Abstract
The actions of 13 general anaesthetics (diethyl ether, enflurane, isoflurane, methoxyflurane, sevoflurane, chloral hydrate, trifluoroethanol, tribromoethanol, tert-butanol, chloretone, brometone, trichloroethylene, and α-chloralose) were studied on agonist-activated Cl− currents at human GABAA α2β1, glycine α1, and GABAC ρ1 receptors expressed in human embryonic kidney 293 cells.
All 13 anaesthetics enhanced responses to submaximal (EC20) concentrations of agonist at GABAA and glycine receptors, except α-chloralose, which did not enhance responses at the glycine α1 receptor. None of the anaesthetics studied potentiated GABA responses at the GABAC ρ1 receptor.
Potentiation of submaximal agonist currents by the anaesthetics was studied at GABAA and glycine receptors harbouring mutations in putative transmembrane domains 2 and 3 within GABAA α2, β1, or glycine α1 receptor subunits: GABAA α2(S270I)β1, α2(A291W)β1, α2β1(S265I), and α2β1(M286W); glycine α1(S267I) and α1(A288W). For all anaesthetics studied except α-chloralose, at least one of the mutations above abolished drug potentiation of agonist responses at GABAA and glycine receptors.
α-Chloralose produced efficacious direct activation of the GABAA α2β1 receptor (a ‘GABA-mimetic' effect). The other 12 anaesthetics produced minimal or no direct activation of GABAA and glycine receptors. A non-anaesthetic isomer of α-chloralose, β-chloralose, was inactive at GABAA and glycine receptors and did not antagonize the actions of α-chloralose at GABAA receptors.
The implications of these findings for the molecular mechanisms of action of general anaesthetics at GABAA and glycine receptors are discussed.
Keywords: GABA, GABAA receptor, glycine, glycine receptor, GABAC ρ receptor, general anaesthetic, sevoflurane, diethyl ether, chloral hydrate, α-chloralose
Introduction
γ-aminobutyric acid type A (GABAA) and glycine receptors are the major inhibitory neurotransmitter receptors in the vertebrate central nervous system. Ligand-gated ion channels have emerged as strong molecular candidates to mediate the effects of general anaesthetics (Franks & Lieb, 1994; Harris et al., 1995; Krasowski & Harrison, 1999). GABAA receptor function is allosterically enhanced by a wide range of chemically diverse anaesthetic compounds at clinically relevant concentrations. These include halogenated volatile ethers and alkanes (Nakahiro et al., 1989; Wakamori et al., 1991; Jones et al., 1992), n-alcohols (Nakahiro et al., 1991; Dildy-Mayfield et al., 1996), chloral derivatives (Lovinger et al., 1993; Peoples & Weight, 1994), propofol (Hales & Lambert, 1991), etomidate (Belelli et al., 1997), the barbiturates (Thompson et al., 1996), and α-chloralose (Garrett & Gan, 1998). Strychnine-sensitive glycine receptors are positively modulated by volatile ether and alkane anaesthetics (Wakamori et al., 1991; Harrison et al., 1993; Downie et al., 1996), n-alcohols (Mascia et al., 1996), and chloral derivatives (Pistis et al., 1997; Krasowski et al., 1998a), but are much less sensitive to barbiturates, propofol, and etomidate (Koltchine et al., 1996; Mascia et al., 1996).
GABAA and glycine receptors are both part of a ligand-gated ion channel gene superfamily that also includes the serotonin3, GABA ρ (‘GABAC') and nicotinic acetylcholine receptors. The GABAA receptor is a heteromeric complex assembled from different glycoprotein subunits (α1–6, β1–4, γ1–4 δ, ε, π) which combine to form a chloride channel (reviewed by Barnard et al., 1998). GABAA receptors in vivo probably consist in general of pentameric complexes of α, β, and γ subunits with a stoichiometry of 2α: 2β: 1γ (Chang et al., 1996; Farrar et al., 1999). GABAA receptors lacking the γ subunit (αβ receptors) can be studied in heterologous expression systems and retain sensitivity to general anaesthetics and n-alcohols (Pritchett et al., 1989; Harrison et al., 1993; Mihic et al., 1994a,1994b). GABAA αβ receptors are very useful for molecular pharmacology studies, as they reduce the number of subunits to consider.
Native glycine receptors consist either of homomeric assemblies of α subunits, or of heteromeric complexes of α and β subunits, which assemble in vivo with a proposed stoichiometry of 3α : 2β (Kuhse et al., 1995). Glycine α subunits, which contain the agonist binding sites, can also be expressed in heterologous systems as functional receptor complexes. These homomeric glycine α receptors are sensitive to allosteric modulation by alcohols and general anaesthetics (Harrison et al., 1993; Mascia et al., 1996) and have proven to be very helpful for molecular pharmacology studies.
In contrast to GABAA receptors, ‘GABAC' receptors assembled from ρ1–3 subunits (Cutting et al., 1991) are insensitive to ether anaesthetics (Harrison et al., 1993; Mihic & Harris, 1996). N-alcohols inhibit GABA-mediated currents at the GABAC ρ1 receptor (Mihic & Harris, 1996).
Specific amino acid residues within putative transmembrane domains (TM) 2 and 3 have been shown to be critically important for the positive modulatory actions of enflurane (Mihic et al., 1997), isoflurane, propofol (Krasowski et al., 1998b), n-alcohols (Mihic et al., 1997; Wick et al., 1998; Ueno et al., 1999), and etomidate (Belelli et al., 1997; McGurk et al., 1998) at GABAA and glycine receptors. In this study, we examined whether mutations within TM2 and TM3 of GABAA and glycine receptors alter sensitivity to a panel of 13 general anaesthetics, including ethers, alkanes, primary and tertiary alcohols, and α-chloralose. We also compared the potentiation of agonist responses produced by the anaesthetics at GABAA and glycine receptors with their anaesthetic potencies in vivo.
Some of the results presented here have been reported previously in preliminary form (Krasowski et al., 1998c).
Methods
Site-directed mutagenesis of receptor cDNA
The GABAA α2 (Hadingham et al., 1993a), β1 (Hadingham et al., 1993b), GABAC ρ1 (Cutting et al., 1991), and glycine α1 (Grenningloh et al., 1987) receptor subunit cDNAs are all of human origin. Preparation of the S270I and A291W mutations of the GABAA α2 subunit, the S265I and M286W mutations of the GABAA β1 subunit, and the S267I and A288W mutations of the glycine α1 subunit has been described in detail elsewhere (Krasowski et al., 1998a,1998b).
Cell culture and transfection of receptor cDNAs
Wild-type or mutated receptor cDNAs were expressed via the vector pCIS2 which contains one copy of the strong promoter from cytomegalovirus and a polyadenylation sequence from SV40. Human embryonic kidney (HEK) 293 cells (American Type Culture Collection, Rockville, MD, U.S.A.) were maintained in culture on glass coverslips and transfected with the appropriate cDNAs by the calcium phosphate precipitation method to achieve transient expression (Harrison et al., 1993; Krasowski et al., 1998a).
Electrophysiological recordings
HEK 293 cells were used for electrophysiological experiments 2–5 days following cDNA transfection. Recordings were performed at room temperature using the whole-cell patch clamp technique (Harrison et al., 1993; Krasowski et al., 1998a). The extracellular medium contained (in mM): NaCl 145, KCl 3, CaCl2 1.5, MgCl2 1, D-glucose 5.5 and HEPES 10, pH 7.4, osmolarity 320–330 mosmol. The electrode solution contained (in mM): N-methyl-D-glucamine hydrochloride 145, K2ATP 5, HEPES/KOH 5, MgCl2 2, CaCl2 0.1 and EGTA 1.1, pH 7.2, osmolarity 315 mosmol. Pipette-to-bath resistance was 4–6 MΩ. Cells were voltage-clamped at −60 mV.
All drugs were rapidly (<50 ms exchange time) applied to the cell by local perfusion (Koltchine et al., 1996; Krasowski et al., 1997) using a motor-driven solution exchange device (Bio Logic Rapid Solution Changer RSC-100; Molecular Kinetics, Pullman, WA, U.S.A.). Laminar flow was maintained by applying all solutions at identical flow rates via a multi-channel infusion pump (Stoelting, Wood Dale, IL, U.S.A.). The solution changer was driven by protocols in the acquisition program pCLAMP5 (Axon Instruments, Foster City, CA, U.S.A.). Responses were digitized (TL-1-125 interface; Axon Instruments) using pCLAMP5 and stored for off-line analysis.
