Abstract
Previous studies have indicated the expression of multiple P2Y receptors by rat hepatocytes although they have not been identified. Here we show by reverse transcriptase-polymerase chain reaction (RT–PCR) that rat hepatocytes express mRNA encoding all of the four cloned rat P2Y receptors (P2Y1, P2Y2, P2Y4 and P2Y6).
The effects of UTP have been examined on single aequorin-injected rat hepatocytes. The [Ca2+]i transients induced by UTP were indistinguishable from those induced by ATP in the same cell. The modulatory effects of elevated intracellular cyclic AMP concentration were the same on both UTP- and ATP-induced [Ca2+]i transients.
UDP, an agonist at the P2Y6 receptor, failed to induce transients in hepatocytes, indicating that functional P2Y6 receptors coupled to increased [Ca2+]i are not expressed.
The transients evoked by ADP were more sensitive to inhibition by suramin than those induced by either ATP or UTP. Within an individual cell, the transients induced by ATP and UTP were inhibited by the same concentration of suramin. This sensitivity of ATP and UTP responses to suramin suggests action through P2Y2 rather than P2Y4 receptors.
Co-application of 30 μM pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) caused a decrease in frequency and amplitude of transients induced by ADP. ATP- and UTP-induced transients also displayed a decrease in amplitude in response to addition of PPADS, but this was accompanied by an increase in frequency of transients.
In conclusion the data presented here are consistent with the co-expression of P2Y1 and P2Y2 receptors by rat hepatocytes.
Keywords: Rat hepatocytes, P2Y receptors, intracellular free calcium concentration, calcium transients, suramin, PPADS, ADP, ATP, UTP, RT–PCR
Introduction
The nucleotides ADP, ATP, UDP and UTP act as extracellular agonists at P2 receptors, a family of cell-surface receptors which has been divided into two classes based on modes of signal transduction and subsequently molecular cloning: the ligand-gated ion channels, or P2X receptors; and G-protein-coupled, P2Y receptors (Burnstock, 1996). There are a number of subdivisions of both classes which display differential responsiveness to nucleotide agonists (see Boarder & Hourani, 1998), and in the absence of selective antagonists this has formed the basis of pharmacological characterization of these receptors. The five mammalian P2Y receptor subtypes cloned to date are all coupled to hydrolysis of phosphatidylinositol 4,5-bisphosphate and hence to inositol 1,4,5-trisphosphate-mediated release of intracellular Ca2+ stores (Boarder & Hourani, 1998).
In common with many other cell types (Berridge, 1990), single rat hepatocytes generate repetitive transients in cytosolic free calcium concentration ([Ca2+]i) when stimulated with agonists acting through the phosphoinositide signalling pathway (Woods et al., 1986; 1987), including ADP and ATP (Cobbold et al., 1988; Dixon et al., 1990). The duration of the [Ca2+]i transients is dependent on the receptor species being activated, so that transients of very different duration can be recorded from the same individual hepatocyte when stimulated with agonists acting at different receptors (Woods et al., 1987). Increasing the agonist concentration increases the frequency of transients without affecting the duration of individual transients (Woods et al., 1986). The observation that ADP and ATP induce [Ca2+]i transients of very different duration provided the first indication that these nucleotides were acting at distinct receptors on rat hepatocytes (Dixon et al., 1990).
The proposal that hepatocytes express multiple P2Y receptors was strengthened by reports of further differences in the actions of nucleotides both on single cells (Dixon et al., 1993; 1995a; Green et al., 1994) and populations of rat hepatocytes (Keppens & De Wulf, 1991; Keppens et al., 1993; Keppens, 1993). However, the subtypes present have not been identified. Here we have investigated by RT–PCR, the expression of mRNA species encoding the four cloned rat P2Y receptor subtypes. In single cell studies we have investigated the effect of extracellular UTP and compared it with the effect of ATP in the same aequorin-injected hepatocyte. We have also examined the effect of UDP, the most potent agonist at the P2Y6 receptor subtype, and the antagonists suramin and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS). These studies provide information about the P2Y subtypes expressed by rat hepatocytes.
Methods
Hepatocyte preparation
Hepatocytes were isolated from fed, male Wistar-strain rats (150–250 g) by collagenase perfusion as described previously (Woods et al., 1987). Briefly, the hepatic portal vein was cannulated and during an initial Ca2+-free perfusion, the liver was dissected out of the animal. The liver was subsequently perfused on a re-circulating basis for 15 min with collagenase (0.04% w v−1) and Ca2+ (3.8 mM). The perfusion rate was 30 ml min−1 throughout. A purified population (95–98%) of parenchymal cells was obtained through differential centrifugation.
