Abstract
The interaction between nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) was investigated in isolated circular smooth muscle cells and strips of the guinea-pig gastric fundus.
VIP induced a concentration-dependent inhibition of carbachol-induced contraction in smooth muscle cells with a maximum at 10−6 M. The relaxation by 10−6 M VIP was inhibited for 79.1±5.8% (mean±s.e.mean) by the NO-synthase (NOS) inhibitor L-NG-nitroarginine (L-NOARG; 10−4 M) in a L-arginine reversible way. Also the inducible NOS (iNOS) selective inhibitor N-(3-(acetaminomethyl)-benzyl)acetamide (1400 W; 10−6 M) inhibited the VIP-induced relaxation, but its inhibitory effect was not reversed by L-arginine.
When cells were incubated with the guanylyl cyclase inhibitor 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ, 10−6 M), the protein kinase A-inhibitor (R)-p-cyclic adenosine-3′,5′-monophosphothioate ((R)-p-cAMPS, 10−6 M) and the glucocorticoid dexamethasone (10−5 M), the relaxant effect of VIP was decreased by respectively 80.9±7.6, 77.0±11.6 and 87.1±4.5%.
In circular smooth muscle strips of the guinea-pig gastric fundus, the VIP (10−9–10−7 M)-induced relaxations were not significantly influenced by 10−4 M L-NOARG, 10−6 M 1400 W, 10−6 M ODQ and 10−5 M dexamethasone.
These results suggest that iNOS, possibly induced by the procedure to prepare the smooth muscle cells, is involved in the relaxant effect of VIP in isolated smooth muscle cells but not in smooth muscle strips of the guinea-pig gastric fundus. This study illustrates the importance of the experimental method when studying the influence of NOS inhibitors on the relaxation induced by VIP in gastrointestinal smooth muscle preparations.
Keywords: Guinea-pig, gastric fundus, nitric oxide, vasoactive intestinal polypeptide
Introduction
There is considerable evidence that nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) are both involved in inhibitory non-adrenergic non-cholinergic (NANC) neurotransmission causing relaxation of gastrointestinal smooth muscle (Brookes, 1993; Shuttleworth & Keef, 1995). VIP is thought to stimulate membranous VIP receptors and to induce relaxation via an elevation of intracellular adenosine 3′5′ cyclic monophosphate (cyclic AMP), while NO activates soluble guanylyl cyclase and induces relaxation via increases of guanosine 3′5′ cyclic monophosphate (cyclic GMP). NO is derived from L-arginine by the catalytic activity of three different isoforms of nitric oxide synthase: the constitutive Ca2+/calmodulin-dependent neuronal (nNOS or NOS1) and endothelial (eNOS or NOS3) isoforms, and the inducible isoform (iNOS or NOS2), that can be induced in macrophages and many other cell types including smooth muscle in reaction to bacterial endotoxin and cytokines (Förstermann et al., 1995). Immunohistochemistry with antibodies against nNOS illustrates the presence of nNOS in the myenteric plexus of the gastrointestinal tract (Bredt et al., 1990). VIP and nNOS are colocalized in many neurons of the myenteric plexus (Furness et al., 1992; Berezin et al., 1994; Lefebvre et al., 1995), favouring the idea that NO and VIP are parallel cotransmitters. Functional evidence in the rat and ferret gastric fundus suggests that NO and VIP respectively initiate and sustain NANC relaxation (Li & Rand, 1990; Boeckxstaens et al., 1992; D'Amato et al., 1992; Grundy et al., 1993), while NO seems the major neurotransmitter, also during sustained NANC relaxation, in the cat and the pig gastric fundus (Barbier & Lefebvre, 1993; Lefebvre & Vandekerkhove, 1998).
The interaction between NO and VIP in the gastrointestinal tract might be more complex. In vitro studies in the guinea-pig gastric fundus and the rat colon have shown that the relaxant effect of VIP is antagonized by NOS inhibitors and that VIP stimulates NO production, as measured by the amount of 3H-citrulline produced from 3H-arginine, in both classic smooth muscle strips and isolated smooth muscle cells (Grider et al., 1992; Grider, 1993; Jin et al., 1993), suggesting that at least part of the relaxation by VIP is due to NO synthesis in the smooth muscle cells and that VIP and NO are linked sequentially. The presence of a membrane-bound NO synthase was indeed described by the same group in plasma membranes of rabbit gastric smooth muscle cells (Murthy & Makhlouf, 1994) and a similar observation was recently reported for the canine lower oesophageal sphincter (Salapatek et al., 1998); the latter did not recognize antibodies against neural, endothelial and inducible NOS as assessed via immunohistochemistry. nNOS immunoreactivity has been reported to be present in some canine gastrointestinal muscle cells (Berezin et al., 1994), while nNOS mRNA was shown in gastrointestinal muscle cells of the opossum (Chakder et al., 1997). The group of Makhlouf recently reported the expression of eNOS in rabbit gastric and human intestinal smooth muscle cells (Teng et al., 1998). Opposing to the sequential link between VIP and NO is the observation that the relaxation by VIP in many gastrointes-tinal tissues is not blocked by NOS inhibitors (Tøttrup et al., 1991; Boeckxstaens et al., 1992; Barbier & Lefebvre, 1993; Keef et al., 1994; Lefebvre et al., 1995). This was also reported for guinea-pig gastric fundus strips (Lefebvre et al., 1992) and whole stomach (Desai et al., 1994) in complete contrast to the results reported above for the same tissue.
The aim of the present study was therefore to further investigate the mechanism of interaction between NO and VIP in isolated smooth muscle cells and smooth muscle strips from the guinea-pig gastric fundus. Preliminary accounts of these results have been given (Dick & Lefebvre, 1998a,1998b).
Methods
Preparation of isolated smooth muscle cells
Circular smooth muscle cells were isolated from the guinea-pig gastric fundus by collagenase digestion as previously described (Bitar & Makhlouf, 1982; Botella et al., 1994). Briefly, guinea-pigs of either sex (400–700 g) were killed by a blow to the head and bleeding. The gastric fundus was isolated immediately and after removal of the mucosa and submucosa, the circular muscle layer was carefully dissected from the rest of the stomach wall. Small sheets from the circular muscle layer were incubated for two successive periods of 40 min at 31°C, in 15 ml of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered medium (25 mM), containing 150 U ml−1 collagenase (Type II) and 0.01% soybean trypsin inhibitor and gassed with a mixture of 95% O2 and 5% CO2. The medium consisted of (mM): NaCl, 98; KCl, 6; NaH2PO4, 2.5; CaCl2, 1.8; D(+)-glucose, 11.5; bovine serum albumin, 0.2% (w v−1) and was supplemented with (mM): sodium pyruvate, 5; sodium fumarate, 5; sodium glutamate, 5; glutamin, 2; amino acid mixture, 1% (v v−1); vitamin mixture, 1% (v v−1); penicillin G, 50 μg ml−1 and streptomycin, 50 μg ml−1. The pH of the buffered medium was adjusted to 7.4. At the end of the second incubation, the medium was filtered through a 500-μm Nitex filter and the partly digested tissues were washed with 30 ml enzyme-free medium, whereafter they were allowed to disperse spontaneously in enzyme free medium for 30 min. Finally the spontaneously dissociated muscle cells were harvested by filtration and used for functional measurements.
Viability tests by exclusion of trypan blue (Collins & Gardner, 1982), showed that at least 85% of the cells in suspension were viable at the time of contraction experiments. Each stomach yielded about 104 ml−1 muscle cells. Cell suspensions were studied usually within 30 min at 31°C. On two occasions, the whole procedure was performed under sterile conditions.
The length of the isolated smooth muscle cells was determined by Image Splitting after fixation with glutaraldehyde. An aliquot of 50 μl treated cell suspension was placed on a Malassez slide. The first 50 randomly encountered and morphologically intact cells were measured using a Carl Zeiss eyepiece at a magnification of at least 200 times. For the vials with control cells and carbachol treated cells, two different aliquots were taken and two times 50 cells were measured. The absolute cell length measurement was performed with a scale mask placed on a video screen, connected to a video camera. Magnification due to the video camera had been first calculated by use of a micrometer.
Measurement of relaxation (inhibition of contraction) in isolated smooth muscle cells
Untreated cells served as controls. Cells can be maximally contracted by incubation with 10−8 M carbachol for 30 s, followed by fixation of the cells with glutaraldehyde (pH 7.4) to a final concentration of 2.5%. In relaxation experiments, the relaxant agent atrial natriuretic peptide (ANP; 10−6 M), VIP (10−14–10−6 M), forskolin (10−6 M), isoprenaline (10−4 M), pinacidil (10−5 M), 3-morpholinosydnonimine (SIN-1; 10−6 M) or sodium nitroprusside (SNP; 10−6 M) was added 60 s before carbachol. The inhibition of the carbachol-induced contraction was considered as relaxation as previously described (Grider et al., 1992; Jin et al., 1993; Rekik et al., 1996). The term relaxation will be used throughout the manuscript. To study the mechanism of relaxation, the cells were incubated before addition of the relaxant agents with: the NOS inhibitors L-NG-nitroarginine (L-NOARG), S-methyl-L-thiocitrulline, S-isopropyl isothiourea (S-isopropyl ITU), aminoguanidine and N-(3-(aminomethyl)-benzyl)acetamide (1400 W), with or without L-arginine or D-arginine (incubation time 5 min), the cyclic AMP antagonist (R)-p-cyclic adenosine-3′,5′-monophosphothioate ((R)-p-cAMPS; 5 min), tetrodotoxin (TTX, 5 min), the guanylyl cyclase inhibitor 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ; 20 min), the adenylyl cyclase inhibitor 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ 22,536; 20 min) and the glucocorticoid dexamethasone (30 min). In parallel control vials, the cells were incubated with the solvent of these agents.
