
Group II mGlu mRNA is expressed in the STN (Testa et al., 1994) and corresponding group II immunoreactivity is localized to presynaptic terminal regions in the SNr (Yung, 1998), suggesting that group II mGlu autoreceptors might be present on STN terminals. If this is so, their activation is predicted to reduce glutamate release in basal ganglia output regions and thereby alleviate Parkinsonian symptoms. Therefore, the aim of this study was to assess whether injection of a group II mGlu receptor agonist, either directly into the SNr, or away from the target site (i.e. into the cerebral ventricles), could reverse the akinesia in the reserpine-treated rat model of PD. Some of this work has been published previously in abstract form (Dawson et al., 1999).
Methods
Intranigral/intraventricular cannulation and induction of akinesia
Male, Sprague Dawley rats (260–300 g) were housed in a temperature- and humidity-maintained environment with a 12 h light/dark cycle and free access to food and water. All procedures were performed in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and all efforts were made to minimize animal suffering and the number of animals used. Under general anaesthesia (sodium pentobarbitone, 60 mg kg−1 i.p.), rats were stereotaxically implanted with 23 gauge stainless steel guide cannulae positioned 2 mm above either the SNr (3.7 mm anterior, 2.0 mm lateral and 3.6 mm ventral to interaural line) or the third ventricle (4.3 mm posterior to, 0 mm lateral and 3.7 mm ventral to bregma), according to the standard rat brain atlas of Paxinos & Watson (1986). Following a recovery period of at least 4 days, animals were treated with reserpine (5 mg kg−1, s.c.) to induce catecholamine depletion and subsequent akinesia. Eighteen hours later, when animals displayed a stable level of akinesia, the effects of the group II mGlu receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) were assessed on motor behaviours.
Assessments of rotational behaviour following intranigral drug administration
For intranigral studies, visual behavioural assessments were performed in 40 cm diameter, flat-bottomed hemispheric bowls. Following a 15 min acclimatization period, baseline activity was videotaped for 30 min. Animals then received a single, unilateral injection of the group II mGlu receptor agonist, DCG-IV (0.125, 0.25, 0.5, 0.75 or 1 nmol) in 0.1 μl phosphate-buffered saline (PBS; mM): NaCl 137, KCl 2.7, KH2PO4 1.8, Na2HPO4 10; pH 7.4 or vehicle (0.1 μl PBS) into the SNr. Injections were made over a 1 min period via 30 gauge stainless steel needles inserted through, and extending 2 mm below the tip of the guide cannulae and attached with flexible tubing (Portex) to a 5 μl Hamilton microsyringe. Animals were videotaped for a further 90 min. Net contraversive rotations (360°) were assessed as an index of unilateral relief of akinesia. These rotations were counted manually from the videotape recordings, in 5 min time bins over the entire 90 min period (n=6–8 animals per dose). In order to confirm the receptor specificity of the effects of DCG-IV, the effects of the group II mGlu receptor antagonist, (2S)-α-ethyglutamic acid (EGLU), were examined against a single effective dose of DCG-IV (0.5 nmol). In these experiments, 6 h after 60 min monitoring of the effects of an initial intranigral injection of DCG-IV (0.5 nmol in 0.1 μl), rats were injected with either EGLU (100 nmol in 0.2 μl) or vehicle (0.2 μl of 0.3 mM NaOH in PBS; pH 7.0) into the same site. Owing to limitations of solubility, this dose of EGLU (100 nmol) was the maximum achievable with such a small injection volume (0.2 μl). Rotational behaviour was monitored throughout the 30 min equilibration period for EGLU and for a further 60 min following a repeat dose of DCG-IV (0.5 nmol in 0.1 μl). At the end of each experiment, fast blue dye (0.1 μl) was injected via the guide cannula to allow histological verification of injection sites. Approximately 5 min after dye injection, animals were killed by enflurane overdose and cervical dislocation. The brains were rapidly frozen in isopentane (cooled to −45°C with solid CO2) and stored desiccated at −70°C until subsequent cryostat sectioning (20 μm) and cresyl violet staining.