Reverse transcriptase-polymerase chain reaction
Total RNA was prepared from ∼4×106 primary rat hepatocytes (n=3) using Trizol, according to the manufacturer's instructions, and was treated with RNase-free DNaseI for 15 min at 37°C and then re-extracted with Trizol. First strand cDNA was prepared from 5 μg RNA using an oligo(dT) 18 primer and Moloney-murine leukaemia virus reverse transcriptase in a 40 μl reaction volume, according to the manufacturer's instructions. Reverse transcriptions were also performed in the absence of reverse transcriptase as a control for contaminating DNA. PCR reactions for the four rat P2Y receptors (P2Y1, P2Y2, P2Y4 and P2Y6) were performed with specific primer pairs designed to amplify partial cDNAs from each sequence: P2Y1F 5′-TG GT GG CC AT CT CC CC TA TT CT CT T-3′; P2Y1R 5′-ATCT CG TG CC TT CA CA AA CT C-3′; P 2Y2F5′-TTCCAC GT CACC CG CA CC CT CT TA TT AC T-3′; P2Y2R5′-CG AT TC CCCAAC TCACAC AT AC AA ATG AT TG-3′; P2Y4 F5′ CTTC TCTG CC TG GG TG TTT GG TT GG TAGTA-3′; P2Y4R 5′-TCCCCCGT GA AG AGATAGAGCACTGGA-3′; P2Y6F 5′-GC CA GT TA TG GA GC GG GA CA AT GG -3′; P2Y6R 5′-AGGAACAGGATGCTGCCG TGTAGGTT G-3′.
PCR reactions were performed using 7.5% (v v−1) of each first strand cDNA reaction and 15 pmol of each subtype-specific forward and reverse primer, using 2.5 units of Biotaq with the buffer supplied, 200 μM of each deoxy-nucleotide triphosphate and 1.5 mM MgCl2. Amplification conditions were 60 s at 94°C, 30 s at 60°C, 45 s at 72°C for 35 cycles and finally 10 min at 72°C. Control amplifications were performed in parallel using plasmid DNA (5 ng) encoding each receptor subtype, the −RT cDNA synthesis and a reaction from which template was excluded. Amplification products (10 μl) were resolved on a 2% (w v−1) agarose gel by electrophoresis. PCR products were sequenced directly using an automated DNA sequencer to confirm their identity.
Preparation of single cells
After harvesting the cells were incubated at 37°C at low density (∼103 cells ml−1) in 2% type IX agarose in William's medium E (WME). Single hepatocytes were transferred to 0.1 mm path length microslides containing 1% type VII agarose which was subsequently gelled at 4°C for 2 min. The cells were then held at 37°C under a layer of liquid paraffin.
Aequorin preparation
An exhaustive description of the aequorin technique is provided in Cobbold & Lee (1991). A stock solution of aequorin was prepared by dissolving 1 mg aequorin in 15 μl of Ca2+-free buffer (EDTA 10 mM, PIPES 10 mM, pH 7.0). Small aliquots (∼200 nl) were dialysed on a micro-scale against injection buffer (KCl 150 mM, PIPES 1 mM, EDTA 100 μM, EGTA 25 μM), to reduce the concentrations of KCl and EDTA. The dialysed aequorin was held as a droplet under liquid paraffin.
Microinjection procedure and data acquisition
Freshly-pulled pipettes were filled with aequorin by dipping the tip for a few seconds in the aequorin droplet. Individual hepatocytes were injected to approximately 0.5% of the cell volume, and transferred in the microslide to a perfusable cup held at 37°C, positioned under a cooled, low-noise photomultiplier, and continuously superfused with WME, to which agonists were added. Photon counts were sampled every 50 ms by computer. At the end of an experiment, the total aequorin content of each cell was determined by discharging the aequorin by lysing the cell. The data were normalized retrospectively by computer, by calculating the photon counts per second divided by the total counts remaining. In vitro calibration data of the aequorin signal in terms of [Ca2+]i were calculated by determining the rate of consumption of aequorin in Ca2+-EGTA buffers mimicking the intracellular milieu, with [Ca2+] ranging from 10−8 M to 10−5 M, and 1 mM free Mg2+ (Cobbold & Lee, 1991). The computed fractional rate of aequorin consumption could then be plotted as [Ca2+]i using exponential smoothing with time constants: for resting [Ca2+]i, 12 s; for transients, 1 s.
Materials
Aequorin was provided by Prof O. Shimomura (Marine Biological Laboratory, Woods Hole, MA, U.S.A.). Collagenase was obtained from Boehringer and WME from Gibco BRL. Agarose and nucleotides were purchased from Sigma, suramin from Calbiochem and PPADS from Tocris Cookson. Intracellular cyclic AMP levels were raised by addition of the cell permeant analogue of cyclic AMP, dibutyryl cyclic AMP. A stock solution of UDP (1 mM) was pre-incubated with hexokinase (10 units ml−1; Sigma) and 22 mM glucose for 1 h to remove contaminating UTP (Nicholas et al., 1996). Biotaq was purchased from Bioline U.K., Moloney-murine leukaemia virus reverse transcriptase, RNase-free DNaseI and Trizol were all obtained from Gibco BRL. Oligonucleotides were synthesized by Genosys. All other molecular biology reagents were obtained from Sigma.