Preparation of smooth muscle strips
Guinea-pigs of either sex (400–700 g) were fasted for 12 h and killed by a blow to the head and bleeding. The stomach was immediately excised and after careful removal of the mucosa by sharp dissection, four muscle strips (15×3 mm) were prepared from the fundus by cutting in the direction of the circular muscle layer. The strips were suspended between two platinum plate electrodes under a load of 1 g in 5 ml organ baths containing Krebs solution of the following composition (mM): NaCl, 118.5; KCl, 4.8; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 1.9; NaHCO3, 25.0 and D(+)-glucose, 10.1. The Krebs solution always contained 10−6 M atropine and 4×10−6 M guanethidine to inhibit cholinergic and noradrenergic responses; it was maintained at 37°C and gassed with a mixture of 95% O2 and 5% CO2. The guinea-pig gastric fundus is a spontaneous tone preparation, thus it is not necessary to raise tone in order to observe relaxant responses (Sahyoun et al., 1982). Changes in length were recorded isotonically via Hugo Sachs B40 Lever transducers type 373 on a Graphtec Linearcorder 8 WR 3500. Electrical field stimulation (EFS) was performed by means of a Hugo Sachs Stimulator I type 215/I.
Measurement of relaxation in muscle strips
Once a stable basal tone was obtained after an equilibration period of at least 1 h 30 min with rinsing every 15 min in the first 45 min of the equilibration, electrical field stimulation was performed or relaxant agents were administered. Frequency-response curves to EFS (40 V, 1 ms, 0.125–16 Hz) were obtained by stimulating the tissues with 10 s trains at 5 min intervals. VIP, isoprenaline, SNP, forskolin and pinacidil were administered in a cumulative way. To study the influence of L-NOARG, aminoguanidine, S-isopropyl ITU, 1400 W, ODQ, dexamethasone and TTX on the relaxant responses, these drugs were added 30 min (10 min for TTX) before a second frequency-response curve or concentration-response curve. Between the first frequency-response curve or concentration-response curve and the addition of the drug, an interval of 30 min with regular rinsing was inserted. In an additional set of experiments an interval of 4 h was respected between the first and the second concentration-response curve. Half an hour after this second curve, drugs were added and 30 min later a third concentration-response curve was constructed. In parallel control strips, only the solvent of the tested drug was incubated. None of the solvents influenced the tone of the tissues; the responses to electrical stimulation or to the relaxant agents were reproducible in the control strips unless otherwise stated. At the end of each experiment a maximal relaxation was induced by administration of 10−4 M papaverine.
Data analysis
The contraction of the isolated smooth muscle cells was expressed as the percentage decrease in cell length from untreated controls, using the following formula: ((L0−Lx) L0−1)×100 where L0 is the mean length of cells in control state and Lx the mean length of carbachol-treated cells. In relaxation experiments, the degree of inhibition of contraction was expressed as the percentage decrease in maximal contractile response, as observed in carbachol-treated cells in the absence of relaxant agent.
Relaxations in the smooth muscle strips were expressed as percentage of the papaverine-induced relaxation at the end of the experiment.
Results are given as means±s.e.mean and n refers to material from different animals. Responses in parallel vials with isolated smooth muscle cells were compared by analysis of variance (ANOVA) and the t-test corrected for multiple comparisons (Bonferroni procedure). Responses during the first and second curves in the strips were compared by a paired t-test. If a statistically significant difference was reached in the control strips, these differences were compared with the differences obtained in the strips treated with the tested drug by an unpaired t-test. P values of less than 0.05 were considered statistically significant.
Immunocytochemistry
Preparation of cytospins
A smooth muscle cell suspension was made as described above. Cells were washed three times with PBS. Cytospins were prepared and air-dry fixed. In preliminary experiments it was tested whether supplementary fixation was necessary. No difference was seen between air-dry-fixed and paraformaldehyde-fixed preparations. Therefore, results of air-dry-fixed preparations were calculated except when otherwise indicated.
Immunostaining
Cytospin preparations of smooth muscle cells were preincubated for 30 min at room temperature in 0.01 M PBS (pH 7.4) containing 10% normal serum, 0.5% Boseral 20T, 0.5% thimerosal, 0.01% NaN3 and 0.1% Triton X-100 and subsequently incubated with primary antiserum for 17 h at room temperature or 65–72 h at 4°C. After several washes in PBS, they were labelled with a corresponding biotinylated secondary antiserum for 2 h at room temperature, followed by an incubation with streptavidin conjugated to the fluorochrome Texas Red for another 2 h at room temperature. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), 0.5 μg ml−1 in 0.01 M PBS for 1 min. After a final rinsing in PBS, the preparations were mounted in Vectashield® (H-1000, Vector Laboratories, CA, U.S.A.) and examined under a Zeiss Axiophot fluorescence microscope equipped with the appropriate filtersets for DAPI and Texas Red. To check the method specificity, in some preparations the primary antiserum was omitted or replaced by non-immune serum.
Antibodies
All antibodies as well as the streptavidin-Texas Red complex (1 : 100 diluted, RPN 1233, Amersham, Brussels, Belgium) were diluted in preincubation medium without Triton X-100. Cells were incubated with mouse monoclonal or rabbit polyclonal antibodies raised against nNOS (monoclonal, 1 : 1000 diluted, N-2280, Sigma, St Louis, MO, U.S.A.; this antibody stained neuronal cell bodies in the myenteric plexus along with a fibre pattern of relative density in the circular and longitudinal muscle layer in gastric fundus cryosections), eNOS (monoclonal, 1 : 100 diluted, N-30020, Transduction Laboratories, Lexington, U.S.A.; this antibody stained the cell bodies of ECV304 cells, a human endothelial cell line [ECACC number: 92091712], and the inner wall of arteries in gastric fundus cryosections), iNOS (polyclonal, 1 : 100 diluted, sc-651, Santa Cruz Biotechnology, CA, U.S.A.; this antibody recognized on Western blot a 135 kDa iNOS specific band in cell lysate of RAW264.7 cells [ATCC number: TIB-71] induced with 1 μg ml−1 LPS and 10 ng ml−1 IFNγ), neuron specific enolase (NSE; monoclonal, 1 : 100 diluted, CY-083B, Innogenetics, Gent, Belgium; this antibody stained neuronal cell bodies in the submucous and myenteric plexuses along with a fibre pattern of relative density in the circular and longitudinal muscle layer in gastric fundus cryosections), c-Kit (polyclonal, 1 : 250 diluted, sc-168, Santa Cruz Biotechnology, CA, U.S.A.; this antibody stained the interstitial cells of Cajal in the circular and longitudinal muscle layer in gastric fundus cryosections), von Willebrandt factor (vWF; polyclonal, 1 : 400 diluted, A0082, Dako, Glostrup, Denmark; this antibody stained the cell bodies of ECV304 cells and the inner wall of arteries in gastric fundus cryosections), lysozyme (1 : 200 diluted, A0099, Dako, Glostrup, Denmark; this antibody stained the cell bodies of human macrophages and macrophages of guinea-pig bronchial alveolar liquid), α-smooth muscle actin (α-SM actin; monoclonal, 1 : 10 diluted, 128M, BioGenex, CA, U.S.A.; this antibody stained the circular and longitudinal muscle layer in gastric fundus cryosections). For the primary mouse monoclonal or rabbit polyclonal antibody, a biotinylated sheep anti-mouse (polyclonal, 1 : 100 diluted, RPN 1001, Amersham, Brussels, Belgium) respectively biotinylated goat anti-rabbit (polyclonal, 1 : 100 diluted, E0432, Dako, Glostrup, Denmark) antibody was used as second antibody.
Semiquantification
Cells were only counted when DAPI staining revealed a sharply delineated intact oval or corkscrew shaped nucleus. The staining was assessed semiquantitatively as follows: 0/+: no to weak staining, ++/+++: moderate to strong staining. For each primary antibody, at least three cytospins obtained from different animals were rated independently by two investigators (100 cells cytospin−1). The interobserver correlation was ⩾93%.
Chemicals
Collagenase was purchased from Worthington Biochemical Corporation (Freehold, NJ, U.S.A.). Penicillin G and streptomycin were from ICN Pharmaceuticals Inc. (Costa Mesa, U.S.A.). Vasoactive intestinal polypeptide (VIP) was obtained from Genosys (Cambridgeshire, U.K.) and isoprenaline hydrochloride from Sanofi-Winthrop (France). N-(3-(aminomethyl)-benzyl)acetamide (1400 W), S-isopropyl isothiourea hydrobromide (S-isopropyl ITU), S-methyl-L-thiocitrulline hydrochloride and 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ 22,536) were obtained from Alexis Corporation (Nottingham, U.K.) and 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) from Tocris Cookson Ltd. (Bristol, U.K.). Aminoguanidine hemisulphate, atrial natriuretic peptide (ANP), D-arginine hydrochloride, L-arginine hydrochloride, atropine sulphate, bovine serum albumin, dexamethasone, essential amino acid mixture, forskolin, glutamin, glutaraldehyde, guanethidine sulphate, L-NG-nitroarginine (L-NOARG), (R)-p-cyclic adenosine-3′,5′-monophosphothioate ((R)-p-cAMPS), sodium nitroprusside (SNP), sodium fumarate, sodium glutamate, sodium pyruvate, trypan blue and vitamin mixture were from Sigma Chemicals (St. Louis, MO, U.S.A.). N - 2 - hydroxyethylpiperazine - N′ - 2 - ethanesulphonic acid (HEPES) and soybean trypsin inhibitor were from Boehringer Mannheim Biochemicals (Indianapolis, IN, U.S.A.). Pinacidil monohydrate was from Leo Pharmaceuticals (Ballerup, Denmark) and carbamoylcholine chloride (carbachol) from Fluka (Switzerland). Tetrodotoxin citrate (TTX) was purchased from Alomone Labs (Jerusalem, Israel) and 3-morpholinosydnonimine (SIN-1) was a gift from Therabel Research (Brussels, Belgium).
