Abstract
Wistar rats with streptozotocin-induced diabetes (STZ-diabetic rats), which is similar to human insulin-dependent diabetic mellitus (IDDM), were employed to investigate the antihyperglycemic action of isoferulic acid.
A single intravenous injection of isoferulic acid decreased the plasma glucose in a dose-dependent manner in the STZ-diabetic rats. Repeated intravenous administration of STZ-diabetic rats with isoferulic acid (5.0 mg kg−1) also resulted in the lowering of plasma glucose after one day.
Stimulatory effects of isoferulic acid on the glucose uptake and glycogen synthesis in soleus muscles isolated from STZ-diabetic rats were also obtained indicating an increase of glucose utilization following isoferulic acid treatment which was not dependent on insulin.
The mRNA level of glucose transporter subtype 4 form (GLUT4) in soleus muscle was raised by isoferulic acid after repeated treatment for 1 day in STZ-diabetic rats. Similar repeated treatment with isoferulic acid reversed the elevated mRNA level of phosphoenolpyruvate carboxykinase (PEPCK) in liver of STZ-diabetic rats to the normal level. However, expression of GLUT4 and PEPCK genes in nondiabetic rats were not influenced by similar treatment with isoferulic acid.
These results suggest that isoferulic acid can inhibit hepatic gluconeogenesis and/or increase the glucose utilization in peripheral tissue to lower plasma glucose in diabetic rats lacking insulin.
Keywords: Isoferulic acid, glucose uptake, glycogen synthesis, skeletal muscle
Introduction
Diabetes mellitus (DM), both insulin-dependent DM (IDDM) and non-insulin-dependent DM (NIDDM) (Keen, 1986), is a common and serious disorder throughout the world (Harris et al., 1987). This chronic disease often leads to disability from the vascular complications of coronary artery disease and cerebrovascular disease, renal failure, blindness, and limb amputation in addition to neurological complications and premature death (Goldstein & Massry, 1978; Weidmann et al., 1993). Treatment of diabetes mellitus by insulin and/or oral drugs fails to prevent these complications in many patients indicating that additional treatment would be helpful.
Cimicifugae rhizoma is a constituent in the prescription of ‘Bu-Zhong-I-Chi-Tang', a medication said to be effective in the treatment of diabetes mellitus in traditional Chinese medicine. Isoferulic acid, one of active principles in Cimicifugae Rhizoma with an antihyperglycemic action on spontaneously diabetic BioBreeding/Worcester (BB/W) rats, a model of insulin-dependent diabetes, has been studied in our previous report (Liu et al., in press). Studies were performed to examine the effect of isoferulic acid on glucose utilization, glucose uptake and glycogen synthesis in isolated soleus muscles of streptozotocin (STZ)-diabetic rats. Also, we examined whether levels of mRNA for phosphoenolpyruvate carboxykinase (PEPCK), a rate-limiting enzyme of gluconeogenesis, in the liver and the levels of mRNA for the glucose transporter (GLUT4) in skeletal muscle can be influenced by isoferulic acid in STZ-diabetic rats after 1-day repeated treatment.
Methods
Animals
Male Wistar rats, weighing 220–250 g, were used in the present study. They were obtained from the animal center of the National Cheng Kung University Medical College. Rats were housed in a temperature-controlled room (25±1°C) and kept on a 12 : 12 light-dark cycle (light on at 0600 h). Food (Purina Rat Chow) and water were available ad libitum throughout the experiment.
Induction of diabetes
Insulin-dependent diabetes mellitus (IDDM) was induced according to the previous method (Forman et al., 1988). In brief, an intravenous injection of STZ (Sigma Chemical Co., St. Louis, U.S.A.) at 60 mg kg−1 dissolved in 1% citrate buffer into the femoral vein was performed under pentobarbital anaesthesia (30 mg kg−1, i.p.) in adult Wistar rats fasted for 3 days. Control rats received a similar injection of vehicle. Plasma samples taken from the tail vein of fasting rats under pentobarbital anaesthesia (30 mg kg−1, i.p.) were employed to determine the glucose concentrations as described previously (Hsu et al., 1997). Rats with plasma glucose concentrations of 20.0 mmol l−1 or greater in addition to polyuria, hyperphagia and decrease of body weight were considered as diabetic. All studies were carried out 2 weeks after the injection of STZ.
