Abstract
The spontaneous activity of the rat isolated colon is suppressed by prostacyclin analogues such as cicaprost (IC50=4.0 nM). Activation of prostanoid IP1-receptors located on NANC inhibitory neurones is involved. However, several non-prostanoids, which show medium to high IP1 agonist potency on platelet and vascular preparations, exhibit very weak inhibitory activity on the colon. The aim of the study was to investigate this discrepancy.
Firstly, we have demonstrated the very high depolarizing potency of cicaprost on the rat isolated vagus nerve (EC50=0.23 nM). Iloprost, taprostene and carbacyclin were 7.9, 66, and 81 fold less potent than cicaprost, indicating the presence of IP1 as opposed to IP2-receptors. Three non-prostanoid prostacyclin mimetics, BMY 45778, BMY 42393 and ONO-1301, although much less potent than cicaprost (195, 990 and 1660 fold respectively), behaved as full agonists on the vagus nerve.
On re-investigating the rat colon, we found that BMY 45778 (0.1–3 μM), BMY 42393 (3 μM) and ONO-1301 (3 μM) behaved as specific IP1 partial agonists, but their actions required 30–60 min to reach steady-state and only slowly reversed on washing. This profile contrasted sharply with the rapid and readily reversible contractions elicited by a related non-prostanoid ONO-AP-324, which is an EP3-receptor agonist.
The full versus partial agonism of the non-prostanoid prostacyclin mimetics may be explained by the markedly different IP1 agonist sensitivities of the two rat neuronal preparations. However, the slow kinetics of the non-prostanoids on the NANC system of the colon remain unexplained, and must be taken into account when characterizing neuronal IP-receptors.
Keywords: Prostacyclin, prostanoid IP-receptors, prostanoid EP3-receptors, cicaprost, iloprost, BMY 45778, ONO-1301, vagus nerve, colon, NANC innervation
Introduction
The non-prostanoid prostacyclin mimetics activate prostanoid IP1-receptors on platelets and vascular smooth muscle to elicit inhibitory effects characteristic of prostacyclin (see Jones et al., 1993; Meanwell et al., 1994 for reviews). One of the most potent of these agents is BMY 45778 (Figure 1), which completely inhibits aggregation in human platelet-rich plasma and has a Kd of 7 nM for the IP1-receptor on human platelet membranes (Seiler et al., 1997). It also fully relaxes human pulmonary artery rings with an IC50 of about 3 nM (Jones et al., 1997). The non-prostanoids tend to be less potent inhibitors on rat and rabbit platelet suspensions (Armstrong et al., 1989; Merritt et al., 1991). However, we were surprised by the very weak inhibitory effects of BMY 45778 on the spontaneous motility of the rat isolated colon (10–15% inhibition at 2–10 μM) in comparison to the complete inhibition induced by the prostacyclin analogue cicaprost (IC50=4 nM) (Wise et al., 1995). In addition, two other non-prostanoids, BMY 42393 (Figure 1, Seiler et al., 1994) and EP 185 (Jones et al., 1993) had minimal inhibitory activity at a concentration of 10 μM. Cicaprost's action on the rat colon is abolished by tetrodotoxin (TTX), and it appears that activation of IP-receptors on non-adrenergic non-cholinergic (NANC) neurones in the colon leads to the release of transmitters that inhibit the pacemaker/smooth muscle system (Figure 3a) (Qian & Jones, 1995).
Figure 1.
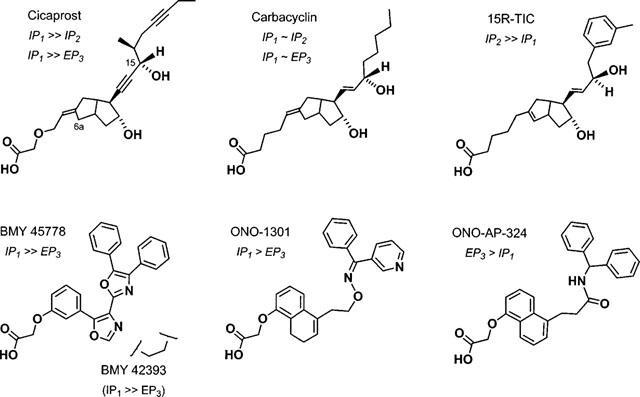
6a-Carba analogues of prostacyclin (upper row) and non-prostanoid prostacyclin mimetics (lower row) with differing agonist specificities. IP1/IP2 receptor selectivity is derived from ligand binding measurements in rat brain (Takechi et al., 1996) and IP1/EP3 receptor selectivity from functional studies on rat colon (this study) and human platelets/guinea-pig vas deferens (Armstrong et al., 1989; Lawrence et al., 1992). The prostacyclin analogues are drawn in the IP1-receptor-bound conformation (anti-parallel chains) proposed by Tsai et al. (1991).
Figure 3.
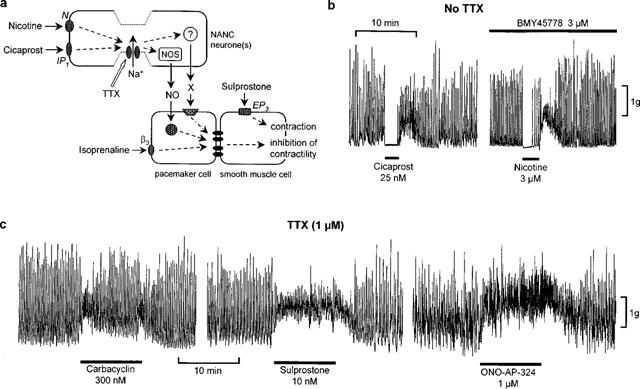
Rat colon: (a) Schematic arrangement of the NANC neurone/pacemaker/smooth muscle system and the proposed sites of action of agonists for IP1, EP3, nicotinic (N) and β3-adrenergic receptors. β3-Adrenoceptors may also be located on the smooth muscle cell. NOS=nitric oxide synthase; X=unknown, apamin-sensitive transmitter. The pacemaker and smooth muscle cells are coupled by gap junctions. TTX inhibits the action of cicaprost and nicotine by abolishing action potential conduction in NANC neurones. (b) Typical effects on spontaneous motility of cicaprost and nicotine after 50 min exposure to BMY 45778. (c) Effects of carbacyclin, sulprostone and ONO-AP-324 in the presence of 1 μM TTX.