Throughout this study, potentiation by the various general anaesthetic agents was always assessed with test concentrations of agonist that correspond to the EC20 value on the concentration-response curve for the particular receptor under study (i.e., the concentration of agonist that produces 20% of the maximal response to agonist). Detailed concentration-response curves for agonist alone were determined for each receptor combination, in order to control for differences in agonist sensitivity between different receptors. At the end of each potentiation experiment, a maximal agonist response was elicited by an appropriately high concentration of agonist. The test concentration of agonist was adjusted as appropriate if the responses differed significantly from 20% of the maximal agonist response. As in previous studies (Krasowski et al., 1998a,1998b), the average percentage of maximal agonist responses for the test concentrations used did not differ significantly from 20% for any of the receptor combinations studied.
Data analysis
Anaesthetic-induced ‘potentiation' of an agonist-induced current was defined as the percentage increase of the control agonist response (defined as the average of the pre-drug and post-drug agonist induced currents). Concentration response data were fitted (KaleidaGraph; Reading, PA, U.S.A.) with the Hill equation: I/Imax=[A]nH ([A]nH+[EC50]nH)−1, where [A]=concentration of anaesthetic, I/Imax=percentage of the maximum obtainable response, EC50=concentration producing a half-maximal response, and nH=Hill coefficient. Pooled data are presented throughout as mean±s.e.mean. Statistical significance was determined by one-way analysis of variance with Dunnet's post-hoc test, unless stated otherwise.
Drugs
Stock solutions of GABA, glycine (Sigma Chemical Co., St. Louis, MO, U.S.A.), and anaesthetic compounds were diluted into extracellular solution each day before use. The sources of the anaesthetics (see Figure 1 for chemical structures) were as follows: chloral hydrate, trichloroethylene, 1,1,1-trichloro-2-methyl-2-propanol (chloretone), 2,2,2-trichloroethanol (TCEt), 2,2,2-trifluoroethanol (TFEt) [Sigma]; methoxyflurane (Metofane®) [Mallinckrodt Veterinary, Inc., Mundelein, IL, U.S.A.]; diethyl ether [J.T. Baker, Inc., Phillipsburgh, NJ, U.S.A.]; β-chloralose, 2-methyl-2-propanol (tert-butanol) [Aldrich Chemical Co., Milwaukee, WI, U.S.A.]; α-chloralose, 2,2,2-tribromoethanol (TBrEt) [Fluka, Ronkonkoma, NY, U.S.A.]; 1,1,1-tribromo-2-methyl-2-propanol (brometone) [Sigma-Aldrich Rare Chemicals Library, Milwaukee, IL, U.S.A.]; sevoflurane (Ultane®) [Abbott Laboratories, North Chicago, IL, U.S.A.]; isoflurane (Forane®) [Ohmeda Caribe, Inc., Guayama, Puerto Rico]; and enflurane (Ethrane®) [Anaquest Caribe, Inc., Guayama, Puerto Rico]. Chloretone, α-chloralose, β-chloralose, TBrEt, and brometone were first prepared as stock solutions in dimethyl sulphoxide (Sigma) before being dissolved in the extracellular medium. The maximum final concentration of dimethyl sulphoxide was 0.1% (v v−1), which was determined during carrier control experiments to have no significant effect on agonist-induced currents in the receptor constructs analysed in this study.
Figure 1.
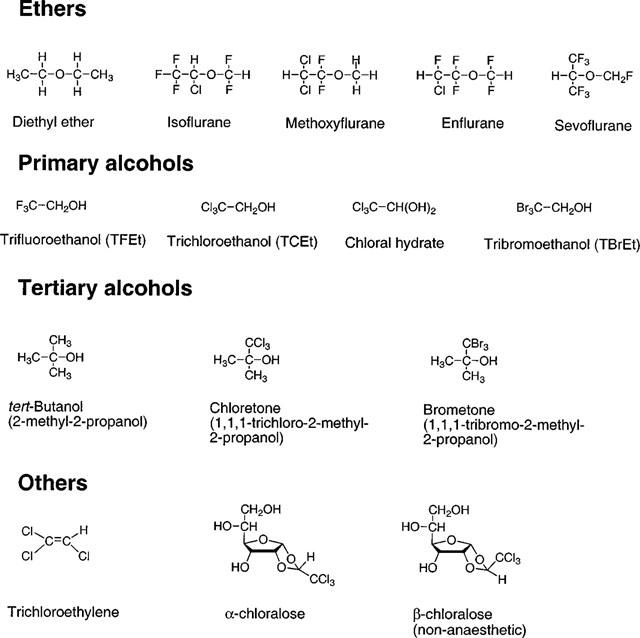
Chemical structures of the anaesthetic compounds investigated in this study.
Preparation of volatile anaesthetic solutions and determination of anaesthetic concentrations by gas chromatography
Volatile anaesthetic solution preparation and gas chromatography measurements were adapted from methods previously described (Jones et al., 1992). All volatile anaesthetic solutions were prepared by injection of pure liquid anaesthetic with gastight syringes (Hamilton, Reno, NV, U.S.A.) into evacuated intravenous drip bags containing defined volumes of extracellular solution. The nature of the local perfusion method used to apply solutions during electrophysiological recordings precluded direct determination of the concentration of volatile anaesthetic solutions reaching a cell during patch-clamp recording. Consequently, a series of control experiments were undertaken to estimate the amount of volatile agents (diethyl ether, enflurane, isoflurane, methoxyflurane, sevoflurane, and trichloroethylene) lost prior to cellular perfusion. Losses of volatile anaesthetics from aqueous solution in our experiments can result only from escape of the anaesthetic from the intravenous drip reservoirs, or from manipulations involved in the local perfusion application. To address the first concern, anaesthetic solutions were prepared in the intravenous drip bags and left for up to 4 h. At various time-points, solutions were withdrawn for later chromatographic analysis. To determine losses resulting from the perfusion method, anaesthetic solutions were loaded into syringes and perfused through the catheter tubing exactly as during electrophysio-logical recordings. The effluent flowing out of the solution changer head was immediately withdrawn for analysis.
All samples destined for gas chromatography analysis consisted of 100 μl of aqueous anaesthetic solution injected into a 2 ml septum-capped autosampler vial (Wheaton, Millville, NJ, U.S.A.). For gas headspace chromatography analysis on a Sigma 2000 gas chromatogram (Perkin-Elmer, Norwalk, CT, U.S.A.), the gas phase (100 μl) above the sample was injected (100°C) onto a 2.74 m long 5% SE-30 packed column (Ohio Specialty Chemical, Marietta, OH, U.S.A.; 110°C) and detected by flame ionization (110°C) with N2 as the carrier gas (10–20 ml min−1 flow rate). Analysis of trichloro-ethylene solutions required higher column (200°C), detector (200°C), and oven temperatures (175°C) for optimal detection. Peak heights from the gas chromatograph output were used to quantify concentrations, and experimental concentrations were determined by comparison with peak heights from standard samples.
The control experiments demonstrated that losses of volatile agents were, for the most part, small (<10% total losses) and would minimally influence the pharmacology experiments. The agents requiring special attention were diethyl ether, trichloroethylene, and enflurane. Following 4 h in the intravenous drip bags, roughly 20% of the trichloroethylene, enflurane, and diethyl ether solutions were lost. For these agents, the losses were much less at 2 h or shorter (<5%); consequently, during actual electrophysiological experiments, fresh aqueous solutions of diethyl ether, trichloroethylene, and enflurane were prepared every 2 h. With these added precautions for trichloroethylene, diethyl ether, and enflurane, loss in the intravenous drip bags should be <5–10% for all agents for the pharmacology data presented in this study. Loss due to perfusion was substantial for both diethyl ether (52.1±7.6% loss, 16 control experimental trials) and trichloroethylene (35.2±7.4% loss, 19 trials). Concentrations of diethyl ether and trichloroethylene referred to in this study are adjusted to reflect this perfusion loss.
Clinically relevant concentrations of the general anaesthetics
Clinically relevant concentrations for the various anaesthetic compounds were derived to facilitate comparison with the potencies of the anaesthetics in potentiating agonist responses at GABAA and glycine receptors. Immobility, a lack of purposeful response to a noxious stimulus, was considered here to represent the anaesthetic endpoint, and represents an easily determined and consistent endpoint across a large variety of different animal species. For some of the anaesthetic compounds, previous studies have determined accurate EC50 plasma concentrations for the agents in producing immobility in mammals. These include the volatile anaesthetics (see below), along with α-chloralose (Nattel et al., 1990) and TCEt (Garrett & Lambert, 1973), for which studies have assayed EC50 plasma concentrations following intravenous administration (see Table 1).