Experimental protocol
The diabetic rats were divided into two groups for investigation. Group I; animals received a single injection of isoferulic acid and plasma glucose concentrations were measured by tail vein sampling. In brief, STZ-diabetic rats were fasted overnight before receiving an intravenous injection of isoferulic acid at the desired doses (1.0, 5.0 and 10.0 mg kg−1). Plasma glucose 30 min after the injection of STZ-diabetic rats was compared as the maximal action of isoferulic acid was seen 30 min following injection in preliminary experiments.
Group II; STZ-diabetic rats and nondiabetic rats received injections of isoferulic acid (5.0 mg kg−1) every 8 h, three times daily, into the tail vein for 1 day. After the final treatment, animals were sacrificed. Liver and soleus muscle were immediately removed, frozen in liquid nitrogen and stored at −70°C for subsequent mRNA extraction and Northern blot analysis. Blood samples were also collected from the femoral vein of these rats before sacrificing.
Determination of plasma glucose
A blood sample (0.2 ml) was collected with a chilled syringe containing 10 IU heparin from the femoral vein of rats under sodium pentobarbital anaesthesia (30 mg kg−1, i.p.). Blood samples were then centrifuged at 13,000 r.p.m. for 3 min and an aliquot (15 μl) of plasma was added to 1.5 ml of the Glucose Kit Reagent (Biosystems S.S., Barcelona, Spain) and incubated at 37°C in a water bath (Yamato-BT-25, Tokyo, Japan) for 10 min. The concentration of plasma glucose was then estimated in duplicate using an analyzer (Quik-Lab, Ames, Miles Inc., Elkhart, Indiana 46515, U.S.A.).
Measurement of glucose uptake into soleus muscle
Glucose uptake was determined using the uptake of the radioactive glucose analogue, 2-[1-14C]-deoxy-D-glucose (2-DG) (NEN Research, Boston, U.S.A.), as described previously (Chang et al., 1996). Animals were sacrificed by cervical dislocation and the soleus muscle was quickly excised, dissected free of any adjoining connective tissue, blotted, and divided into long longitudinal strips (35–25 mg per strip). Muscles were placed in 3 ml of Krebs-Ringer bicarbonate buffer (KRB) (37°C, pH 7.4) containing 1 mmol l−1 glucose, 1% fatty acid-free bovine serum albumin (BSA) under aeration of 5% CO2 in O2. After pre-incubation for 30 min, the muscle tissue was incubated with 1.0 nmol l−1 bovine insulin (Novo Nordisk A/S, DK-2880 Bagsvaerd, Denmark) or isoferulic acid at the indicated concentrations for 30 min and then with 50 μl KRB containing 2-DG (1 μCi ml−1) for 5 min at 37°C in the shaking water bath under aeration. Reactions were terminated by quickly blotting the muscles and dissolving them in 0.5 ml of 0.5 N NaOH for 45 min before neutralization with 0.5 ml 0.5 N HCl. After centrifugation, 800 μl of each supernatant was added to 1 ml of aqueous counting scintillant (ASC, Amersham, Arlington Heights, IL, U.S.A.) and the radioactivity was determined using a β-counter (Beckman LS6000). Uptake of 2-DG, assessed after preincubation of the muscle with 20 μmol l−1 cytochalasin B (Sigma Chemical Co., St. Louis, U.S.A.), was subtracted from the total muscle-associated radioactivity (Gliemann et al., 1972). Specific 2-DG uptake was expressed as the percentage of basal uptake that was obtained from soleus muscle incubated with KRB only.
Measurement of glycogen synthesis in the soleus muscle
The maximal rate of insulin and isoferulic acid-stimulated [14C]-glucose incorporation into glycogen was measured as described by Challis et al., (1988) using soleus muscles prepared as described above. After the 30 min pre-incubation period of KRB at 37°C, as described previously (Challis et al., 1988), the muscles were transferred to fresh incubation flasks containing [U-14C]-glucose (0.25 μCi ml−1) (NEN Research, Boston, U.S.A.) with appropriate concentrations of the test drugs. The incubation period was 30 min in a shaking water bath with continuous gassing. This optimal time was obtained from preliminary experiments. The muscles were then removed, rapidly blotted and freeze-clamped. Glycogen synthesis was measured following alkaline digestion of the freeze-clamped muscles to obtain the ethanolic precipitation of glycogen for determination of radioactivity (as above). The results are expressed as the percentage of basal uptake that was obtained from soleus muscle incubated with KRB only.