The question therefore arose as to whether non-prostanoid prostacyclin mimetics are genuinely poor agonists at IP-receptors on rat peripheral neurones, or have other actions that interfere with IP agonist activity. There are two related reasons for considering the latter possibility. Firstly, the high lipophilicity of the non-prostanoids may affect cell membrane function, and their diaryl-heteroatomic units resemble moieties found in other pharmacologically active agents (e.g. methadone analogues and diphenylmethane antihistamines; see Foye, 1990). Secondly, the control of colonic motility through the interaction of enteric neurones, pacemaker cells (interstitial cells of Cajal) and smooth muscle cells is known to be complex (Huizinga & Thuneberg, 1997). In the case of IP agonist action, at least two inhibitory neurotransmitter systems are involved (Figure 3a); nitric oxide (NO) is one transmitter while the other remains unidentified, but does not appear to be ATP, VIP or pituitary adenylate cyclase activating peptide (PACAP) (Qian & Jones, 1995). As a result, there are a number of steps at which a non-prostanoid might exert an additional action.
Consequently, we have looked for an anatomically and mechanistically simpler neuronal preparation from the rat. The rat cervical vagus nerve is depolarized by 5-HT and has been used previously to characterize excitatory 5-HT-receptors (mainly 5-HT3) involved in vagal afferent nerve activity in emesis (Ireland & Tyers, 1987; Rhodes et al., 1992; Minami et al., 1997). We now report that this preparation is depolarized by cicaprost at subnanomolar concentrations, and that BMY 45778, BMY 42393, and a third non-prostanoid prostacyclin mimetic, ONO-1301 (Figure 1, Kondo & Hamanaka, 1995; Kondo et al., 1995) also show strong depolarizing activity in the nanomolar range. These findings prompted us to reinvestigate the actions of the nonprostanoids on the rat colon preparation.
Methods
Vagus nerve preparation
The method is essentially that of Ireland & Tyers (1987). Male Sprague-Dawley rats (250–350 g) were anaesthetized with diethyl ether and exsanguinated by incising the ventricles through the chest wall. A 20–25 mm length of cervical vagus was dissected and placed in ice-cold Krebs-Henseleit solution (in mM): NaCl 118, KCl 4.75, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 , glucose 11. The nerve was desheathed under a dissecting microscope and then carefully mounted on filter paper strips in a perspex ‘grease-gap' chamber, contained in a water jacket at 27°C. Silver/silver chloride recording electrodes with cotton-wool wicks were placed in contact with the surface of the nerve in both halves of the chamber and the potential difference was recorded with a MacLab GP amplifier/4s interface/PowerMac 7200/120 computer (sampling rate 40 Hz). Each half of the nerve was superfused at 1.5–2.0 ml min−1 with Krebs-Henseleit solution, aerated with 95% O2/5% CO2 and maintained at 27°C. Drugs were applied in the superfusate to one half of the nerve.
Preparations that were depolarized to less than 2 mV by 20 mM KCl were abandoned. Preparations remained usable for at least 3 h: three sequences of KCl doses (10–30 mM) 75 min apart gave highly consistent responses; a fourth sequence revealed a slight loss of sensitivity. 5-HT-receptor agonists were added non-cumulatively (3 min contact/15 min interval) and IP-receptor agonists cumulatively (15–20 min contact). The correlation between initial responses to 20 mM KCl (range=2.0–6.3 mV) and maximum responses to cicaprost was not good enough to persuade us to normalize all depolarization responses to the 20 mM KCl depolarization. In some experiments, TTX or Ca2+-free bathing fluid was applied for 30 min before agonist addition.
Rat colon preparation
Spontaneous motility of the rat isolated colon was recorded as described by Qian & Jones (1995). Male Sprague-Dawley rats (250–350 g) were killed by a sharp blow to the head followed by exsanguination. Segments of colon were removed and set up for isometric recording of longitudinal muscle contraction (resting tension=0.5 g) in 10 ml organ baths containing Krebs-Henseleit solution (see earlier), 95% O2/5% CO2 and maintained at 37°C. Inhibitory agonists were added to the organ bath for 2.5 min and removed by overflow washing. For dose-response relationships obtained on the same preparation, motility (%) was calculated by comparing the (area under the trace) min−1 for the 2 min period prior to agonist application with the corresponding value over 0.5–2.5 min of cicaprost/isoprenaline application and 0.5–1.5 min of nicotine application. In experiments involving repeated application of a fixed dose of inhibitory agonist, all motility values were calculated relative to the resting motility for the last agonist application before the start of the vehicle/non-prostanoid treatment. In some experiments, TTX was applied for 10 min before agonist addition.
Data analysis
Values in the text and figures are means±s.e.mean. Sigmoidal curves were fitted to log concentration-response data using GraphPad Prism software (GraphPad Software Inc., San Diego, U.S.A.); the low-concentration asymptote was constrained to zero mV in the vagus nerve experiments and to 100% in the colon experiments. Statistical analysis of the non-prostanoid interaction data on the rat colon was performed by repeated-measures 2-factor ANOVA; factor 1=time, factor 2=vehicle/non-prostanoid treatment. Comparison of means at each time point was performed by pre-planned contrast (SuperANOVA, Abacus Concepts, California, U.S.A.); differences were considered significant when P<0.05.