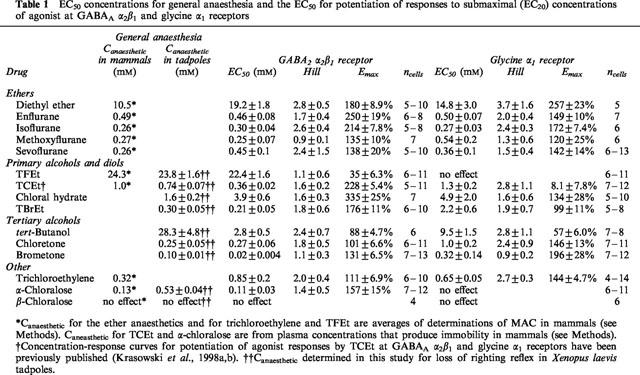
Table 1.
EC50 concentrations for general anaesthesia and the EC50 for potentiation of responses to submaximal (EC20) concentrations of agonist at GABAA α2β1 and glycine α1 receptors
The most commonly used measure of volatile anaesthetic potency in vivo is minimum alveolar concentration (MAC), the partial pressure of volatile anaesthetic required to produce immobility in response to a noxious stimulus in 50% of trials (Eger et al., 1965). MAC values for the various volatile agents were averaged from a range of different mammalian studies. MAC values (in partial pressures) were first converted into aqueous concentrations using the appropriate saline/gas partition coefficients after which temperature corrections were then applied to the aqueous concentrations to obtain average aqueous MAC equivalents at 20°C (Franks & Lieb, 1993; 1996). Average aqueous MAC equivalent values for diethyl ether, enflurane, isoflurane, methoxyflurane, and sevoflurane have been reviewed by Krasowski & Harrison (1999) (see Table 1). MAC values for trichloroethylene (Allott et al., 1973; Halsey, 1980) and TFEt (Eger et al., 1999) were obtained from published studies (see Table 1).
For the remaining compounds, no published studies have reported EC50 concentrations for anaesthesia. The anaesthetic potencies for these compounds were therefore determined by using the loss of righting reflex assay in Xenopus laevis tadpoles.
Determination of anaesthetic potencies for loss of righting reflex in tadpoles
General anaesthetic in vivo potencies were determined for Xenopus laevis tadpoles (Xenopus 1, Ann Arbor, MI, U.S.A.) in the pre-limb-bud stage of development, corresponding to stages 43–50 of the standard nomenclature for Xenopus laevis development (Nieuwkoop & Faber, 1956). Tadpoles were maintained in de-chlorinated tap water in an aerated aquarium at room temperature.
The assay for loss of righting reflex in tadpoles has historically been a very popular assay for determining the in vivo potency of general anaesthetics (Downes & Courogen, 1996). The anaesthetic endpoint of loss of righting reflex, a measure of immobility, is defined as a lack of purposeful and sustained swimming response after a gentle inversion with a smooth glass rod (Downes & Courogen, 1996; Tomlin et al., 1998). During randomized blind experiments, approximately ten tadpoles were placed in each of a number of beakers containing 300 ml of tap water, with or without the addition of anaesthetic compounds. Except for a tap water control, all beakers contained 0.1% dimethyl sulphoxide to control for the highest dimethyl sulphoxide concentration that would be present in any beaker. No anaesthetic actions or mortality were ever observed at 0.1% dimethyl sulphoxide or lower. The number of anaesthetized tadpoles was recorded every 10 min until equilibrium was reached, after which the tadpoles were returned to fresh tap water, where recovery was monitored. Equilibrium with respect to loss of righting reflex was usually reached within 80 min, except for α-chloralose which required approximately 4 h to reach equilibrium.
Tadpole concentration-response data were fitted to a quantal analysis equation of the form p=(100* In) [In+(EC50)n]−1 where p is the percentage of the population anaesthetized, I is the anaesthetic concentration, n is the slope factor, and EC50 is the concentration for a half-maximal anaesthetic effect (Waud, 1972). Quantal analysis used software written by Dr Andrew Jenkins (Cornell University).
Results
Potentiation of agonist responses by the general anaesthetics at wild-type human GABAA α2β1 and glycine α1 receptors
Agonist concentration-response relationships for wild-type human GABAA α2β1 and glycine α1 receptors expressed in HEK 293 cells have been previously described in detail (Koltchine et al., 1996; Krasowski et al., 1998a). The modulatory effects of a panel of chemically diverse anaesthetic compounds (Figure 1) at these two receptors were assessed on responses to EC20 concentrations of agonist. Figures 2 and 3 show representative examples of potentiation of agonist responses by sevoflurane, methoxyflurane, and chloral hydrate at GABAA α2β1 and glycine α1 receptors. Figure 4A shows a representative example of α-chloralose potentiating submaximal GABA responses at the GABAA α2β1 receptor. In contrast to the ethers and alcohols, α-chloralose did not potentiate glycine-mediated currents at glycine α1 receptors (Figure 4C).
Figure 2.
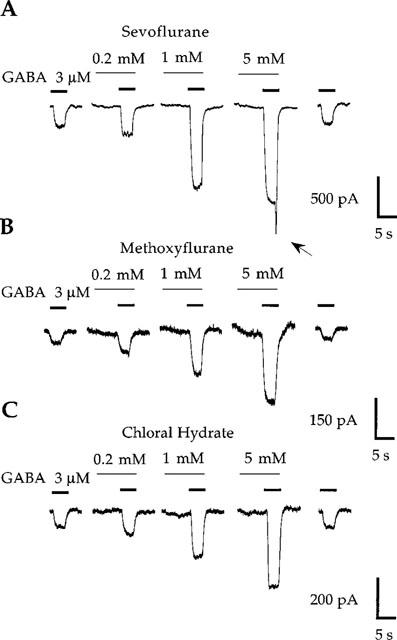
Potentiation of current responses to EC20 concentrations of GABA at wild-type human GABAA α2β1 receptors by sevoflurane (A), methoxyflurane (B), and chloral hydrate (C). Note the ‘rebound' current (arrow) following washout of 5 mM sevoflurane. Both sevoflurane and methoxyflurane directly activate the GABAA α2β1 receptor, particulary noticeable during the pre-application of 5 mM sevoflurane or methoxyflurane. Traces shown are individual recordings from HEK 293 cells transfected with cDNAs encoding the GABAA α2 and β1 receptor subunits.
Figure 3.
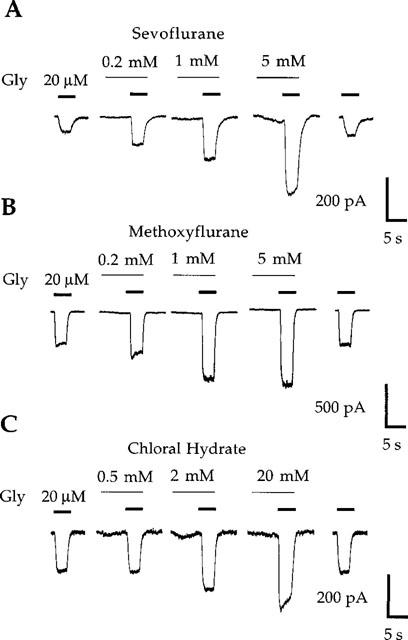
Potentiation of current responses to EC20 concentrations of glycine (Gly) at wild-type human glycine α1 receptors by sevoflurane (A), methoxyflurane (B), and chloral hydrate (C). Sevoflurane (5 mM) produces slight direct activation of the glycine α1 receptor. Traces shown are individual recordings from HEK 293 cells transfected with cDNA encoding the glycine α1 receptor subunit.
Figure 4.
