Abstract
The effects on the responses to coronary artery occlusion of a combined ACE/NEP inhibitor (Z13752A) were examined in anaesthetized dogs.
A 1 h infusion of Z13752A (128 μg kg−1 min−1 intravenously) decreased arterial blood pressure (by 11±3%; P<0.05) and increased coronary blood flow (by 12±4%, P<0.05). There were no other significant haemodynamic changes.
Z13752A inhibited both NEP and ACE enzymes both in dog plasma and in tissue (lung ACE; kidney NEP). Pressor responses to angiotensin I in vivo were inhibited and systemic vasodilator responses to bradykinin were potentiated.
When the left anterior descending coronary artery was occluded for 25 min, Z13752A markedly reduced the severity of the resultant ventricular arrhythmias. No ventricular fibrillation (VF) occurred (compared to 7/16 in the controls; P<0.05), and ventricular tachycardia (VT) was reduced (VT in 2/9 dogs treated with Z13752A cp. 16/16 of controls; episodes of VT 0.2±0.1 c.p. 10.7±3.3; P<0.05).
Reperfusion of the ischaemic myocardium led to VF in all control dogs but occurred less frequently in dogs given Z13752A (survival from the combined ischaemia-reperfusion insult 67% c.p. 0% in controls; P<0.05).
Z13752A reduced two other indices of ischaemia severity; epicardial ST-segment elevation and inhomogeneity of electrical activation.
These protective effects of Z13752A during ischaemia and reperfusion were abolished by the administration of icatibant (0.3 mg kg−1, i.v.) a selective antagonist of bradykinin at B2 receptors; the ischaemic changes in dogs given both icatibant and Z13752A were similar to those in the controls.
We conclude that this ACE/NEP inhibitor is effective at reducing the consequences of coronary artery occlusion in this canine model and that this protection is primarily due to potentiation of released bradykinin.
Keywords: Myocardial ischaemia, ventricular arrhythmias, Z13752A, bradykinin, combined ACE/NEP inhibition, anaesthetized dogs
Introduction
The cardioprotective effects of ACE inhibition in myocardial ischaemia are well documented in both experimental animal models (Parratt, 1994; Linz et al., 1995; Liu et al., 1996) and in clinical situations (e.g. Lonn et al., 1994; Ikram, 1996), and a clear role for bradykinin in mediating these protective effects has been demonstrated based mainly on studies in which its effects on bradykinin (B2) receptors were antagonized (Schölkens et al., 1989; Linz et al., 1990; Martorana et al., 1990; 1991; Ehring et al., 1994; Liu et al., 1996; Shimada & Avkiran, 1996).
There have been relatively few studies with inhibitors of the other major enzyme responsible for kinin degradation, neutral endopeptidase 24.11 (NEP; Erdõs & Skidgel, 1989). This is a membrane bound metallopeptidase present in endothelial cells (Graf et al., 1995) and in (rat) cardiomyocytes (Piedimonte et al., 1994) which has a high affinity for a variety of vasoactive peptides including substance P, bradykinin, atrial natriuretic peptide and endothelin (for references see Graf et al., 1995). In the heart this is a particularly important enzyme responsible for kinin degradation (Piedimonte et al., 1994; Yang et al., 1997; Kokkonen et al., 1999) but there are few studies dealing with the effects of inhibition of this enzyme, especially under conditions of ischaemia. In rat hearts inhibition of neutral endopeptidase increases myocardial blood flow (Maxwell et al., 1995), reduces infarct size (Yang et al., 1997) and prevents isoprenaline-induced myocardial hypoperfusion (Piedimonte et al., 1994), effects mediated primarily through inhibition of kinin breakdown.
The paucity of studies involving NEP inhibition, especially in large animal models, is surprising in view of the considerable evidence for the cardioprotective effects of both administered and endogenously produced kinins. Kinins reduce ischaemia-induced cell necrosis (Daniell et al., 1984; Noda et al., 1993; Richard et al., 1993), enhance recovery of contractile function after a period of ischaemia and reperfusion (Grocott-Mason et al., 1993; Ehring et al., 1994) and have a particularly pronounced effect in reducing the severity of ischaemia and reperfusion-induced ventricular arrhythmias (Tobé et al., 1991; Végh et al., 1991a). Further there are studies suggesting a role for bradykinin release in the protection of the heart afforded by ischaemic preconditioning (Végh et al., 1994; Wall et al., 1994; Goto et al., 1995; Parratt et al., 1995; 1997; Bouchard et al., 1998) and by cardiac pacing (Kaszala et al., 1997).