Drugs
Cicaprost, iloprost and sulprostone from Schering AG, Berlin, Germany, and taprostene (CG 4203) from Grunenthal GmBH, Aachen, Germany, were gifts. Carbacyclin was purchased from Cayman Chemicals, Ann Arbor, U.S.A. Primary stocks were prepared in absolute ethanol at 2.5–10 mM. BMY 45778 (3-[4-(4,5-diphenyl-2-oxazolyl)-5-oxazolyl] phenoxy] acetic acid), and BMY 42393 (2-[3-[2-(4,5-diphenyl–2oxazolyl)ethyl]phenoxy] acetic acid) from Bristol-Myers Squibb Pharmaceutical Research Institute, Wallingford, U.S.A., and ONO-1301 (formerly ONO-AP-500-02; 7,8-dihydro-5-(2-(1-phenyl-1-pyrid-3-yl-methiminoxy)-ethyl)-α-naphthyloxyacetic acid) and ONO-AP-324 (5-(2-diphenylmethyl aminocarboxy)-ethyl)-α-naphthyloxyacetic acid) from ONO Pharmaceuticals, Osaka, Japan, were also gifts. Primary stocks were prepared in dimethylsulphoxide (DMSO) at 10 mM. All substocks were prepared in 0.9% NaCl solution, with a trace of solid NaHCO3 added to the primary substock to aid dissolution as necessary. Thus, 3 μM solutions of the non-prostanoids contain 4.2 mM DMSO. Tetrodotoxin and 5-hydroxytryptamine (5-HT) maleate were purchased from Sigma Chemical Co., U.S.A., and 2-methyl 5-HT maleate from Research Biochemicals International, U.S.A.
Results
Characteristics of the depolarizing action of IP agonists on the rat vagus nerve
Rat vagus nerve preparations were depolarized by 5-HT3-receptor agonists in a manner similar to that reported previously (Ireland & Tyers 1987; Kilpatrick et al., 1990). Responses to 5-HT and 2-methyl 5-HT (a specific 5-HT3 agonist) were rapid in onset and offset, with EC50 values of 1.08±0.12 and 2.7±0.3 μM respectively and maximum responses of about 0.5 mV (n=4; first dose sequences). Sensitivity was reasonably well maintained over a period of 6 h, as shown by mean EC50 values of 2.3, 4.8 and 3.7 μM and relative maxima of 104, 83 and 80% for three further dose sequences of 2-methyl 5-HT.
The prostacyclin analogue cicaprost was a highly potent depolarizing agent (EC50=0.23 nM), with a maximum response similar to that of 2-methyl 5-HT (Figure 2). Cicaprost responses took 10–15 min to reach a stable level, and showed no fade over 90 min. However, the most striking characteristic of cicaprost's action was its persistence on continuous washing of the preparation. For example, near-maximal depolarization induced by 10 nM cicaprost had only decayed to about 40% of its initial value after 3 h of continuous washing. The other IP agonists listed in Table 1 behaved similarly, and this meant that only a single cumulative dose sequence (15–20 min contact times) could be obtained on each preparation, and ‘within-preparation' comparison of agonist potencies was not possible.
Figure 2.
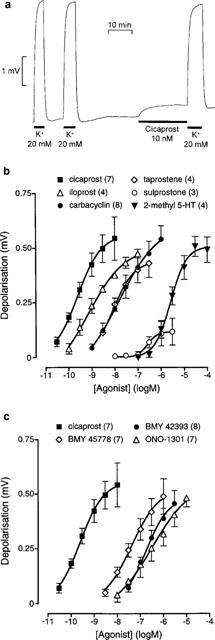
Rat isolated vagus nerve: (a) experimental record showing depolarization induced by KCl and the prostacyclin analogue cicaprost, (b) log concentration-response curves for four prostacyclin analogues, the EP1/EP3 agonist sulprostone, and the selective 5-HT3 agonist 2-methyl 5-HT, and (c) log concentration-response curves for cicaprost and three non-prostanoid prostacyclin mimetics. Numbers of preparations are shown in brackets; vertical bars are s.e.means.
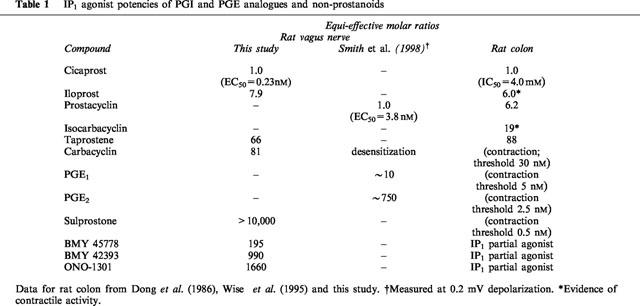
Table 1.
IP1 agonist potencies of PGI and PGE analogues and non-prostanoids
Removal of Ca2+ from the bathing fluid caused a rapid hyperpolarization of 0.41±0.02 mV (n=4), but no change in the depolarization induced by 20 mM KCl. Depolarization to 10 nM cicaprost was also unaffected by this procedure (0.41±0.02 versus 0.41±0.05 mV, n=4, P<0.05). TTX (1 μM) affected neither the resting potential nor the depolarization to 10 nM cicaprost (0.41±0.02 versus 0.45±0.03 mV, n=4, P>0.05).
Comparison of IP agonist potencies on the vagus nerve
Log concentration-response curves for cicaprost and three other prostacyclin analogues, iloprost, carbacyclin and taprostene, are shown in Figure 2b. Equi-effective molar ratios (EMRs, Table 1) were calculated relative to cicaprost (EC50=0.23 nM, EMR=1.0). Similar data for the three non-prostanoids BMY 45778, BMY 42393 and ONO-1301 are also shown in Figure 2c and Table 1. BMY 45778 depolarized the vagus preparation at concentrations as low as 3 nM and was about 5 and 8.5 times more potent than BMY 42393 and ONO-1301 respectively. Addition of 300 nM BMY 45778 to preparations already depolarized with 10 nM cicaprost resulted in a slight hyperpolarization (91% of control, n=4) after 40 min. Vehicle-treated preparations showed no change in potential (101% of control, n=4).
Sulprostone, a potent agonist at prostanoid EP1 and EP3-receptors, induced small depolarization responses between 0.3 and 3 μM (n=3) (Figure 2b).