Locomotor assessments following intraventricular drug administration
For intraventricular studies, visual locomotor assessments were performed in rectangular cages with 5 cm square grid lines covering the base. Following an initial 15 min period of acclimatization, baseline activity was videotaped for 30 min. Animals (n=6–7 per dose) then received a single intraventricular injection of DCG-IV (0.125, 0.25, 0.5, 0.75 or 1.5 nmol in 2 μl PBS, pH 7.4) or vehicle (2 μl PBS) over a 2 min period and were videotaped for a further 60 min. Locomotor activity was measured manually, by observation of videorecording. Activity was quantified in 5 min time-bins over a 60 min period in arbitrary locomotor units (ALUs), where one ALU refers to both front paws crossing a grid line. The effects of EGLU were examined against 0.5 nmol DCG-IV (n=5 animals per group). Higher doses of EGLU (>100 nmol) could be achieved via the intraventricular route since larger injection volumes were used. Thus, doses of 200 and 400 nmol EGLU were examined. Six hours after 30 min monitoring of the effects of an initial intraventricular injection of DCG-IV (0.5 nmol in 2 μl), intraventricular injections of either EGLU (200 or 400 nmol in 2 μl) or vehicle (2 μl of 0.3 mM NaOH in PBS; pH 7.0) were made. Locomotor activity was monitored throughout the 30 min equilibration period and for a further 30 min following a repeat dose of DCG-IV (0.5 nmol in 2 μl). The positioning of cannulae was checked in all cases by examination of needle tracts in freshly dissected brain.
Data analysis
Differences between the various doses of DCG-IV or vehicle were compared using a 1-way Analysis of Variance with a Student-Newman-Keuls post-hoc analysis. The effects of DCG-IV prior to and following EGLU or vehicle treatment were compared using a 2-tailed paired t-test. In all cases, P<0.05 was taken to represent a significant difference.
Drugs
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) and 2S-α-ethylglutamic acid (EGLU) were obtained from Tocris Cookson Ltd., U.K. Reserpine and PBS reagents were obtained from Sigma, U.K.
Results
Effects of intranigral injection of the group II mGlu receptor agonist and antagonist
Light microscopic examination of injection sites revealed that approximately 80% of those animals included in this part of the study were correctly injected into the SNr. Where aberrant injections of the group II mGlu receptor agonist, DCG-IV, were made in regions outside of the SNr, animals remained fully akinetic and no contraversive rotations were observed. Only data from those animals with correctly positioned cannulae were included in the data analyses below.
During the baseline periods, all the reserpine-treated rats exhibited negligible rotational behaviour (between 0 and 4 rotations 30 min−1) and were thus considered suitably akinetic for inclusion in the study. Unilateral injection of the groups II mGlu receptor agonist, DCG-IV, into the SNr of reserpine-treated rats produced an increase in net contraversive rotations. This activity, shown in Figure 1a for a maximally effective dose of DCG-IV (0.75 nmol), commenced within 5 min of administration, peaked at around 30 min and had fully subsided within 60 min. Subsequent quantification of rotational behaviour induced by the full dose range of DCG-IV was, therefore, made over 60 min. The net contraversive rotations induced by intranigral DCG-IV (0.125–0.75 nmol) or vehicle over 60 min are shown in Figure 1b (n=6–8 animals per dose). Neither vehicle nor low-dose DCG-IV (0.125 nmol) produced any significant net contraversive rotations over this period. In contrast, between 0.25 and 0.75 nmol, DCG-IV produced a dose-dependent increase in net contraversive rotations 60 min−1. At the highest dose administered, (1 nmol), DCG-IV produced central excitation, in the form of wet dog shakes and intermittent barrel rolling, in all five animals tested. Quantification of rotational behaviour at this dose was therefore not possible.
Figure 1.