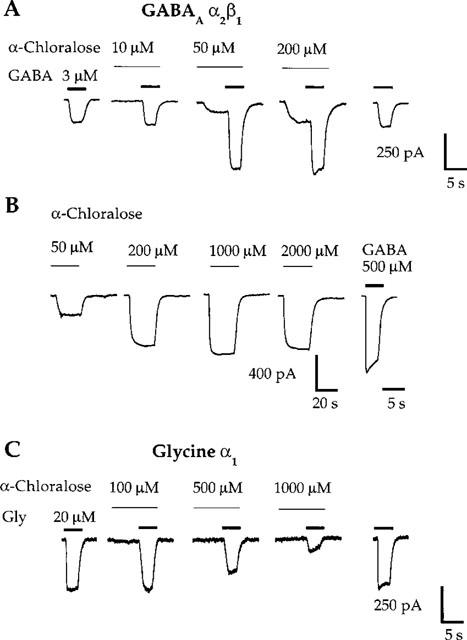
α-Chloralose produces potentiation of GABA responses and direct activation at the GABAA α2β1 receptor, yet inhibits submaximal glycine responses at the glycine α1 receptor. (A) α-Chloralose potentiates current responses to EC20 concentrations of GABA at the GABAA α2β1 receptor. Direct activation is evident during the pre-application of 50 and 200 μM α-chloralose. (B) α-Chloralose directly activates the GABAA α2β1 receptor. The first four traces are for α-chloralose applied in the absence of any GABA in the perfusion system. The response to a maximal concentration of GABA (500 μM) is shown for comparison. Note the slow time course of the α-chloralose-mediated currents. (C) α-Chloralose, at concentrations greater than 100 μM, inhibits current responses to EC20 concentrations of glycine (Gly) at the glycine α1 receptor. Traces shown are individual recordings from HEK 293 cells transfected with cDNAs encoding the GABAA α2 and β1 receptor subunits (A,B) or the glycine α1 receptor subunit (C).
Complete concentration-response curves for potentiation of agonist responses by the anaesthetics were constructed by assessing the effects of at least five anaesthetic concentrations on submaximal agonist currents. Figure 5 illustrates concentration-response curves for potentiation of agonist responses by sevoflurane, diethyl ether, chloral hydrate, and α-chloralose at GABAA α2β1 and glycine α1 receptors. Note that 0.2, 0.5, and 1 mM α-chloralose inhibited submaximal glycine responses (Figures 4C and 5D).
Figure 5.
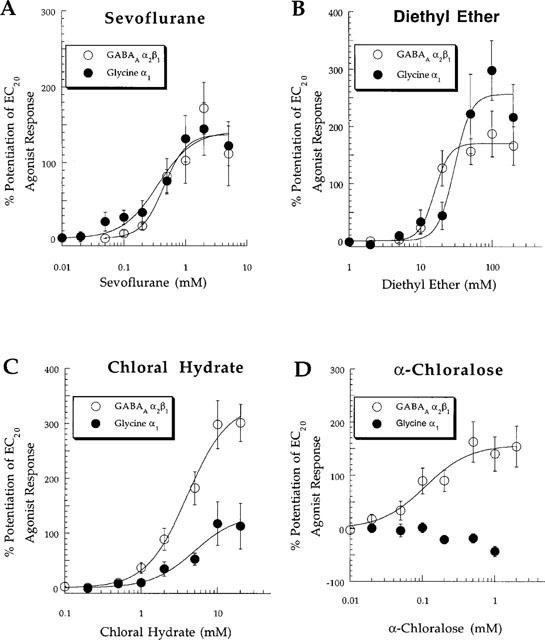
Pooled concentration-response relationships for (A) sevoflurane, (B) diethyl ether, (C) chloral hydrate, and (D) α-chloralose modulation of currents in response to submaximal (EC20) concentrations of agonist at wild-type GABAA α2β1 and glycine α1 receptors. Note the lack of effect of α-chloralose at the glycine α1 receptor at concentrations ⩽0.1 mM with inhibition of glycine responses evident at 0.2, 0.5, and 1 mM. See Table 1 for EC50, Hill slope, Emax, and ncells values.
Table 1 summarizes the anaesthetic effects on submaximal agonist responses at GABAA α2β1 and glycine α1 receptors. The anaesthetics generally had a higher potency for potentiation of agonist responses at GABAA α2β1 than at glycine α1 receptors. α-chloralose and TFEt were the only anaesthetics which did not potentiate submaximal glycine responses at glycine α1 receptors. TFEt potentiated GABA-mediated currents although with weak efficacy (approximately 35% potentiation of responses to EC20 concentrations of GABA); no effect of TFEt was noted on submaximal currents at glycine α1 receptors at TFEt concentrations up to 100 mM (Table 1).
Direct activation and blocking effects by the anaesthetics at GABAA α2β1 and glycine α1 receptors
A small degree of direct activation (i.e., a GABA-mimetic effect) is noticeable during anaesthetic pre-application in some of the current traces at the GABAA α2β1 receptor in Figure 2. With the exception of α-chloralose (see Figure 4A,B), the maximal magnitude of direct activation for any anaesthetic at the GABAA α2β1 receptor did not exceed 10–15% of the maximal GABA current. Direct activation of glycine α1 receptors by the anaesthetics was usually absent, although some of the anaesthetics directly activated glycine receptors with maximal magnitudes less than 5–10% of the maximal glycine response, similar to previous reports (see Figure 3; Downie et al., 1996). Other than α-chloralose (Figure 4A,B; see below), direct activation of GABAA and glycine receptors by the anaesthetics was not studied in detail.
A ‘rebound' current is evident following washout of 5 mM sevoflurane in experiments with the GABAA α2β1 receptor (Figure 2A, arrow). This phenomenon has been reported previously in studies of potentiation of GABA-mediated currents by sevoflurane in hippocampal neurons (Wu et al., 1996), and also has been observed in studies of several other general anaesthetics at GABAA receptors (Robertson, 1989; Adodra & Hales, 1995; Banks & Pearce, 1999). The rebound current is believed to reflect rapid relief of low-affinity anaesthetic block of GABA-induced current (Robertson, 1989; Wu et al., 1996; Banks & Pearce, 1999). Rebound currents were also observed following washout of high concentrations of other general anaesthetics at the GABAA α2β1 receptor (e.g., α-chloralose, chloral hydrate; data not shown). Rebound currents were never observed at the glycine α1 receptor, indicating that they are receptor-specific.
Lack of potentiation of agonist responses by the general anaesthetics at GABAC ρ1 receptors
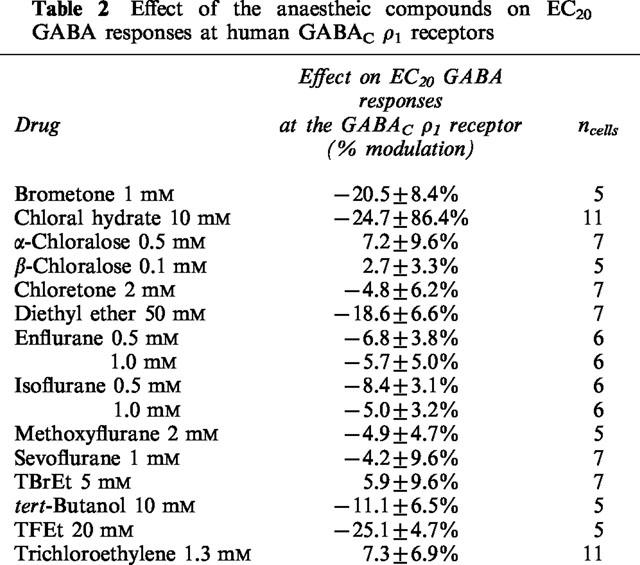
The GABA concentration-response curve for the wild-type human GABAC ρ1 receptor expressed in HEK 293 cells has been previously published (Krasowski et al., 1998a). None of the anaesthetic compounds studied produced potentiation of GABA responses or direct activation at GABAC ρ1 receptors (Table 2). In fact, some anaesthetics inhibited GABA responses. Diethyl ether (50 mM), TFEt (20 mM), and chloral hydrate (10 mM) each produced significant inhibition of EC20 GABA responses at the GABAC ρ1 receptor (P<0.05; Table 2).
Table 2.
