Abstract
In the present work we studied the effect of protein phosphatase inhibitors on the phosphorylation state and function of α1b-adrenoceptors.
Okadaic acid increased receptor phosphorylation in a time- and concentration-dependent fashion (maximum at 30 min, EC50 of 30 nM). Other inhibitors of protein phosphatases (calyculin A, tautomycin and cypermethrin) mimicked this effect.
Staurosporine and Ro 31-8220, inhibitors of protein kinase C, blocked the effect of okadaic acid on receptor phosphorylation. Neither genistein nor wortmannin altered the effect of okadaic acid.
The intense adrenoceptor phosphorylation induced by okadaic acid altered the adrenoceptor-G protein coupling, as evidenced by a small decreased noradrenaline-stimulated [35S]GTPγS binding. Okadaic acid did not alter the noradrenaline-stimulated increases in intracellular calcium or the production of inositol trisphosphate.
Our data indicate that inhibition of protein phosphatases increases the phosphorylation state of α1b-adrenoceptors; this effect seems to involve protein kinase C. In spite of inducing an intense receptor phosphorylation, okadaic acid alters α1b-adrenergic actions to a much lesser extent than the direct activation of protein kinase C by phorbol myristate acetate.
Keywords: α1-Adrenoceptors, α1-adrenergic receptors, phosphorylation, desensitization
Introduction
The α1-adrenoceptors (α1-ARs) are a heterogeneous subgroup of receptors, members of the seven transmembrane domains/G protein-coupled family of receptors that mediate important physiological actions of adrenaline and noradrenaline. Three α1-ARs have been cloned and expressed, i.e. the α1a-, α1b- and α1d-ARs (Hieble et al., 1995). The hamster α1b subtype was the first to be cloned (Cotecchia et al., 1988), it is the best characterized and, therefore, considered prototypic of this subgroup. These receptors are mainly coupled to the phosphoinositide/calcium mobilization signal transduction pathway, although they can also activate other signalling processes (Graham et al., 1996).
Modulation of G protein coupled receptor function (desensitization/resensitization) is a key event in the adaptation of cells to the changes in the internal milieu of an organism and to the overall homeostasis. Different cellular processes are involved (receptor uncoupling from G proteins, internalization, degradation, regulation of receptor gene expression, etc.) with different time frames (Lefkowitz, 1998). An initial event seems to be receptor phosphorylation. Three groups of protein kinases are the major modulators of G protein-coupled receptors: (a) second messenger-activated kinases, such as protein kinase A and protein kinase C (PKC) (Clark et al., 1988; Houslay, 1991, (b) members of the G-protein receptor kinase (GRK) family (Benovic et al., 1990) and (c) some receptors with tyrosine kinase activity (Hadcock et al., 1992).
Regarding α1b-ARs, it is known that activation of PKC blocks the actions of these receptors and that such effect is associated to receptor phosphorylation (Corvera & García-Sáinz, 1984; Corvera et al., 1986; Diviani et al., 1996; 1997; Lattion et al., 1994; Leeb-Lundberg et al., 1985; Vázquez-Prado & García-Sáinz, 1996; Vázquez-Prado et al., 1997). Similarly, there is also evidence indicating that GRKs phosphorylate these receptors and participate in their homologous desensitization (Diviani et al., 1996). The PKC- and GRK-phosphorylation sites were recently identified at the α1b-AR carboxyl terminus (Diviani et al., 1996; 1997).
The phosphorylation state of a receptor results from the balance of activities of protein kinases and protein phosphatases. In contrast to what is known about the roles of protein kinases on G protein coupled receptor phosphorylation, little is known about the role of protein phosphatases. In this regard, Pitcher et al. (1995) have shown that a latent oligomeric form of protein phosphatase 2A actively dephosphorylate the β2-AR in vitro and Shih et al. (1999) reported that protein phosphatase 2A and 2B are associated with β2-ARs. It has also been observed that okadaic acid, an inhibitor of protein phosphatases, induces both augmentation and inhibition of β2-AR-mediated stimulation of cyclic AMP accumulation (Clark et al., 1993). Inhibition of the serine/threonine protein phosphatase, calcineurin, enhances desensitization and phosphorylation of adipocyte β1-AR (Bahouth et al., 1996).
In the case of the α1b-ARs there is no information available. Regulation of protein phosphatase activity is a potentially important point of regulation of receptor function. Low molecular weight protein phosphatase inhibitors that are able to penetrate living cells are important tools in the study of receptor function (Holmes & Boland, 1993). Okadaic acid, tautomycin and calyculin-A are selective inhibitors, in vitro, for the catalytic subunits of protein phosphatase 1 and protein phosphatase 2A (Holmes & Boland, 1993) whereas cypermethrin is specific, in vitro, for protein phosphatase 2B (Wang & Stelzer, 1994). In the present work we explore the role(s) of protein phosphatases on receptor phosphorylation and function by the use of inhibitors.