All drugs were dissolved in distilled water, except for SNP which was dissolved in 0.9% NaCl solution and for forskolin, ODQ and pinacidil which were dissolved in pure ethanol up to 10−2 M; further dilutions were made in physiological salt solution. The solvents, diluted in physiological salt solution till the final concentration given to the strips or the cells had no effect per se on the tone of the strips or on control isolated smooth muscle cells. Stock solutions of S-isopropyl ITU, S-methyl-L-thiocitrulline, 1400 W, all up to 10−2 M, SQ 22,536 and TTX up to 10−3 M and ANP and VIP up to 10−4 M were prepared in distilled water and stored at −20°C. All other solutions were prepared on the day of the experiment.
Results
Isolated smooth muscle cells
Relaxant effect of VIP
Untreated cells, obtained after dispersion of the circular muscle layer of the guinea-pig gastric fundus, had a mean cell length of 135.2±1.9 μm (n=6) and were taken as controls. Incubation with increasing concentrations of carbachol for 30 s, contracted the cells in a concentration-dependent manner, with a maximal effect at 10−8 M (results not shown). This concentration of carbachol produced a 19.0±0.8% shortening of the cells to 109.5±2.2 μm (Table 1). When cells were preincubated for 60 s with increasing concentrations of VIP (10−14–10−6 M), the contraction was inhibited in a concentration-dependent manner (Figure 1). Full relaxation was obtained at 10−6 M VIP and this concentration was selected for further investigation. When muscle cells were incubated with 10−10 M VIP (inducing approximately 60% relaxation when incubated for 60 s, Figure 1) during 60, 120 and 180 s, the relaxant effect of VIP was not changed when incubation periods longer than 60 s were tested (n=6), so that this incubation period was used in further experiments. In the absence of carbachol, VIP did not affect the cell length of the isolated smooth muscle cells (n=6).
Table 1.
Effect of L-NOARG on VIP-induced relaxation in isolated smooth muscle cells
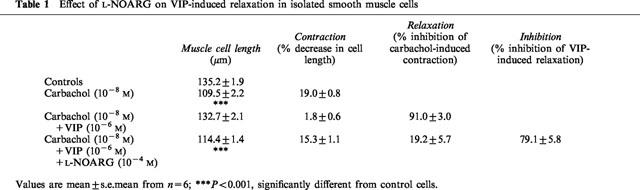
Figure 1.
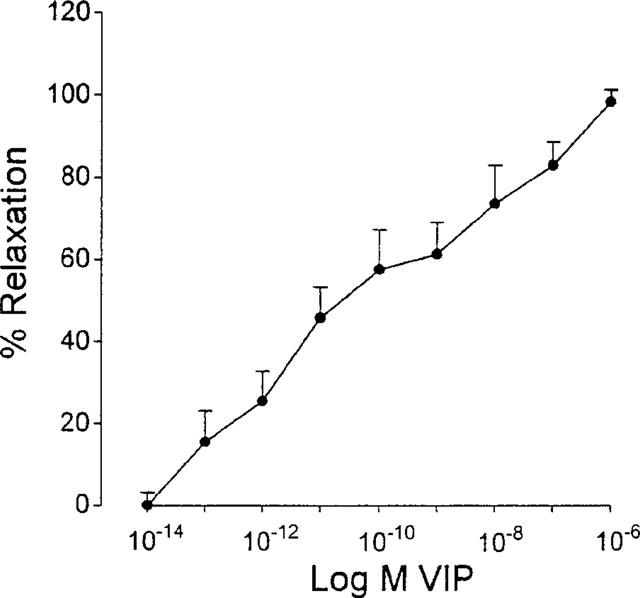
Concentration-response curve to VIP (10−14–10−6 M) in isolated smooth muscle cells from the circular muscle layer of the guinea-pig gastric fundus. Values are mean±s.e.mean from n=6.
Influence of NOS inhibitors on the relaxant effect of VIP
When cells were incubated with 10−4 M of the NOS inhibitor L-NOARG, the relaxant effect of 10−6 M VIP was inhibited by 79.1±5.8% (Table 2, Figure 2). The inhibition of the VIP-induced relaxation was fully reversed by preincubation for 5 min with 10−4 M L-arginine, but not by 10−4 M D-arginine (Figure 2). Two other non-selective NOS inhibitors S-methyl-L-thiocitrulline (10−4 M) and S-isopropyl ITU (10−4 M) inhibited the relaxant effect of VIP to a similar extent as L-NOARG (Table 2). The influence of two selective iNOS inhibitors aminoguanidine and 1400 W on the relaxation induced by VIP was as follows. At 10−4 M, aminoguanidine nearly abolished the relaxant effect of VIP and its inhibitory effect was fully reversed by 10−4 M L-arginine, but not by 10−4 M D-arginine (Figure 2). Preincubation of the cells with 1400 W inhibited the relaxant effect of VIP in a concentration-dependent manner from 10−9 M on with maximal inhibition obtained at 10−6 M 1400 W (results not shown). In contrast to L-NOARG and aminoguanidine, the inhibitory effect of 10−6 M 1400 W on the VIP-induced relaxation was only partially reversed by 10−4 M L-arginine (Figure 2). Even when L-arginine was incubated 20 min before the addition of 1400 W, the reversion was not more pronounced: the inhibition of the VIP-induced relaxation was 83.5±6.3, 67.7±5.0 and 56.3±13.1% (n=6) with 10−6 M 1400 W per se, or preceded by L-arginine for 5 or 20 min respectively. None of these NOS inhibitors nor L-arginine or D-arginine altered the mean cell length of the control resting circular muscle cells, nor the degree of the carbachol-induced contraction.
Table 2.
Inhibition (%) of the relaxation induced by different relaxant agents under the influence of NOS inhibitors, ODQ, (R)-p-cAMPS, dexamethasone and SQ 22,536 in isolated smooth muscle cells
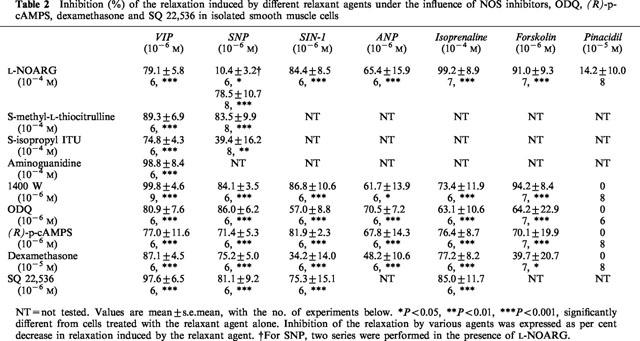
Figure 2.
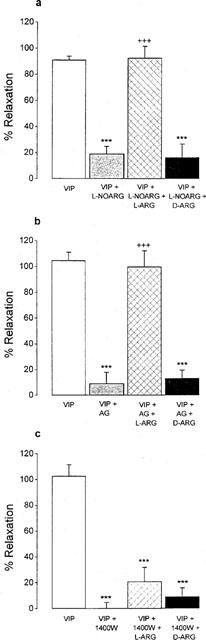
Effect of L-NOARG (10−4 M, a), aminoguanidine (AG, 10−4 M, b) and 1400 W (10−6 M, c) with or without L-arginine (L-ARG, 10−4 M) and D-arginine (D-ARG, 10−4 M) on VIP-induced relaxation in isolated smooth muscle cells of the guinea-pig gastric fundus. Values are mean±s.e.mean from n=6. ***P<0.001, significantly different from cells treated with VIP. +++P<0.001, significantly different from cells treated with VIP and NOS inhibitor.
In two experiments, performed under sterile conditions, L-NOARG (10−4 M) and 1400 W (10−6 M) inhibited the VIP-induced relaxation to the same extent (57.5 and 89.7% for L-NOARG and 90.1 and 69.0% for 1400 W) as when the experiments were performed under standard conditions.
Influence of ODQ, SQ 22,536, (R)-p-cAMPS and dexamethasone on the relaxant effect of VIP
The guanylyl cyclase inhibitor ODQ (10−6 M), the adenylyl cyclase inhibitor SQ 22,536 (10−6 M), the cyclic AMP antagonist (R)-p-cAMPS (10−6 M) and the glucocorticoid dexamethasone (10−5 M) did not alter the mean length of resting smooth muscle cells nor the contraction induced by carbachol. When cells were incubated for 20 min with 10−6 M ODQ, the relaxant effect of VIP was decreased by 80.9±7.6% (Table 2). Preincubation (5 min) of the cells with 10−6 M (R)-p-cAMPS, decreased the effect of VIP by 77.0±11.6%. Also dexamethasone (10−5 M, 30 min), reduced the relaxant effect of VIP by 87.1±4.5% (Table 2). SQ 22,536, incubated for 20 min, inhibited the VIP-induced relaxation in a concentration-dependent manner with maximal inhibition obtained at 10−7 M (Figure 3). This concentration of SQ 22,536 antagonized the relaxant effect of VIP by 94.9±15.6%.
Figure 3.
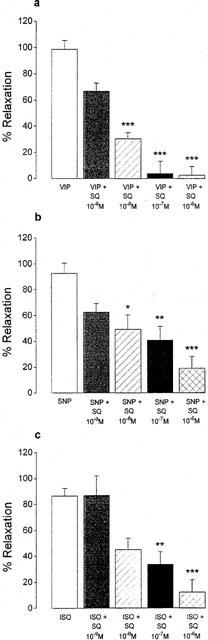
Effect of increasing concentrations of SQ 22,536 (10−9–10−6 M) on the relaxant effect of VIP (10−6 M), SNP (10−6 M) and isoprenaline (ISO, 10−4 M) in isolated smooth muscle cells of the guinea-pig gastric fundus. Values are mean±s.e.mean from n=6. *P<0.05, **P<0.01, ***P<0.001, significantly different from cells treated with VIP, SNP or ISO.