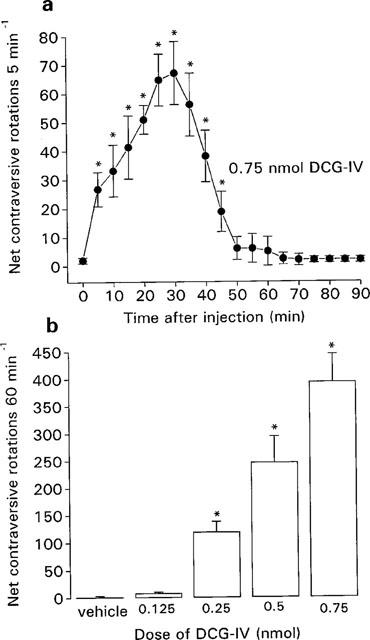
(a) Time-course of locomotor activity induced by a maximally-effective dose of the group II mGlu receptor agonist, DCG-IV (0.75 nmol in 0.1 μl) and (b) Dose-related locomotor effects of DCG-IV (0.125–0.75 nmol in 0.1 μl) or vehicle (0.1 μl PBS), following unilateral injection into the SNr of the reserpine-treated rat. Values represent mean±s.e.mean (n=6–8 animals per dose). *Indicates a significant difference compared to (a) baseline activity or (b) the previous dose (1-way ANOVA, P<0.05 in all cases).
Pre-treatment with the group II-selective mGlu receptor antagonist, EGLU (100 nmol) significantly inhibited the DCG-IV (0.5 nmol)-induced net contraversive rotations 60 min−1 by 63±9% (mean±s.e.mean, n=6). In contrast, pre-treatment with vehicle for EGLU (0.3 mM NaOH in PBS: pH 7.4) did not affect the subsequent response to DCG-IV (n=6) (Figure 2). No rotational behaviour was observed during the equilibration period with EGLU alone.
Figure 2.
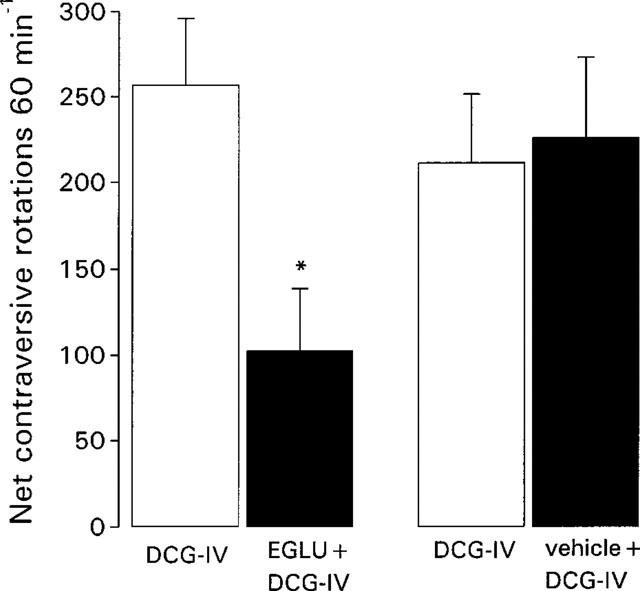
Comparison of locomotor activity induced by intranigral injection of the group II mGlu receptor agonist, DCG-IV (0.5 nmol in 0.1 μl) prior to (open bars) and 30 min following (closed bars) treatment with the group II mGlu receptor antagonist, EGLU (100 nmol in 0.1 μl) or vehicle (0.1 μl of 0.3 mM NaOH in PBS). Data represent mean±s.e.mean (n=6 animals per treatment). *Indicates a significant difference between pre-treatment and post-treatment responses to DCG-IV (paired t-test, P<0.05).
Effects of intraventricular injection of the group II mGlu receptor agonist and antagonist
Visual examination in freshly dissected brain confirmed the correct positioning of cannulae in the dorsal tip of the third ventricle. Data from all animals were thus included in the final data analyses below.