Methods
Cell line and culture
Rat-1 fibroblasts transfected with the hamster α1b-AR (Cotecchia et al., 1988), generously provided to us by Drs R.J. Lefkowitz, M.G. Caron and L. Allen (Duke University), were cultured as described previously (Vázquez-Prado & García-Sáinz, 1996; Vázquez-Prado et al., 1997). In the present experiments, cells at confluence were serum-deprived in unsupplemented Dulbecco's modified Eagle's medium (DMEM) for 18–24 h.
Receptor phosphorylation
Experiments were performed as described in detail (Vázquez-Prado et al., 1997). In brief, cells were maintained in phosphate-free DMEM during 1 h and then incubated in 3 ml of the same medium containing [32P]Pi (50 μCi ml−1) for 3 h at 37°C. Labelled cells were stimulated as indicated, then they were washed and solubilized with 1 ml of ice-cold solubilization buffer, containing 1% Triton X-100 and 0.05% sodium dodecyl sulphate (Vázquez-Prado et al., 1997). The extracts were centrifuged and the supernatants transferred to tubes containing a rabbit antiserum generated against the carboxyl terminus decapeptide of the hamster α1b-AR and sepharose-coupled protein A and immunoprecipitated (Vázquez-Prado et al., 1997). The immunoprecipitates were subjected to electrophoresis and phosphorylated receptor was determined by PhosphorImager analysis.
Intracellular calcium concentration ([Ca2+]i)
Determinations were as reported previously (Vázquez-Prado et al., 1997). Briefly, cells were incubated overnight in G418-free DMEM without serum, loaded with 5 μM Fura 2/AM at 37°C for 1 h, detached and washed. Cells were resuspended at a concentration of approximately 106 cells ml−1 in Krebs-Ringer-HEPES, pH 7.4, containing 0.05% bovine serum albumin. When okadaic acid or phorbol 12-myristate 13-acetate (PMA) were used, the cells were in contact with these agents for 15 min and remained present during the assay. Fluorescence measurements were performed in an AMINCO-Bowman spectrofluorometer, with excitation monochromators set at 340 and 380 nm, with a chopper interval of 0.5 s, and the emission monochromator set at 510 nm. The [Ca2+]i was calculated according to Grynkiewicz et al. (1985) using the software provided by AMINCO-Bowman; traces were directly exported to the graphs.
[3H]inositol trisphosphate production
Determinations were as previously reported (Vázquez-Prado & García-Sáinz, 1996) with minor modifications. In brief, cells were labelled with [3H]inositol (6 μCi ml−1) for 18–24 h in inositol-free DMEM. On the day of the experiment, cells were washed twice with Krebs-Ringer-HEPES buffer containing 1.3 mM CaCl2 and preincubated for 30 min in 2 ml of the same buffer containing 10 mM LiCl. When okadaic acid or PMA were used they were added during this preincubation and remained present during the assay. Incubations continued a further 15 min (in the absence or presence of noradrenaline) and were terminated by the addition of ice-cold perchloric acid. Supernatants were neutralized and [3H]inositol trisphosphate was separated by Dowex AG1-X8 chromatography (Berridge et al., 1983).
Membrane preparation and [35S]GTPγS binding
Confluent cells were incubated in the absence (control) or presence of noradrenaline (10 μM) for 5 min or with PMA (1 μM), okadaic acid (1 μM) or okadaic acid (1 μM) plus PMA (1 μM) for 15 min at 37°C. Washing with ice-cold phosphate buffered saline terminated the reaction and cells were scraped with 1 ml of ice-cold buffer (50 mM Tris, 150 mM NaCl, pH 7.5, 5 mM EDTA, 100 μM Na3VO4, 10 mM β-glycerophosphate, 10 mM sodium pyrophosphate, plus protease inhibitors) (Vázquez-Prado et al., 1997). Membranes were prepared according to the method of Mattingly et al. (1992). [35S]GTPγS binding was performed as described by Wieland & Jakobs (1994) with minor modifications. Briefly, membranes were resuspended in binding buffer (in mM): Tris 50, MgCl2 10, EDTA 1, NaCl 100, DTT 1, pH 7.4, 1 μM GDP, 0.1% bovine serum albumin. Binding was performed at 25°C for 30 min in a volume of 250 μl of binding buffer containing 0.2 nM [35S]GTPγS. Membranes were stimulated with 10 μM noradrenaline. The reaction was initiated by the addition of membranes (25 μg of protein/tube) and terminated by rapid filtration through Whatman GF/C filters followed by three washes of the filters with ice-cold buffer (50 mM Tris, 10 mM MgCl2, pH 7.5). The filters were dried and the radioactivity was quantified by liquid scintillation. Nonspecific binding was determined in the presence of unlabelled GTPγS (30 μM) and represented 10% of total binding.