Non-prostanoid prostacyclin mimetics on the rat colon
Our previous experiments on the rat colon (Wise et al., 1995) clearly showed that the slight inhibition of spontaneous motility (5–15%) induced by BMY 45778 (2–10 μM) was accompanied by modest block of the inhibitory action of cicaprost (10 nM), but no effect on the inhibitory action of nicotine, which also stimulates NANC inhibitory neurones. A partial agonist action of BMY 45778 on the neuronal IP system was proposed. However, on re-examination of the data we noticed that in some experiments the cumulative log concentration-response curve to cicaprost in the presence of BMY 45778 was quite shallow, as though the antagonistic action of BMY 45778 had increased during the cicaprost dose sequence. This raised the possibility that 5 min pre-exposure to BMY 45778 had been insufficient for its equilibration with the IP-receptor pool.
We have therefore examined the time courses of the inhibitory actions of BMY 45778, BMY 42393 and ONO-1301 and their interactions with cicaprost, and in the case of BMY 45778 its interaction with nicotine and isoprenaline. The inhibitory action of isoprenaline on the rat colon is mediated through post-junctional β3-adrenoceptors (Bianchetti & Manara, 1990). For cicaprost, a dose (usually 10 nM) that consistently induced 80–90% inhibition of colonic motility was determined, and then applied for 3 min periods at 10, 30, 50 and 70 min after the start of continuous exposure of the preparation to either the non-prostanoid or the appropriate vehicle (DMSO/saline). The results are shown in Figure 4a–c,f–h; statistical analysis was performed by repeated-measures 2-factor ANOVA (see Methods). In vehicle-treated preparations, the resting motility and the inhibitory action of cicaprost were both well maintained throughout the experimental period. Continuous exposure of the colon to BMY 45778 (0.1–3 μM) slowly and partially inhibited colonic motility, accompanied by a correspondingly slow loss of responsiveness to cicaprost (Figure 4a–c). With 3 μM BMY 45778, an inhibitory effect was discernible at 10 min in some preparations, but a statistically significant difference from the vehicle-treated preparation was only found after 20–30 min exposure. After 50 min exposure to 3 μM BMY 45778, the response to cicaprost was abolished. With 0.1 μM BMY 45778, the absolute motility values for cicaprost in vehicle and non-prostanoid-treated prepartions were not significantly different, but it was clear that the inhibitory actions of cicaprost and BMY 45778 were less than additive. Treatment with 1 μM BMY 45778 almost abolished the response to cicaprost after 50 min (data not shown); construction of a cumulative concentration-response curve to cicaprost between 70–80 min showed that the BMY 45778 antagonism was surmountable (Figure 4f).
Figure 4.
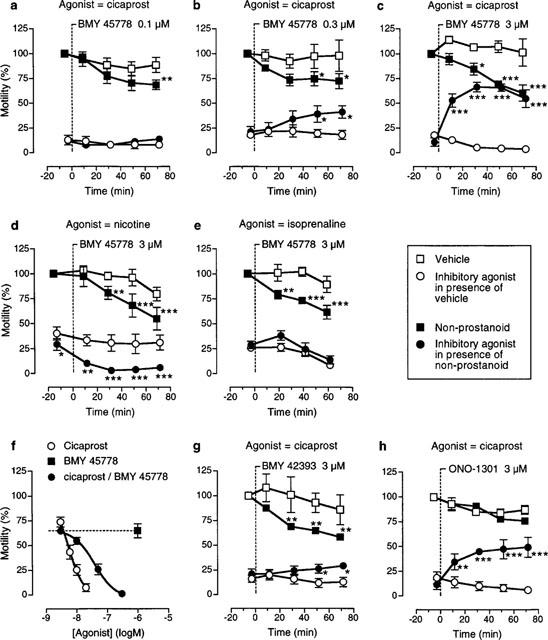
Spontaneous motility of the rat isolated colon: concentration- and time-dependencies of the actions of the non-prostanoids BMY 45778, BMY 42393 and ONO-1301 and their effects on inhibitory responses elicited by cicaprost, nicotine and isoprenaline. A vertical broken line indicates the start of continuous exposure to non-prostanoid (or vehicle). (f) shows that cicaprost can overcome the antagonistic action of BMY 45778 (1 μM, 70–80 min exposure). Vertical bars are s.e.means; n=4–8 for all experiments; statistical comparisons of motilities of vehicle and non-prostanoid treated preparations (upper asterisks) and of inhibitory agonist-induced responses of vehicle and non-prostanoid treated preparations (lower asterisks): *P<0.05, **P<0.01, ***P<0.001.
To study the effects of continuous exposure to 3 μM BMY 45778 on inhibitory responses to nicotine (3 μM) and isoprenaline (30 nM), the protocol was altered so that nicotine doses were alternated with isoprenaline doses in the same experiment. Nicotine's inhibitory action starts to fade after 1–2 min, but in the long-term desensitization is minimal provided that at least 20 min is allowed between consecutive doses. In these experiments, there was some reduction of resting motility in the vehicle-treated preparations after 60 min (Figure 4d,e). However, with 50 min exposure to BMY 45778, at which time cicaprost responses were abolished, nicotine (Figure 3b) and isoprenaline still gave large inhibitory effects.
With cicaprost as the inhibitory agonist, BMY 42393 at 3 μM (Figure 4g) showed a similar profile to 0.3 μM BMY 45778; 1 μM BMY 42393 had no significant effect of its own or on cicaprost responses (data not shown). ONO-1301 at 3 μM also suppressed the action of cicaprost, but did not appear to have an inhibitory effect of its own (P>0.05) (Figure 4h).
Continuous washing of 3 μM BMY 45778-treated preparations for 30 min neither reversed the BMY 45778-induced inhibition nor re-established the cicaprost response. In contrast, the BMY 45778 response was reversed within 1–2 min of addition of 1 μM TTX; this concentration of TTX abolishes maximal inhibition of motility induced by cicaprost, and nicotine, but not that due to isoprenaline (data not shown).