During the baseline recording period, all reserpine-treated rats exhibited little locomotor activity (between 0 and 6 ALUs) and were thus considered sufficiently akinetic for inclusion in the study. Following intraventricular injection of an effective dose of DCG-IV, locomotor activity in the reserpine-treated rats increased within 5 min of administration, peaked at around 10 min and had fully subsided within 30 min. This time-course, shown for the maximally-effective dose of DCG-IV (0.5 nmol) in Figure 3a, led to the subsequent quantification of locomotor activity over a 30 min period. The locomotor activity induced by intraventricular DCG-IV (0.125–1.5 nmol) or vehicle over 30 min is shown in Figure 3b (n=6–7 animals per dose). Neither vehicle nor low-dose DCG-IV (0.125 nmol) produced any increase in locomotor activity. In contrast, 0.25 and 0.5 nmol DCG-IV induced a dose-related increase in locomotor activity that failed to increase further with administration of the higher doses. The mean locomotor score achieved here with 0.5 nmol DCG-IV (180±21 ALUs (n=6)) amounted to some 27.9% of that achieved by non-akinetic animals under this same measurement paradigm (645±88 ALUs (n=5)). In contrast to the central excitation seen following intranigral administration of 1 nmol DCG-IV, none was observed following intraventricular administration of 1.5 nmol DCG-IV. However, at higher doses (3 nmol) intraventricular administration did induce similar behavioural patterns (wet dog shakes and barrel rolling) of central excitation.
Figure 3.
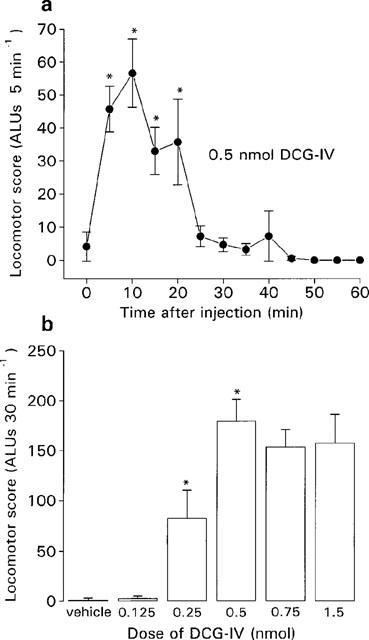
(a) Time-course of locomotor activity induced by a maximally-effective dose of DCG-IV (0.5 nmol in 2 μl) and (b) dose-related locomotor effects of DCG-IV (0.125–1.5 nmol in 2 μl) or vehicle (2 μl of PBS), following intraventricular injection in the reserpine-treated rat. Values represent mean±s.e.mean (n=6–7 animals per dose). *Indicates a significant difference compared to (a) baseline activity or (b) the previous dose (1-way ANOVA, P<0.05 in all cases).
Pre-treatment with EGLU (200 nmol), but not vehicle, significantly inhibited the locomotor activity induced by DCG-IV (0.5 nmol) by 68.2±12.3% (mean±s.e.mean, n=5; Figure 4). Increasing the dose of EGLU to 400 nmol did not produce any further inhibition of the DCG-IV-induced locomotor activity (data not shown). No locomotor activity was observed during the equilibration period with either dose of EGLU.
Figure 4.