Effect of the anaestheic compounds on EC20 GABA responses at human GABACρ1 receptors
Effects of mutations within TM2 and TM3 of GABAA and glycine receptor subunits on anaesthetic potentiation of agonist responses
Anaesthetic potentiation of agonist responses was evaluated in four GABAA and two glycine receptors with mutations in TM2 or TM3 of individual receptor subunits: GABAA α2(S270I)β1, α2(A291W)β1, α2β1(S265I), and α2β1(M286W); glycine α1(S267I) and α1(A288W). Detailed agonist concentration-response data for these mutated GABAA and glycine receptors have been reported previously (Krasowski et al., 1998a,1998b). Figure 6 illustrates the actions of three volatile either anaesthetics (methoxyflurane, sevoflurane, and diethyl ether) at the GABAA α2(S270I)β1, α2(A291W)β1, and α2β1(S265I) receptors. Concentrations of methoxyflurane, sevoflurane, and diethyl ether that produced substantial potentiation of GABA-mediated currents at wild-type GABAA α2β1 receptors (c.f., Figures 2A,B and 5A,B) had no effect on GABA responses at GABAA α2(S270I)β1 and α2(A291W)β1 receptors (Figure 6A,B). In contrast, potentiation of GABA responses by these three volatile anaesthetics at the GABAA α2β1(S265I) receptor was similar to that at the wild-type GABAA α2β1 receptor (Figure 6C).
Figure 6.
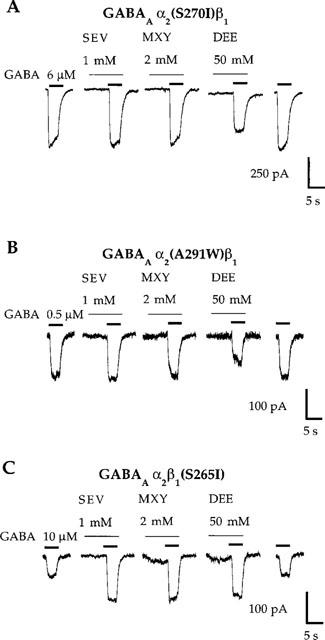
Mutations in TM2 or TM3 of the GABAA α2 subunit abolish potentiation of responses to EC20 concentrations of GABA by the ether anaesthetics sevoflurane (SEV), methoxyflurane (MXY), and diethyl ether (DEE). (A,B) Sevoflurane (1 mM) and methoxyflurane (2 mM) fail to potentiate submaximal GABA currents, while diethyl ether (50 mM) inhibits GABA responses at GABAA α2(S270I)β1 and GABAA α2(A291W)β1 receptors. (C) In contrast, sevoflurane, methoxyflurane, and diethyl ether potentiate submaximal GABA responses at the GABAA α2β1(S265I) receptor. Note that the concentrations of GABA required to produce 20% of a maximal response (i.e., the EC20 concentration) are different for each of the receptors depicted in (A), (B), and (C). Some of the receptors containing mutated subunits have apparent affinities for GABA that differ from that at the wild-type GABAA α2β1 receptor. Traces shown are individual recordings from HEK 293 cells transfected with cDNAs encoding the indicated receptor subunit combinations.
Figure 7 summarizes data for the modulatory effects of all 13 anaesthetics studied at wild-type and mutated GABAA α2β1 receptors. Previously published data for isoflurane, propofol (Krasowski et al., 1998b), and TCEt (Krasowski et al., 1998a) has been included in Figure 7 for comparison. For all the anaesthetics tested except α-chloralose, at least one of this group of mutations abolished the potentiation of GABA responses at GABAA α2β1 receptors.
Figure 7.
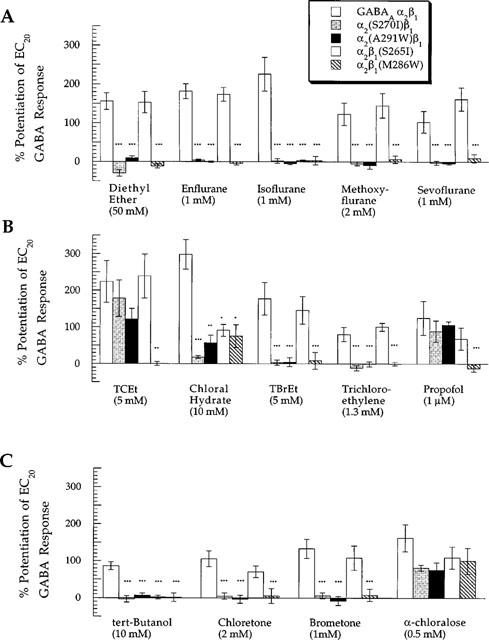
Summary of the effects of mutations in TM2 or TM3 of GABAA α2 or β1 receptor subunits on potentiation of GABA responses by general anaesthetics. The general anaesthetics are sorted into (A) ethers, (B) primary alcohols along with trichloroethylene and propofol, and (C) tertiary alcohols and α-chloralose. The ordinate depicts percentage change of a control response to an EC20 concentration of GABA by co-application with anaesthetic (where 0%=no effect). The amount of potentiation produced by an anaesthetic at a given mutated receptor was compared to the corresponding potentiation produced at the wild-type GABAA α2β1 receptor. *, **, or *** indicates that the amount of potentiation at the mutated receptor was different from that at the wild-type GABAA α2β1 receptor with a significance value of P<0.05, P<0.01, or P<0.005, respectively. Data for isoflurane, propofol (Krasowski et al., 1998b) and TCEt (Krasowski et al., 1998a) have been previously published.
We also tested the actions of the anaesthetics at glycine α1(S267I) and α1(A288W) receptors. Figure 8 demonstrates that the glycine α1(A288W) receptor was insensitive to positive modulation by all anaesthetics tested. In contrast, all five ether anaesthetics potentiated submaximal glycine responses at the glycine α1(S267I) receptor, while most of the halogenated and non-halogenated alcohols failed to potentiate glycine responses at this receptor. The effects of enflurane on the mutated GABAA and glycine receptors analysed in this study have been previously described for receptors expressed in Xenopus laevis oocytes (Mihic et al., 1997); the results presented here for receptors expressed in HEK 293 cells are qualitatively similar.
Figure 8.
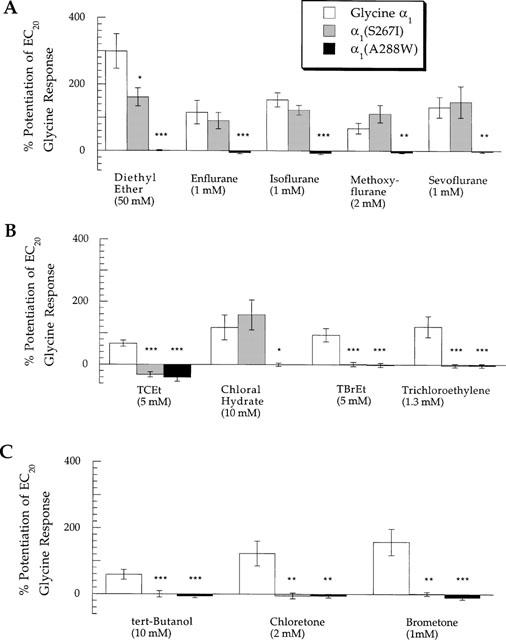
Summary of the effects of mutations in TM2 or TM3 of the glycine α1 receptor subunit on potentiation of submaximal glycine currents by (A) ethers, (B) primary alcohols along with the alkane trichloroethylene, and (C) tertiary alcohols. The ordinate depicts percentage change of a control response to an EC20 concentration of glycine by co-application with anaesthetic. The amount of potentiation produced by an anaesthetic at a given mutated receptor was compared to the corresponding potentiation produced at the wild-type glycine α1 receptor. *, **, or *** indicates that the amount of potentiation at the mutant receptor was different from that at the wild-type glycine α1 receptor with a significance value of P<0.05, P<0.01, or P<0.005, respectively. Data for TCEt has been previously published (Krasowski et al., 1998a).
Direct activation by α-chloralose at the GABAA α2β1 receptor and effects of the non-anaesthetic isomer β-chloralose
α-chloralose elicited profound direct activation at GABAA α2β1 receptors (Figure 4A,B), as noted in previous studies of neuronal GABAA receptors (Ishizuka et al., 1989; Robertson, 1989). No direct activation by α-chloralose was detected at either glycine α1 (see Figure 4C; n=6–11) or GABAC ρ1 receptors (n=5) at concentrations up to 2 mM. α-chloralose directly activated GABAA α2β1 receptors with an EC50 of 0.12±0.01 mM (nH=3.6±0.4, Emax=66±2.3% of the maximal GABA response, n=6). The direct activating effects of α-chloralose were mediated via the GABAA receptor, as the α-chloralose-induced current was blocked by the GABAA receptor antagonist picrotoxin. Application of 10 μM picrotoxin blocked 89.0±2.2% of the current elicited by 200 μM α-chloralose at the wild-type GABAA α2β1 receptor (n=4). α-chloralose also directly activated all four mutated GABAA receptors with EC50 values similar to that for the wild-type GABAA α2β1 receptor (n=5–7 for all experiments): GABAA α2(S270I)β1 (EC50=0.15±0.02 mM, Emax=64±3.9%), α2(A291W)β1 (EC50=0.10±0.02 mM, Emax=43±2.4%), α2β1(S265I) (EC50=0.15±0.02 mM, Emax=35±1.9%) and α2β1(M286W) receptors (EC50=0.11±0.02 mM, Emax=35±1.6%).