Results
Expression of mRNA for P2Y receptor subtypes
cDNA derived from rat hepatocytes was subjected to 35 cycles of PCR amplification using oligonucleotide primers specific for the four cloned rat P2Y subtypes (P2Y1, P2Y2, P2Y4 and P2Y6). PCR products with the predicted sizes for each of the P2Y receptor subtypes were apparent in the reactions that included RT, but were absent from those that did not (Figure 1), demonstrating expression of transcripts encoding all four receptors by rat hepatocytes. Products were also absent from reactions where exogenous template was not included (data not shown). The identity of the amplified products was confirmed by sequence analysis.
Figure 1.
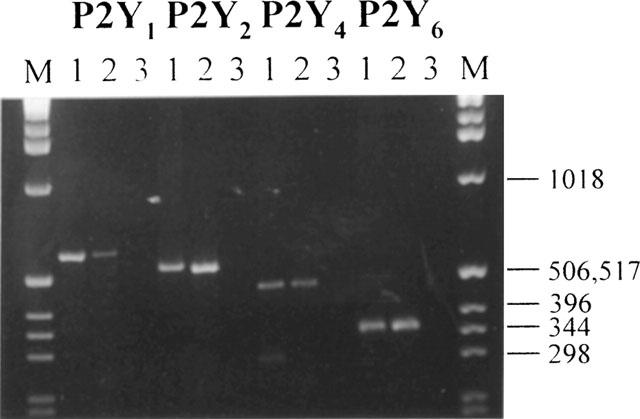
RT–PCR analysis of P2Y receptor transcripts present in primary rat hepatocytes. Agarose gel electrophoresis of PCR products, see Methods for further details. (M), size markers: 1 Kbp ladder, appropriate sizes are indicated. For each receptor amplification lane 1 is a PCR reaction using the appropriate plasmid construct as template, lanes 2 and 3 incorporate cDNA syntheses where reverse transcriptase was present or absent respectively. The figure is representative of three independent experiments.
[Ca2+]i responses of single rat hepatocytes to UTP and UDP
The application of UTP (0.4–8 μM) to 20 single aequorin-injected hepatocytes resulted in the generation of repetitive [Ca2+]i transients in every cell. Here we compared the effects of UTP and ATP in the same cell; in 18 out of 20 cells the transients evoked by UTP were indistinguishable from those evoked by ATP. This is illustrated in Figure 2 which additionally depicts the contrasting transients of short duration which are invariably induced by ADP.
Figure 2.
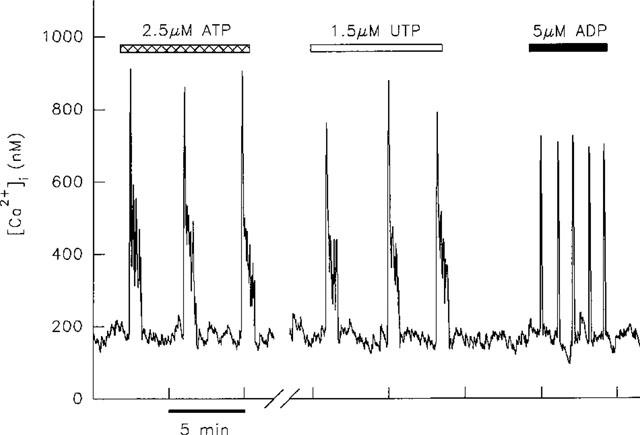
[Ca2+]i transients induced by 1.5 μM UTP in a single aequorin-injected hepatocyte are indistinguishable in terms of duration from those induced by 2.5 μM ATP. In contrast 5 μM ADP induced transients of much shorter duration. The break in the x-axis represents a period of 15 min during which the cell was superfused with WME alone. This result is representative of records from 18 cells.
We then investigated the effects of elevated cyclic AMP levels on [Ca2+]i transients evoked by UTP. We have previously shown that this experimental protocol has different modulatory effects on ADP- and ATP-induced [Ca2+]i transients (Green et al., 1994). Thus the peak height and frequency of transients induced by ADP are enhanced, whereas ATP-induced transients undergo either an increase in duration of individual transients or conversion into a sustained rise (Green et al., 1994). In the present study the effect of co-application of 10 μM dibutyryl cyclic AMP on UTP-induced transients was investigated in seven cells. In six cells the effect was found to be the same as that previously observed for ATP-induced transients; UTP-induced transients were prolonged (four cells), or converted into a sustained rise (two cells) as illustrated in Figure 3. In our previous study we showed that raising cyclic AMP concentrations by direct activation of adenylyl cyclase with forskolin had the same modulatory effect on ATP-induced [Ca2+]i transients, thus arguing against a non-specific action of dibutyryl cyclic AMP.