In the present study we have examined the effects of a combined ACE/NEP inhibitor Z13752A (N-[(2S)-3-mercapto-2-phenylmethylpropionyl]-4-(2-thiazolyl)-L-phenylalanine; Pradella et al., 1998; Morazzoni et al., 1998a) on the responses of anaesthetized dogs to acute coronary artery occlusion, with particular reference to ischaemia and reperfusion-induced ventricular arrhythmias. Z13752A is a newly developed ACE/NEP inhibitor with an IC50 of 3.2 nM on ACE and of 1.8 nM on NEP (Morazzoni et al., 1998a). Z13752A has been found to potently inhibit plasma and tissue ACE, as well as tissue NEP activity in various in vitro and in vivo experiments. Z13752A resulted in a long lasting antihypertensive effect in both SHR and DOCA-salt hypertensive rats after intravenous or oral administration (Morazzoni et al., 1998b; Pradella et al., 1998). A preliminary account of these results was presented at the Fourth European Congress of Pharmaceutical Sciences in Milan (Morazzoni et al., 1998a, 1998b; Pradella et al., 1998) and at the World Congress of the International Society for Heart Research (Rastegar et al., 1998).
Methods
Evaluation of the effects of Z13752A on plasma and tissue ACE and NEP activities
These experiments were performed at the Zambon Group in Milan, Italy, using beagle dogs of either sex and weighing between 8–12 kg, anaesthetized with sodium pentobarbitone (30 mg kg−1 i.v.) Following experimental preparation for the measurement of arterial blood pressure and heart rate and also collecting blood, Z13752A was infused for 3 h at a flow rate of 0.5 ml min−1 in six dogs in a dose of 0.3 mol kg−1 min−1 (i.e. 128 μg kg−1 min−1) and in eight dogs in a dose of 1.0 μmol kg−1 min−1 (426 μg kg−1 min−1). In a further six and eight dogs respectively, captopril 0.3 μmol kg−1 min−1 (65 μg kg−1 min−1) or vehicle (saline plus NaOH 0.1 N) was infused for 3 h at a flow rate of 0.5 ml min−1. The haemodynamic effects were measured at various times over the 3 h observation period and blood samples (3 ml) for the determination of plasma ACE were collected every 30 min. At the end of the experiments, and before sacrificing the animals, the left kidney was removed for the measurement of tissue NEP activity and an apical portion of the lung for the determination of tissue ACE activity. These tissues were immediately frozen in liquid nitrogen. ACE activity, both in the plasma and in the lung homogenates in the presence of 300 mM NaCl and using [H3]-hippuryl-glycyl-glycine as the substrate, was determined by a radiochemical method according to Ryan and colleagues (1977). NEP activity was determined in kidney homogenates at pH 7.6 containing 0.1% Triton X100 by using spectrophotometric kinetic determination in which glutaryl-ala-ala-phe-2naphtylamide was the reaction substrate (Orlowsky & Wilk, 1981).
In vivo studies in anaesthetized dogs
Mongrel dogs with a body weight in excess of 17 kg (see Végh et al., 1991a) were anaesthetized with a mixture of chloralose and urethane (60 and 200 mg kg−1, i.v. respectively), and ventilated with room air using a Harvard Respirator at a rate and volume sufficient to maintain arterial blood gases and pH within normal limits (Végh et al., 1992). The animals were thoracotomized at the fifth intercostal space and the anterior descending branch of the left coronary artery (LAD) prepared for occlusion just proximal to the first main diagonal branch. This gives an area at risk, as assessed by infusing blue V dye into the occluded artery at the end of the experiment, of around 35–42% (see Results).
Blood flow was measured on both the anterior descending (LAD) and circumflex (LCX) branches of the left coronary artery (Doppler flow probe; Triton, U.S.A. and a 2.0 mm electromagnetic flow probe attached to a Statham SP 2202 flowmeter, respectively). Epicardial ST-segment changes and the degree of inhomogeneity of electrical activation were measured from the left ventricular wall distal to the occlusion site using a ‘composite' electrode described previously (Williams et al., 1974; Végh et al., 1987; 1992). This gives a summarized recording of R-waves from 30 epicardial measuring points. In the adequately perfused and oxygenated myocardium all sites are activated almost simultaneously, resulting in a single large spike. However, following occlusion, widening and fractionation of the summarized R-waves occurs indicating that adjacent fibres are not simultaneously activated because of inhomogeneity of conduction. We expressed this as the greatest delay in activation (ms) within the ischaemic area. This reflects, in part, local changes in myocardial blood flow. The composite electrode also contains four unipolar electrodes by which epicardial ST-segment changes are measured and meaned within the ischaemic area.