Statistical analysis between comparable groups was performed using analysis of variance with Newman-Keuls post test.
Materials
(−)-Noradrenaline, PMA, dl-propranolol, staurosporine, bovine serum albumin, wortmannin and protease inhibitors were obtained from Sigma Chemical Co. Genistein were from Research Biochemicals Inc. DMEM, foetal bovine serum, trypsin, antibiotics, and other reagents used for cell culture were from Gibco BRL. Okadaic acid, cypermethrin, tautomycin, calyculin A and Ro 31-8220 were from Calbiochem. [2,3-3H]myo-inositol (22.9 Ci mmol−1), [35S]GTPγS (1250 Ci mmol−1) and [32P]Pi (8500-9120 Ci mmol−1) were from New England Nuclear. Sepharose-coupled protein A was from Upstate Biotechnology. Fura-2/AM was from Molecular Probes.
Results
The effect of okadaic acid on α1b-AR phosphorylation was studied. As shown in Figure 1 (left panel), okadaic acid (1 μM) increased the phosphorylation of α1b-ARs. A clear increase was evident as early as 5 min after the addition of the protein phosphatase inhibitor and the effect reached its maximum (∼2.5 fold) at 30 min; this effect remained constant up to 60 min. The effect of okadaic acid was concentration-dependent with an EC50 of 30 nM (Figure 1, right panel). The effect of okadaic acid on α1b-AR phosphorylation (P<0.001 vs control) was comparable in magnitude to that induced by 1 μM PMA (280±40% of basal, n=17). Other protein phosphatase inhibitors were tested at a concentration of 1 μM and the effect is shown in Figure 2. Tautomycin (P<0.001 vs control), calyculin A (P<0.001 vs control) and to a lesser extent cypermethrin (P<0.05 vs control) increased α1b-AR phosphorylation.
Figure 1.
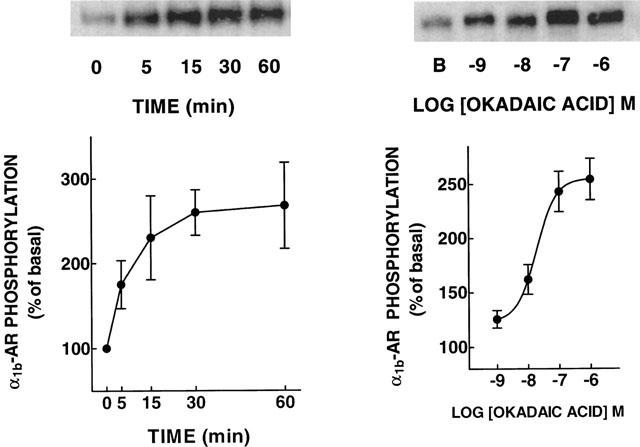
Effects of okadaic acid on α1b-adrenoceptor phosphorylation. Left panel: cells were incubated with 1 μM okadaic acid for the times indicated. Right panel: cells were incubated with the indicated concentrations of okadaic acid for 30 min. Plotted are the means and vertical lines represent the s.e.mean of three determinations using different cell preparations. Representative autoradiographs are shown. B, basal phosphorypatium.
Figure 2.
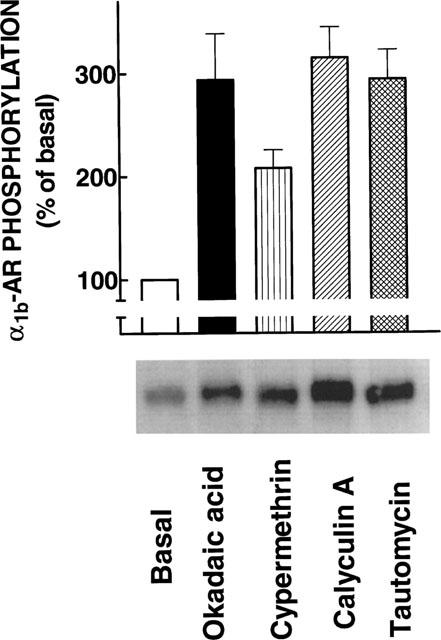
Effects of protein phosphatase inhibitors on α1b-adrenoceptor phosphorylation. Cells were incubated in the absence of any inhibitor (Basal) or in the presence of 1 μM of the indicated inhibitors for 30 min. Plotted are the means and vertical lines represent the s.e.mean of four determinations using different cell preparations. A representative autoradiograph is shown.