A structural isomer of α-chloralose, β-chloralose (see Figure 1), possesses almost no sedative/hypnotic, anti-convulsant, or general anaesthetic activity (Monroe et al., 1963a,1963b). β-chloralose produced no loss of righting reflex in tadpoles, even when applied at saturating concentrations for up to 24 h.
β-chloralose did not produce potentiation of agonist responses or direct activation of GABAA α2β1 receptors, glycine α1, or GABAC ρ1 receptors (Tables 1 and 2). At GABAA α2β1 receptors, 100 μM β-chloralose produced −2.4±3.9% alteration of an EC20 GABA response (n=4) with no direct activation evident even with applications up to 3 min (n=4). Further, 100 μM β-chloralose did not antagonize potentiation of GABA responses or direct receptor activation by 100 μM α-chloralose at wild-type GABAA α2β1 receptors. Potentiation of an EC20 concentration of GABA by 100 μM α-chloralose was 87.6±14.3% (n=13) and 83.9±18.4% (n=6) in the absence and presence of pre- and co-application of 100 μM β-chloralose. Direct activation of wild-type GABAA α2β1 receptors by 100 μM α-chloralose was 19.9±2.1% (n=13) and 18.9±4.0% (n=7) of the maximal GABA current in the absence and presence of pre- and co-application of 100 μM β-chloralose. Thus, β-chloralose does not antagonize potentiation of GABA responses or direct activation by α-chloralose at the GABAA α2β1 receptor.
In vivo anaesthetic potencies of the compounds
Table 1 lists the EC50 concentrations for some of the anaesthetic compounds in producing immobility in mammals, using values reported in the literature. For some of the anaesthetics in this study, particularly the alcohols, there are no published data for anaesthetic potencies in mammals. Anaesthetic potencies for these compounds were thus determined for loss of righting reflex in Xenopus laevis tadpoles (Table 1). Determination of the anaesthetic potency of chloral hydrate is not possible in mammals, because chloral hydrate is very rapidly metabolized to TCEt (Marshall & Owens, 1954; Garrett & Lambert, 1973). Drug metabolism is inefficient in tadpoles (Brodie & Maickel, 1962), so presumably very little chloral hydrate is converted to TCEt.
Discussion
Relevance to general anaesthesia
The results of this study demonstrate a qualitative correlation between the potencies of the general anaesthetics for potentiating GABA responses at GABAA α2β1 receptors and the in vivo potencies of these agents for producing immobility in mammals or loss of righting reflex in tadpoles. The potencies of the anaesthetics for potentiating glycine responses at the glycine α1 receptors correlated less impressively with the in vivo anaesthetic potencies, primarily because α-chloralose and TFEt did not potentiate glycine responses at the glycine α1 receptor. The results presented here and in other studies suggest that the glycine receptor is most sensitive to ether, alkane, and alcohol anaesthetics, but is much less sensitive or insensitive to other anaesthetic agents such as etomidate, barbiturates, propofol, α-chloralose, and steroidal anaesthetics (Prince & Simmonds, 1992; Koltchine et al., 1996; Mascia et al., 1996; Pistis et al., 1997).
Actions of α-chloralose and β-chloralose
The modulatory actions of α-chloralose at inhibitory ligand-gated ion channels differ markedly from those of the ether, alcohol, and alkane general anaesthetics. This is somewhat surprising because α-chloralose structurally resembles the halogenated alcohols, being formed essentially from the condensation of chloral hydrate with glucose (see Figure 1). The main differences are the complete absence of potentiation of submaximal glycine currents by α-chloralose at glycine α1 receptors, and the lack of effect of the GABAA receptor mutations studied here on potentiation of GABA responses by α-chloralose. In addition, α-chloralose directly activates GABAA receptors with high potency and efficacy, something not seen with the ether, alkane, and alcohol general anaesthetics analysed in this study. The total pattern of α-chloralose activity more closely resembles that of the barbiturates and steroidal anaesthetics (Prince & Simmonds, 1992; Koltchine et al., 1996; Mascia et al., 1996; Pistis et al., 1997).
The α- and β-chloralose structural isomers exhibit substantial potency differences for general anaesthesia (Monroe et al., 1963a,1963b), which are paralleled by similar potency differences for potentiation of GABA responses and direct activation of GABAA α2β1 receptors (Table 1). Interestingly, the non-anaesthetic β-chloralose is also unable to antagonize the potentiation of GABA responses or direct activation produced by α-chloralose. This suggests the presence of a specific binding site on the GABAA receptor for α-chloralose that excludes β-chloralose. These results also are consistent with the GABAA receptor playing a role in the anaesthetic actions of α-chloralose.
Interpretation of the effects of the mutations within TM2 and TM3 of GABAA and glycine receptor subunits on potentiation of agonist responses by general anaesthetics
Mihic et al. (1997) demonstrated that specific mutations within TM2 and TM3 of GABAA and glycine receptor subunits affected the potentiation of agonist responses by enflurane and ethanol. These findings were later extended to long-chain n-alcohols and isoflurane (Krasowski et al., 1998b; Wick et al., 1998). The effects of the receptor mutations studied were qualitatively similar for all five ethers, with the sole exception that potentiation of submaximal GABA-evoked currents by isoflurane, but not by the other four ethers, was abolished at the GABAA α2β1(S265I) receptor (Table 1). This indicates subtle differences between structurally related ethers with respect to the effects of the mutations in TM2 and TM3 of the GABAA receptor subunits; for example, enflurane and isoflurane are positional isomers of one another (Figure 1). Trichloroethylene, a halogenated alkane, displayed a response pattern to mutations similar to that of the ethers, with the exception that trichloroethylene did not potentiate submaximal glycine responses at the glycine α1(S267I) receptor (Figure 8).
This leads to the question of how these mutations abolish the potentiating actions of the general anaesthetics. Two possibilities are: (1) the mutations alter the characteristics of the binding sites for the general anaesthetics or (2) the mutations perturb an allosteric mechanism necessary for the positive modulatory effects of the anaesthetics. In possibility (1), the residues which are mutated need not necessarily be physically involved in binding the anaesthetics, but may control the dimensions of a nearby anaesthetic binding pocket. In possibility (2), the mutated amino acids may be quite distant from the actual binding sites for the general anaesthetics. The GABAA and glycine receptor subunit mutations described in this study have already been shown to alter the apparent affinity for agonist (Mihic et al., 1997; Krasowski et al., 1998a,1998b), presumably by an alteration of a gating mechanism.
A previous study has suggested that certain mutations in TM2 of the glycine α1 receptor (e.g., S267Q) change the dimensions of a ‘binding pocket' for n-alcohols, manifested by an alteration of the n-alcohol ‘cut-off' for the regulation of glycine responses (Wick et al., 1998). Straight-chain alcohols up to n-dodecanol potentiate glycine responses at the wild-type glycine α1 receptor (Mascia et al., 1996), whereas only alcohols up to n-propanol inhibit submaximal glycine currents at the glycine α1(S267Q) receptor (Wick et al., 1998). A similar alteration of cut-off by the GABAA receptor mutations may be evident for the primary and tertiary alcohols examined in this study. TCEt potentiates submaximal GABA responses at the GABAA α2(S270I)β1 receptor while the larger chloral hydrate, TBrEt, chloretone, and brometone molecules all fail to enhance GABA responses at this receptor. Similarly, TCEt and chloral hydrate both enhance submaximal GABA responses at the GABAA α2(A291W)β1 receptor while the other alcohols do not potentiate GABA responses at this receptor (Table 1). It is perhaps noteworthy that none of the tertiary alcohols potentiate GABA responses at the GABAA α2(S270I)β1 and α2(A291W)β1 receptors. The differences in molecular geometries between primary and tertiary alcohols may aid molecular modeling studies of alcohol interactions with GABAA and glycine receptors.