All these parameters, together with a limb lead electrocardiogram, systemic arterial and left ventricular (LV) systolic (S) and end-diastolic (ED) pressures (Statham P23XL transducers) and LVdP/dt were recorded on an eight channel Medicor R81 recorder.
Ventricular arrhythmias during a 25 min coronary artery occlusion (i.e. ischaemia) were assessed and analysed as outlined previously (Végh et al., 1992) i.e. total ventricular premature beats (VPBs), the incidence and number of episodes of ventricular tachycardia (VT) and the incidence of ventricular fibrillation (VF). At the end of the period of ischaemia the area supplied by the occluded vessel was rapidly reperfused. The only reperfusion arrhythmia that was determined was VF. Survival (from the combined ischaemia-reperfusion insult) was defined in terms of those dogs which were predominantly in sinus rhythm 10 min after the commencement of reperfusion.
Although these experiments were carried out in Szeged the protocol complied with U.K. Home Office regulations (Project Licence No. 60/00307).
Experimental protocol
There were four groups of animals. Nine dogs were infused with Z13752A, in a dose of 128 μg kg−1 min−1 intravenously over a 1 h period. At the end of this infusion time the left anterior descending coronary artery was occluded for 25 min, and the artery was then re-opened rapidly to allow reperfusion. A second group of 11 animals was given icatibant, an antagonist of bradykinin at B2 receptors, in a dose of 0.3 mg kg−1 as an i.v. bolus, 10 min prior to coronary artery occlusion. This dose of icatibant was sufficient to abolish the protection against ventricular arrhythmias afforded by ischaemic preconditioning (Végh et al., 1994). A third group of 12 additional dogs were also infused with Z13752A, in the dose given above, and after 50 min (i.e. 10 min prior to coronary artery occlusion) were also given icatibant. The responses were compared with those of 16 control dogs which were infused with a similar volume (60 ml) of the vehicle for 1 h and then subjected to coronary artery occlusion followed by reperfusion. The protocol for these four groups is illustrated in Figure 1.
Figure 1.
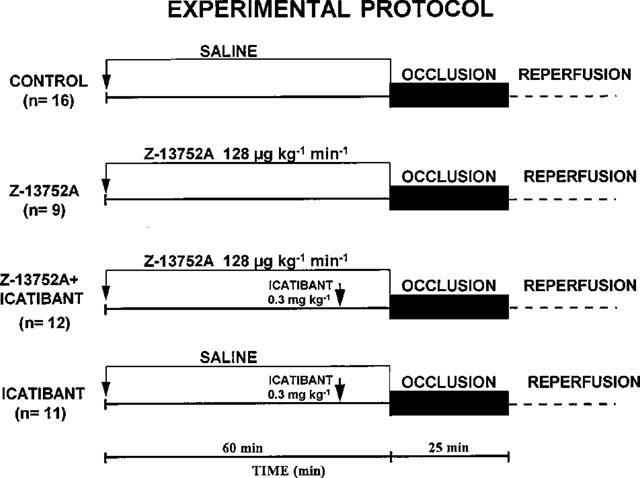
Experimental protocol for the studies involving Z13752A, and its modification by icatibant, an antagonist of bradykinin at B2 receptors. The duration of the Z13752A infusion was 1 h, the occlusion time was 25 min and icatibant was given 10 min prior to occlusion.
In order to determine the effect of Z13752A on the blood pressure responses to angiotensin I (A1), angiotensin II (A2) and bradykinin, separate groups of dogs were given either A1 and A2 (in doses of 5, 10, 15 and 20 ng kg−1 intravenously, n=11) or bradykinin (in doses of 0.1, 0.25, 0.5 and 1 μg kg−1; n=4) both before and at the end of the 1 h infusion period of Z13752A. These responses were compared to those in control dogs given either A1 and A2 (n=6) or bradykinin (n=4), in the doses outlined above, but in which Z13752A was replaced by infusion of the vehicle.