The data indicated that when protein phosphatases were inhibited the phosphorylated receptor accumulated. In order to gain insight into the protein kinase(s) involved in this effect, the effect of protein kinase inhibitors was tested. As shown in Figure 3, staurosporine (10 μM), an inhibitor of protein serine/threonine kinases with relative selectivity for PKC, decreased basal receptor labelling and blocked the effect of okadaic acid (P<0.001 vs okadaic acid alone). In contrast genistein (10 μM), a protein tyrosine kinase inhibitor, blocked neither the basal labelling nor the effect of okadaic acid on the state of phosphorylation of the receptor. The effect of wortmannin (10 μM), an inhibitor of phosphatidyl inositol 3-kinase, was also tested; this inhibitor altered neither the basal phosphorylation of the α1b-AR nor the effect of okadaic acid. In order to define more clearly the role of PKC the effects of staurosporine and of the selective PKC inhibitor, Ro 31-8220, were studied in more detail. The inhibitors decreased the basal phosphorylation of the receptor at concentrations of 1 μM and higher and blocked, in a concentrationdependent fashion, the effect of okadaic acid (Figure 4). As shown, staurosporine was slightly, but consistently, more potent than Ro 31-8220.
Figure 3.
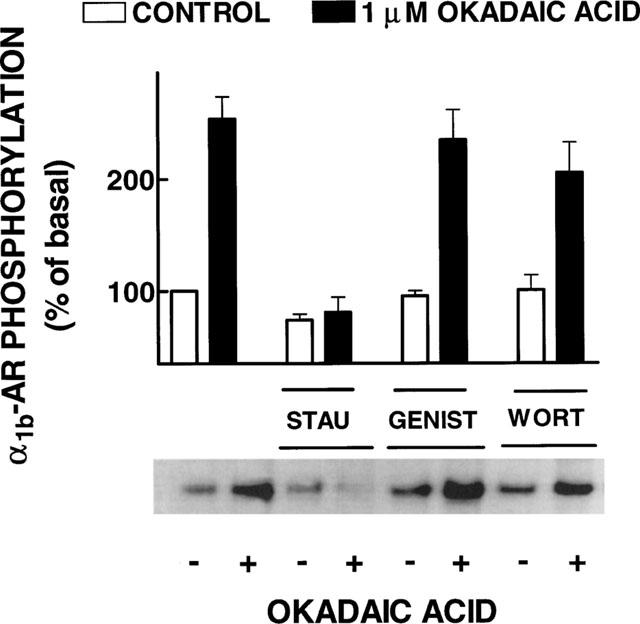
Effects of kinase inhibitors on the effect of okadaic acid on α1b-adrenoceptor phosphorylation. Cells were incubated in the absence or presence of 1 μM okadaic acid and the inhibitors: 10 μM staurosporine (STAU), 10 μM genistein (GENIST) or 10 μM wortmannin (WORT). Plotted are the means and vertical lines represent the s.e.mean of 4–5 determinations using different cell preparations. A representative autoradiograph is shown.
Figure 4.
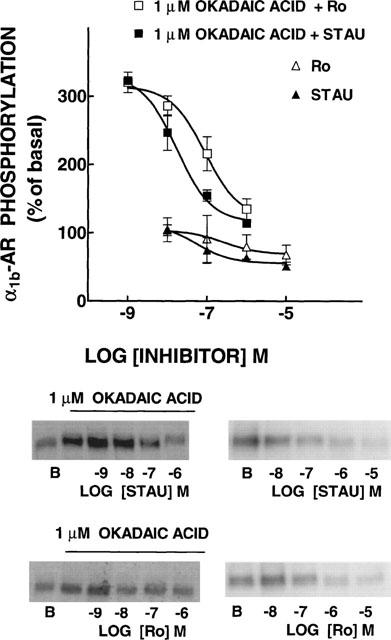
Effects of protein kinase C inhibitors on basal and okadaic acid-induced α1b-adrenoceptor (α1b-AR) phosphorylation. Cells were incubated in the absence or presence of 1 μM okadaic acid and different concentrations of staurosporine (STAU) or Ro-31-8220 (Ro). B, basal phosphorylation. Plotted are the means and vertical lines represent the s.e.mean of 3–4 determinations using different cell preparations. Representative autoradiographs are shown.
It has been previously shown that PMA, noradrenaline and endothelin induce α1b-AR phosphorylation (Diviani et al., 1996; 1997; Lattion et al., 1994; Leeb-Lundberg et al., 1985; Vázquez-Prado et al., 1997). The possibility that the action of okadaic acid could be additive to that of the previously mentioned agents was examined. However, we were unable to observe any clear additivity (data not shown).