EP3 agonism on the rat colon
ONO-1301 is known to have weak EP3 agonist activity (K. Kondo, personal communication), and since the rat colon has a post-junctional EP3 contractile system, it was necessary to determine whether this activity was interfering with the IP agonist activity of any of the non-prostanoids. In the presence of 1 μM TTX to abolish neuronal IP actions, BMY 45778, BMY 42392 and ONO-1301, all at 3 μM, gave less than 10% increase in motility of the rat colon (n=4). In contrast, ONO-AP-324, which is related to ONO-1301 and has much greater EP3 agonist activity (see Discussion), consistently elicited contractile activity between 30 and 1000 nM; its action was rapid in onset and offset (Figure 3). The PGE analogue sulprostone (0.5–30 nM) and the prostacyclin analogue carbacyclin (30–1000 nM) also contracted the colon in the presence of TTX (Figure 3). Carbacyclin also contracted the colon in the absence of TTX and showed no sign of IP inhibitory activity.
Discussion
Interest in the neuronal stimulant actions of prostacyclin has increased of late, based on the recognition that it may exert some of the pro-inflammatory actions traditionally attributed to prostaglandin E2 (see Wise & Jones, 1996; Bley et al., 1998). Of special note is the finding that mice with knock-out of the IP-receptor gene show reduced sensitivity to pain and less inflammatory oedema than wild-type mice (Murata et al., 1997). In addition, it has been shown that rat brain contains two subtypes of IP-receptor that mediate neuronal excitation, and these have different anatomical distributions and different structural requirements for activation by prostanoid ligands (Figure 1) (Takechi et al., 1996). In the nucleus tractus solitarius (NTS), IP1-receptors are activated by prostacyclin analogues having a 15-hydroxy group with the natural (S) configuration. The ranking for competition for [3H]-isocarbacyclin binding is cicaprost=isocarbacyclin=iloprost>carbacyclin>PGE1>15R-16-m-tolyl-ω-tetranor isocarbacyclin (15R-TIC)>PGE2. This agonist profile is similar to that previously reported for functional assays on blood platelets and vascular smooth muscle: cicaprost consistently showed inhibitory potency slightly greater than iloprost and greater than prostacyclin and carbacyclin (Armstrong et al., 1989), and the natural 15S epimers were much more potent than the corresponding 15R epimers (Morton et al., 1979; Suzuki et al., 1996). The IP2-receptor present in the thalamus has the following affinity ranking: isocarbacyclin=15RTIC>carbacyclin>iloprost>PGE2>PGE1>cicaprost, with cicaprost showing very low potency; configuration at C15 is not critical to the binding process. Indeed, 15-deoxy-TIC, which has no oxygen function at C15, binds well to the IP2-receptor in the thalamus (Suzuki et al., 1999).
IP-receptors in the rat vagus nerve
The IP-receptor in the rat vagus nerve is clearly an IP1 subtype. Firstly, it is ‘cicaprost-sensitive'; the EC50 of 0.23 nM for cicaprost makes the vagus nerve one of the most sensitive IP-receptor preparations reported so far. Secondly, cicaprost is more potent than iloprost, which is more potent than carbacyclin (Table 1). [3H]-Iloprost binding has been demonstrated in rat nodose ganglia and the IP1-receptors travel towards and away from the brain by axoplasmic flow (Matsumura et al., 1995). The IP1-receptors in the vagus nerve preparation are therefore probably located on afferent neurones that have their cell bodies in the nodose ganglia and project to the NTS in the caudal medulla.
During our studies, a report of the depolarizing action of prostanoids on the rat vagus nerve appeared in this journal (Smith et al., 1998). Of the primary prostaglandins tested, prostacyclin was found to be the most potent, with an EC50 value of 3.8 nM and a maximum response of about 0.5 mV. The potency of prostacyclin fits reasonably well with our potency data for an IP1 system (Table 1), bearing in mind the chemical instability of prostacyclin under physiological conditions. There were however significant differences between our study and that of Smith et al. (1998) Firstly, in our study depolarization responses to IP agonists decayed slowly after washing, while in the Smith et al. (1998) study the return to baseline was fast enough for a discrete-dosing protocol to be used. Secondly, Smith et al. (1998) reported desensitization to IP agonist action. This was seen as fade of depolarization responses, and as a lower response to the highest concentration of prostacyclin in a non-cumulative dose sequence compared to the response to a single matching dose of prostacyclin (0.24 versus 0.54 mV). Desensitisation to carbacyclin was more severe (0.10 versus 0.47 mV), making its relative potency difficult to assess. Desensitization was not a problem in our study, with established depolarization responses remaining stable or decaying only slowly (Figure 2a), and a single high concentration of cicaprost (10 nM) showing a similar response to the same concentration in the cumulative sequence. The reasons for the differences between the two studies are unclear, given that the experimental conditions were similar.
EP3-receptor messenger RNA has been detected in cell bodies in the rat nodose ganglion (Ek et al., 1998), raising the question of the presence of EP-receptors in vagal (sensory) axons. Smith et al. (1998) showed that a 20 min exposure to 10 μM prostacyclin or carbacyclin drastically reduced the response to 10 μM PGE2 (0.05 and 0.02 versus 0.28 mV), suggesting that PGE2 activates IP1-receptors in the vagus preparation. Smith et al. (1998) also found PGE1 to be a much more potent depolarizer than PGE2; from their data we estimate PGE1 and PGE2 to be about 10 and 750 times less potent than prostacyclin respectively (Table 1). These values are entirely consistent with activation of IP1-receptors (see Matthews & Jones, 1993 and references therein). In addition, Smith et al. (1998) showed that several EP1 antagonists (SC 19220, SC 25469, SC 51322 and AH 6809) did not affect the concentration-response curve for prostacyclin. In our study, we have found that sulprostone, which is a highly potent EP3 agonist and a moderately potent EP1 agonist (Coleman et al., 1987; Lawrence et al., 1992), weakly depolarized the vagus preparation at concentrations of 0.3–3 μM. These observations suggest, but in no way prove, that EP1 and EP3-receptors are absent from the vagus nerve preparation.