Northern blot analysis
Total RNA was extracted from liver or soleus muscle of experimental animals using the Ultraspec™-II RNA extraction system (Bioteck, Houston, TX, U.S.A.). The concentration of RNA was measured using the absorbance at 260 nm. For Northern blot analysis, RNA (20 μg) was denatured in a solution containing 2.2 mmol l−1 formaldehyde and 50% formamide (v v−1) by heating at 55°C for 15 min. Aliquots of total RNA were then size-fractionated in a 1.2% agarose/formaldehyde gel (Lehrach et al., 1977). Ethidium bromide staining was used to identify the position of the 18S and 28S rRNA subunits and to confirm that equivalent amounts of undegraded RNA had been loaded. The fractionated RNA was transferred to a Hybond-N membrane (Amersham, Bucks, U.K.) and crosslinked to U.V. irradiation (Stratagene, CA, U.S.A.).
The plasmid containing cDNA (Iynedjian & Hanson, 1977) for phosphoenolpyruvate carboxykinase (PEPCK) was kindly supplied by Professor R.W. Hanson (Cleveland, OH, U.S.A.). The plasmid containing cDNA of glucose transporter subtype 4 form (GLUT4) was obtained from Professor C. Makepeace (St. Louis, MO, U.S.A.) and the β-actin cDNA was from Professor S.S. Liu (Taiwan, R.O.C.). Probes were labeled with [α-32P]dCTP using the Medaprime labelling system kit (Amersham, Bucks, U.K.). Hybridizations were carried out in medium containing denatured salmon sperm DNA (100 μg ml−1) at 65°C for 2 h. The membrane was washed twice for 20 min in 2× sodium saline citrate (SSC)/0.1% SDS at room temperature and once for 20 min in 0.1×SSC/0.1% SDS at 40°C. Autoradiograms were prepared on Kodak X-ray film (Rochester, NY, U.S.A.) using a single enhancing screen at −70°C (laid down for 16 h). Intensity of the mRNA bands on the blot were quantified by scanning densitometry (Hoefer, San Francisco, CA, U.S.A.). The response of β-actin was used as an internal standard.
Statistical analysis
Data, as mean±s.e.mean, were obtained from the number (n) of animals in each group as indicated. Repeated measure analysis of variance (ANOVA) was used to analyse the difference of tested samples from control. Dunnett's range post-hoc comparisons were used to determine the source of significant differences where appropriate. A P value of 0.05 or less was considered as statistically significant.
Results
Effect of isoferulic acid on the plasma glucose level
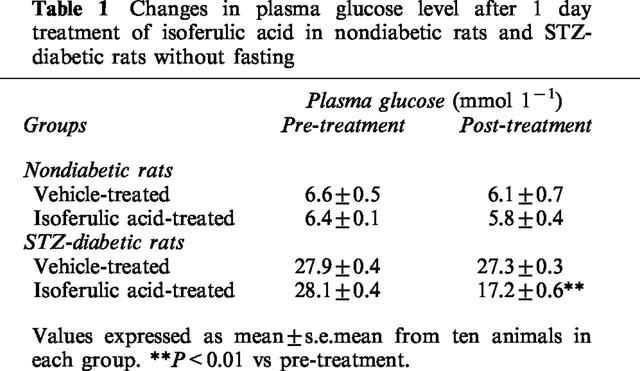
Isoferulic acid (1.0, 5.0, 10.0 mg kg−1, i.v.) was administered to fasted STZ-diabetic rats and blood samples were obtained from the femoral vein 30 min post-injection. A dose-dependent reduction of plasma glucose was observed in STZ-diabetic rats after the injection of isoferulic acid at 5.0 mg kg−1 and 10.0 mg kg−1 (Figure 1). However, no statistical difference was obtained in STZ-diabetic rats receiving isoferulic acid at 1.0 mg kg−1 (Figure 1). Plasma glucose concentrations of non-fasted STZ-diabetic rats were also decreased by 10.9±0.5 mmol l−1 after repeated treatment with isoferulic acid (5.0 mg kg−1) for 1 day (P<0.01 vs vehicle-treated STZ-diabetic rats) (Table 1). However, the plasma glucose concentration in isoferulic acid-treated rats was still markedly higher than that from the vehicle-treated nondiabetic rats (6.1± 0.7 mmol l−1, P<0.01). Isoferulic acid did not affect the plasma glucose level in nondiabetic rats even with repeated treatments over 1 day (P>0.05).
