Abstract
Calcitonin gene-related peptide (CGRP) is one of the most potent endogenous vasodilators known. This peptide is increased during migraine attacks and has been implicated in the pathogenesis of migraine headache. Here we report on the first small molecule selective CGRP antagonist: BIBN4096BS. In vitro, this compound is extremely potent at primate CGRP receptors exhibiting an affinity (Ki) for human CGRP receptors of 14.4±6.3 (n=4) pM. In an in vivo model, BIBN4096BS in doses between 1 and 30 μg kg−1 (i.v.) inhibited the effects of CGRP, released by stimulation of the trigeminal ganglion, on facial blood flow in marmoset monkeys. It is concluded that BIBN4096BS is a potent and selective CGRP antagonist.
Keywords: BIBN4096BS, CGRP-antagonist, migraine
Introduction
CGRP, a 37 amino acid neuropeptide first identified in 1982 (Amara et al., 1982) belongs to a family of peptides including calcitonin, adrenomedullin and amylin. Localization studies have shown a wide distribution of CGRP-like immunoreactive structures including receptors in the periphery and central nervous system (Sexton, 1991; Brain & Cambridge, 1996). In many tissues, CGRP containing nerves are closely associated with blood vessels. Although CGRP has a number of effects, its most pronounced action is vasodilation. It is one of the most potent endogenous vasodilators known and its vasodilatory effects have been shown in a variety of vessels, e.g. CGRP released from sensory fibres originating in the trigeminal ganglia dilates cerebral vessels (Goadsby & Edvinsson, 1993).
Migraine headache is thought to be associated with dilatation of cranial vessels and activation of the trigemino-vascular system (Moskowitz & Macfarlane, 1993; Moskowitz, 1984; Goadsby et al., 1991). In man stimulation of the trigeminal ganglion results in the release of CGRP (Goadsby et al., 1988). Moreover, during a migraine attack levels of CGRP are increased (Goadsby et al., 1990; Gallai et al., 1995). Thus, CGRP has been implicated in the pathogenesis of migraine headache. Accordingly, inhibition of the CGRP-induced vasodilatation could be expected to attenuate migraine symptoms.
So far, only C-terminal fragments, example given CGRP(8–37), have been described to act as antagonists (Chiba et al., 1989; Rist et al., 1998), however these compounds are not very potent and their peptidic nature limit their use, especially for in vivo or clinical investigations. Our goal was to identify small molecule antagonists for the CGRP receptor and to use them to assess the role of CGRP in migraine headache.
Our high throughput screening led to the identification of dipeptide-like compounds that showed weak but unequivocal inhibition of binding to the CGRP receptor. Lead optimization led to potent CGRP antagonists, the prototype being BIBN4096BS, 1-Piperidinecarboxamide, N-[2-[ [5-amino-1-[ [4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[ (3,5-dibromo - 4 - hydroxyphenyl)methyl]- 2-oxoethyl]- 4- (1,4- dihydro-2-oxo-3(2H)-quinazolinyl)-, [R-(R*,S*)]- (Figure 1). The present study was undertaken to examine the pharmacological profile of BIBN4096BS and to investigate its potential as an anti-migraine drug.
Figure 1.
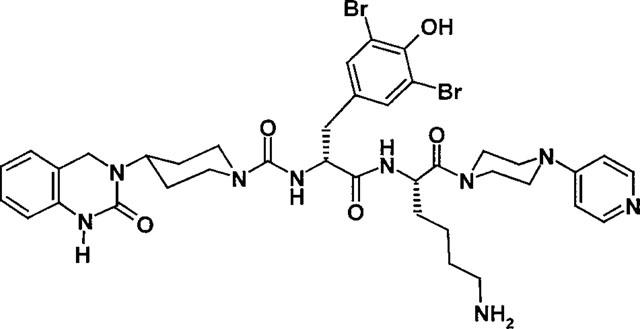
Structural formula of BIBN4096BS.
Methods
In-vitro experiments
Binding studies investigating CGRP-receptors from human or rat origin were performed using SK-N-MC (neuroblastoma cell line of human origin) cell membranes (approximately 0.175 Mio cells) and rat spleen homogenates (approximately 50 μg of protein per assay). 125I-hCGRP was used as the radioligand. The incubation buffer contained (in mM): Tris 50, NaCl 150, MgCl2 5 and EDTA 1, (ethylene diamine tetra-acetic acid) pH 7.4. Membrane homogenates were incubated for 180 min at room temperature with 50 pM 125I-hCGRP and increasing concentrations of displacing compound. The incubation was terminated by filtration through GF/B glass fibre filters using a cell harvester. The protein-bound radioactivity was determined in a gamma counter. The nonspecific binding was defined as radioactivity bound in the presence of 1 μM CGRP. The IC50 values were obtained by non-linear regression analysis on the basis of a one binding site model. Ki values were calculated according to the equation Ki=IC50/1+L/Kd, according to Cheng & Prusoff (1973), where L and Kd represent the concentration and the dissociation constant of the radioligand. The Kd was 0.05 nM and 0.12 nM for the human and rat CGRP receptor, as determined by saturation binding experiments with 0.5–500 pM 125I-CGRP.