Statistical evaluation
All the data were analysed statistically as previously described (Végh et al., 1992) i.e. data were expressed as means (±s.e.mean) and the differences between means were compared by analysis of variance (ANOVA for repeated measures) or the Student's t-test as appropriate. A one-way ANOVA was undertaken to determine whether or not there were significant haemodynamic differences between the groups. Ventricular premature beats were compared by using the Mann-Whitney Rank Sum test, and the incidence of arrhythmias was compared using the Fisher Exact test. Differences between groups were considered significant when P<0.05.
Results
The effect of Z13752A on plasma and lung ACE and on kidney NEP activities
The effects of two doses of Z13752A, in comparison with captopril and vehicle controls, were examined on plasma ACE activity (Figure 2a) and on tissue ACE and NEP activities (Figure 2b). Both doses of Z13752A completely inhibited plasma ACE activity within the first 30 min of the infusion and this inhibition was maintained throughout the entire infusion period (Figure 2a). Following a 3 h infusion of the 0.3 and 1.0 μmol kg−1 min−1 doses of Z13752A, both lung ACE and kidney NEP activities were markedly reduced (Figure 2b) whereas captopril (0.3 μmol kg−1 min−1) inhibited ACE activity in the lung but was without effect on renal NEP activity (Figure 2b). Infusion of the vehicle did not influence the activity of either of these enzymes (Figure 2b).
Figure 2.
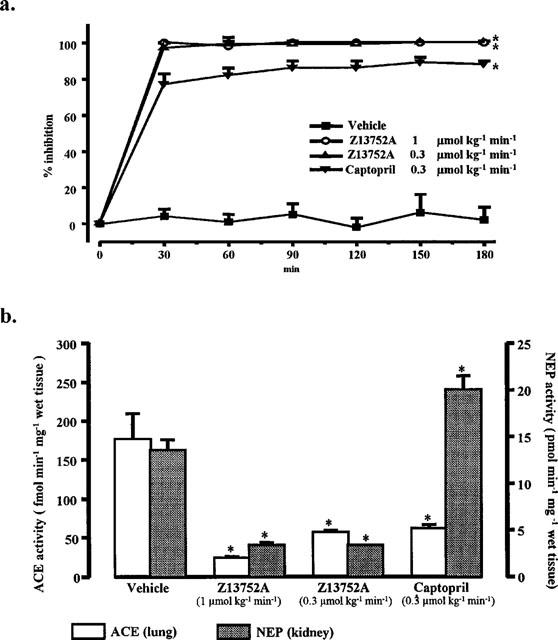
(a) Plasma ACE activity in anaesthetized dogs given intravenous infusion of vehicle (saline+NaOH 1N; n=6), Z13752A in doses of 0.3 μmol kg−1 ml−1 (128 μg kg−1 ml−1; n=6) and 1.0 μmol kg−1 ml−1 (426 μg kg−1 ml−1; n=6) and of captopril in a dose of 0.3 μmol kg−1 ml−1 (65 μg kg−1 ml−1; n=6) over a period of 3 h. Values are means±s.e.mean; *P<0.05 compared to the vehicle controls. (b) Ex vivo determination of tissue ACE and NEP activities, measured in the lung and in the kidney, respectively, following intravenous infusion of vehicle (saline+NaOH 1N; n=8), Z13752A in doses of 0.3 μmol kg−1 ml−1 (128 μg kg−1 ml−1; n=6) and 1.0 μmol kg−1 ml−1 (426 μg kg−1 ml−1; n=8) and of captopril in a dose of 0.3 μmol kg−1 ml−1 (65 μg kg−1 ml−1; n=6) over a period of 3 h. Values are means±s.e.mean; *P<0.05 compared to the vehicle controls.
Haemodynamic effects of Z13752A
At the end of a 1 h infusion of Z13752A (total dose 7.68 mg kg−1) the only significant haemodynamic changes, immediately prior to coronary artery occlusion, were reductions in arterial blood pressure (systolic 129±4 to 117±3 mmHg; diastolic 80±3 to 71±4 mmHg; mean 97±3 to 86±3 mmHg; P<0.05) and in negative LVdP/dtmax (3242±150 to 2879±252 mmHg s−1; P<0.05) and a slight increase in coronary blood flow (and a reduction in coronary vascular resistance; Figure 3). There were no significant changes in LVEDP (5.6±0.6 to 5.3±0.3 mmHg), in heart rate (142±6 to 138±8 beats min−1) or in positive LVdP/dtmax (3666±151 to 3533±225 mmHg s−1). In control dogs a 1 h infusion of the vehicle resulted in no significant haemodynamic changes.