We next examined the functional relevance of the effect of okadaic acid on the α1b-AR phosphorylation state. Two parameters were examined: [Ca2+]i and [3H]inositol trisphosphate production. In these experiments okadaic acid and/or PMA were preincubated with the cells for 15 min ([Ca2+]i) or 30 min ([3H]inositol trisphosphate production) before noradrenaline (in the presence of 10 μM propranolol to block any β-adrenergic action) was added. It can be observed in Figure 5 (upper panel) that noradrenaline induced a concentration dependent increase in [Ca2+]i. Okadaic acid, by itself, did not alter basal [Ca2+]i and it did not alter in any significant way the effect of different concentrations of noradrenaline. In contrast, preincubation with PMA markedly decreased the effect of noradrenaline (P<0.001 vs control at noradrenaline concentrations of 100 mM and above). When the cells were preincubated with okadaic acid and PMA no effect of noradrenaline on [Ca2+]i was observed at all (Figure 5, upper panel) (P<0.05 vs PMA alone at noradrenaline concentrations of 1 and 10 μM; P<0.001 vs control at noradrenaline concentrations of 100 nM and above).
Figure 5.
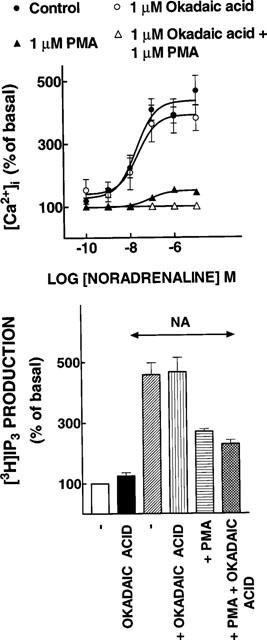
Functional consequences of the effects of okadaic acid and phorbol 12-myristate 13-acetate (PMA) on α1b-adrenergic action. Upper panel, [Ca2+]i; concentration-response curves to noradrenaline of cells preincubated in the absence of any agent, 1 μM okadaic acid, 1 μM PMA or 1 μM PMA plus 1 μM okadaic acid. Lower panel, [3H]IP3. Cells were preincubated without any agent or with 1 μM of the agents indicated under the bars. After this preincubation the cells were further incubated for 15 min in the absence or presence of 10 μM noradrenaline plus 10 μM propranolol (NA). Plotted are the means and vertical lines represent the s.e.mean of 6–7 determinations using different cell preparations.
Similarly, noradrenaline (in the presence of 10 μM propranolol) induced a marked increase in the production of [3H]inositol trisphosphate from [3H]inositol-labelled cells (Figure 5, lower panel) (P<0.001 vs control). Okadaic acid (Figure 5, lower panel) and PMA (not shown) by themselves did not alter the basal production of this second messenger. PMA (P<0.01 vs control), but not okadaic acid, markedly decreased the effect of noradrenaline (Figure 5, lower panel). When the cells were incubated with PMA, the addition of okadaic acid only slightly further decreased the α1b-adrenergic effect, but did not block it; this further decrease was not statistically significant (Figure 5, lower panel).
In order to study more directly the effect of the different treatments on α1b-AR-G protein coupling, noradrenaline-stimulated [35S]GTPγS binding was studied. It can be observed in Figure 6 that noradrenaline increased the guanine nucleotide binding to membranes of control cells; such increase was of smaller magnitude in membranes obtained from cells incubated with 10 μM noradrenaline (P<0.001 vs control), or 1 μM PMA (P<0.001 vs control). Okadaic acid induced a small but statistically significant (P<0.05 vs control) decrease in agonist-stimulated binding. In membranes from cells incubated in the presence of PMA and okadaic acid, the decrease in agonist-stimulated guanine nucleotide binding was bigger than that observed with okadaic acid alone (P<0.001) but similar to that observed with PMA alone (Figure 6).
Figure 6.