Antagonism of BIBN4096BS was determined by measuring the formation of cyclic AMP in SK-N-MC cells. For cyclic AMP (adenosine 3′5′-cyclic monophosphate) measurements SK-N-MC cells were incubated with CGRP alone or in the presence of 10 nM BIBN4096BS for 15 min at 37°C. Cyclic AMP was extracted with 0.1 M HCl and determined by radioimmunoassay.
An apparent pKb value was calculated by the equation pKb=−log[B]+log (CR-1) (Schild, 1949), where [B] is the molar concentration of the antagonist and CR is the ratio of the EC50 values in the presence and absence of the antagonist.
In vivo experiments
Marmosets of either sex (300–400 g) were anaesthetized with sodium pentobarbitone (induction with 30 mg kg−1, i.p. and maintained with an infusion of 6 mg kg−1 h−1, i.m). Body temperature was kept at 37°C by automatic control of a heating pad. The animals were placed in a stereotaxic frame and a longitudinal incision was made in the scalp. A small hole was drilled in the skull and a bipolar electrode (Rhodes SNEX 100) was lowered, using a micromanipulator, in the trigeminal ganglion.
We used a radiographic approach to identify bony landmarks as guide in placing probes in the ganglion. An X-ray was taken using a Trophy CCX digital X-Ray apparatus and the Pars petrosa was identified as guide for placing the electrodes. The positions of the electrodes in the trigeminal ganglion were checked visually at the end of each experiment following removal of the brain. The trigeminal ganglion was stimulated at 10 Hz, 2 mA, 2 ms for 30 s using a Hugo Sachs Elektronik stimulator. Neuromuscular blockade, 15 min before electrical stimulation, was achieved using pancuronium bromide (initial 1 mg kg−1, i.v., followed by 0.5 mg kg−1 every hour).
Microvascular blood flow changes in the facial skin were measured by Laser Doppler flowmetry with a PeriFlux laser doppler system (wave length 780 nM; Time Constant 3 s) as flux in arbitrary units and expressed as area under the flux-curve (mm2) according to Escott et al., 1995.
Animals were subjected to two or three periods of electrical stimulations, separated by a 30 min interval. The first stimulation was a control for the subsequent stimulations. Test compounds were administered intravenously 5 min prior to the second or third stimulation.
Results
BIBN4096BS completely inhibited the specific binding of 125I-CGRP to SK-N-MC cells and displayed an affinity (Ki) of 14.4±6.3 pM (n=4) for the human CGRP receptor (Figure 2A). The endogenous ligand CGRP itself and the peptidic antagonist CGRP(8-37) displayed affinities (Ki) of 31.7±1.6 pM (n=15) and 3.6±0.7 nM (n=4), respectively. Employing the same cell line it was shown that BIBN4096BS is a pure antagonist. In the presence of antagonist the concentration-response curve of CGRP to increase cyclic AMP levels was shifted to the right in a parallel fashion, indicating competitive antagonism (Figure 2B). The pKb value calculated at an antagonist concentration of 10 nM was 11.0±0.3 (n=3). In addition, concentrations of BIBN4096BS up to 100 μM did not show any agonistic effects. The affinity (Ki) for CGRP binding sites in rat spleen was 3.4±0.5 nM (n=3) and 96±4 pM (n=9) for BIBN4096BS and CGRP, respectively. Accordingly, BIBN4096BS possessed a 236 fold higher affinity for human (SK-N-MC cells) than for rat (spleen) receptors.
Figure 2.
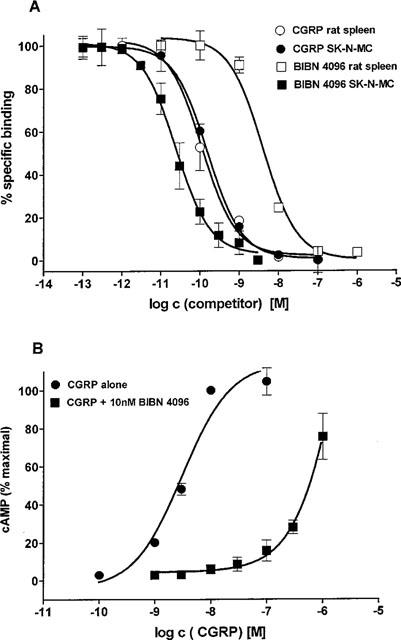
(A) Concentration-dependent inhibition of BIBN4096BS and CGRP of the specific binding of 125I-CGRP to membranes obtained from the human neuroblastoma cell line SK-N-MC or from rat spleen. (B) Stimulation of cyclic AMP formation in SK-N-MC cells by CGRP alone and in the presence of 10 nM BIBN4096BS. Data are means±s.e.mean.