Figure 3.
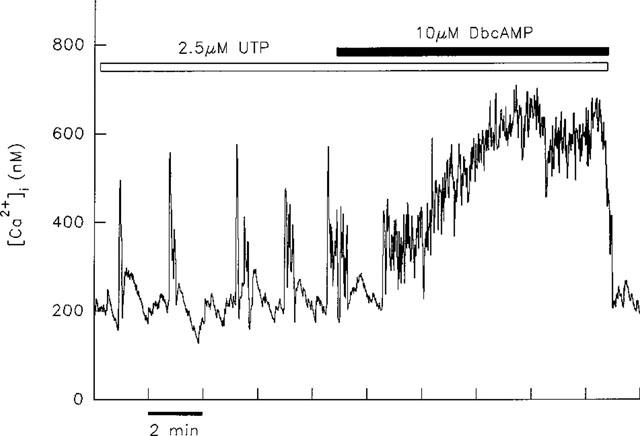
Effect of elevated intracellular cyclic AMP concentration on [Ca2+]i transients induced by UTP. Application of the cell-permeant analogue of cyclic AMP, dibutyryl cyclic AMP (dbcAMP), resulted in the conversion of transients induced by 2.5 μM UTP into a sustained rise in [Ca2+]i.
The effect of UDP was investigated in three cells. Due to the high concentrations of agonist used, the UDP stock was treated with hexokinase to remove contaminating UTP (Nicholas et al., 1996). In all three cells 100 μM UDP had no effect on [Ca2+]i (data not shown).
Effects of suramin on [Ca2+]i transients induced by ADP, ATP and UTP
Experiments with suramin revealed that ADP-evoked [Ca2+]i transients were more sensitive to inhibition by this antagonist than those evoked by either ATP or UTP. Thus co-application of 25 μM suramin to hepatocytes stimulated with ADP resulted in the inhibition of transients in five out of ten cells as illustrated in Figure 4. In three out of ten cells this dose of suramin caused a decrease in frequency of transients and the remaining two cells were unaffected by this treatment. In contrast, the same concentration of suramin inhibited ATP-induced transients in only one/five hepatocytes. In three out of five cells the oscillatory response to ATP was unaffected by 25 μM suramin, with the remaining cell displaying transients at a reduced frequency. UTP-induced transients were unaffected by 25 μM suramin in two cells as shown in Figure 5.
Figure 4.
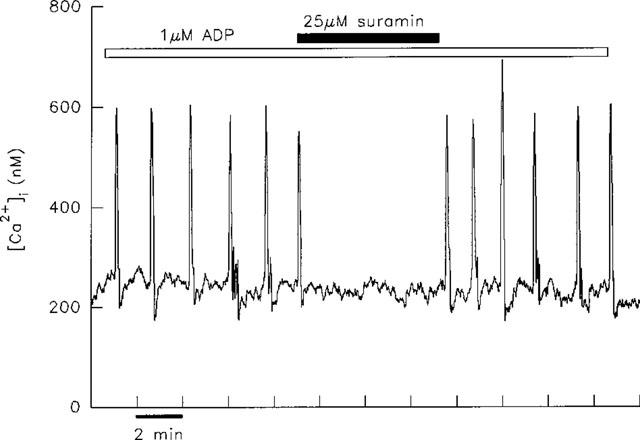
Inhibition of ADP-induced [Ca2+]i transients by suramin. A single hepatocyte responded to 1 μM ADP with typical transients of short duration. The application of 25 μM suramin led to the prompt inhibition of transients. Transients were restored when suramin was removed.
Figure 5.
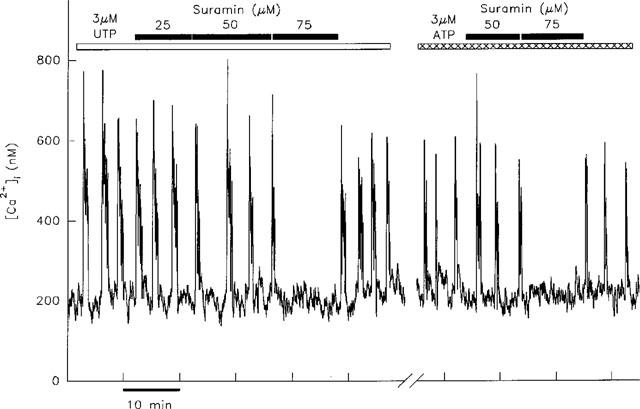
Effect of suramin on transients induced by UTP and ATP. Transients evoked by 3 μM UTP in a single hepatocyte were unaffected by the application of 25 or 50 μM suramin, but were inhibited when the concentration was increased to 75 μM. In the same cell the transients induced by 3 μM ATP were similarly unaffected by 50 μM suramin, but were blocked by 75 μM suramin. Inhibition of transients induced by ATP and UTP by the same concentration of suramin was observed in three out of three cells. The break in the x-axis represents a period of 30 min during which the cell was superfused with WME alone.