These size-dependent, agent-specific effects on the mutated receptors are difficult to reconcile with the idea of an ‘allosteric switch' that is altered by the mutations. Instead, the mutations might alter the dimensions of binding pockets for the anaesthetics, akin to the idea proposed by Wick et al. (1998) for the n-alcohols at the glycine α1 receptor. In accounting for the differential actions of the GABAA β1(S265I) mutation on potentiation of GABA responses by the ether anaesthetics (Figure 7), it seems extravagant to envision that enflurane, methoxyflurane, sevoflurane, and diethyl ether utilize allosteric mechanisms distinct from that for isoflurane.
Definitive identification of general anaesthetic binding pockets probably awaits the determination of high-resolution three-dimensional structures for the ligand-gated ion channels. Structural biology approaches have already been applied to the study of general anaesthetic interactions with ‘model' soluble proteins (Eckenhoff & Johansson, 1997), including the 2.2 Å resolution three-dimensional structure of firefly luciferase complexed with the general anaesthetic bromoform (Franks et al., 1998). This structure provides a striking example of an amphipathic anaesthetic binding cavity of defined dimensions. It will be interesting to see whether amino acid residues within TM2 and TM3 of GABAA and glycine receptors may yet prove to control a similar binding pocket for ether, alkane, and alcohol anaesthetics.
Acknowledgments
We are grateful to Amiinah Kung, Steve Lopez, Matthew Ruan, Suzanne Finn, Qing Ye, and Audrey Lin for invaluable technical support. We thank Dr Andrew Jenkins for assistance with the tadpole loss of righting reflex experiments. We also thank Dr Mathew Jones for advice on gas chromatographic analysis. We also appreciate the expertise and suggestions of Drs Edmond Eger and Donald Koblin. Funding for this study was generously provided by National Institutes of Health grants GM45129 and GM56850 to N.L.Harrison by GM47818 to Dr Egar, and by National Institute of Mental Health training fellowship MH11504 to M.D.Krasowski.
Abbreviations
- MAC
minimum alveolar concentration
- TBrEt
2,2,2-tribromoethanol
- TCEt
2,2,2-trichloroethanol
- TFEt
2,2,2-trifluoroethanol
- TM
transmembrane domain
References
- ADODRA S., HALES T.G. Potentiation, activation and blockade of GABAA receptors of clonal murine hypothalamic GT1-7 neurones by propofol. Br. J. Pharmacol. 1995;115:953–960. doi: 10.1111/j.1476-5381.1995.tb15903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLOTT P.R., STEWARD A., FLOOK V., MAPLESON W.W. Variation with temperature of the solubilities of inhaled anaesthetics in water, oil and biological media. Br. J. Anaesth. 1973;45:294–300. doi: 10.1093/bja/45.3.294. [DOI] [PubMed] [Google Scholar]
- BANKS M.I., PEARCE R.A. Dual actions of volatile anesthetics on GABAA IPSCs: dissociation of blocking and prolonging effects. Anesthesiol. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- BARNARD E.A., SKOLNICK P., OLSEN R.W., MOHLER H., SIEGHART W., BIGGIO G., BRAESTRUP C., BATESON A.N., LANGER S.Z. International union of pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- BELELLI D., LAMBERT J.J., PETERS J.A., WAFFORD K., WHITING P.J. The interaction of the general anesthetic etomidate with the γ-aminobutyric acid type A receptor is influenced by a single amino acid. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODIE B.B., MAICKEL R.P.Comparative biochemistry of drug metabolism Proceedings of the First International Pharmacological Meeting 1962MacMillan Co.: New York; 299–324.Brodie, B.B.P & Erdos, E.G. (eds.) [Google Scholar]
- CHANG Y., WANG R., BAROT S., WEISS D.S. Stoichiometry of a recombinant GABAA receptor. J. Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTTING G.R., LU L., O'HARA B.F., KASCH L.M., MONTROSE-RAFIZADEH C., DONOVAN D.M., SHIMADA S., ANTONARAKIS S.E., GUGGINO W.B., UHL G.R., KAZAZIAN H.H. Cloning of the γ-aminobutyric acid (GABA) ρ1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., MIHIC S.J., LIU Y., DEITRICH R.A., HARRIS R.A. Actions of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. Br. J. Pharmacol. 1996;118:378–384. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWNES H., COUROGEN P.M. Contrasting effects of anesthetics in tadpole bioassays. J. Pharmacol. Exp. Ther. 1996;278:284–296. [PubMed] [Google Scholar]
- DOWNIE D.L., HALL A.C., LIEB W.R., FRANKS N.P. Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. Br. J. Pharmacol. 1996;118:493–502. doi: 10.1111/j.1476-5381.1996.tb15430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKENHOFF R.G., JOHANSSON J.S. Molecular interactions between inhaled anesthetics and proteins. Pharmacol. Rev. 1997;49:343–367. [PubMed] [Google Scholar]
- EGER E.I., IONESCU P., LASTER M.J., GONG D., HUDLICKY T., KENDIG J.J., HARRIS R.A., TRUDELL J.R., POHORILLE A. Minimum alveolar anesthetic concentration of fluorinated alkanols in rats: relevance to theories of narcosis. Anesth. Analg. 1999;88:867–876. doi: 10.1097/00000539-199904000-00035. [DOI] [PubMed] [Google Scholar]
- EGER E.I., SAIDMAN L.J., BRANDSTATER B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiol. 1965;26:756–763. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- FARRAR S.J., WHITING P.J., BONNERT T.P., MCKERNAN R.M. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J. Biol. Chem. 1999;274:10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- FRANKS N.P., JENKINS A., CONTI E., LIEB W.R., BRICK P. Structural basis for the inhibition of firefly luciferase by a general anesthetic. Biophys. J. 1998;75:2205–2211. doi: 10.1016/S0006-3495(98)77664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKS N.P., LIEB W.R. Selective actions of volatile general anaesthetics at molecular and cellular levels. Br. J. Anaesth. 1993;71:65–76. doi: 10.1093/bja/71.1.65. [DOI] [PubMed] [Google Scholar]
- FRANKS N.P., LIEB W.R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- FRANKS N.P., LIEB W.R. Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology. 1996;84:716–720. doi: 10.1097/00000542-199603000-00027. [DOI] [PubMed] [Google Scholar]
- GARRETT E.R., LAMBERT H.J. Pharmacokinetics of trichloroethanol and metabolites and interconversions among variously referenced pharmacokinetic parameters. J. Pharm. Sci. 1973;62:550–572. doi: 10.1002/jps.2600620404. [DOI] [PubMed] [Google Scholar]
- GARRETT K.M., GAN J.P. Enhancement of γ-aminobutyric acidA receptor activity by α-chloralose. J. Pharmacol. Exp. Ther. 1998;285:680–686. [PubMed] [Google Scholar]
- GRENNINGLOH G., RIENITZ A., SCHMITT B., METHFESSEL C., ZENSEN M., BEYREUTHER K., GUNDELFINGER E.D., BETZ H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- HADINGHAM K.L., WINGROVE P., LE BOURDELLES B., PALMER K.J., RAGAN C.I., WHITING P.J. Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3-, and α5-containing human γ-aminobutyric acidA receptors. Mol. Pharmacol. 1993a;43:970–975. [PubMed] [Google Scholar]
- HADINGHAM K.L., WINGROVE P.B., WAFFORD K.A., BAIN C., KEMP J.A., PALMER K.J., WILSON A.W., WILCOX A.S., SIKELA J.M., RAGAN C.I., WHITING P.J. Role of the β subunit in determining the pharmacology of human γ-aminobutyric acid type A receptors. Mol. Pharmacol. 1993b;44:1211–1218. [PubMed] [Google Scholar]
- HALES T.G., LAMBERT J.J. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br. J. Pharmacol. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALSEY M.J.Physicochemical properties of inhalational anaesthetics General Anaesthesia 1980Butterworths: London; 45–65.Gray, T.C., Nunn, J.F. & Utting, J.E. (eds.) [Google Scholar]