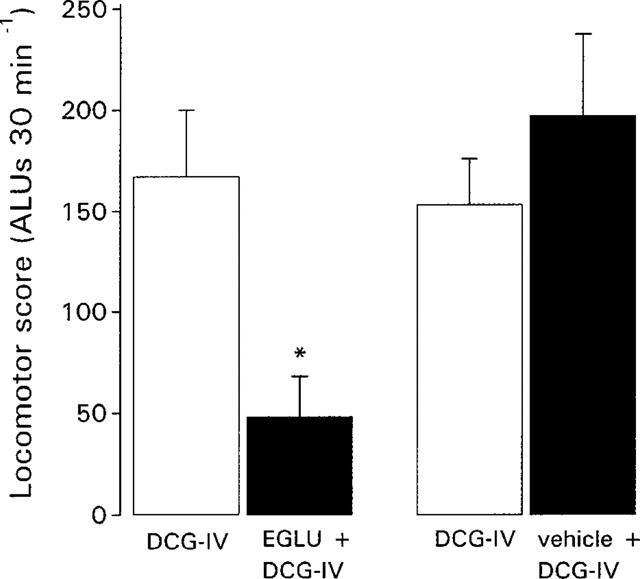
Comparison of locomotor activity induced by intraventricular injection of the group II mGlu receptor agonist, DCG-IV (0.5 nmol in 2 μl) prior to (open bars) and 30 min following (closed bars) treatment with the group II mGlu receptor antagonist, EGLU (200 nmol in 2 μl) or vehicle (2 μl of 0.3 mM NaOH in PBS). Data represent mean±s.e.mean (n=5 animals per treatment). *Indicates a significant difference between pre-treatment and post-treatment responses to DCG-IV (paired t-test, P<0.05).
Discussion
Reversal of reserpine-induced akinesia by the group II mGlu receptor agonist, DCG-IV
This study has shown that unilateral injection of the group II mGlu receptor agonist, DCG-IV, into the SNr of the reserpine-treated rat produces contraversive rotational behaviour, indicative of a unilateral reversal of akinesia. The dose-response relationship of this effect of DCG-IV was very narrow, with a threshold dose of 0.25 nmol and a maximally-effective dose of 0.75 nmol. Higher doses of DCG-IV failed to reverse the akinesia, but rather caused central excitation, as evidenced by wet dog shakes and barrel rolling. The narrow dose-response relationship and central excitation at higher doses are in keeping with previous in vivo studies on the neuroprotective actions of intracerebral administration of DCG-IV (Miyamoto et al., 1997). This central excitation may reflect the appearance of NMDA receptor agonist activity with high-dose DCG-IV, previously observed in neonatal rat spinal cord (Ishida et al., 1993) with an ED50 some 100 times greater than that for activation of mGlu receptors. Similarly, since antagonism of NMDA receptors in the SNr alleviates Parkinsonian akinesia (Starr et al., 1997), this NMDA agonist activity might be expected to counteract any anti-akinetic effects of group II mGlu receptor activation, thereby explaining the narrow dose-response relationship of DCG-IV.
Although the locomotor activity produced following intraventricular administration of DCG-IV exhibited a similar, narrow dose-response relationship, both a lower maximally-effective dose and shorter duration of action of DCG-IV were observed. Metabolism and dilution of the effective dose of DCG-IV in ventricular fluid might account for the shorter duration of action, but would appear to contradict the lower maximally-effective dose. The most likely explanation for this is that following intraventricular administration, DCG-IV may act not only in the SNr but also in additional sites such as the EPN. Since the EPN functions in an homologous manner to the SNr (Smith et al., 1998), any effect of DCG-IV within this region would provide additional locomotor activity to complement that produced by DCG-IV in the SNr. In any event, the maximum relief of akinesia obtained following intraventricular administration of DCG-IV only restored locomotor activity to approximately one-third that of non-akinetic animals. The reasons behind this failure to restore full locomotor activity remain to be explored. As described for the intranigral studies, the NMDA agonist component of DCG-IV may compromise the level of akinesia obtainable. However, considering that neither wet dog shakes nor barrel rolling were observed following intraventricular injection of up to 1.5 nmol DCG-IV, it seems unlikely that NMDA agonist activity contributed to this restricted maximum response achieved at 0.5 nmol.