The differential sensitivity to suramin of ADP-induced [Ca2+]i transients compared with those induced by ATP (or UTP) is more clearly illustrated at 50 μM suramin. This concentration of antagonist abolished the ADP-induced transients in six out of eight cells, with the remaining cells displaying transients at lower frequency. However, ATP-induced transients were inhibited in only two out of eight hepatocytes by 50 μM suramin. The remaining six cells continued to produce transients, (Figure 5) although their frequency was decreased in five. Similarly, UTP-induced [Ca2+]i transients were unaffected by 50 μM suramin in two cells as shown in Figure 5. This figure additionally illustrates that in an individual cell the same concentration of suramin was required to inhibit transients induced by both ATP and UTP.
At higher concentrations (75–100 μM) suramin abolished the ATP- and UTP-induced [Ca2+]i transients in all cells (see Figure 5). This was not due to a non-specific effect of suramin on the phosphoinositide signalling pathway as transients induced by the α1-adrenergic agonist, phenylephrine were unaffected by the application of 100 μM suramin in three cells (data not shown). The effects of suramin were largely reversible; the observed decrease in frequency or abolition of transients was reversed in 12 out of 16 cells, with transients produced at the original frequency upon removal of suramin (see Figures 4 and 5).
Effects of PPADS on [Ca2+]i transients induced by ADP, ATP and UTP
The effect of 30 μM PPADS was investigated in five cells producing transients in response to ADP. This concentration of PPADS has previously been shown to competitively block the rat P2Y1 receptor (Schachter et al., 1997). As illustrated in Figure 6, co-application of PPADS resulted in a prompt decrease in frequency of transients in all five cells, and a concomitant decrease in peak height was seen in four of these cells. The effects of PPADS were largely reversible; peak height was restored in all five cells upon removal of PPADS. However, as depicted in Figure 6, transient frequency only partially recovered.
Figure 6.
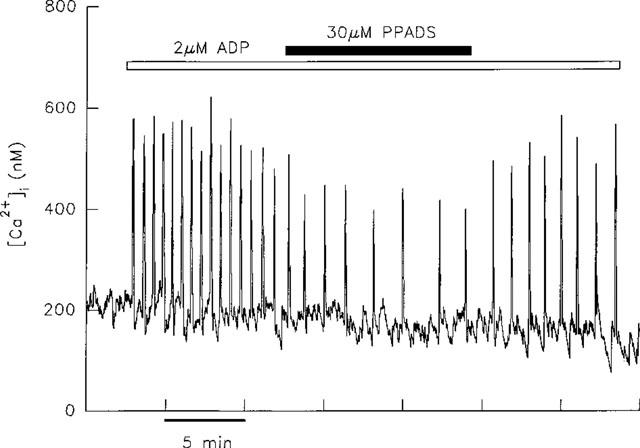
Effect of PPADS on transients induced by ADP. The application of 30 μM PPADS to a single hepatocyte responding to 2 μM ADP resulted in a decrease in frequency and amplitude of transients. When PPADS was removed transient amplitude was restored, but recovery of transient frequency was only partial.
A similar decrease in peak height was observed for transients induced by ATP (two out of three cells) or UTP (three out of three cells). However, in contrast to the decrease in frequency of ADP-induced transients, co-application of 30 μM PPADS led to an increase in frequency of ATP- (three out of three cells) or UTP-induced [Ca2+]i transients (three out of three cells) as illustrated in Figure 7. These effects were not reversed upon removal of PPADS in the continued presence of agonist. However, following a 10–15 min wash, re-application of agonist gave rise to transients at the original frequency and amplitude in every cell (data not shown). The same concentration of PPADS had no effect on either the frequency or peak height of transients induced by phenylephrine (three out of three cells; data not shown).
Figure 7.
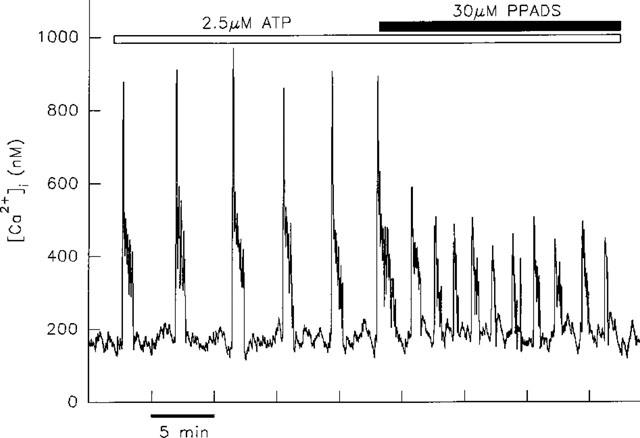
Effect of PPADS on ATP-induced transients. The application of 30 μM PPADS to a single cell led to a decrease in amplitude of the transients induced by 2.5 μM ATP. This was accompanied by an increase in the frequency of transients.