Figure 3.
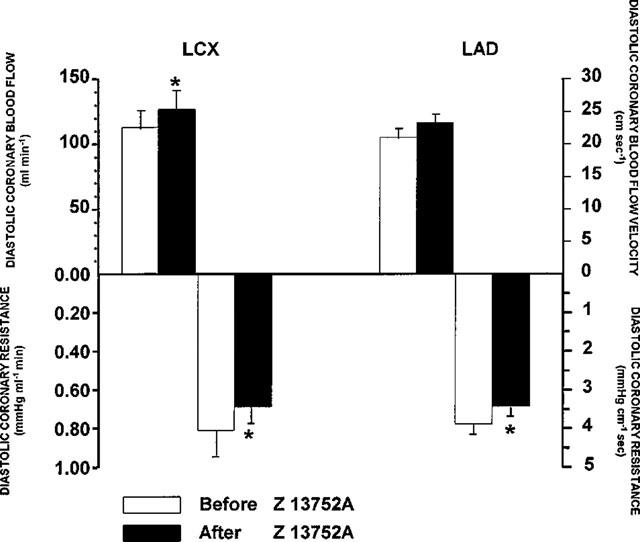
Changes in diastolic coronary blood flow and resistance induced at the end of a 1 h infusion of Z13752A in a dose of 128 μg kg−1 min−1. There is an increase in blood flow in both the circumflex (LCX) and anterior descending (LAD) branches of the left coronary artery and a decrease in coronary vascular resistance. Values are means±s.e.mean; *P<0.05 compared to the identical values before giving Z13752A.
Angiotensin and bradykinin responses before and after Z13752A
In a separate group of dogs the effects of intravenous bolus injections of angiotensin and bradykinin were examined prior to, and at the end of, an infusion of Z13752A in the doses outlined above. These responses were compared to those obtained from control dogs in which the Z13752A was replaced by the vehicle. The results are illustrated in Figures 4 and 5. The vasodepressor response to bradykinin was significantly augmented at all dose levels (Figure 4), particularly so with the lowest doses (100 and 250 ng kg−1). In contrast, angiotensin I responses were significantly reduced and those to angiotensin II slightly potentiated (Figure 5).
Figure 4.
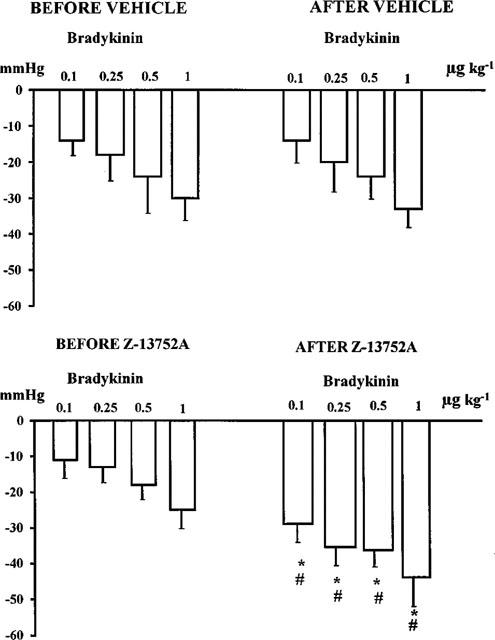
Changes in blood pressure induced by bolus injections of bradykinin in control, vehicle treated dogs (upper panels) and in dogs before and after the administration of Z13752A (lower panels). Values are means±s.e.mean; *P<0.05 compared to the values before giving Z13752A. #P<0.05 compared to the values of the vehicle-treated controls.
Figure 5.