Relaxant effect of ANP, SIN-1, SNP, isoprenaline, forskolin and pinacidil
The particulate guanylyl cyclase stimulant ANP, the NO donors SIN-1 and SNP, the β-receptor agonist isoprenaline, the adenylyl cyclase stimulant forskolin and the K+ channel opener pinacidil, all elicited relaxation in dispersed smooth muscle cells with full inhibition of carbachol-induced contraction obtained at 10−6 M for ANP, forskolin, SIN-1 and SNP, at 10−5 M for pinacidil and at 10−4 M for isoprenaline; these maximally effective concentrations were selected for further investigation. None of these relaxant agents affected the mean length of control resting smooth muscle cells.
In a first set of experiments we tested the effect of the different NOS inhibitors on the relaxation elicited by 10−6 M SNP (Table 2). L-NOARG (10−4 M) reduced only moderately, but significantly, the SNP-induced relaxation by 10.4±3.2%. However, 10−6 M 1400 W was able to antagonize the relaxant effect of SNP by 84.1±3.5% and 10−4 M S-methyl-L-thiocitrulline was just as effective as 1400 W to inhibit the action of SNP. S-isopropyl ITU (10−4 M) reduced the relaxant effect of SNP by 39.4±16.2%. Because of these contradictory results, the influence of L-NOARG (10−4 M) was again tested on the relaxant effect of SNP. In contrast to the first set of experiments, L-NOARG now markedly antagonized the SNP-induced relaxation by 78.5±10.7%; we have no explanation for these different results. L-NOARG and 1400 W also markedly reduced the relaxant effect of 10−6 M SIN-1 (Table 2). When tested versus the relaxant effect of ANP, isoprenaline, forskolin and pinacidil, both inhibitors clearly reduced the relaxant effect of ANP, isoprenaline and forskolin, but had no significant influence on the effect of pinacidil.
Pretreatment of the cells with ODQ (10−6 M) and (R)-p-cAMPS (10−6 M) equally impaired the relaxant effect induced by ANP, SIN-1, SNP, isoprenaline and forskolin. The inhibitory effect of dexamethasone (10−5 M) was clearly less pronounced versus SIN-1 and forskolin. In contrast, none of these substances influenced the pinacidil-induced relaxation. SQ 22,536 inhibited the relaxant effect of SNP and isoprenaline in a concentration-dependent manner, but neither was fully inhibited by 10−6 M SQ 22,536, that abolished the relaxant effect of VIP (Figure 3).
Isolated smooth muscle strips
Influence of NOS inhibitors, ODQ, dexamethasone and TTX on the relaxant effect of VIP
VIP (10−9–10−7 M) induced concentration-dependent relaxations of circular smooth muscle strips of the guinea-pig gastric fundus (Figure 4a). These VIP-induced relaxations were not affected by the axonal blocker TTX (3×10−6 M, n=8) implicating that VIP has a direct effect on smooth muscle. In the presence of 10−4 M L-NOARG, the relaxations induced by 10−9 M and 3×10−9 M VIP were significantly decreased (n=7, Figure 5a), but this decrease was not significantly different from that observed in the parallel control tissues. Similarly, when the tonus of the strips was increased with 10−5 M carbachol, L-NOARG did not influence the relaxations induced by VIP in comparison to parallel control strips. Aminoguanidine (10−4 M, n=7, not shown), S-isopropyl ITU (10−4 M, n=8, not shown), 1400 W (10−6 M, n=7, Figure 5b) and dexamethasone (10−5 M, n=6, not shown) had no influence on the relaxant effect of VIP in gastric smooth muscle strips. When ODQ (10−6 M, n=6) was administered to the strips, the tone of the tissues decreased sharply by 68.3±18.4%, but recovered quickly for about 50% of the decline. Just before adding VIP again, the tone of the tissues was still decreased by 33.6±4.8% (n=5). ODQ did not influence significantly the relaxant effect of VIP as measured from the tone level just before adding VIP (Figure 5c). Final experiments were performed by inserting an interval of 4 h before testing the effect of L-NOARG (10−4 M, n=3), 1400 W (10−6 M, n=4) and dexamethasone (10−5 M, n=4) on the relaxations induced by VIP. The relaxations by VIP after an interval of 4 h were not different from those obtained before, and were still not influenced by L-NOARG, 1400 W and dexamethasone.
Figure 4.
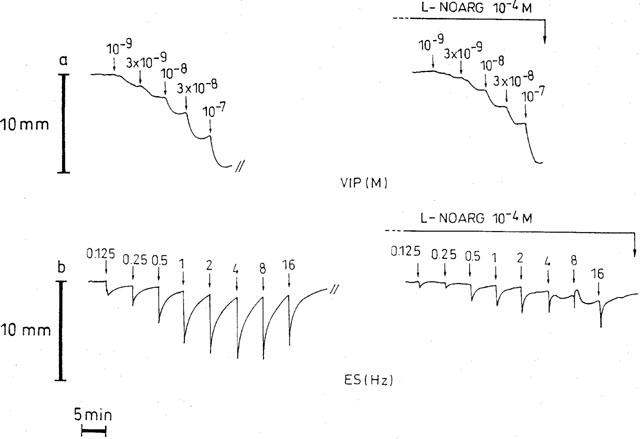
Representative traces from circular smooth muscle strips of the guinea-pig gastric fundus showing the responses to VIP (a) and to EFS (40 V, 0.1 ms, 0.125–16 Hz) with 10 s trains (b) before and after addition of L-NOARG (10−4 M).
Figure 5.
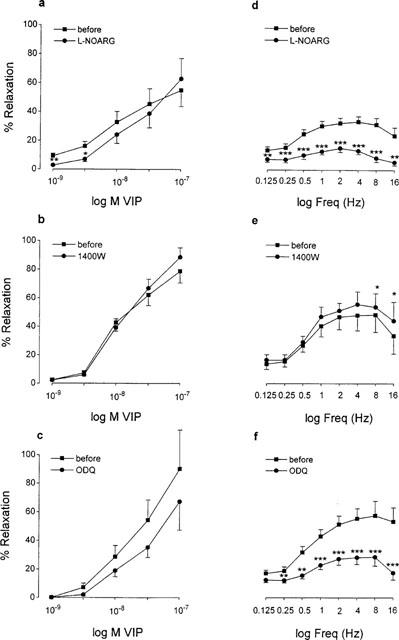
Concentration-response curves to VIP (a–c) and frequency-response curves to EFS (40 V, 0.1 ms, 0.125–16 Hz) with 10 s trains (d–f) in circular smooth muscle strips of the guinea-pig gastric fundus in the absence and presence of L-NOARG (10−4 M, a, d), 1400 W (10−6 M, b, e) and ODQ (10−6 M, c, f). Values are mean±s.e.mean from n=6, *P<0.05, **P<0.01, ***P<0.001, significantly different from the response before the addition of L-NOARG, 1400 W or ODQ.
Influence of NOS inhibitors, ODQ, dexamethasone and TTX on the relaxant effect of electrical stimulation and other relaxant agents than VIP
Train stimulation with 10 s trains at 5 min intervals, in the presence of atropine and guanethidine, induced short-lasting frequency-dependent (0.25–16 Hz) relaxations (Figure 4b). Maximal relaxation was obtained at 4 to 8 Hz. TTX (3×10−6 M) reduced but did not abolish the relaxation induced by train stimulation at all frequencies tested. The relaxations obtained at 0.125, 0.25, 0.5, 1, 2, 4, 8 and 16 Hz were 12.0±2.7, 15.3±2.8, 27.2±4.5, 35.5±4.6, 40.8±5.0, 41.8±6.2, 42.5±6.3, 38.0±7.6% before and 4.9±1.9 (P<0.05), 6.5±2.3 (P<0.01), 9.4±3.2 (P<0.001), 11.7±3.4 (P<0.001), 13.3±3.8 (P<0.001), 15.0±4.0 (P<0.001), 14.9±3.6 (P<0.001), 13.9±3.5% (P<0.001) in the presence of TTX (n=8). We have no explanation for this incomplete blockade by TTX. A representative experiment showing the influence of L-NOARG on NANC relaxations induced by train stimulation is given in Figure 4b, the mean results are given in Figure 5d; L-NOARG (10−4 M) clearly reduced the electrically-induced short-lasting frequency-dependent relaxations. S-Isopropyl ITU (10−4 M) was just as effective as L-NOARG to reduce this type of relaxations (n=10, not shown), but the iNOS inhibitors aminoguanidine (10−4 M, n=6, not shown) and 1400 W (10−6 M, n=6, Figure 5e) and the glucocorticoid dexamethasone (10−5 M, n=6, not shown) had no influence. Also ODQ (10−6 M, n=6) was able to inhibit the electrically induced relaxations (Figure 5f). In the same manner as described above, administration of ODQ decreased the basal tone of the tissue by 24.2±6.6%.
Isoprenaline (10−9–10−5 M), forskolin (10−9–10−7 M), SNP (10−9–10−5 M) and pinacidil (10−7–10−4 M), all caused concentration-dependent relaxations (Figure 6) that were not influenced by 10−4 M L-NOARG (n=6 for each agonist, results not shown).
Figure 6.