Electrical stimulation of the trigeminal ganglion in marmosets caused an ipsilateral increase in facial skin blood flow. Following stimulation there was a rapid increase in flow which returned to base line values after approximately 10 min. This response was obtained three times in the same animal with no significant difference between the first and third stimulation. The area under the curve for the increase in flux observed was used to determine the effects of BIBN4096BS and amounted to 112±11 mm2. Administration of BIBN4096BS significantly and dose-dependently inhibited the response evoked by trigeminal ganglion stimulation (Figure 3). A 50% inhibition was achieved with a dose of approximately 3 μg kg−1 (n=4) applied intravenously. An almost complete blockade was observed with a dose of 30 μg kg−1 (i.v.). BIBN4096BS in doses up to 1 mg kg−1 (i.v.) had no intrinsic cardiovascular effects.
Figure 3.
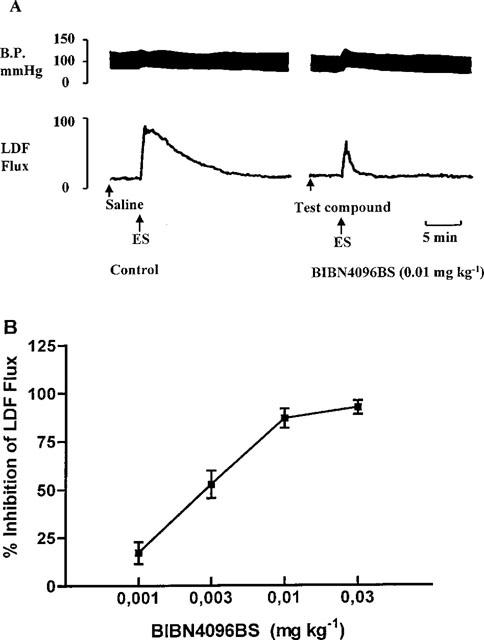
(A) Representative recording to illustrate the changes in blood pressure and LDF-Flux following electrical trigeminal ganglion stimulation. (B) Dose-dependent inhibition of the facial skin vasodilation induced by trigeminal ganglion stimulation in the marmoset by BIBN4096BS. Data are means±s.e.mean.
Discussion
In order to determine the affinity of BIBN4096BS for human CGRP receptors we decided to use SK-N-MC cells since the receptor expressed in this cell line is identical to the cloned human CGRP receptor (Aiyar et al., 1996). The affinity of BIBN4096BS for the human CGRP receptor is even higher than that of the endogenous ligand CGRP itself and 150 fold higher compared to the peptidic antagonist CGRP(8-37). Moreover, BIBN4096BS proved to be a competitive antagonist without any intrinsic agonistic activity. BIBN4096BS had no significant affinity for a set of 75 different receptor or enzyme system (IC50>>1000 nM) including calcitonin, amylin or adrenomedullin receptors (for complete list of assays, see Rudolf et al., 1997). Therefore, it can be concluded that BIBN4096BS is a selective CGRP antagonist with high affinity. We also demonstrated that BIBN4096BS possesses a remarkable species selectivity, exhibiting an approximately 200 fold higher affinity for primate CGRP receptors compared to rat CGRP receptors. The same was observed for other non-primate species, such as dog, guinea-pig and rabbit. Having established that BIBN4096BS is a potent and selective CGRP antagonist we addressed the question whether BIBN4096BS has the potential to be an anti-migraine drug. Measuring facial blood flow following antidromic stimulation of the trigeminal ganglion has been suggested as a model to evaluate drugs that target the trigemino-vascular system during migraine headache (Escott et al., 1995). Antidromic stimulation will result in the release of neuropeptides such as CGRP. Concomitantly, an increase in blood flow will be observed in blood vessel innervated by sensory nerves. As the facial skin is innervated by the trigeminal nerve, we measured the blood flow in this area. Because BIBN4096BS has a much higher affinity for primate CGRP receptors compared to rat CGRP receptors, we used marmosets. BIBN4096BS completely inhibited the neurogenic vasodilation, demonstrating that CGRP plays a major role in the vasodilation observed following stimulation of the trigeminal nerve in the marmoset. This is in agreement with experiments performed in the rat by Escott et al. (1995). Although CGRP itself is a strong vasodilator, i.v. administration of the antagonist had no effect on basal blood pressure and heart rate. This might indicate that CGRP plays a minor role in basal cardiovascular homeostasis under normal conditions, and that CGRP is only involved in (human) pathophysiology. Moreover, it is of interest to mention that in our model anti-migraine drugs like sumatriptan and zolmitriptan, both 5-HT1B/D agonists, inhibited the increase in facial blood flow due to trigeminal stimulation not completely, but only approximately seventy per cent (data not shown). Accordingly, it could be hypothesized that blockade of postsynaptically located vascular CGRP receptors is a more powerful way to attenuate CGRP mediated effects than inhibiting the release of the peptide by stimulating presynaptic 5-HT-receptors.