Figure 1.
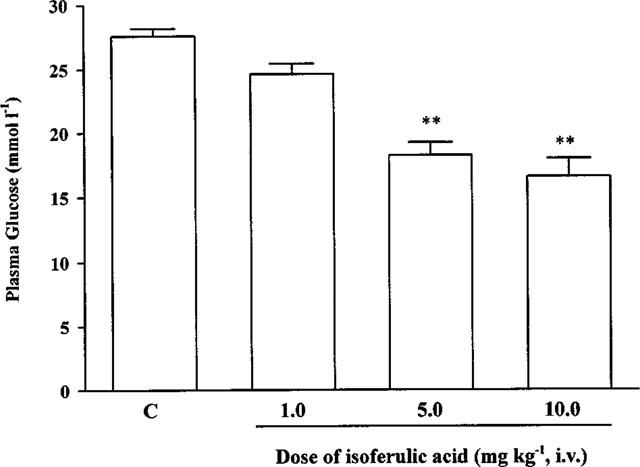
The effects of isoferulic acid on plasma glucose concentrations in STZ-diabetic rats. Values are expressed as mean±s.e.mean and were obtained from ten rats. STZ-diabetic rats treated with vehicle at the same volume were used as control (C). **P<0.01 vs the control.
Table 1.
Changes in plasma glucose level after 1 day treatment of isoferulic acid in nondiabetic rats and STZ-diabetic rats without fasting
Effect of isoferulic acid on glucose uptake
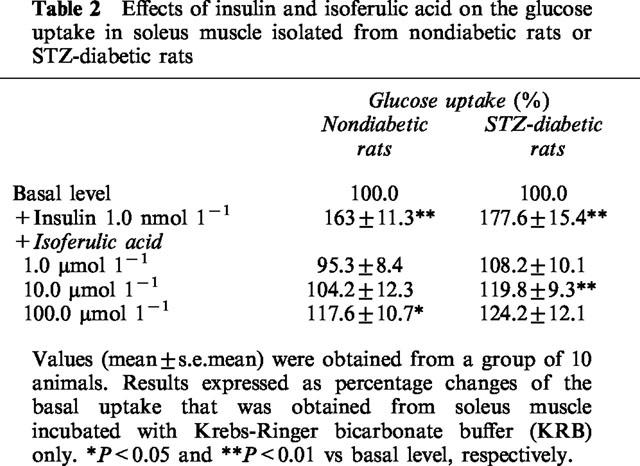
Glucose utilization was investigated by measuring glucose uptake into isolated soleus muscles from nondiabetic and STZ-diabetic rats. Changes of 2-DG uptake are indicated as a percentage of basal level. Table 2 shows that insulin at a concentration of 1.0 nmol l−1 significantly (P<0.05) stimulated glucose uptake into isolated soleus muscles of STZ-diabetic rats given a level similar to that in nondiabetic rats. Isoferulic acid significantly increased glucose uptake into isolated soleus muscles of STZ-diabetic rats at 10.0 μmol l−1 and 100.0 μmol l−1, but not at 1.0 μmol l−1 (Table 2). However, increase of glucose uptake in samples from nondiabetic rats incubated with isoferulic acid was observed only at 100.0 μmol l−1 (Table 2).
Table 2.