IP-receptors on NANC neurones in rat colon
We have previously reported that the EP agonist activity of iloprost, and isocarbacyclin affects the estimation of their IP agonist potency on the rat colon (Table 1) (Dong et al., 1986). In this study, we have found that carbacyclin at concentrations of 3–30 nM shows no inhibitory effect and therefore appears to be less potent than cicaprost. How much less potent is impossible to say, since at higher concentrations (up to 1000 nM) it exhibits contractile activity. Since the latter action remains in the presence of TTX, it is likely that the prostanoid receptors involved are located on the pacemaker/smooth muscle system (Figure 3a). Sulprostone was about 30 times more potent than carbacyclin as a contractile agent. Using radioligand binding techniques, Woodward et al. (1997) have shown that both EP3 and FP-receptors are present in the rat colon. It is likely that carbacyclin activates the former based on binding data for mouse cloned prostanoid receptors expressed in Chinese hamster ovary cells: Ki for carbacyclin at EP3 and FP-receptors=31 and 1200 nM respectively (Kiriyama et al., 1997). Taprostene, like cicaprost, showed only inhibitory activity on the colon (Table 1), but its potency was only moderate, in agreement with previous studies on human platelets (Flohe et al., 1983) and human pulmonary artery (Jones et al., 1997). From the agonist ranking shown in Table 1, it would appear that the IP-receptors present on NANC neurones in the rat colon correspond to the IP1 subtype.
Neuronal activation by non-prostanoid prostacyclin mimetics
There is no information on the activities of non-prostanoid prostacyclin mimetics on the IP2-receptor, while the IP1-receptor has an interesting complexity. Two species divisions can be compiled based on the relative potencies of non-prostanoids for inhibition of platelet aggregation. In the first division, IP1-receptors on human, monkey, pig and horse platelets are relatively sensitive to the non-prostanoids, whereas in the second division, rat, rabbit, cow, dog, cat and guinea-pig platelet IP1-receptors are relatively insensitive to the non-prostanoids (Armstrong et al., 1989; Merritt et al., 1991). High relative potency for the non-prostanoids also extends to smooth muscle relaxation in the human pulmonary artery (Jones et al., 1997). The IP1-receptors in the rat vagus nerve clearly belong to the second division (Table 1).
Turning to the rat colon, BMY 45778 and BMY 42393 showed more pronounced inhibition of motility in the current study compared to our previous report (Wise et al., 1995). The reason for this discrepancy is the slow onset of non-prostanoid action, which we did not appreciate at the time of our previous studies. Are the observed responses due to actions of the non-prostanoids at neuronal IP1-receptors in the rat colon? We believe so, for several reasons. (i) The highest concentration of each non-prostanoid was restricted to 3 μM in an attempt to reduce the likelihood of additional actions. This limit was based on information from a colleague that in Chinese hamster ovary cells transfected with the mouse cloned IP1-receptor, cyclic AMP accumulation induced by BMY 42393 and BMY 22389 (octimibate) fell away at concentrations in excess of 3 μM (H. Wise, personal communication). (ii) TTX rapidly reversed an established inhibitory response to BMY 45778, indicating that the non-prostanoid-induced inhibition is neuronal in nature and not due to depression of the pacemaker/smooth muscle system. (iii) Prolonged application of BMY 45778 did not suppress the inhibitory actions of either nicotine, which activates NANC neurones, or isoprenaline, which acts on the pacemaker/smooth muscle system. (iv) Raising the concentration of cicaprost overcame the inhibitory effect of BMY 45778. (v) BMY 42393 and ONO-1301 were about an order of magnitude less potent than BMY 45778, roughly in agreement with their potencies on the vagus nerve.
There is good evidence from second messenger studies that the non-prostanoids have lower efficacies on IP1-receptor systems compared to prostacyclin analogues such as cicaprost and iloprost, and this applies to tissues from species in both divisions (Armstrong et al., 1989; Seiler et al., 1994; 1997). Whether one will see full or partial agonism in functional assays usually depends on the agonist sensitivity of the preparations under test (see Kenakin, 1984; 1985; 1999). Thus, on the highly sensitive rat vagus nerve preparation (cicaprost threshold concentration ∼0.03 nM), BMY 45778 is moderately active (threshold concentration ∼3 nM) and exhibits full (or nearly full) agonism. In contrast, on the rat colon, BMY 45778 has a higher threshold concentration (∼100 nM) and behaves as a partial agonist, correlating with a lower sensitivity to cicaprost (threshold concentration ∼2 nM). We now suggest that there is no conflict between the activities of the non-prostanoid prostacyclin mimetics on the NANC innervation of the rat colon and other IP1-receptor systems.
The non-prostanoids ONO-AP-324 and ONO-1301 (Figure 1) suppress twitch responses of the guinea-pig vas deferens to electrical field stimulation, being 22 and 3100 times less potent than PGE2 (Jones et al., 1998). This is an EP3-receptor-mediated response involving inhibition of neurotransmitter release from postganglionic sympathetic varicosities. In the present study, we have also observed EP3-like contractile activity on the rat colon with ONO-AP-324, but not with ONO-1301 (or BMY 45778 and BMY 42393). The reason for this difference probably lies with the lower sensitivity of the colon to EP3 agonists compared to the vas deferens (threshold concentration 1 nM versus 0.02 nM). The important point is that EP3 agonism by the three non-prostanoid prostacyclin mimetics is unlikely to interfere with their IP agonist activities on the rat colon.
The fast-onset/fast-offset excitatory action of ONO-AP-324 on the rat colon compared to the slow inhibitions induced by the other non-prostanoids is somewhat puzzling. All four compounds have similar structures, molecular weight and lipophilicity, and were used over similar concentration ranges (0.1–3 μM). In addition, access to IP1-receptors on NANC neurones and EP3-receptors on pacemaker/smooth muscle cells would both seem to be relatively unhindered as judged by the rapid actions of cicaprost and sulprostone respectively (Figure 3). So the slow action of the non-prostanoid prostacyclin mimetics on the rat colon may lie at the receptor level, but its nature remains elusive at this time.