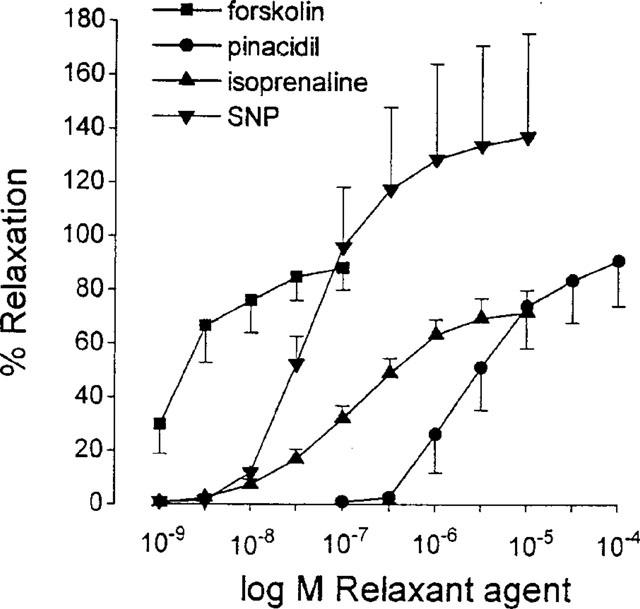
Concentration-response curve to forskolin (10−9–10−7 M), pinacidil (10−7–10−4 M), isoprenaline (10−9–10−5 M) and SNP (10−9–10−5 M) in isolated smooth muscle strips of guinea-pig gastric fundus. Values are mean±s.e.mean from n=6.
Immunocytochemistry
The results are given in Table 3. Only the antibody against α-smooth muscle actin yielded consistently strong staining of a major proportion of the cells. Cells with moderate to strong staining for vWF and NSE amounted to 15 and 20% of the total number of counted cells respectively. None of the cells was positive for nNOS and eNOS while cells with strong staining for c-Kit were occasionally found. The staining for iNOS (n=7) was negative in three preparations of different animals but in four other preparations moderate to strong staining was observed; the results of the latter four preparations are given in Table 3.
Table 3.
Scoring of the immunocytochemistry with different antibodies in cytospins
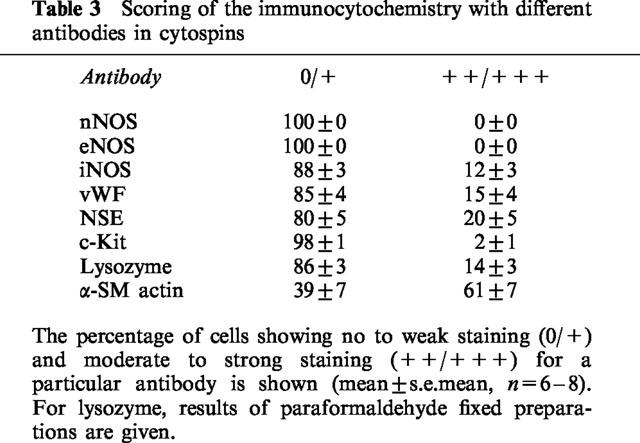
Cells staining for lysozyme amounted to 14% of total, 12±3% being spindle-shaped and 2±1% round-shaped. Round-shaped cells were not detected with any other antibody.
Discussion
Because of contradictory reports on the interaction between VIP and NO in the guinea-pig stomach, the aim of this study was to investigate the mechanism of interaction between VIP and NO in isolated smooth muscle cells and smooth muscle strips from the guinea-pig gastric fundus.
Involvement of iNOS in the relaxant effect of VIP in isolated smooth muscle cells
L-NOARG reduced the relaxant effect of VIP in a L-arginine reversible way and this inhibitory effect was mimicked by S-isopropyl ITU and S-methyl-L-thiocitrulline. These results confirm previously described data by Grider et al. (1992), suggesting that the VIP-induced relaxation in isolated smooth muscle cells of the guinea-pig gastric fundus is related to NOS activation and production of NO. This is corroborated by the results with the selective guanylyl cyclase inhibitor ODQ (Garthwaite et al., 1995) that also inhibited the relaxant effect of VIP, as NO usually acts through activation of cytosolic guanylyl cyclase to relax smooth muscle cells (Ignarro, 1991). The group of Grider and Makhlouf recently reported that the NOS isoform expressed in rabbit gastric and human intestinal smooth muscle cells is eNOS (Teng et al., 1998). The NOS inhibitors L-NOARG, S-isopropyl ITU and S-methyl-L-thiocitrulline do not show any preference for a particular NOS isoform (Klatt et al., 1994; Southan et al., 1995; Narayanan & Griffith, 1994; Joly et al., 1995) and their effect on the VIP-induced relaxation might thus be related to eNOS inhibition. However, in our experiments, the effect of VIP was also inhibited by two selective iNOS inhibitors aminoguanidine and 1400 W (Griffiths et al., 1993; Southan & Szabó, 1996; Garvey et al., 1997). In the concentrations used, both agents did not interfere with the electrically induced nitrergic relaxations in the smooth muscle strips (see below), illustrating that they do not inhibit nNOS in the guinea-pig gastric fundus. Even at 10−4 M, aminoguanidine did not influence eNOS in isolated rat aorta (Misko et al., 1993) and the selectivity window of 1400 W is larger versus eNOS than versus nNOS (Garvey et al., 1997). Still, in the isolated smooth muscle cells of the guinea-pig stomach, aminoguanidine and 1400 W were just as effective as L-NOARG to inhibit the action of VIP, suggesting that an inducible NO synthase might be involved in the effect of VIP in the isolated smooth muscle cells of the guinea-pig gastric fundus. Many L-arginine-based inhibitors are competitive inhibitors of NOS and their blockade can be overcome by L-arginine but not by D-arginine, thereby indicating the specificity of the inhibitory effect. However, some NOS inhibitors, such as L-N-iminoethylornithine (L-NIO) and L-N-iminoethyllysine (L-NIL), show inhibition that cannot be reversed by L-arginine (McCall et al., 1991; Moore et al., 1994). The inhibitory effect of aminoguanidine versus VIP was reversed by L-arginine, while that of 1400 W was not, even when L-arginine was added 20 min before the addition of 1400 W. This might be related to the very tight binding of 1400 W to iNOS (Garvey et al., 1997).
iNOS is usually expressed under the influence of bacterial endotoxin and cytokines, which were not added in our experiments. Contamination of the material used during the preparation of the isolated smooth muscle cells seems unlikely as a reason for the iNOS expression, since the involvement of iNOS in the relaxant effect of VIP remained when the cells were prepared under sterile conditions. A possible explanation for the involvement of iNOS in the relaxant effect of VIP in the isolated gastric smooth muscle cells might be the induction of iNOS in response to the stress of the dissociating procedure; indeed the induction of iNOS in response to ischaemia-reperfusion stress has been shown in vivo (Imagawa et al., 1999). Cells are used approximately 3 h after starting the dissociation, which is sufficient for the expression of iNOS. In rat vascular preparations, the threshold time point for iNOS mRNA induction was between 20 and 40 min after lipopolysaccharide administration, whereafter the quantity of mRNA increased progressively (Liu et al., 1997). Chen et al. (1996) reported detection of iNOS mRNA in several rat gastrointestinal tissues 1 h after lipopolysaccharide administration, although not in the stomach. iNOS activity was already measurable in vascular endothelial cells activated with interferon-γ and LPS after a lag period of 2 h (Radomski et al., 1990). Also the manifest reduction in the relaxant effect of VIP with the glucocorticoid dexamethasone supports the involvement of iNOS. Dexamethasone is known to suppress iNOS gene expression in different cell types (Di Rosa et al., 1990; Radomski et al., 1990; Imai et al., 1994). To inhibit the iNOS expression, most authors administer dexamethasone before treatment of the tissues with endotoxines, while we were able to inhibit the VIP-induced relaxation by incubating the smooth muscle cells with dexamethasone after dissociation, only 30 min before the administration of VIP. This has also been reported by Rekik et al. (1996) in isolated smooth muscle cells of the guinea-pig ileum. In time course experiments, Hall et al. (1994) have shown in macrophages that dexamethasone inhibited iNOS-dependent NO production when adding it after activation with lipopolysaccharide. This can probably be explained by the multiple actions of dexamethasone. Walker et al. (1996) demonstrated that dexamethasone not only decreases iNOS gene transcription and iNOS mRNA stability, but also reduces the translation of iNOS mRNA and increases the degradation of the iNOS protein. Posttranslational modifications of iNOS due to the glucocorticoid might inactivate iNOS and thus reduce the iNOS-dependent relaxation by VIP.
Immunocytochemistry did not reveal systematic iNOS expression in the smooth muscle cells, although some iNOS-positive cells were observed in about half of the preparations tested. This might be related to a low level of expression of iNOS in the gastric smooth muscle cells, around the limit of immunological detection. Although eNOS transcripts were localized by in situ RT–PCR in dispersed rabbit gastric smooth muscle cells, Western and immunoblot analysis did not detect eNOS protein (Teng et al., 1998). In our study, eNOS immunoreactivity was also not observed. As endothelial cells express sufficient eNOS for immunological detection, this result suggests that the cells staining for von Willebrand factor are not endothelial cells. vWF staining might correspond to some non-specific staining of smooth muscle cells, which might also account for NSE staining in 20% of the cells, as these cells were all spindle-shaped. Still, as far as we know, such non-specific staining with this type of antibodies was never observed in whole mount preparations. VIP-receptors are present on macrophages (Sakakibara et al., 1994), that are a possible source of iNOS. However, the antibody for lysozyme only stained very few round-shaped cells.