Effects of insulin and isoferulic acid on the glucose uptake in soleus muscle isolated from nondiabetic rats or STZ-diabetic rats
Effect of isoferulic acid on glycogen synthesis
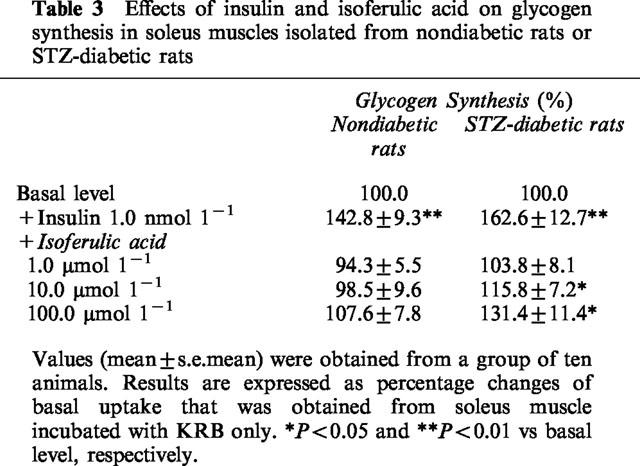
Insulin caused a marked increase in [14C]-glucose incorporation into glycogen, as compared to basal level (P<0.05) (Table 3). In soleus muscles from STZ-diabetic rats, isoferulic acid enhanced the glycogen synthesis in a concentration-related manner. As shown in Table 3, a marked increase of glycogen synthesis was observed in samples treated with isoferulic acid at concentrations of 10.0 μmol l−1 and 100.0 μmol l−1 in STZ-diabetic rats (P<0.05). However, isoferulic acid did not stimulate the glycogen synthesis in soleus muscles from nondiabetic rats at similar concentrations; the level of [14C]-glucose incorporation into glycogen was not significantly (P>0.05) different from the basal level.
Table 3.
Effects of insulin and isoferulic acid on glycogen synthesis in soleus muscles isolated from nondiabetic rats or STZ-diabetic rats
Effect of isoferulic acid on the mRNA level for glucose transporter subtype 4 form (GLUT4) in soleus muscle of STZ-diabetic rats
Figure 2 shows the representative response of mRNA level for GLUT4 in isolated soleus muscle from the vehicle or isoferulic acid-treated animals determined by Northern blot analysis using β-actin as the internal standard. The mRNA level for GLUT4 in isolated soleus muscle from the vehicle-treated STZ-diabetic rats decreased to about 1.6 fold of that from the vehicle-treated nondiabetic rats. Repeated treatment of STZ-diabetic rats with isoferulic acid (5.0 mg kg−1) for 1 day resulted in a marked elevation of GLUT4 mRNA level in isolated soleus muscle to a level near to that of vehicle-treated nondiabetic rats. However, similar treatment with isoferulic acid in nondiabetic rats did not (P>0.05) modify the GLUT4 mRNA level of isolated soleus muscle as compared with the vehicle-treated nondiabetic rats.
Figure 2.
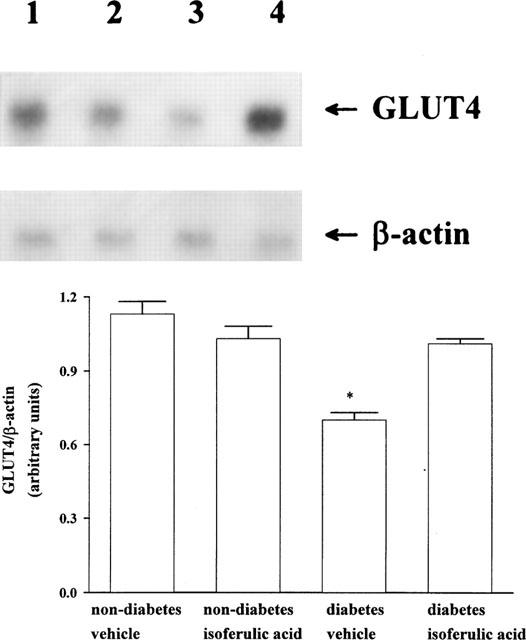
Upper picture shows the representative response of the level of mRNA for GLUT4 or β-actin in soleus muscle isolated from nondiabetic or STZ-diabetic rats receiving repeated treatment with isoferulic acid (5.0 mg kg−1) or the same volume of vehicle three times per day for 1 day. Lane 1, vehicle-treated nondiabetic rats (non-diabetes vehicle); lane 2, isoferulic acid-treated nondiabetic rats (non-diabetes isoferulic acid); lane 3, vehicle-treated STZ-diabetic rats (diabetes vehicle); lane 4, isoferulic acid-treated STZ-diabetic rats (diabetes isoferulic acid). Quantification of the mRNA level using the GLUT4/β-actin ratio is expressed as the mean±s.e.mean (n=4 per group) in each column is indicated in the lower picture. *P<0.05 vs data for the vehicle-treated nondiabetic rats.