- HARRIS R.A., MIHIC S.J., DILDY-MAYFIELD J.E., MACHU T.K. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. FASEB J. 1995;9:1454–1462. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- HARRISON N.L., KUGLER J.L., JONES M.V., GREENBLATT E.P., PRITCHETT D.B. Positive modulation of human γ-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol. Pharmacol. 1993;44:628–632. [PubMed] [Google Scholar]
- ISHIZUKA S., SIKDAR S.K., YASUI S., OYAMA Y., AKAIKE N. α-chloralose opens the chloride channel of frog isolated sensory neurons. Brain Res. 1989;498:181–184. doi: 10.1016/0006-8993(89)90418-6. [DOI] [PubMed] [Google Scholar]
- JONES M.V., BROOKS P.A., HARRISON N.L. Enhancement of γ-aminobutyric acid-activated Cl− currents in cultured rat hippocampal neurones by three volatile anaesthetics. J. Physiol. 1992;449:279–293. doi: 10.1113/jphysiol.1992.sp019086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLTCHINE V.V., YE Q., FINN S.E., HARRISON N.L. Chimeric GABAA/glycine receptors: Expression and barbiturate pharmacology. Neuropharmacology. 1996;35:1445–1456. doi: 10.1016/s0028-3908(96)00088-3. [DOI] [PubMed] [Google Scholar]
- KRASOWSKI M.D., FINN S.E., YE Q., HARRISON N.L. Trichloroethanol modulation of recombinant GABAA, glycine, and GABA ρ1 receptors. J. Pharmacol. Exp. Ther. 1998a;284:934–942. [PubMed] [Google Scholar]
- KRASOWSKI M.D., HARRISON N.L. General anaesthetic actions on ligand-gated ion channels. Cell. Mol. Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRASOWSKI M.D., KOLTCHINE V.V., RICK C.E., YE Q., FINN S.E., HARRISON N.L. Propofol and other intravenous anesthetics have sites of action on the γ-aminobutyric acidA receptor distinct from that for isoflurane. Mol. Pharmacol. 1998b;53:530–538. doi: 10.1124/mol.53.3.530. [DOI] [PubMed] [Google Scholar]
- KRASOWSKI M.D., O'SHEA S.M., RICK C.E.M., WHITING P.J., HADINGHAM K.L., CZAJKOWSKI C., HARRISON N.L. α subunit isoform influences GABAA receptor modulation by propofol. Neuropharmacology. 1997;36:941–949. doi: 10.1016/s0028-3908(97)00074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRASOWSKI M.D., YE Q., FINN S.E., LIN A., HARRISON N.L. Actions of volatile anesthetics and a volatile convulsant at inhibitory ligand-gated receptors [abstract] Soc. Neurosci. Abstr. 1998c;28:795.6. [Google Scholar]
- KUHSE J., BETZ H., KIRSCH J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr. Opin. Neurobiol. 1995;5:318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., ZIMMERMAN S.A., LEVITIN M., JONES M.V., HARRISON N.L. Trichloroethanol potentiates synaptic transmission mediated by γ-aminobutyric acidA receptors in hippocampal neurons. J. Pharmacol. Exp. Ther. 1993;264:1097–1103. [PubMed] [Google Scholar]
- MARSHALL E.K., OWENS A.H. Absorption, excretion, and metabolic fate of chloral hydrate and trichloroethanol. Bull. Johns Hopkins Hosp. 1954;95:1–18. [PubMed] [Google Scholar]
- MASCIA M.P., MACHU T.K., HARRIS R.A. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br. J. Pharmacol. 1996;119:1331–1336. doi: 10.1111/j.1476-5381.1996.tb16042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGURK K.A., PISTIS M., BELELLI D., HOPE A.G., LAMBERT J.J. The effect of a transmembrane amino acid on etomidate sensitivity of an invertebrate GABA receptor. Br. J. Pharmacol. 1998;124:13–20. doi: 10.1038/sj.bjp.0701787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHIC S.J., HARRIS R.A. Inhibition of ρ1 receptor GABAergic currents by alcohols and volatile anesthetics. J. Pharmacol. Exp. Ther. 1996;277:411–416. [PubMed] [Google Scholar]
- MIHIC S.J., MCQUILKIN S.J., EGER E.I., IONESCU P., HARRIS R.A. Potentiation of γ-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol. Pharmacol. 1994a;46:851–857. [PubMed] [Google Scholar]
- MIHIC S.J., WHITING P.J., HARRIS R.A. Anaesthetic concentrations of alcohols potentiate GABAA receptor-mediated currents: lack of subunit specificity. Eur. J. Pharmacol. 1994b;268:209–214. doi: 10.1016/0922-4106(94)90190-2. [DOI] [PubMed] [Google Scholar]
- MIHIC S.J., YE Q., WICK M.J., KOLTCHINE V.V., KRASOWSKI M.D., FINN S.E., MASCIA M.P., VALENZUELA C.F., HANSON K.K., GREENBLATT E.P., HARRIS R.A., HARRISON N.L. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- MONROE R.R., BALIS G.U., EBERSBERGER E. Anticonvulsant activity of alpha and beta chloralose in rats. Curr. Ther. Res. 1963a;5:154–165. [PubMed] [Google Scholar]
- MONROE R.R., BALIS G.U., EBERSBERGER E. The hypnotic effects of alpha and beta chloralose in rats. Curr. Ther. Res. 1963b;5:141–153. [PubMed] [Google Scholar]
- NAKAHIRO M., ARAKAWA O., NARAHASHI T. Modulation of γ-aminobutyric acid receptor-channel complex by alcohols. J. Pharmacol. Exp. Ther. 1991;259:235–240. [PubMed] [Google Scholar]
- NAKAHIRO M., YEH J.Z., BRUNNER E., NARAHASHI T. General anesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons. FASEB J. 1989;3:1850–1854. doi: 10.1096/fasebj.3.7.2541038. [DOI] [PubMed] [Google Scholar]
- NATTEL S., WANG Z.G., MATTHEWS C. Direct electrophysiological actions of pentobarbital at concentrations achieved during general anesthesia. Am. J. Physiol. 1990;259:H1743–H1751. doi: 10.1152/ajpheart.1990.259.6.H1743. [DOI] [PubMed] [Google Scholar]
- NIEUWKOOP P.D., FABER J. Normal table of Xenopus laevis (daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. North-Holland Publishing Company: Amsterdam; 1956. [Google Scholar]
- PEOPLES R.W., WEIGHT F.F. Trichloroethanol potentiation of γ-aminobutyric acid-activated chloride current in mouse hippocampal neurones. Br. J. Pharmacol. 1994;113:555–563. doi: 10.1111/j.1476-5381.1994.tb17025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISTIS M., BELELLI D., PETERS J.A., LAMBERT J.J. The interaction of general anaesthetics with recombinant GABAA and glycine receptors expressed in Xenopus laevis oocytes: a comparative study. Br. J. Pharmacol. 1997;122:1707–1719. doi: 10.1038/sj.bjp.0701563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINCE R.J., SIMMONDS M.A. Steroid modulation of the strychnine-sensitive glycine receptor. Neuropharmacology. 1992;31:201–205. doi: 10.1016/0028-3908(92)90168-o. [DOI] [PubMed] [Google Scholar]
- PRITCHETT D.B., SONTHEIMER H., SHIVERS B.D., YMER S., KETTENMANN H., SCHOFIELD P.R., SEEBURG P.H. Importance of a novel GABAA receptor subunit for benzo-diazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br. J. Pharmacol. 1989;98:167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON S.A., WHITING P.J., WAFFORD K.A. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br. J. Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLIN S.L., JENKINS A., LIEB W.R., FRANKS N.P. Stereoselective effects of etomidate optical isomers on gamma-aminobutyric acid type A receptors and animals. Anesthesiology. 1998;88:708–717. doi: 10.1097/00000542-199803000-00022. [DOI] [PubMed] [Google Scholar]
- UENO S., WICK M.J., YE Q., HARRISON N.L., HARRIS R.A. Subunit mutations affect ethanol actions on GABAA receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 1999;127:377–382. doi: 10.1038/sj.bjp.0702563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKAMORI M., IKEMOTO Y., AKAIKE N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J. Neurophysiol. 1991;66:2014–2021. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- WAUD D.R. On biological assays involving quantal responses. J. Pharmacol. Exp. Ther. 1972;183:577–607. [PubMed] [Google Scholar]
- WICK M.J., MIHIC S.J., UENO S., MASCIA M.P., TRUDELL J.R., BROZOWSKI S.J., YE Q., HARRISON N.L., HARRIS R.A. Mutations of γ-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU J., HARATA N., AKAIKE N. Potentiation by sevoflurane of the γ-aminobutyric acid-induced chloride current in acutely dissociated CA1 pyramidal neurones from rat hippocampus. Br. J. Pharmacol. 1996;119:1013–1021. doi: 10.1111/j.1476-5381.1996.tb15772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