In conclusion, the non-prostanoid prostacyclin mimetics are potentially useful agents for characterizing IP1-receptors, including those on enteric neurones, but the experimenter should be aware that their kinetics of action may be slow.
Acknowledgments
This work was supported by a grant from the University Grants Council of Hong Kong. We thank the pharmaceutical companies listed in the Methods section for their generous gifts of compounds. The excellent technical assistance of Messrs M.P. Ngan, K.M. Chan and K.L. Wong is highly appreciated.
Abbreviations
- EMR
equi-effective molar ratio
- DMSO
dimethylsulphoxide
- 5-HT
5-hydroxytryptamine
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- PACAP
pituitary adenylate cyclase activating peptide
- TTX
tetrodotoxin
References
- ARMSTRONG R.A., LAWRENCE R.A., JONES R.L., WILSON N.H., COLLIER A. Functional and ligand binding studies suggest heterogeneity of platelet prostacyclin receptors. Br. J. Pharmacol. 1989;97:657–668. doi: 10.1111/j.1476-5381.1989.tb12001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCHETTI A., MANARA L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical beta-adrenoceptors in rat colon. Br. J. Pharmacol. 1990;100:831–839. doi: 10.1111/j.1476-5381.1990.tb14100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEY K.R., HUNTER J.C., EGLEN R.M., SMITH J.A. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol. Sci. 1998;19:141–147. doi: 10.1016/s0165-6147(98)01185-7. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I., SHELDRICK R.L.G. New evidence with selective agonists and antagonists for the subclassification of PGE2-sensitive (EP) receptors. Adv. Prost. Thromb. Leukot. Res. 1987;17A:467–470. [PubMed] [Google Scholar]
- DONG Y.J., JONES R.L., WILSON N.H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable prostacyclin analogues. Br. J Pharmacol. 1986;87:97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EK M., KUROSAWA M., LUNDEBERG T., ERICSSON A. Activation of vagal afferents after injection of interleukin-1β: role of endogenous prostaglandins. J. Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOHE H., BOHLKE H., FRANKUS E., KIM S.M.A., LINTZ W., LOSCHEN G., MICHEL G., MULLER B., SCHNEIDER J., SEIPP U., VOLLENBERG W., WILSMANN K. Designing prostacyclin analogues. Drug Res. 1983;33:1240–1248. [PubMed] [Google Scholar]
- FOYE W.O. Principles of Medicinal Chemistry. Lea & Febiger: London; 1990. [Google Scholar]
- HUIZINGA J.D., THUNEBERG L. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal disorders. Trends Pharmacol. Sci. 1997;18:393–403. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- IRELAND S.J., TYERS M.B. Pharmacological characterisation of 5-hydroxytryptamine-induced depolarisation of the rat isolated vagus nerve. Br. J. Pharmacol. 1987;90:229–238. doi: 10.1111/j.1476-5381.1987.tb16844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES R.L., QIAN Y.M., CHAN Y.M., YIM A.P.C. Characterisation of a prostanoid EP3-receptor in guinea-pig aorta: partial agonist action of the non-prostanoid ONO-AP-324. Br. J. Pharmacol. 1998;125:1288–1296. doi: 10.1038/sj.bjp.0702189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES R.L., QIAN Y.M., WISE H., WONG H.N.C., LAM W.L., CHAN H.O., YIM A.P.C., HO J.K.S. Relaxant actions of non-prostanoid prostacyclin mimetics on human pulmonary artery. J. Cardiovasc. Pharmacol. 1997;29:525–535. doi: 10.1097/00005344-199704000-00015. [DOI] [PubMed] [Google Scholar]
- JONES R.L., WILSON N.H., MARR C.G., MUIR G., ARMSTRONG R.A. Diphenylmethylazine prostanoids with prostacyclin-like actions on human platelets. J. Lipid. Mediators. 1993;6:405–410. [PubMed] [Google Scholar]
- KENAKIN T.P. The relative contribution of affinity and efficacy to agonist activity: organ selectivity of noradrenaline and oxymetazoline with reference to classification of drug receptors. Br. J. Pharmacol. 1984;81:131–141. doi: 10.1111/j.1476-5381.1984.tb10753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P. The quantification of relative efficacy of agonists. J. Pharmacol. Meth. 1985;13:281–308. doi: 10.1016/0160-5402(85)90011-7. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P. Efficacy in drug receptor theory: outdated concept or under-valued tool. Trends Pharmacol. Sci. 1999;20:400–405. doi: 10.1016/s0165-6147(99)01361-9. [DOI] [PubMed] [Google Scholar]
- KILPATRICK G.J., BUNCE K.T., TYERS M.B. 5-HT3 receptors. Med. Res. Rev. 1990;10:441–475. doi: 10.1002/med.2610100404. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:17–24. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONDO K., HAMANAKA N. Prostacyclin mimetics with non-prostanoid structures. Folia Pharmacol. Jpn. 1995;106:181–191. doi: 10.1254/fpj.106.181. [DOI] [PubMed] [Google Scholar]
- KONDO K., MACHII K., NARITA M., KAWAMOTO A., YAMASAKI S., HAMANAKA N. ONO-AP-500-02: A non prostanoid prostaglandin I2 mimetic with inhibitory activity against thromboxane synthase. Adv. Prost. Thromb. Leuko. Res. 1995;23:401–403. [PubMed] [Google Scholar]