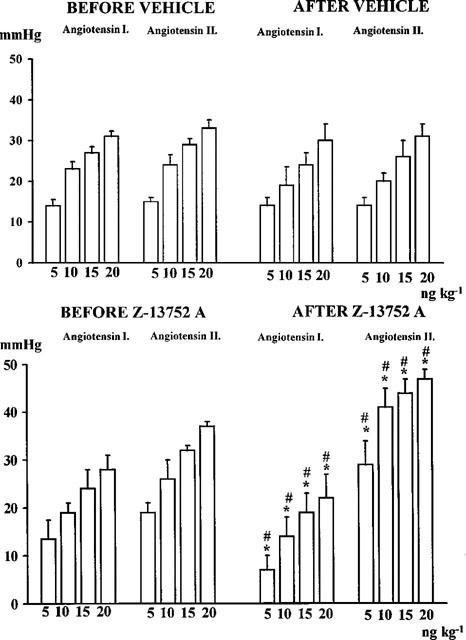
Changes in blood pressure induced by bolus injections of angiotensin I and angiotensin II in vehicle-treated control dogs (upper panels) and in dogs before and after the administration of Z13752A (lower panels). Values are means±s.e.mean; *P<0.05 compared to the values before giving Z13752A. #P<0.05 compared to the values of the vehicle-treated controls.
Modification of the haemodynamic effects of Z13752A by icatibant
Icatibant was given, in a dose of 0.3 mg kg−1 i.v. in two series of experiments (see protocol Figure 1). The only significant haemodynamic effects of icatibant were a slight (4±1 mmHg) increase in mean arterial pressure (95±3 to 99±3 mmHg) and a reduction in heart rate (of 4±1 beats min−1 from 153±7 beats min−1). When given to dogs infused for 1 h with Z13752A there was a small, but significant (P<0.05), increase in mean arterial blood pressure (of 5±1 mmHg) and a decrease in LVdP/dtmax (positive of 336±130 mmHg s−1; negative of 80±150 mmHg s−1) and in heart rate (of 5±2 beats min−1). These icatibant-induced changes were somewhat more pronounced in dogs given Z13752A than in dogs not infused with this drug.
Haemodynamic changes induced by coronary artery occlusion in control dogs, and in dogs given icatibant, Z13752A or Z13752A together with icatibant
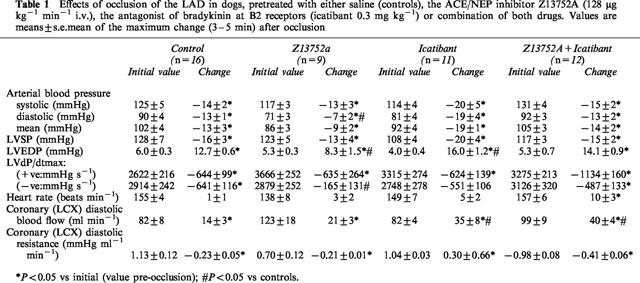
The results are shown in Table 1. In all dogs coronary artery occlusion resulted in decreases in arterial blood pressure and LVdP/dtmax. The marked increase in LVEDP was significantly (P<0.05) less marked in dogs given Z13752A (from 5.3±0.3 to 13.7±1.5 mmHg) than in either the controls (increase from 6.0±0.3 to 18.4±0.6 mmHg) or in dogs given icatibant, either alone (from 4.0±0.4 to 20.1±0.8 mmHg) or in the presence of Z13752A (from 5.3±0.7 to 18.4±1.1 mmHg). The less pronounced increase in LV filling pressure (and the less marked reduction in negative LVdP/dtmax) following occlusion in dogs given Z13752A was not apparent in dogs infused with the drug and then given icatibant (Table 1).
Table 1.
Effects of occlusion of the LAD in dogs, pretreated with either saline (controls), the ACE/NEP inhibitor Z13752A (128 μg kg−1 min−1 i.v.), the antagonist of bradykinin at B2 receptors (icatibant 0.3 mg kg−1) or combination of both drugs. Values are means±s.e.mean of the maximum change (3–5 min) after occlusion
Occlusion of the anterior descending coronary artery led to an immediate and sustained increase in blood flow (maximal at 2 min) in the other major (circumflex) branch of the left coronary artery. This compensatory blood flow increase was unaffected by Z13752A, whether or not icatibant had been administered (Table 1).
Effects of Z13752A, of icatibant and of a combination of Z13752A and icatibant on ventricular arrhythmias following coronary artery occlusion and reperfusion
In this canine model occlusion of the left anterior descending coronary artery leads to pronounced ventricular ectopic activity. In the 16 control dogs in this study all had ventricular premature beats (with a mean of 353±79 over the 25 min occlusion period) and all exhibited VT at some stages during the period of ischaemia. Seven of the dogs fibrillated, usually between 14 and 17 min of the commencement of the occlusion. The distribution of these arrhythmias is illustrated in Figure 6 and the results are summarized in Figure 7.
Figure 6.