Effect of isoferulic acid on the mRNA level for hepatic PEPCK in STZ-diabetic rats
The expression of the genes for enzymes involved in the key steps of hepatic glucose metabolism was investigated by the determination of mRNA level for PEPCK in liver isolated from STZ-diabetic rats which had received repeated treatment with isoferulic acid as above. Figure 3 shows the representative data of Northern blot analysis of the mRNA for PEPCK. The mRNA level of PEPCK in liver from the vehicle-treated STZ-diabetic rats increased to about 1.4 fold of that in the vehicle-treated nondiabetic rats. Treatment of STZ-diabetic rats with isoferulic acid three times per 1 day resulted in a marked reduction of the mRNA level for PEPCK in liver. However, the value was still higher than that of vehicle-treated nondiabetic rats (P<0.05). Similar treatment with isoferulic acid in nondiabetic rats did not (P>0.05) modify the mRNA level for hepatic PEPCK as compared with the vehicle-treated nondiabetic rats.
Figure 3.
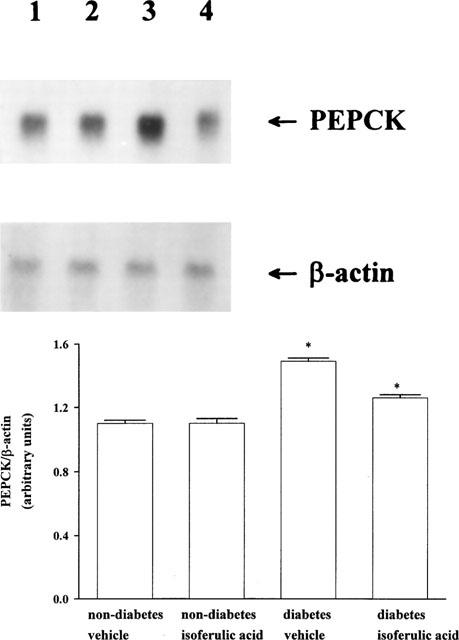
Upper picture shows the representative response of the level of mRNA for PEPCK or β-actin in soleus muscle isolated from nondiabetic or STZ-diabetic rats receiving the repeat treatment with isoferulic acid (5.0 mg kg−1) or the same volume of vehicle three times daily for 1 day. Lane 1, vehicle-treated nondiabetic rats (non-diabetes vehicle); lane 2, isoferulic acid-treated nondiabetic rats (non-diabetes isoferulic acid); lane 3, vehicle-treated STZ-diabetic rats (diabetes vehicle); lane 4 isoferulic acid-treated STZ-diabetic rats (diabetes isoferulic acid). Quantification of the mRNA level using the PEPCK/β-actin ratio is expressed as the mean±s.e.mean (n=4 per group) in each column is indicated in the lower picture. *P<0.05 vs data for the vehicle-treated nondiabetic rats.
Discussion
We have demonstrated that isoferulic acid lowers plasma glucose concentrations in STZ-diabetic rats in a dose-dependent manner. This is consistent with our previous report that intravenous injection of isoferulic acid lowered the plasma glucose level in BB/W rats (Liu et al., in press).
Skeletal muscles are a major site of glucose disposal (Baron et al., 1988). Glucose transportation, which depends on insulin-stimulated translocation of glucose carriers to the cell membrane, is the rate-limiting step in carbohydrate metabolism in skeletal muscle (Ziel et al., 1988). Under basal conditions, the rate of glucose uptake in skeletal muscle is low. Insulin-stimulated glucose disposal (Hollenbeck & Reaven, 1987) is the major site for regulation of plasma glucose concentrations. We found that the stimulatory effect of 1.0 nmol l−1 insulin on 2-DG uptake was similar in nondiabetic rats and STZ-diabetic rats and that isoferulic acid caused an increase in both 2-DG uptake and glycogen incorporation in the STZ-diabetic rats. The effects of isoferulic acid on 2-DG uptake and glycogen incorporation were produced from 10.0 μmol l−1 in STZ-diabetic rats but only from 100.0 μmol l−1 in nondiabetic rats.