In conclusion, our results demonstrate that BIBN4096BS is a high affinity and selective CGRP antagonist. Moreover, BIBN4096BS is a very potent inhibitor of neurogenic vasodilation. Since several lines of evidence indicate that CGRP might be a key factor in the initiation of migraine headache, we expect that CGRP antagonists will be effective anti-migraine drugs. Much remains to be investigated to understand the origin of migraine and to validate the concept that CGRP is a major player in its pathophysiology. The availability of a potent and well tolerated CGRP antagonist is a prerequisite to examine whether CGRP antagonism is a new therapeutic concept. BIBN4096BS is such a tool which is presently under clinical investigation for the acute treatment of migraine headache.
Abbreviations
- BIBN4096BS
1-Piperidinecarboxamide, N-[2-[ [5-amino-1-[ [4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[ (3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-, [R-(R*,S*)]-
- cAMP
adenosine 3′5′-cyclic monophosphate
- CGRP
calcitonin gene-related peptide
- EDTA
ethylenediaminetetraacetic acid
- LDF
laser doppler flow
- SK-N-MC
neuroblastoma cell line of human origin
- 5-HT
5-hydroxytryptamine
References
- AIYAR N., RAND K., ELSHOURBAGY N.A., ZENG Z., ADAMOU J.E., BERGSMA D.J., LI Y. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J. Biol. Chem. 1996;271:11325–11329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- AMARA S.G., JONES V., ROSENFELD M.G., ONG E.S., EVANS R.M. Alternative RNA processing in calcitonin gene expression generates mRNA encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D., CAMBRIDGE H. Calcitonin Gene-Related Peptide: Vasoactive Effects and Potential Therapeutic Role. Gen. Pharmacol. 1996;27:607–611. doi: 10.1016/0306-3623(95)00125-5. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3105. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIBA T., YAMAGUCHI A., YAMATANI T., NAKAMURA A., MORISHITA T., INUI T., FUKASE M., NODA T., FUJITA T. Calcitonin gene-related peptide receptor antagonist human CGRP(8-37) Am. J. Physiol. 1989;256:E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- ESCOTT K.J., BEATTIE D.T., CONNOR H.E., BRAIN S.D. Trigeminal ganglion stimulation increases facial skin blood flow in the rat: a major role for calcitonin gene-related peptide. Brain Research. 1995;669:93–99. doi: 10.1016/0006-8993(94)01247-f. [DOI] [PubMed] [Google Scholar]
- GALLAI V., SARCHIELLI P., FLORIDI A., FRANCESCHINI M., CODINI M., GLIOTI G., TREQUATTRINI A., PALUMBO R. Vasoactive peptide levels in the plasma of young migraine patients with and without aura assessed both interictally and ictally. Cephalalgia. 1995;15:384–390. doi: 10.1046/j.1468-2982.1995.1505384.x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. The trigemino-vascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., ZAGAMI A.S., LAMBERT G.A. Neural processing of craniovascular pain: a synthesis of the central structures involved in migraine. Headache. 1991;31:365–371. doi: 10.1111/j.1526-4610.1991.hed3106365.x. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A., MACFARLANE R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc. Brain Metab. Rev. 1993;5:159–177. [PubMed] [Google Scholar]
- RIST B., ENTZEROTH M., BECK-SICKINGER A.G. From micromolar to nanomolar affinity: a systematic approach to identify the binding site of CGRP at the human calcitonin gene-related peptide 1 receptor. J. Med. Chem. 1998;41:117–123. doi: 10.1021/jm970533r. [DOI] [PubMed] [Google Scholar]
- RUDOLF K., EBERLEIN W., ENGEL W., BECK-SICKINGER A.G., WITTNEBEN H., WIELAND H.A., DOODS H.N.BIBP3226, a potent and selective neuropeptide Y Y1-receptor antagonist. Structure-activity studies and localization of the human Y1 receptor binding site Neuropeptide Y and drug development 1997175–190.In: Grundemar, L. & Bloom, S.R. (eds)
- SCHILD H.O. pAx and competitive drug antagonism. Brit. J. Pharmacol. 1949;4:277–280. doi: 10.1111/j.1476-5381.1949.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEXTON P.M. Central nervous binding sites for calcitonin and calcitonin gene-related peptide. Mol. Neurobiol. 1991;5:251–273. doi: 10.1007/BF02935550. [DOI] [PubMed] [Google Scholar]