Proposed cellular mechanism underlying anti-akinetic effects of DCG-IV
The group II mGlu receptor antagonist, EGLU, inhibited the behavioural responses to intranigral and intraventricular administration of DCG-IV by approximately two-thirds, confirming that this action of DCG-IV is mediated primarily through activation of group II mGlu receptors. Group II mGlu receptors are believed to exist on STN terminals in the SNr (Testa et al., 1994; Yung, 1998), although their functional role in the SNr remains to be examined. In other brain regions, such as the striatum, group II mGlu receptors have an autoreceptor function. Thus, activation of group II mGlu receptors has been shown to inhibit presynaptic striatal glutamate release both in vitro and in vivo (Lombardi et al., 1993; Battaglia et al., 1997) and to provide presynaptic inhibition of striatal excitatory post synaptic potentials (Calabresi et al., 1999). In the midbrain of the rat, the maximum DCG-IV-induced depression of glutamate-mediated excitatory post synaptic potentials (27%, Wigmore & Lacey, 1998) is extremely close to the maximum degree of restoration of locomotor activity seen here following intraventricular administration of DCG-IV (27.9%). This correlation may indicate that the cellular mechanism underlying the present locomotor activity is similar to that responsible for the inhibition of synaptic glutamate release. Therefore, it is proposed that restricting glutamate release through activation of group II mGlu autoreceptors on STN efferents in the SNr (and EPN in the case of intraventricular administration) is the most likely cellular mechanism underlying the present anti-akinetic effects of DCG-IV. The nature of the remaining one-third EGLU-insensitive component of the DCG-IV-induced reversal of akinesia remains to be determined.
Therapeutic implications for Parkinson's disease (PD)
Activation of group II mGlu receptors in the basal ganglia output regions (SNr and GPi) may provide a useful alternative to dopamine replacement therapies in the treatment of PD. Although no ataxia or sedation, as seen with AMPA and NMDA receptor antagonists (see Starr et al., 1997), was apparent following intraventricular administration of DCG-IV, the side-effects related to long-term use of group II mGlu receptor agonists remain to be examined. Since the STN also projects to the SNc, overactivity of STN neurones has been further implicated in the excitotoxic degeneration of SNc neurones in PD. Therefore, by also reducing glutamate release from STN terminals in the SNc, group II mGlu receptor agonists may provide both symptomatic relief and neuroprotection in PD. The outcome of in vivo studies examining possible SNc neurone protection by group II mGlu receptor agonists is eagerly awaited.
In conclusion, these data indicate that DCG-IV displays antiparkinsonian potential in the reserpinized rodent model of PD via activation of group II mGlu receptors in at least one region, the SNr. Moreover, the successful alleviation of reserpine-induced akinesia achieved following intraventricular injection of DCG-IV indicates that activation of group II mGlu receptors may be a useful pharmacological approach in the treatment of PD even following systemic administration.
Acknowledgments
This work was funded by the Nuffield Foundation, U.K. and the Varga Foundation, Canada. A. Chadha is an MRC Research Student.
Abbreviations
- DCG-IV
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- EGLU
(2S)-α-ethylglutamic acid
- mGlu receptor
metabotropic glutamate receptor
- PD
Parkinson's disease
- SNr
substantia nigra pars reticulata
References
- BATTAGLIA G., MONN J.A., SCHOEPP D.D. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY35470 in rats. Neurosci. Lett. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- BENAZZOUZ A., GROSS C., FEGER J., BORAUD T., BIOULAC B. Reversal of rigidity and improvement of motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur. J. Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- BERGMAN H., WICHMANN T., DELONG M.R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1346–1348. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- BROTCHIE J.M., CROSSMAN A.R. D-[3H]Aspartate and [14C]GABA uptake in the basal ganglia of rats following lesions in the subthalamic region suggest a role for excitatory amino acids but not GABA-mediated transmission in subthalamic nucleus efferents. Exp. Neurol. 1992;113:171–181. doi: 10.1016/0014-4886(91)90173-a. [DOI] [PubMed] [Google Scholar]