A family of glucose transporters (GLUT) mediate glucose transport across the cell membrane and subtype 4 (GLUT4) is predominant in skeletal muscle (Fukumoto et al., 1989; James et al., 1989; Pessin & Bell, 1992). Insulin induces a translocation of GLUT4 from microsomal membranes to plasma membranes (Goodyear et al., 1991; Klip & Paquet, 1990). Thus, it is possible that isoferulic acid could enhance the glucose uptake via an effect on GLUT4 translocation. We found that the level of mRNA for GLUT4 can be raised by isoferulic acid after repeated injection for 1 day. It has been documented that long term exposure is required for the activation of mRNA levels in cells (Baque et al., 1998). Because more protein is generally formed following an increase in the mRNA level (Henriksen et al., 1990), it is reasonable to speculate that an increase in the level of mRNA for GLUT4 may stimulate glucose uptake. However, this view needs a further experimentation.
In STZ-diabetic rats, isoferulic acid was also effective in lowering plasma glucose after 1-day repeat treatment. This action of isoferulic acid was associated with a marked reduction of the level of mRNA for PEPCK in the liver. The enhanced expression of PEPCK genes in diabetic rats was suppressed by the repeated treatment for 1 day with isoferulic acid. The expression of PEPCK genes in liver is regulated by a number of hormones and physiological conditions and glucagon and insulin act as possible and negative regulators, respectively (Petrack et al., 1981). An effect on the hepatic level of mRNA for PEPCK in STZ-diabetic rats by isoferulic acid is of special interest and more work needs to be performed to ascertain the mechanism.
In conclusion, the data obtained suggest that intravenous injections of isoferulic acid can lower plasma glucose in STZ-diabetic rats through the increase of glucose utilization and a reduction of hepatic gluconeogenesis.
Acknowledgments
We are grateful to Professor R.W. Hanson, Professor C. Makepeace, and Professor S.S. Liu for kindly providing us with the plasmids containing cDNAs. The present study is supported in part by a grant from the National Science Council of the Republic of China (NSC87-2314-B006-104).
Abbreviations
- GLUT4
glucose transporter subtype 4 form
- IDDM
insulin-dependent diabetic mellitus
- PEPCK
phosphoenolpyruvate carboxykinase
- STZ-diabetic rat
streptozotocin-induced diabetic rat
References
- BAQUE S., MONTELL E., CAMPS M., GUINOVART J.J., ZORZANO A., GOMEZ-FOIX A.M. Overexpression of glycogen phosphorylase increases GLUT4 expression and glucose transport in cultured skeletal human muscle. Diabetes. 1998;47:1185–1192. doi: 10.2337/diab.47.8.1185. [DOI] [PubMed] [Google Scholar]
- BARON A.D., BRECHTEL G., WALLACE P., EDELMAN S.V. Rates and tissue sites of non-insulin-and insulin-mediated glucose uptake in human. Am. J. Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- CHALLIS R.A.J., LEIGHTON B., WILSON S., THURLBY P.L., ARCH J.R.S. An investigation of the β-adrenoceptor that mediates metabolic responses to the novel agonist BRL 28410 in rat soleus muscle. Biochem. Pharmacol. 1988;37:947–950. doi: 10.1016/0006-2952(88)90186-4. [DOI] [PubMed] [Google Scholar]
- CHANG C.J., KAO J.T., LEE T.L., LAI C.W., CHENG J.T. Comparison of isoproterenol with BRL37344 in activation of beta 3-adrenoceptors to inhibit the uptake of [14C]-deoxy-D-glucose and translocation of glucose transport (GLUT4) to membrane fraction in rat adipocytes. J. Auton. Nerv. Syst. 1996;61:191–194. doi: 10.1016/s0165-1838(96)00068-9. [DOI] [PubMed] [Google Scholar]