Discussion
The molecular biology studies described here demonstrate that transcripts for all four of the cloned rat P2Y receptors (P2Y1, P2Y2, P2Y4 and P2Y6) are expressed by rat hepatocytes. However, results from single cell studies suggest that not all of these transcripts are translated into functional receptors, or that these receptors are expressed but are coupled to a signalling pathway other than [Ca2+]i. A similar finding was reported by Schofl et al. (1999) for two human hepatoma cell lines, Hep G2 and HuH-7; mRNA for all four of the P2Y receptors for which they tested was detected, yet the results of Ca2+i studies demonstrated that not all of these were functionally expressed.
Evidence for expression of P2Y1 receptors by rat hepatocytes
Similarities in the [Ca2+]i transients evoked by ADP and 2-MeSATP in single aequorin-injected rat hepatocytes indicate that these nucleotides act at a common receptor, which has an agonist profile characteristic of the P2Y1 receptor (Dixon et al., 1995b). In populations of rat hepatocytes the effects of ADPβS resemble those of 2-MeSATP, again consistent with action through a P2Y1 receptor (Keppens & DeWulf, 1991; Keppens et al., 1993). P2Y1 receptor mRNA has been detected in rat liver (Tokuyama et al., 1995) and the RT–PCR studies reported here confirm the expression of transcripts for the P2Y1 receptor by rat hepatocytes. Schofl et al. (1999) detected mRNA for the P2Y1 receptor in the human hepatoma cell lines, Hep G2 and HuH-7. These cells were however, unresponsive to ADP, indicating that functional receptors were not expressed. Cell lines derived from hepatomas differ substantially in their properties to primary hepatocytes, and differences in receptor expression are therefore not unexpected. Unfortunately, although primary human hepatocytes were also included in their study, the effect of ADP on this cell type was not determined (Schofl et al. 1999).
[Ca2+]i transients induced by ADP were more readily abolished by suramin than those evoked by either ATP or UTP. This is consistent with ADP acting at the P2Y1 receptor; antagonism by suramin is most potent at this P2Y subtype (Boarder & Hourani, 1998). The rat P2Y1 receptor is competitively inhibited by PPADS in both native (Hansmann et al., 1997; Ralevic & Burnstock, 1996; Vigne et al., 1998) and transfected systems (Schachter et al., 1997). The inhibitory effects of PPADS on ADP-induced transients therefore also indicate that the effects of ADP are mediated by a P2Y1 receptor. In a recent study Vigne et al. (1998) reported that in rat brain capillary endothelial cells and rat ileal myocytes, inhibition of the P2Y1 receptor by PPADS was only observed when cells were pre-equilibrated with the antagonist; PPADS was inactive when added at the same time as ADP. Here, PPADS acted promptly to modulate ADP-induced [Ca2+]i transients when co-applied with agonist; the reason for this difference is unclear.
Evidence for expression of P2Y2 receptors
Keppens et al. (1992) reported similarities in the effects of ATP and UTP on populations of rat hepatocytes and concluded that these agonists act at a common receptor. Primary human hepatocytes have also been shown to respond similarly to ATP and UTP (Schofl et al., 1999). Here we show that within the same individual hepatocyte, ATP and UTP induce [Ca2+]i transients that are indistinguishable in terms of duration. Furthermore, transients induced by both nucleotides were modulated similarly by elevated intracellular cyclic AMP levels. The observed modulatory effect is in contrast to the effect on transients induced by either ADP (Green et al., 1994) or 2-MeSATP (Dixon et al., 1995b). The possible mechanisms underlying the receptor-specific regulation of the hepatocyte Ca2+ oscillator by increased cyclic AMP levels have been discussed previously (Green et al., 1994). The similarity in the modulation of ATP- and UTP-induced transients is consistent with the proposal that these nucleotides share a receptor(s) on rat hepatocytes. This is further strengthened by the demonstration that the same concentration of suramin is required to inhibit transients induced by both ATP and UTP in an individual cell, and also by the similarity of the effect of PPADS.
It has recently been shown that in common with the P2Y2 receptor, the rat homologue of the P2Y4 receptor is activated equipotently by ATP and UTP (Bogdanov et al., 1998; Webb et al., 1998). This is in contrast to the human P2Y4 receptor which displays a marked preference for UTP over ATP (Communi et al., 1995; Nguyen et al., 1995). Expression by rat hepatocytes of either (or both) of these subtypes could then account for the effects of ATP and UTP. Indeed transcripts for both of these receptor subtypes were detected in the RT–PCR studies described here. However, the antagonist suramin offers some means of discriminating between the involvement of P2Y2 or P2Y4 receptors. Thus the rat P2Y4 receptor has been shown to be insensitive to suramin (Bogdanov et al., 1998). In contrast, the rat P2Y2 receptor is inhibited by suramin (Chen et al., 1996) albeit at higher concentrations than required to antagonize P2Y1-mediated effects (Schachter et al., 1997). The inhibition of ATP- and UTP-induced [Ca2+]i transients by 75–100 μM suramin therefore suggests that these responses are mediated by P2Y2 rather than P2Y4 receptors, although a contribution to the ATP/UTP response by the P2Y4 receptor can not be completely discounted.
mRNA encoding the P2Y6 receptor was detected by RT–PCR. However, the lack of effect of UDP, the most potent agonist at this P2Y subtype (Nicholas et al., 1996), suggests that these transcripts are not translated into functional receptors in rat hepatocytes.