VIP induces relaxation through stimulation of VIP-receptors and activation of the cyclic AMP-protein kinase A (PKA)-dependent pathway in both smooth muscle strips (Bolton, 1979; Scheid et al., 1979; Said, 1992) and dispersed smooth muscle cells (Bitar & Makhlouf, 1982; Chijiiwa et al., 1993). The relaxant effect of VIP in the isolated smooth muscle cells of the guinea-pig was reduced by the adenylyl cyclase inhibitor SQ 22,536 (Fabbri et al., 1991) and the cyclic AMP antagonist (R)-p-cAMPS to the same extent as the NOS inhibitors and ODQ. These results are similar to those reported by Rekik et al. (1996) in isolated smooth muscle cells of the guinea-pig ileum and suggest that VIP activates the iNOS-NO-cyclic GMP pathway of relaxation via cyclic AMP and protein kinase A. This link might be present for all agents activating adenylyl cyclase as also the relaxant effect of isoprenaline, activating adenylate cyclase via β-receptors (Honeyman et al., 1977) and the direct adenylyl cyclase activator forskolin (Laurenza et al., 1989) were inhibited by the NOS inhibitors, ODQ, SQ 22,536, (R)-p-cAMPS and dexamethasone, in contrast to that of the potassium channel opener pinacidil, which is not mediated via cyclic nucleotides (Rhim & Hong, 1994). We have no explanation why the influence of dexamethasone versus SIN-1 and forskolin was less pronounced. Cyclic AMP and cyclic AMP generating agents have been shown to induce iNOS expression in vascular smooth muscle cells (Koide et al., 1993; Imai et al., 1994) but this is not comparable to the actual finding in the guinea-pig gastric smooth muscle cells, as the cyclic AMP generating agents seem able to acutely switch on iNOS activity. Tyrosine phosphorylation by tyrosine kinases has been reported to increase the activity of iNOS (Pan et al., 1996). In contrast to nNOS and eNOS, the activity of iNOS is largely or completely calcium-independent (Förstermann et al., 1995) and upon induction of iNOS, basal NO production can be expected. This is not the case in the isolated gastric smooth muscle cells as the NOS inhibitors did not influence the length of untreated cells, whereas a contraction should be obtained if NO were contributing to the relaxed state of these cells.
As expected, the relaxant effect of the NO donors SNP and SIN-1, which induce smooth muscle cell relaxation by activation of soluble guanylyl cyclase (Feelisch & Noack, 1987; Waldman & Murad, 1987) was antagonized by ODQ. However, their relaxant effect was also inhibited by the NOS inhibitors, dexamethasone and the agents interfering with the adenylyl cyclase-protein kinase A pathway. Recently, Murthy et al. (1998) reported that the relaxant effect of VIP in isolated rabbit and human intestinal smooth muscle cells is related to dual signalling cascades: 1/activation of VIP-receptors and subsequently adenylyl cyclase; 2/activation of natriuretic peptide clearance receptors and a membrane-bound eNOS. As explained above, our results suggest the involvement of iNOS. Still, the relaxant effect of ANP, which is usually accepted to induce smooth muscle cell relaxation by activating particulate guanylyl cyclase (Anand-Srivastava & Trachte, 1993), was inhibited by L-NOARG, 1400 W and the other inhibitors tested, suggesting that the adenylyl cyclase-protein kinase A-iNOS-NO-guanylyl cyclase pathway is also involved in the relaxation by SIN-1, SNP and ANP. This might be related to an increase in cyclic AMP by SIN-1, SNP and ANP, by a decrease in the breakdown of cyclic AMP through cyclic GMP-inhibited phosphodiesterase type III. In vascular smooth muscle cells and in platelets, an increase in cyclic AMP-mediated relaxation respectively suppression of aggregation through the inhibition of phosphodiesterase type III by cyclic GMP has been reported (Maurice & Haslam, 1990; Komas et al., 1991; Eckly & Lugnier, 1994).
An integrating scheme is represented in Figure 7. VIP and isoprenaline, via their receptor, and forskolin, directly, activate adenylyl cyclase, raise the concentration of cyclic AMP and stimulate protein kinase A. This will activate the iNOS-NO-guanylyl cyclase pathway. Through an increase in the concentration of cyclic GMP and the inhibition of phosphodiesterase III, SIN-1, SNP and ANP can also activate this cyclic AMP-iNOS linked pathway. The scheme allows to explain why ODQ, that is specific for soluble guanylyl cyclase (Cellek et al., 1996), is able to inhibit the effect of ANP. The inhibitory effect of SQ 22,536 on the relaxation by SIN-1 and SNP might be explained by some non-selective inhibition at the level of guanylyl cyclase. SQ 22,536 was indeed clearly less effective versus SNP than versus VIP (Figure 3). It is difficult to explain why it was also less effective versus isoprenaline. As (R)-p-cAMPS has also been shown to inhibit protein kinase G (Boese et al., 1996), an alternative possibility is that cyclic AMP acts through protein kinase G, that in turn could activate iNOS assuring a positive feedback loop. Several reports suggest that cyclic AMP may act through protein kinase G to induce smooth muscle relaxation (Jiang et al., 1992; Boese et al., 1996; Jackson, 1996).
Figure 7.
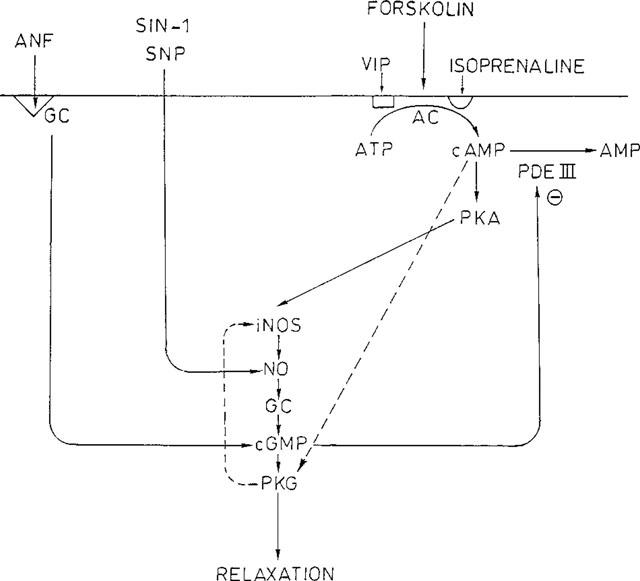
Signalling pathway proposed for the relaxant agents VIP, isoprenaline, forskolin, SIN-1, SNP and ANF in isolated smooth muscle cells of the guinea-pig gastric fundus. The interrupted line shows an alternative possibility whereby cyclic AMP is able to act on protein kinase G, that activates iNOS through a positive feedback loop.
Relaxation by VIP in isolated smooth muscle strips
To investigate whether the link between VIP and NO observed in isolated cells is also relevant in intact tissues, we studied the effect of VIP in classic smooth muscle strips of the guinea-pig gastric fundus. The involvement of NO in NANC neurotransmission in these tissues has been shown before (Lefebvre et al., 1992) and this was confirmed in this study by the inhibition of the electrically induced NANC relaxations by L-NOARG, S-isopropyl ITU and ODQ. This nitrergic mechanism, with NO released from neurones, is clearly different from that observed in isolated smooth muscle cells as aminoguanidine, 1400 W and dexamethasone did not influence the NANC relaxations by short train stimulation.
Also the relaxant effect of VIP was not influenced by the NOS inhibitors. It is unlikely that the difference between strips and cells is related to the different mechanism of contractile activation in cells (cholinergic) and strips (non-cholinergic) when testing the inhibitory action of VIP. In the mouse anococcygeus, the relaxant potency of nitrergic relaxant stimuli was dependent on the agent used to induce tone but the relaxant mechanism was the same (Gibson et al., 1994). Furthermore, even when the strips were further contracted with carbachol to obtain the same conditions as when VIP was tested in isolated smooth muscle cells, the relaxant effect of VIP was not influenced by NOS inhibitors. This corresponds with the non-effect of NOS inhibitors on the relaxant effect of VIP in many gastrointestinal smooth muscle preparations such as the opossum lower oesophageal sphincter (Tøttrup et al., 1991), the canine lower oesophageal sphincter (De Man et al., 1991), the rat gastric fundus (Boeckxstaens et al., 1992; D'Amato et al., 1992), the pig gastric fundus (Lefebvre et al., 1995) and the canine proximal colon (Keef et al., 1994). Our results also confirm previous results in the same tissues (Lefebvre et al., 1992) and in the whole stomach preparation of the guinea-pig (Desai et al., 1994) but contrast with the report of Grider et al. (1992) that the relaxant effect of VIP in the guinea-pig gastric fundus strips is partly reduced by L-NOARG. In our study, the relaxation by VIP was also not influenced by ODQ and dexamethasone, suggesting that there is no sequential link between VIP and NO in our conditions. Similarly, the relaxations by forskolin, isoprenaline and SNP, all inhibited by L-NOARG in the isolated smooth muscle cells, were not influenced by the NOS inhibitor in the strips. Induction of iNOS by a prolonged stay of the tissues in the organ bath was also excluded as L-NOARG, 1400 W and dexamethasone still did not influence the relaxation by VIP when the tissues were left in the bath for more than 4 h. These results thus clearly illustrate that the experimental model is important for the outcome with regard to the interaction between NO and VIP. In the smooth muscle strips, that do not undergo the collagenase treatment of the cells, NO does not seem to be involved in the relaxant effect of VIP. The mechanism of relaxation by VIP in the smooth muscle strips is probably related to the influence of increased cyclic AMP and activated protein kinase A on intracellular calcium levels and on the sensitivity of the contractile apparatus to calcium (Ozaki et al., 1992). This effect of protein kinase A seems not to occur in isolated smooth muscle cells as the effect of VIP was nearly completely inhibited by the NOS inhibitors.