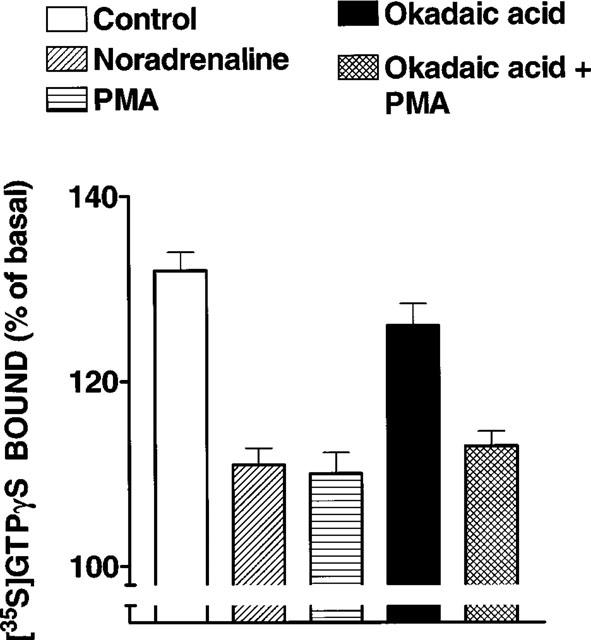
Effect of cell treatment with noradrenaline, okadaic acid and phorbol 12-myristate 13-acetate (PMA) on noradrenaline-stimulated [35S]GTPγS binding. Cells were incubated in the absence of any agent (Control) or presence of 10 μM noradrenaline, 1 μM PMA, 1 μM okadaic acid or 1 μM okadaic acid plus 1 μM PMA. Membranes were stimulated with 10 μM noradrenaline. Plotted are the means and vertical lines represent the s.e.mean of 18–25 determinations using six different membrane preparations, for each condition.
Discussion
Our present data clearly indicate that inhibition of serine/threonine protein phosphatases markedly increases the phosphorylation state of the α1b-AR. To the best of our knowledge this is the first demonstration of such an effect for an α1-AR.
As indicated in the introduction, considerable insight on the roles that different protein kinases play in the modulation/phosphorylation of G protein coupled receptors has been gained, but there is very little information on the role(s) of protein phosphatases. Nevertheless, it has been observed that peroxovanadate enhances desensitization and phosphorylation of adipocyte β1-AR by inhibiting the activity of the serine/threonine protein phosphatase, calcineurin (Bahouth et al., 1996). A latent oligomeric form of protein phosphatase 2A has been shown to actively dephosphorylate the β2-AR in vitro (Pitcher et al., 1995).
Current evidence suggest that scaffold, anchoring and adapter proteins contribute to the specificity of signal transduction by recruiting active enzymes into signalling complexes (Pawson & Scott, 1997). This has been extensively observed in tyrosine kinase receptor signalling (Pawson & Scott, 1997) and was very elegantly extended for G protein coupled receptors by Shih et al. (1999). Very recently, these authors reported that protein phosphatase 2A and 2B are associated with β2-ARs in the basal state and that the latter protein phosphatase and PKA display a robust association with these receptors following challenge with the agonists. The anchoring protein gravin participates in the formation of these dynamic complexes with protein kinases, protein phosphatases and the β2-AR (Shih et al., 1999).
A complex interplay seems to exist between PKC and protein phosphatases to modulate α1b-AR phosphorylation and function. Our studies with protein phosphatase inhibitors suggest that protein phosphatases-1, 2A and 2B could be involved in modulating receptor phosphorylation. However, although these inhibitors show selectivity in vitro, their selectivity in vivo, is questionable. Therefore, we cannot define the type(s) of protein phosphatase(s) involved.
It is possible that basal PKC activity was sufficient to phosphorylate α1b-ARs. This is supported by the observation that PKC inhibitors decreased basal phosphorylation at concentrations above 1 μM. The marked increase in α1b-AR phosphorylation in the presence of okadaic acid suggests that protein phosphatase activity exert a tonic action dephosphorylating these adrenoceptors. Alternatively, it is possible that inhibition of protein phosphatases may activate PKC; in fact, there is evidence that protein phosphatases reversibly inhibit PKC activity in vitro (Ricciarelli & Azzi, 1998). The present data do not allow us to distinguish between these not mutually exclusive possibilities.
A particularly interesting finding was that despite the fact that okadaic acid increased α1b-AR phosphorylation, neither the [Ca2+]i increase nor the production of [3H]inositol trisphosphate induced by noradrenaline were significantly altered. This is in marked contrast with the results obtained with PMA, which greatly reduced both receptor responses. We have previously observed that bradykinin induced α1b-AR phosphorylation without leading to adrenoceptor desensitization (Medina et al., 1998); however, bradykinin induced only a 50% increase in receptor phosphorylation. Okadaic acid induced an increase in α1b-AR phosphorylation of similar magnitude as PMA but the functional repercussions markedly differ.
The results on noradrenaline-stimulated [35S]GTPγS binding indicate that the phosphorylation induced by the treatment with okadaic acid does indeed impair the adrenoceptor-G protein coupling, but to a much lesser extent than PMA or noradrenaline. Such okadaic acid-induced decrease of receptor-G protein coupling does not however seem to affect the adrenergic actions in whole cells, i.e. the intracellular calcium and inositol trisphosphate responses were not decreased.