PPADS has previously been reported to be ineffective at both P2Y2 and P2Y4 receptors (Charlton et al., 1996a,1996b; Bogdanov et al., 1998). The effect of PPADS on ATP- and UTP-induced [Ca2+]i transients described here therefore apparently contradicts these earlier findings. However, the modulatory effects observed in single cells may be masked in a population of cells. Thus considering the total Ca2+ mobilized, the increase in frequency of transients will compensate for the decrease in amplitude of individual transients, so that overall the total Ca2+ released may be unchanged. This study indicates that caution should be exercised in the use of PPADS as a selective antagonist for the P2Y1 receptor; ATP- and UTP-induced effects may not be refractory to this antagonist as suggested by population studies.
With the limited tools available to investigate P2Y receptor expression it is not possible to determine definitively which receptors are expressed by a given cell type. However, the use of single cells as described here avoids some of the complications that afflict this field. The single hepatocyte is held in isolation from any other cells, and is constantly superfused with medium which provides a continuous supply of fresh agonist and simultaneously removes any breakdown products. Hydrolysis and inter-conversion of nucleotides by extracellular enzymes which must be accounted for in a cell population, is therefore not an issue (Harden et al., 1997). In addition, the [Ca2+]i transients studied are generated at concentrations of agonist very close to the threshold for a response. This threshold concentration is similar for all nucleotide agonists which evoke transients. It is therefore extremely unlikely that the effects described are due to nucleotides contaminating the agonist stock which is a complicating factor in the interpretation of studies employing high doses of nucleotides (Nicholas et al., 1996; Leon et al., 1997). In conclusion these studies provide evidence that rat hepatocytes co-express P2Y1 and P2Y2 receptors. However, involvement of the P2Y4 receptor in the response to ATP and UTP can not be fully discounted. The definitive test of these conclusions awaits the advent of receptor-specific antibodies and selective ligands/antagonists.
Acknowledgments
We are grateful to The Wellcome Trust for funding (C.J. Dixon, T.E. Webb and A.K. Green), and to Dr Michael Boarder for providing facilities for the molecular biology studies. This paper is dedicated to the memory of Dr Antonio Sanchez-Bueno.
Abbreviations
- [Ca2+]i
intracellular free Ca2+ concentration
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
- RT–PCR
reverse transcriptase-polymerase chain reaction
- WME
William's medium E
References
- BERRIDGE M.J. Calcium oscillations. J. Biol. Chem. 1990;265:9583–9586. [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOGDANOV Y.D., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G.P2 purinoceptors: historical perspective and classification P2 purinoceptors: localization, function and transduction mechanisms 1996198Chichester: John Wiley & Sons Ltd; 1–29.Ciba Foundation Symposium [DOI] [PubMed] [Google Scholar]
- CHARLTON S.J., BROWN C.A., WEISMAN G.A., TURNER J.T., ERB L., BOARDER M.R. Cloned and transfected P2Y4 receptors: characterization of a suramin and PPADS-insensitive response to UTP. Br. J. Pharmacol. 1996a;119:1301–1303. doi: 10.1111/j.1476-5381.1996.tb16038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARLTON S.J., BROWN C.A., WEISMAN G.A., TURNER J.T., ERB L., BOARDER M.R. PPADS and suramin as antagonists at cloned P2Y- and P2U purinoceptors. Br. J. Pharmacol. 1996b;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Z-P., KRULL N., XU S., LEVY A., LIGHTMAN S.L. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- COBBOLD P.H., LEE J.A.C.Aequorin measurements of cytoplasmic free calcium Cellular Calcium: A Practical Approach 1991Oxford: I.R.L. Press; 55–81.McCormack, J.G. & Cobbold, P.H. (eds). pp [Google Scholar]