Conclusion
In isolated smooth muscle strips of the guinea-pig gastric fundus, the relaxant effect of VIP is not influenced by NOS inhibitors. In contrast, in isolated smooth muscle cells, the relaxation by VIP is inhibited by NOS inhibitors, including the iNOS selective inhibitor 1400 W. This suggests that the procedure to prepare the smooth muscle cells is able to induce the expression of iNOS. This observation might explain the contradictory results with regard to the influence of NOS inhibitors on the relaxant effect of VIP in gastrointestinal smooth muscle.
Acknowledgments
The study was financially supported by grant No. 3G0031.96 from the Fund for Scientific Research Flanders, grant O11A1696 from the Special Investigation Fund of the Gent University and by Interuniversity Pole of Attraction Programme P4/16 (Services to the Prime Minister - Federal Services for Scientific, Technical and Cultural Affairs). The NSE antibody was kindly provided by Innogenetics. The authors thank Mrs E. Coene for her help with the cytospins, Mr F. Terloo for his assistance with immunocytochemistry and Drs L. Bueno, M. Delvaux and M. Rekik (Toulouse) for illustrating to J. Dick the method of the isolated smooth muscle cells during a stay in their laboratory.
Abbreviations
- iNOS
inducible nitric oxide synthase
- L-NOARG
L-NG-nitroarginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one
- (R)-p-cAMPS
(R)-p-cyclic adenosine-3′,5′-monophosphothioate
- VIP
vasoactive intestinal polypeptide
- 1400 W
N-(3-(acetaminomethyl)-benzyl)acetamide
References
- ANAND-SRIVASTAVA M.B., TRACHTE G.J. Atrial natriuretic factor receptor and signal transduction mechanism. Pharmacol. Rev. 1993;45:455–497. [PubMed] [Google Scholar]
- BARBIER A.J., LEFEBVRE R.A. Involvement of the L-arginine: nitric oxide pathway in non-adrenergic non-cholinergic relaxation of the cat gastric fundus. J. Pharmacol. Exp. Ther. 1993;266:172–178. [PubMed] [Google Scholar]
- BEREZIN I., SNYDER S.H., BREDT D.S., DANIEL E.E. Ultrastructural localization of nitric oxide synthase in canine small intestine and colon. Am. J. Physiol. 1994;266:C981–C989. doi: 10.1152/ajpcell.1994.266.4.C981. [DOI] [PubMed] [Google Scholar]
- BITAR K.N., MAKHLOUF G.M. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. Am. J. Physiol. 1982;242:G400–G407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., DE MAN J.G., BULT H., HERMAN A.G., VAN MAERCKE Y.M. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by non-adrenergic non-cholinergic nerves in the rat gastric fundus. Arch. Int. Pharmacodyn. 1992;318:107–115. [PubMed] [Google Scholar]
- BOESE M., BUSSE R., MÜLSCH A., SCHINI-KERTH V. Effect of cGMP-dependent vasodilators on the expression of inducible nitric oxide synthase in vascular smooth muscle cells: role of cAMP. Br. J. Pharmacol. 1996;119:707–715. doi: 10.1111/j.1476-5381.1996.tb15730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Phys. Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- BOTELLA A.M., REKIK M., DELVAUX M., DAVICCO M.J., BARLET J.P., FREXINOS J., BUENO L. Parathyroidhormone (PTH) and PTH-related peptide induce relaxation of smooth muscle cells from guinea-pig ileum: interaction with vasoactive intestinal polypeptide receptors. Endocrinology. 1994;135:2160–2167. doi: 10.1210/endo.135.5.7525262. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., HWANG P.M., SNYDER S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- BROOKES S.J.H. Neuronal nitric oxide in the gut. J. Gastroenterol. Hepatol. 1993;8:590–603. doi: 10.1111/j.1440-1746.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- CELLEK S., KASAKOV L., MONCADA S. Inhibition of nitrergic relaxations by a selective inhibitor of the soluble guanylyl cyclase. Br. J. Pharmacol. 1996;118:137–140. doi: 10.1111/j.1476-5381.1996.tb15376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKDER S., BANDYOADHYAY A., RATTAN S. Neuronal NOS gene expression in gastrointestinal myenteric neurons and smooth muscle cells. Am. J. Physiol. 1997;273:C1868–C1875. doi: 10.1152/ajpcell.1997.273.6.C1868. [DOI] [PubMed] [Google Scholar]
- CHEN K., INOUE M., OKADA A. Expression of inducible nitric oxide synthase mRNA in rat digestive tissues after endotoxin and its role in intestinal mucosal injury. Biochem. Biophys. Res. Com. 1996;224:703–708. doi: 10.1006/bbrc.1996.1087. [DOI] [PubMed] [Google Scholar]
- CHIJIIWA Y., MURTHY J.R., GRIDER J.R., MAKHLOUF G.M. Expression of functional receptors for vasoactive intestinal peptide in freshly isolated and cultured muscle cells. Regul. Pept. 1993;47:223–232. doi: 10.1016/0167-0115(93)90389-p. [DOI] [PubMed] [Google Scholar]
- COLLINS S.M., GARDNER J.D. Cholecystokinin-induced contraction of dispersed smooth muscle cells. Am. J. Physiol. 1982;243:G497–G509. doi: 10.1152/ajpgi.1982.243.6.G497. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., CURRO D., MONTUSCHI P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J. Auton. Nerv. Syst. 1992;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- DE MAN J.G., PELCKMANS P.A., BOECKXSTAENS G.E., BULT H., OOSTERBOSCH L., HERMAN A.G., VAN MAERCKE Y.M. The role of nitric oxide in inhibitory non-adrenergic non-cholinergic neurotransmission in the canine lower oesophageal sphincter. Br. J. Pharmacol. 1991;103:1092–1096. doi: 10.1111/j.1476-5381.1991.tb12305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESAI K.M., WARNER T.D., BISHOP A.E., POLAK J.M., VANE J.R. Nitric oxide, and not vasoactive intestinal polypeptide, as the main neurotransmitter of vagally induced relaxation of the guinea-pig stomach. Br. J. Pharmacol. 1994;113:1197–1202. doi: 10.1111/j.1476-5381.1994.tb17124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICK J.M.C., LEFEBVRE R.A. Investigation of the interaction between NO and VIP in the gastric fundus of the guinea-pig. Neuropeptides. 1998a;32:369. [Google Scholar]
- DICK J.M.C., LEFEBVRE R.A. Mechanism of the relaxant effect of VIP in the guinea-pig gastric fundus. Neurogastroenterol. Motil. 1998b;10:467. [Google Scholar]
- DI ROSA R.M., RADOMSKI M., CARNUCCIO R., MONCADA S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem. Biophys. Res. Commun. 1990;172:1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- ECKLY A.E., LUGNIER C. Role of phosphodiesterases III and IV in the modulation of vascular cAMP content by the NO/cGMP pathway. Br. J. Pharmacol. 1994;113:445–450. doi: 10.1111/j.1476-5381.1994.tb17009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABBRI E., BRIGHENTI L., OTTOLENGHI C. Inhibition of adenylyl cyclase of catfish and rat hepatocyte membranes by 9-(tetrahydro-2-furyl)adenine (SQ 22,536) J. Enzym. Inhib. 1991;5:87–98. doi: 10.3109/14756369109069062. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., NOACK E. Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or non-heme iron. Eur. J. Pharmacol. 1987;142:465–469. doi: 10.1016/0014-2999(87)90090-2. [DOI] [PubMed] [Google Scholar]
- FÖRSTERMANN U., GATH I., SCHWARZ P., CLOSS E.I., KLEINERT H. Isoforms of nitric synthase; properties, cellular distribution and expressional control. Biochem. Pharmacol. 1995;50:1321–1332. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B., BORNSTEIN J.C., MURPHY R., POMPOLO S. Roles of peptides in transmission in the enteric nervous system. Trends Neurosci. 1992;15:361–372. doi: 10.1016/0166-2236(92)90029-8. [DOI] [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GARVEY E.P., OPLINGER J.A., FURFINE E.S., KIFF R.J., LASZLO F., WHITTLE B.J.R., KNOWLES R.G. 1400 W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 1997;172:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., TUCKER J.F., WAYMAN C. Variable potency of nitergic-nitrovasodilator relaxations of the mouse anococcygeus against different forms of induced tone. Br. J. Pharmacol. 1994;113:1494–1500. doi: 10.1111/j.1476-5381.1994.tb17165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIDER J.R. Interplay of VIP and nitric oxide in regulation of the descending relaxation phase of peristalsis. Am. J. Physiol. 1993;264:G334–G340. doi: 10.1152/ajpgi.1993.264.2.G334. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., MURTHY K.S., JIN J.-G., MAKHLOUF G.M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am. J. Physiol. 1992;262:G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M.J.D., MESSENT M., MCALLISTER R.J., EVANS T.W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br. J. Pharmacol. 1993;110:963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDY D., GHARIB-NASERI M.K., HUTSON D. Role of nitric oxide and vasoactive intestinal polypeptide in vagally mediated relaxation of the gastric corpus in the anaesthetized ferret. J. Auton. Nerv. Syst. 1993;43:241–246. doi: 10.1016/0165-1838(93)90330-w. [DOI] [PubMed] [Google Scholar]
- HALL T.J., GASSER J., ULRICH F., GIORGIO FERRINI P. Effects of the cytokine synthesis inhibitor CGP 47969A on nitric oxide production by lipopolysaccharide-stimulated J774A.1 macrophages. Agents Action. 1994;43:60–63. doi: 10.1007/BF02005766. [DOI] [PubMed] [Google Scholar]