- LAWRENCE R.A., JONES R.L., WILSON N.H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br. J. Pharmacol. 1992;105:271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMURA K, , WATANABE Y., ONOE H., WATANABE Y. Prostacyclin receptor in the brain and central terminals of the primary sensory neurons: an autoradiographic study using a stable prostacyclin analogue [3H]-iloprost. Neuroscience. 1995;65:493–503. doi: 10.1016/0306-4522(94)00505-y. [DOI] [PubMed] [Google Scholar]
- MATTHEWS J.S., JONES R.L. Potentiation of aggregation and inhibition of adenylate cyclase in human platelets by prostaglandin E analogues. Br. J. Pharmacol. 1993;108:363–369. doi: 10.1111/j.1476-5381.1993.tb12810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEANWELL N.A., ROMINE J.L., SEILER S.M. Non-prostanoid prostacyclin mimetics. Drugs Future. 1994;19:361–383. [Google Scholar]
- MERRITT J.E., HALLAM T.J., BROWN A.M., BOYFIELD L., COOPER D.G., HICKEY D.M.B., JAXA-CHARMIEC A.A., KAUMANN A.J., KEEN M., KELLY E., KOZLOWSKI U., LYNHAM J.A., MOORES K.E., MURRAY K.J., MACDERMOT J., RINK T.J. Octimibate, a potent non-prostanoid inhibitor of platelet aggregation acts via the prostacyclin receptor. Br. J. Pharmacol. 1991;102:251–259. doi: 10.1111/j.1476-5381.1991.tb12162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAMI M., ENDO T., TAMAKAI H., OGAWA T, , HAMAUE N., MONMA Y., YOSHIOKA M., HAGIHARA K. Antiemetic effects of N-3389, a newly synthesized 5-HT-3 and 5-HT4 receptor antagonist in ferrets. Eur. J. Pharmacol. 1997;321:333–342. doi: 10.1016/s0014-2999(96)00974-0. [DOI] [PubMed] [Google Scholar]
- MORTON D.R., BUNDY G.L., NISHIZAWA E.E.Five-membered ring-modified prostacyclin analogues Prostacyclin 1979Raven Press: New York; 31–41.Vane J.R and Bergström S. (eds.) [Google Scholar]
- MURATA T., USHIKUBI F., MATSUOKA T., HIRATA M., YAMASAKI A., SUGIMOTO Y., ICHIKAWA A., AZE Y., TANAKA T., YOSHIDA N., UENO A., OHISHI S., NARUMIYA S. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- QIAN Y.M., JONES R.L. Inhibition of rat colon contractility by prostacyclin (IP-) receptor agonists: involvement of NANC neurotransmission. Br. J. Pharmacol. 1995;115:163–171. doi: 10.1111/j.1476-5381.1995.tb16334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES K.F., COLEMAN J., LATIMER N. A component of 5-HT-evoked depolarisation of the rat isolated vagus nerve is mediated by a putative 5-HT4 receptor. Naunyn-Schmied. Arch. Pharmacol. 1992;346:496–503. doi: 10.1007/BF00169003. [DOI] [PubMed] [Google Scholar]
- SEILER S., BRASSARD C.L., FEDERICI M.E., BUCHANAN J.O., ZAVOICO G.B., FLEMING J.S., MEANWELL N.A. 2-[3-[2-(4,5-Diphenyl-2-oxazolyl] ethyl] phenoxy] acetic acid (BMY 42393): A new, structurally novel prostacyclin partial agonist: 1) Inhibition of platelet aggregation and mechanism of action studies. Thromb. Res. 1994;74:115–123. doi: 10.1016/0049-3848(94)90004-3. [DOI] [PubMed] [Google Scholar]
- SEILER S., BRASSARD C.L., FEDERICI M.E., ROMINE J., MEANWELL N.A. [3-[4-(4,5-Diphenyl-2-oxazolyl)-5-oxazolyl]phenoxy]acetic acid (BMY 45778) is a potent non-prostanoid prostacyclin partial agonist: effects on platelet aggregation, adenylyl cyclase, cAMP levels, protein kinase, and iloprost binding. Prostaglandins. 1997;53:21–35. doi: 10.1016/s0090-6980(96)00138-4. [DOI] [PubMed] [Google Scholar]
- SMITH J.A.M., AMAGASU S.M., EGLEN R.M., HUNTER J.C., BLEY K.R. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. Br. J. Pharmacol. 1998;124:513–523. doi: 10.1038/sj.bjp.0701853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI M., KATO K., NOYORI R., WATANABE Y., TAKECHI H., MATSUMURA K., LANGSTROM B., WATANABE Y. (15R)-16-m-Tolyl-17,18,19,20-tetranorisocarbacyclin: a stable ligand with high binding affinity and selectivity for a prostacyclin receptor in the central nervous system. Angew. Chem. Int. Ed. Engl. 1996;35:334–336. [Google Scholar]
- SUZUKI M., KATO K., WATANABE Y., MATSUMURA K., WATANABE Y., NOYORI R. 15-Deoxy-16-(m-tolyl)-17,18,19,20-tetranorisocarbacyclin: a simple TIC derivative with potent anti-apoptotic activity for neuronal cells. Chem. Commun. 1999. pp. 307–308.
- TAKECHI H., MATSUMURA K., WATANABE Y., KATO K., NOYORI R., SUZUKI M., WATANABE Y. A novel subtype of the prostacyclin receptor expressed in the central nervous system. J. Biol. Chem. 1996;271:5901–5906. doi: 10.1074/jbc.271.10.5901. [DOI] [PubMed] [Google Scholar]
- TSAI A.L., STROBEL-JAGER E., WU K.K. Conformation of receptor-associated PGI2: An investigation by molecular modelling. J. Computer-aided Mol. Des. 1991;5:135–148. doi: 10.1007/BF00129752. [DOI] [PubMed] [Google Scholar]
- WISE H., JONES R.L. Focus on prostacyclin and its novel mimetics. Trends Pharmacol. Sci. 1996;17:17–21. doi: 10.1016/0165-6147(96)81565-3. [DOI] [PubMed] [Google Scholar]
- WISE H., QIAN Y.M., JONES R.L. A study of prostacyclin mimetics distinguishes neuronal from neutrophil IP receptors. Eur. J. Pharmacol. 1995;278:265–269. doi: 10.1016/0014-2999(95)00173-i. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.R., FAIRBAIRN C.E., LAWRENCE R.A. Identification of the FP-receptor as a discrete entity by radioligand binding in biosystems that exhibit different functional rank orders of potency in response to prostanoids. Adv. Exp. Med. Biol. 1997;400A:223–227. doi: 10.1007/978-1-4615-5325-0_32. [DOI] [PubMed] [Google Scholar]