The differences in the effect of PMA and okadaic acid on the receptor response may also reflect the fact that in addition to receptor phosphorylation other event may underlie desensitization. Among these, PKC-dependent phosphorylation of other molecular entities participating in signalling, such as G protein(s) or phospholipase C might result in desensitization.
The actions of both PMA and okadaic acid on α1b-AR phosphorylation seem to involve PKC activity, but they had very different functional repercussions. This suggests that differences may exist in the sites phosphorylated under the action of PMA or okadaic acid. One possibility that may explain this puzzle is that PMA may activate PKC isoforms that are not active in the basal state or in the presence of okadaic acid. PKC is a multigene family of protein kinase with different sensitivity to activators and substrate selectivity (Newton, 1995). There is no data on the isoforms of PKC that participate in α1b-AR phosphorylation.
As indicated, the sites involved in PKC-mediated α1b-AR phosphorylation have been identified in the carboxyl terminus (Ser394 and Ser400) although a third, yet unidentified, site seems to exist (Diviani et al., 1997). The sites where basal phosphorylation takes place and the kinase(s) involved have not yet been positively identified. The functional significance of such basal phosphorylation is unknown.
In summary, our data indicate that inhibition of protein phosphatases increase α1b-AR phosphorylation. This effect seems to involve PKC activity. In contrast to the effect of PMA, okadaic acid does not block α1b-adrenergic actions in whole cells and only marginally affect receptor coupling to G proteins as evidenced by the noradrenaline-stimulated [35S]GTPγS binding.
Acknowledgments
This research was partially supported by Grants from DGAPA (IN 200596 and IN 205199), CONACyT (27569-N) and Fundación Miguel Alemán.
Abbreviations
- α1-AR
α1-adrenoceptor
- [Ca2+]i
intracellular calcium
- DMEM
Dulbecco's modified Eagle's medium
- GRK
G-protein receptor kinase
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
References
- BAHOUTH S.W., GOKMEN-POLAR Y., CORONEL E.C., FAIN J.N. Enhanced desensitization and phosphorylation of the β1-adrenergic receptor in rat adipocyte by peroxovanadate. Mol. Pharmacol. 1996;49::1049–1057. [PubMed] [Google Scholar]
- BENOVIC J.L., DEBLASI A., STONE W.C., CARON M.G., LEFKOWITZ R.J. β-Adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1990;246:235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J., DAWSON R.M.C., DOWNES P., HESLOP J.P., IRVINE R.F. Changes in the level of inositol phosphates after agonist-dependent hydrolysis of membrane phospholipids. Biochem. J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK R.B., FRIEDMAN J., KUNKEL M.W., JANUARY B.G., SHENOLIKAR S. Okadaic acid induces both augmentation and inhibition of β2-adrenergic stimulation of cAMP accumulation in S49 lymphoma cells. J. Biol. Chem. 1993;268:3245–3250. [PubMed] [Google Scholar]
- CLARK R.B., KUNKEL M.W., FRIEDMAN J., GOKA T.J., JOHNSON J.A. Activation of cAMP-dependent protein kinase is required for heterologous desensitization of adenylyl cyclase in S49 wild-type lymphoma cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1442–1446. doi: 10.1073/pnas.85.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORVERA S., GARCÍA-SÁINZ J.A. Phorbol esters inhibit α1-adrenergic regulation of hepatocyte metabolism. Biochem. Biophys. Res. Commun. 1984;119:1128–1133. doi: 10.1016/0006-291x(84)90892-1. [DOI] [PubMed] [Google Scholar]
- CORVERA S., SCHWARZ K.R., GRAHAM R.M., GARCÍA-SÁINZ J.A. Phorbol esters inhibit α1-adrenergic effects and decreases the affinity of liver α1-adrenergic receptors for epinephrine. J. Biol. Chem. 1986;261:520–526. [PubMed] [Google Scholar]
- COTECCHIA S., SCHWINN D.A., RANDALL R.R., LEFKOWITZ R.J., CARON M.G., KOBILKA B.K. Molecular cloning and expression of the cDNA for the hamster α1-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIVIANI D., LATTION A.-L., COTECCHIA S. Characterization of the phosphorylation sites involved in G protein-coupled receptor kinase- and protein kinase C-mediated desensitization of the α1B-adrenergic receptor. J. Biol. Chem. 1997;272:28712–28719. doi: 10.1074/jbc.272.45.28712. [DOI] [PubMed] [Google Scholar]