- COBBOLD P., WOODS N., WAINWRIGHT J., CUTHBERTSON K.S.R. Single cell measurements in research on calcium-mobilising purinoceptors. J. Receptor Res. 1988;8:481–491. doi: 10.3109/10799898809049006. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., PIROTTON S., PARMENTIER M., BOEYNAEMS J.M. Cloning and functional expression of a human uridine nucleotide receptor. J. Biol. Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- DIXON C.J., COBBOLD P.H., GREEN A.K. Adenosine 5′[αβ-methylene]triphosphate potentiates the oscillatory cytosolic Ca2+ responses of hepatocytes to ATP but not ADP. Biochem. J. 1993;293:757–760. doi: 10.1042/bj2930757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., COBBOLD P.H., GREEN A.K. Oscillations in cytosolic free Ca2+ induced by ADP and ATP in single rat hepatocytes display differential sensitivity to application of phorbol ester. Biochem. J. 1995a;309:145–149. doi: 10.1042/bj3090145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., COBBOLD P.H., GREEN A.K. Actions of ADP, but not ATP, on cytosolic free Ca2+ in single rat hepatocytes mimicked by 2-methylthioATP. Br. J. Pharmacol. 1995b;116:1979–1984. doi: 10.1111/j.1476-5381.1995.tb16401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., WOODS N.M., CUTHBERTSON K.S.R., COBBOLD P.H. Evidence for two calcium-mobilizing purinoceptors on rat hepatocytes. Biochem. J. 1990;269:499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN A.K., COBBOLD P.H., DIXON C.J. Elevated intracellular cyclic AMP exerts different modulatory effects on cytosolic free Ca2+ oscillations induced by ADP and ATP in single hepatocytes. Biochem. J. 1994;302:949–955. doi: 10.1042/bj3020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSMANN G., BULTMANN R., TULUC F., STARKE K. Characterization by antagonists of P2-receptors mediating endothelium-dependent relaxation in rat aorta. Naunyn Schmiedeberg's Arch. Pharmacol. 1997;356:641–652. doi: 10.1007/pl00005101. [DOI] [PubMed] [Google Scholar]
- HARDEN T.K., LAZAROWSKI E.R., BOUCHER R.C. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G-protein-coupled P2 receptor agonist selectivity. Trends Pharmacol. Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- KEPPENS S. The complex interaction of ATP and UTP with isolated hepatocytes. How many receptors. Gen. Pharmacol. 1993;24:283–289. doi: 10.1016/0306-3623(93)90304-g. [DOI] [PubMed] [Google Scholar]
- KEPPENS S., DE WULF H. Characterization of the biological effects of 2-methylthio-ATP on rat hepatocytes: clear-cut differences with ATP. Br. J. Pharmacol. 1991;104:301–304. doi: 10.1111/j.1476-5381.1991.tb12426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPPENS S., VANDEKERCKHOVE A., DE WULF H. Extracellular ATP and UTP exert similar effects on rat isolated hepatocytes. Br. J. Pharmacol. 1992;105:475–479. doi: 10.1111/j.1476-5381.1992.tb14278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPPENS S., VANDEKERCKHOVE A., DE WULF H. Characterization of the effects of adenosine 5(-[β-thio]-diphosphate in rat liver. Br. J. Pharmacol. 1993;108:663–668. doi: 10.1111/j.1476-5381.1993.tb12858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.-P., GACHET C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- NGUYEN T., ERB L., WEISMAN G.A., MARCHESE A., HENG H.H.Q., GARRAD R.C., GEORGE S.R., TURNER J.T., O'DOWD B.F. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J. Biol. Chem. 1995;270:30845–30848. doi: 10.1074/jbc.270.52.30845. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., WATT W.C., LAZAROWSKI E.R., LI Q., HARDEN T.K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, and an ATP- and UTP-specific receptor. Mol. Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Discrimination by PPADS between endothelial P2Y- and P2U-purinoceptors in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., BOYER J.L., LI Q., NICHOLAS R.A., HARDEN T.K. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br. J. Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOFL C., PONCZEK M., MADER T., WARING M., BENECKE H., VON ZUR MUHLEN A., MIX H., CORNBERG M., BOKER K.H.W., MANNS M.P., WAGNER S. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am. J. Physiol. 1999;276:G164–G172. doi: 10.1152/ajpgi.1999.276.1.G164. [DOI] [PubMed] [Google Scholar]
- TOKUYAMA Y., HARA M., JONES E.M.C., FAN Z., BELL G.I. Cloning of rat and mouse P2Y purinoceptors. Biochem. Biophys. Res. Commun. 1995;211:211–218. doi: 10.1006/bbrc.1995.1798. [DOI] [PubMed] [Google Scholar]
- VIGNE P., PACAUD P., LOIRAND G., BREITTMAYER J.P., FRELIN C. PPADS inhibits P2Y1 purinoceptors in rat brain capillary endothelial cells and in rat ileal myocytes by an indirect mechanism. Biochem. Biophys. Res. Comm. 1998;244:332–335. doi: 10.1006/bbrc.1998.8262. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., HENDERSON D.J., ROBERTS J.A., BARNARD E.A. Molecular cloning and characterization of the rat P2Y4 receptor. J. Neurochem. 1998;71:1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- WOODS N.M., CUTHBERTSON K.S.R., COBBOLD P.H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- WOODS N.M., CUTHBERTSON K.S.R., COBBOLD P.H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987;8:79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]