- FORMAN L.J., ESTILOW S., MEAD J., VASILENKO P. Eight weeks of streptozotocin-induced diabetes influences the effects of cold stress on immunoreactive beta-endorphin levels in female rats. Horm. Metabol. Res. 1988;20:555–558. doi: 10.1055/s-2007-1010883. [DOI] [PubMed] [Google Scholar]
- FUKUMOTO H., KAYANO T., BUSE J.B., EDWARDS Y., PILCH P.F., BELL G.I., SEINO S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J. Biol. Chem. 1989;264:7776–7779. [PubMed] [Google Scholar]
- GLIEMANN J., OSTERLIND K., VINTEN J., GAMMELTOFT S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim. Biophys. Acta. 1972;286:1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN D.A., MASSRY S.G. Diabetic nephropathy: clinical course and effect of hemodialysis. Nephron. 1978;20:286–296. doi: 10.1159/000181239. [DOI] [PubMed] [Google Scholar]
- GOODYEAR L.J., HIRSHMAN M.F., SMITH R.J., HORTON E.S. Glucose transporter number, activity and isoform content in plasma membranes of red and white skeletal muscle. Am. J. Physiol. 1991;261:E556–E561. doi: 10.1152/ajpendo.1991.261.5.E556. [DOI] [PubMed] [Google Scholar]
- HARRIS M.I., HADDEN W.C., KNOWLER W.C., BENNETT P.H. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in the U.S. population aged 20–74 yr. Diabetes. 1987;36:523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN E.J., BOUREY R.E., RODNICK K.J., KORANYI L., PERMUTT M.A., HOLLOSZY J.O. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am. J. Physiol. 1990;259:E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- HOLLENBECK C., REAVEN G.M. Variations in insulin-stimulated glucose uptake in healthy individuals with normal glucose tolerance. J. Clin. Endocrinol. Metab. 1987;64:1169–1173. doi: 10.1210/jcem-64-6-1169. [DOI] [PubMed] [Google Scholar]
- HSU F.L., LAI C.W., CHENG J.T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Med. 1997;63:323–325. doi: 10.1055/s-2006-957692. [DOI] [PubMed] [Google Scholar]
- IYNEDJIAN P.B., HANSON R.W. Increase in level of functional messenger RNA coding for phosphoenolpyruvate carboxykinase (GTP) during induction by cyclic adenosine 3′:5′-monophosphate. J. Biol. Chem. 1977;252:655–662. [PubMed] [Google Scholar]
- JAMES D.E., STRUBE M., MUECKLER M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- KEEN H. What's in a name? IDDM/NIDDM, type1/type2. Diabetic Med. 1986;3:11–12. doi: 10.1111/j.1464-5491.1986.tb00697.x. [DOI] [PubMed] [Google Scholar]
- KLIP A., PAQUET M.R. Glucose transport and glucose transporters in muscles and their metabolic regulation. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- LEHRACH H., DIAMOND D., WOZNEY J.M., BOEDTKER H. RNA molecular weight determination by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977;16:4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- LIU I.-M., CHI T.C., HSU F.L., CHEN C.F., CHENG J.T. Isoferulic acid as active principle from the rhizoma of Cimicifugae dahurica to lower plasma glucose in diabetic rats. Planta Med. 1999;65:712–714. doi: 10.1055/s-1999-14048. [DOI] [PubMed] [Google Scholar]
- PESSIN J.E., BELL G.I. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu. Rev. Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- PETRACK B., CZERNIK A.J., ANSELL J., CASSIDY J. Potentiation of arginine-induced glucagon secretion by adenosine. Life Sci. 1981;28:2611–2615. doi: 10.1016/0024-3205(81)90718-9. [DOI] [PubMed] [Google Scholar]
- WEIDMANN P., BOEHLEN L.M., DE COURTEN M. Pathogenesis and treatment of hypertension associated with diabetes mellitus. Am. Heart. J. 1993;125:1498–1513. doi: 10.1016/0002-8703(93)90447-h. [DOI] [PubMed] [Google Scholar]
- ZIEL F.H., VENKATESAN N., DAVIDSON M.B. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988;37:885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]