- HONEYMAN T., MERRIAM P., FAY F.S. The effects of isoproterenol on adenosine cyclic 3′, 5′-monophosphate and contractility in isolated smooth muscle cells. Mol. Pharmacol. 1977;14:86–98. [PubMed] [Google Scholar]
- IGNARRO L.J. Signal transduction mechanisms involving nitric oxide. Biochem. Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- IMAGAWA J., YELLON D.M., BAXTER G.F. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. Br. J. Pharmacol. 1999;126:701–708. doi: 10.1038/sj.bjp.0702368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMAI T., HIRATA Y., KANNO K., MARUMO F. Induction of nitric oxide synthase by cAMP in rat vascular smooth muscle cells. J. Clin. Invest. 1994;93:543–549. doi: 10.1172/JCI117005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON W.F. Rp diastereomeric analogs of cAMP inhibit both cAMP- and cGMP- induced dilation of hamster mesenteric small arteries. Pharmacol. 1996;52:226–234. doi: 10.1159/000139387. [DOI] [PubMed] [Google Scholar]
- JIANG H., COLBRAN J.L., FRANCIS S.H., CORBIN J.D. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J. Biol. Chem. 1992;267:1015–1019. [PubMed] [Google Scholar]
- JIN J.-G., MURTHY K.S., GRIDER J.R., MAKHLOUF G.M. Activation of distinct cAMP- and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. Am. J. Physiol. 1993;264:G470–G477. doi: 10.1152/ajpgi.1993.264.3.G470. [DOI] [PubMed] [Google Scholar]
- JOLY G.A., NARAYANAN K., GRIFFITH O.W., KILBOURN R.G. Characterization of the effects of two new arginine/citrulline analogues on constitutive and inducible nitric oxide synthases in rat aorta. Br. J. Pharmacol. 1995;115:491–497. doi: 10.1111/j.1476-5381.1995.tb16360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEEF K.D., SHUTTLEWORTH C.W.R., XUE C., BAYGUINOV O., PUBLICOVER N.G., SANDERS K.M. Relationship between nitric oxide and vasoactive intestinal polypeptide in enteric inhibitory neurotransmission. Neuropharmacology. 1994;33:1303–1314. doi: 10.1016/0028-3908(94)90030-2. [DOI] [PubMed] [Google Scholar]
- KLATT P., SCHMIDT K., BRUNNER F., MAYER B. Inhibitors of brain nitric oxide synthase. J. Biol. Chem. 1994;269:1674–1680. [PubMed] [Google Scholar]
- KOIDE M., KAWAHARA Y., NAKAYAMA I., TSUDA T., YOKOYAMA M. cAMP-elevating agents induce an inducible type of nitric oxide synthase in cultured vascular smooth muscle cells. J. Biol. Chem. 1993;268:24959–24966. [PubMed] [Google Scholar]
- KOMAS N., LUGNIER C., STOCLET J.-C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br. J. Pharmacol. 1991;104:495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENZA A., SUTKOWSKI E., SEAMON K.B. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action. Trends Pharmacol. Sci. 1989;10:442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., BAERT E., BARBIER A.J. Influence of L-NG-nitroarginine on non-adrenergic non-cholinergic relaxation in the guinea-pig gastric fundus. Br. J. Pharmacol. 1992;106:173–179. doi: 10.1111/j.1476-5381.1992.tb14311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A., SMITS G.J.M., TIMMERMANS J.-P. Study of NO and VIP as non-adrenergic non-cholinergic neurotransmitters in the pig gastric fundus. Br. J. Pharmacol. 1995;116:2017–2026. doi: 10.1111/j.1476-5381.1995.tb16406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A., VANDEKERKHOVE K. Effect of nitroglycerin and longterm electrical stimulation on nitrergic relaxation in the pig gastric fundus. Br. J. Pharmacol. 1998;123:143–149. doi: 10.1038/sj.bjp.0701582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G.C., RAND M.J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur. J. Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- LIU S.F., BARNES P.J., EVANS T.W. Time course and cellular localization of lipopolysaccharide-induced nitric oxide synthase messenger RNA expression in the rat in vivo. Crit. Care Med. 1997;25:512–518. doi: 10.1097/00003246-199703000-00022. [DOI] [PubMed] [Google Scholar]
- MAURICE D.H., HASLAM R.J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylyl cyclase: inhibition of cAMP breakdown by cGMP. Mol. Pharmacol. 1990;37:671–681. [PubMed] [Google Scholar]
- MCCALL T.B., FEELISCH M., PALMER R.M.J., MONCADA S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br. J. Pharmacol. 1991;102:234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISKO T.P., MOORE W.M., KASTEN T.P., ALLEN NICKOLS G., CORBETT J.A., TILTON R.G., MCDANIEL M.L., WILLIAMSON J.R., CURRIE M.G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur. J. Pharmacol. 1993;233:119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- MOORE W.M., WEBBER R.K., JEROME G.M., FOE S.T., THOMAS P.M., CURRIE M.G. N-L-iminoethyllysine: A selective inhibitor of inducible nitric oxide synthase. J. Med. Chem. 1994;37:3886–3888. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Vasoactive intestinal polypeptide/pituitary adenylyl cyclase-activating peptide-dependent activation of membrane-bound NO synthase in smooth muscle mediated by pertussis toxin-sensitive Gil-2. J. Biol. Chem. 1994;269:15977–15980. [PubMed] [Google Scholar]
- MURTHY K.S., TENG B.-Q., JIN J.-G., MAKHLOUF G.M. G protein-dependent activation of smooth muscle eNOS via natriuretic peptide clearance receptor. Am. J. Physiol. 1998;275:C1409–C1416. doi: 10.1152/ajpcell.1998.275.6.C1409. [DOI] [PubMed] [Google Scholar]
- NARAYANAN K., GRIFFITH O.W. Synthesis of L-thiocitrulline, L-homothiocitrulline, and S-methyl-L-thiocitrulline: a new class of potent nitric oxide synthase inhibitors. J. Med. Chem. 1994;37:885–887. doi: 10.1021/jm00033a004. [DOI] [PubMed] [Google Scholar]
- OZAKI H., BLONDFIELD D.P., HORI M., SANDERS K.M., PUBLICOVER N.G. cAMP-mediated regulation of excitation-contraction coupling in canine gastric smooth muscle. J. Physiol. Lond. 1992;447:351–372. doi: 10.1113/jphysiol.1992.sp019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN J., BURGHER K.L., SZCZEPANIK A.M., RINGHEIM G.E. Tyrosine phosphorylation of inducible nitric oxide synthase: implications for potential post-translational regulation. Biochem. J. 1996;314:889–894. doi: 10.1042/bj3140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADOMSKI M.W., PALMER R.M.J., MONCADA S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REKIK M., DELVAUX M., TACK I., FREXINOS J., BUENO L. VIP-induced relaxation of guinea-pig intestinal smooth muscle cells: sequential involvement of cAMP and nitric oxide. Br. J. Pharmacol. 1996;118:477–484. doi: 10.1111/j.1476-5381.1996.tb15428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHIM B.Y., HONG K.W. Relaxation by cromakalim and pinacidil of isolated smooth muscle cells from canine coronary artery, multiple sites of action. Arch. Int. Pharmacodyn. 1994;328:67–81. [PubMed] [Google Scholar]
- SAHYOUN H.A., COSTALL B., NAYLOR R.J. On the ability of domperidone to selectivity inhibit catecholamine-induced relaxation of circular smooth muscle of guinea-pig stomach. J. Pharm. Pharmacol. 1982;34:27–33. doi: 10.1111/j.2042-7158.1982.tb04672.x. [DOI] [PubMed] [Google Scholar]
- SAID S.I. Nitric oxide and vasoactive intestinal peptide: cotransmitters of smooth muscle relaxation. New Physiol. Sci. 1992;7:181–183. [Google Scholar]
- SAKAKIBARA H., SHIMA K., SAID S.I. Characterization of vasoactive intestinal peptide receptors on rat alveolar macrophages. Am. J. Physiol. 1994;267:L256–L262. doi: 10.1152/ajplung.1994.267.3.L256. [DOI] [PubMed] [Google Scholar]
- SALAPATEK A.-M., WANG Y.-F., MAO Y.-K., LAM A., DANIEL E.E. Myogenic nitric oxide synthase activity in canine lower oesophageal sphincter: morphological and functional evidence. Br. J. Pharmacol. 1998;123:1055–1064. doi: 10.1038/sj.bjp.0701701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEID C.R., HONEYMAN T.W., FAY F.S. Mechanism of β-adrenergic relaxation of smooth muscle. Nature. 1979;277:32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- SHUTTLEWORTH C.W.R., KEEF K.D. Roles of peptides in enteric neuromuscular transmission. Reg. Peptides. 1995;56:101–120. doi: 10.1016/0167-0115(95)00013-2. [DOI] [PubMed] [Google Scholar]
- SOUTHAN G.J., SZABO C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- SOUTHAN G.J., SZABO C., THIEMERMANN C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br. J. Pharmacol. 1995;114:510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENG B.-Q., MURTHY K.S., KEUMMERLE J.F., GRIDER J.R., SASE K., MICHEL T., MAKHLOUF G.M. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am. J. Physiol. 1998;275:G342–G351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- TØTTRUP A., SVANE D., FORMAN A. Nitric oxide mediating NANC inhibition in opossum lower oesophageal sphincter. Am. J. Physiol. 1991;260:G385–G389. doi: 10.1152/ajpgi.1991.260.3.G385. [DOI] [PubMed] [Google Scholar]
- WALDMAN S.A., MURAD F. Cyclic GMP synthesis and function. Pharmacol. Rev. 1987;39:163–196. [PubMed] [Google Scholar]
- WALKER G., PFEILSCHIFTER J., KUNZ D. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in interferon (IFN)-γ-stimulated RAW 264.7 cells by dexamethasone. J. Biol. Chem. 1996;271:16679–16687. doi: 10.1074/jbc.272.26.16679. [DOI] [PubMed] [Google Scholar]