- DIVIANI D., LATTION A.L., LARBI N., KUNAPULI P., PRONIN A., BENOVIC J.L., COTECCHIA S. Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the α1B-adrenergic receptor. J. Biol. Chem. 1996;271:5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- GRAHAM R.M., PEREZ D.M., HWA J., PIASCK M.T. α1-Adrenergic receptor subtypes. Molecular structure, function and signaling. Circ. Res. 1996;78:737–749. doi: 10.1161/01.res.78.5.737. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HADCOCK J.R., PORT J.D., GELMAN M.S., MALBON C.C. Cross-talk between tyrosine kinase and G-protein-linked receptors. Phosphorylation of β2-adrenergic receptors in response to insulin. J. Biol. Chem. 1992;267:26017–26022. [PubMed] [Google Scholar]
- HIEBLE J.P., BYLUND D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R. R., JR International Union of Pharmacology. X. Recommendations for nomenclature of α1-adrenoceptors: consensus update. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- HOLMES C.F.B., BOLAND M.P. Inhibitors of protein phosphatase-1 and -2A; two of the major serine/threonine protein phosphatases involved in cellular regulation. Curr. Opin. Struc. Biol. 1993;3:934–943. [Google Scholar]
- HOUSLAY M.D. Crosstalk: a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur. J. Biochem. 1991;195:9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- LATTION A.-L., DIVIANI D., COTECCHIA S. Truncation of the receptor carboxyl terminus impairs agonist-dependent phosphorylation and desensitization of the α1B-adrenergic receptor. J. Biol. Chem. 1994;269:22887–22893. [PubMed] [Google Scholar]
- LEEB-LUNDBERG L.M.F., COTECCHIA S., LOMASNEY J.W., DEBERNARDIS J.F., LEFKOWITZ R.J., CARON M.G. Phorbol esters promote α1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc. Natl. Acad. Sci. U.S.A. 1985;82:5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFKOWITZ R.J. G protein-coupled receptors. III. New Roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- MATTINGLY R.R., WASILENKO W.J., WOODRING P.J., GARRISON J.C. Selective amplification of endothelin-stimulated inositol 1,4,5-trisphosphate and calcium signaling by v-src transformation in rat-1 fibroblast. J. Biol. Chem. 1992;267:7470–7477. [PubMed] [Google Scholar]
- MEDINA L.C., VÁZQUEZ-PRADO J., TORRES-PADILLA M.E., MENDOZA-MENDOZA A., CRUZ-MUÑOZ M.E., GARCÍA-SÁINZ J.A. Crosstalk: phosphorylation of α1b-adrenoceptors induced through activation of bradykinin B2 receptors. FEBS Lett. 1998;422:141–145. doi: 10.1016/s0014-5793(97)01615-3. [DOI] [PubMed] [Google Scholar]
- NEWTON A.C. Protein kinase C: structure, function and regulation. J. Biol. Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- PAWSON T., SCOTT J.D. Signaling through scaffold, anchoring and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- PITCHER J.A., PAYNE E.S., CSORTOS C., DEPAOLI-ROACH A., LEFKOWITZ R.J. The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8343–8347. doi: 10.1073/pnas.92.18.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICCIARELLI R., AZZI A. Regulation of recombinant PKCα activity by protein phosphatase 1 and protein phosphatase 2A. Arch. Biochem. Biophys. 1998;355:197–200. doi: 10.1006/abbi.1998.0732. [DOI] [PubMed] [Google Scholar]
- SHIH M., LIN F., SCOTT J.D., WANG H.-Y., MALBON C.C. Dynamic complexes of β2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J. Biol. Chem. 1999;274:1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- VÁZQUEZ-PRADO J., GARCÍA-SÁINZ J.A. Effect of phorbol myristate acetate on α1-adrenergic action in cells expressing recombinant α1-adrenoceptor subtypes. Mol. Pharmacol. 1996;50:17–22. [PubMed] [Google Scholar]
- VÁZQUEZ-PRADO J., MEDINA L.C., GARCÍA-SÁINZ J.A. Activation of endothelin ETA receptors induce phosphorylation of α1b-adrenoceptors in rat-1 fibroblasts. J. Biol. Chem. 1997;272:27330–27337. doi: 10.1074/jbc.272.43.27330. [DOI] [PubMed] [Google Scholar]
- WANG J.H., STELZER A. Inhibition of phosphatase 2B prevents expression of hippocampal long-term potentiation. Neuroreport. 1994;5:2377–2380. doi: 10.1097/00001756-199411000-00041. [DOI] [PubMed] [Google Scholar]
- WIELAND T., JAKOBS K.H.Measurements of receptor-stimulated guanosine 5′-O-(gama-thio)triphosphate binding by G proteins Methods in Enzymology 1994Academic Press: London; 3–13.Iyengar, R. (ed.) [DOI] [PubMed] [Google Scholar]