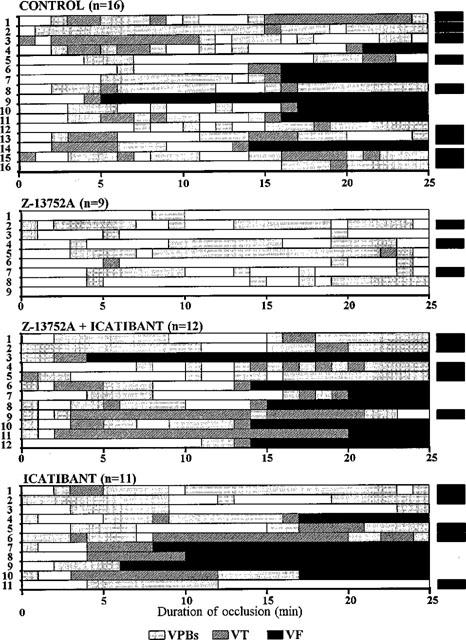
The distribution of ventricular arrhythmias in control dogs, and in dogs infused with Z13752A, with and without icatibant, during a 25 min coronary artery occlusion followed, at the end of this period, by reperfusion. The ACE/NEP inhibitor markedly reduced the severity of these arrhythmias and 6/9 dogs survived the combined ischaemia-reperfusion insult. This protective effect was reversed by icatibant suggesting a role, in the protection, for bradykinin.
Figure 7.
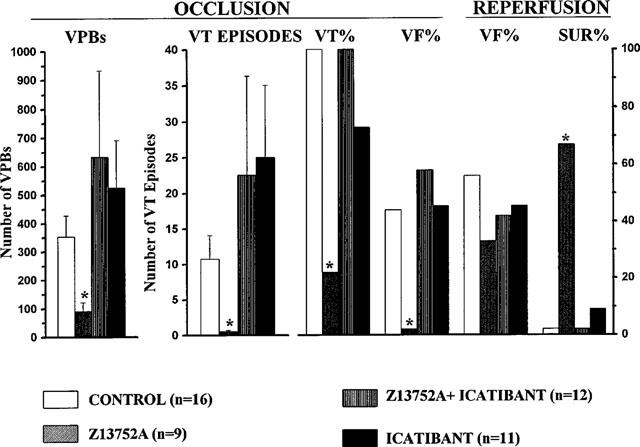
A summary of the effects of Z13752A, with or without icatibant, and of icatibant alone in comparison with control (saline infused) dogs, on ventricular arrhythmias resulting from coronary artery occlusion and subsequent reperfusion. VPBs=ventricular premature beats; VT=ventricular tachycardia; VF=ventricular fibrillation (during occlusion and reperfusion) and SUR=survival. The marked antiarrhythmic effect of Z13752A is abolished by icatibant. Values are means±s.e.mean; *P<0.05 c.p. controls.
These ischaemia-induced arrhythmias were much less pronounced in dogs given Z13752A (Figures 6 and 7). Although all but one of these dogs had some ventricular ectopic activity (mean of 91±41 premature beats; P<0.05 vs control), VT occurred in only two of these nine dogs (P<0.05 vs control) and in both these cases was extremely short-lived (Figure 6). There was no VF during occlusion (P<0.05 vs control).
This marked protection was not seen in dogs infused with Z13752A and then given icatibant (Figures 6 and 7). In these dogs there was again marked ventricular ectopic activity (number of VPBs: 541±256; P<0.05 v Z13752A alone) and a high incidence of (100%) and large number of episodes of VT (32.2±26.8; P<0.05 v Z13752A alone). Arrhythmia severity in these dogs during ischaemia was thus no different to that seen in the controls. Furthermore, seven out of these 12 dogs (58%; P<0.05 v Z13752A alone) fibrillated during the occlusion. Apart from an increase in the number of episodes of VT (to 22.5±13.8), arrhythmia severity after icatibant alone was not significantly different to that in the controls (Figures 6 and 7).
VF occurred following reperfusion in all the control dogs that survived the ischaemic period (Figures 6 and 7) but was less in those dogs given Z13752A (3/9 vs 9/9, P<0.05; Figure 5). This protective effect of Z13752A was abolished by icatibant (reperfusion VF 5/5). Survival from the combined ischaemia-reperfusion insult was thus markedly increased by Z13752A compared to controls (67% vs 0%; P<0.05) and this increase in survival was abolished by icatibant (survival 0%, P<0.05 vs Z13752A alone).