- CALABRESI P., CENTONZE D., PISANI A., BERNARDI G. Metabotropic glutamate receptors and cell-type-specific vulnerability in the rat striatum: implication for ischaemia and Huntington's disease. Exp. Neurol. 1999;158:97–108. doi: 10.1006/exnr.1999.7092. [DOI] [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Ann. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- DAWSON L., CHADHA A., DUTY S. Locomotor effects following injection of the group II mGluR agonist, DCG-IV, into the substantia nigra pars reticulata of the reserpine-treated rat model of Parkinson's disease. Br. J. Pharmacol. 1999;126:60P. [Google Scholar]
- ISHIDA M., SAITOH T., SHIMAMOTO K., OHFUNE Y., SHINOZAKI H. A novel metabotropic glutamate receptor agonist: marked depression of monosynaptic excitation in the isolated newborn rat spinal cord. Br. J. Pharmacol. 1993;109:1169–1177. doi: 10.1111/j.1476-5381.1993.tb13745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMBARDI G., ALESIANI M., LEONARDI P., CHERICI G., PELLICCIARI R., MORONI F. Pharmacological characterisation of the metabotropic glutamate receptor inhibiting D-[3H]-aspartate output in rat striatum. Br. J. Pharmacol. 1993;110:1407–1412. doi: 10.1111/j.1476-5381.1993.tb13977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL I.J., CLARKE C.E., BOYCE S., ROBERTSON R.G., PEGGS D., SAMBROOK M.A., CROSSMAN A.R. Neural mechanisms underlying parkinsonian symptoms based on regional uptake of 2-deoxyglucose in monkeys exposed to 1 - methyl - 4 - phenyl - 1,2,3,6,-tetrahydropyridine (MPTP) Neuroscience. 1989;32:213–226. doi: 10.1016/0306-4522(89)90120-6. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO M., ISHIDA M., SHINOSAKI H. Anti-convulsive and neuroprotective actions of a potent agonist (DCG-IV) for group II metabotropic glutamate receptors against intraventricular kainate in the rat. Neuroscience. 1997;77:131–140. doi: 10.1016/s0306-4522(96)00442-3. [DOI] [PubMed] [Google Scholar]
- PARENT A., HAZRATI L.-N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The rat brain in stereotaxic co-ordinates 1986U.K.: Academic Press; 2nd edn [Google Scholar]
- ROBLEDO P., FEGER J. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulata and the pallidal complex: electrophysiological data. Brain Res. 1990;519:47–54. doi: 10.1016/0006-8993(90)90952-8. [DOI] [PubMed] [Google Scholar]
- SHIGEMOTO R., KINOSHITA A., WADA E., NOMURA S., OHISHI H., TAKADA M., FLOR P., NEKI A., ABE T., NAKANISHI S., MIZUNO N. Different presynaptic localisation of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH Y., BEVAN M.D., SHINK E., BOLAM J.P. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- STARR M.S., STARR B.S., KAUR S. Stimulation of basal and L-DOPA-induced motor activity by glutamate antagonists in animal models of Parkinson's disease. Neurosci. Biobehav. Rev. 1997;21:437–446. doi: 10.1016/s0149-7634(96)00039-5. [DOI] [PubMed] [Google Scholar]
- STOCCHI F., NORDERA G., MARSDEN C.D. Strategies for treating patients with advanced Parkinson's disease with disastrous fluctuation and dyskinesias. Clin. Neuropharmacol. 1997;20:95–115. doi: 10.1097/00002826-199704000-00001. [DOI] [PubMed] [Google Scholar]
- TESTA C.M., FRIBERG I.K., WEISS S.W., STANDAERT D.G. Immunohistochemical localisation of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J. Comp. Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- TESTA C.M., STANDAERT D.G., YOUNG A.B., PENNEY J.B. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J. Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGMORE M.A., LACEY M.G. Metabotropic glutamate receptors depress glutamate-mediated synaptic input to rat midbrain dopamine neurones in vitro. Br. J. Pharmacol. 1998;123:667–674. doi: 10.1038/sj.bjp.0701662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUNG K.K. Localization of ionotropic and metabotropic glutamate receptors in distinct neuronal elements of rat substantia nigra. Neurochem. Int. 1998;33:313–326. doi: 10.1016/s0197-0186(98)00034-5. [DOI] [PubMed] [Google Scholar]