Effect of Z13752A, of icatibant and of a combination of Z13752A and icatibant, on coronary artery occlusion-induced changes in epicardial ST-segment elevation and in the degree of inhomogeneity of electrical activation within the ischaemic area
In control dogs subjected to coronary artery occlusion, the ST-segment recorded from the epicardial electrocardiograms increased within 1 min of the onset of the ischaemia, peaked at 5 min (Figure 8a) and was sustained at this level throughout the 25 min occlusion period. There were similar changes in conduction delay as assessed by the degree of inhomogeneity of electrical activation within the area of ischaemia (Figure 8b). These changes were significantly less marked, and the onset slower, in dogs given Z13752A (Figure 8a,b). Icatibant reversed the protective effect of Z13752A on the degree of inhomogeneity, at least over the initial 15 min (Figure 8b) and, again initially, the ST-segment changes in this group of dogs were similar to that in the controls (Figure 8a).
Figure 8.
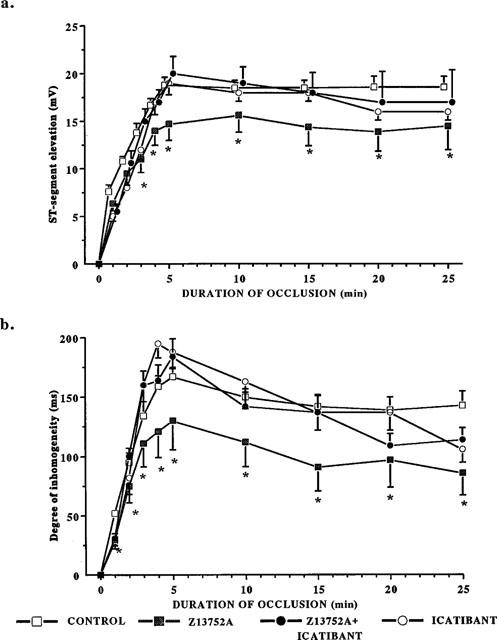
(a) Changes in epicardial ST segment during a 25 min occlusion of the left anterior descending coronary artery in anaesthetized dogs given saline, Z13752A, icatibant and Z13752A in the presence of icatibant. The administration of the ACE/NEP inhibitor leads to a reduction in the severity of the ischaemia, an effect reversed by icatibant. Values are means±s.e.mean; *P<0.05 c.p. control group. (b) Changes in the degree of inhomogeneity of electrical activation during a 25 min occlusion of the left anterior descending coronary artery in control dogs, in dogs given Z13752A, in dogs given icatibant and in dogs given Z13752A in the presence of icatibant. The reduction in this index of the severity of ischaemia is reduced by Z13752A and, again, this is reversed by icatibant. Values are means±s.e.mean; *P<0.05 c.p. control group.
Area at risk in dogs subjected to coronary artery occlusion
There was no significant difference between the four groups in the area at risk from necrosis (i.e. supplied by the occluded artery). These were 38.5±2.0% in the controls, 41.4±2.6% in the dogs infused with Z13752A, 40.8±2.5% in the icatibant group and 45.7±0.8% in the dogs given both Z13752A and icatibant. There were also no significant differences between groups in respect to the weight of the dogs, or in gender distribution. The body weights were 27.2±1.4 kg in the controls, 28.8±0.5 kg in the Z13752A group, 26±1.3 kg in the icatibant alone group and 28.9±1.1 kg in the dogs given both Z13752A and icatibant.
Discussion
The present studies demonstrate that the intravenous administration of the combined ACE/NEP inhibitor Z13752A markedly reduces the detrimental changes that result from coronary artery occlusion and reperfusion in a well documented canine model. Especially pronounced was the marked suppression in the severity of both ischaemia and reperfusion-induced arrhythmias; no dog given the drug fibrillated during the occlusion period and two-thirds of the dogs survived the combined ischaemia-reperfusion insult. This degree of protection against arrhythmias is similar to that previously shown in this model with ischaemic preconditioning (Végh et al., 1990 and reviewed by Parratt & Végh, 1994; 1998), by cardiac pacing (Végh et al., 1991b) and following the local intracoronary infusion of bradykinin (Végh et al., 1991a).
There has been just one study, in Lewis inbred rats, that has examined the effects of an inhibitor of neutral endopeptidase 24.11 on myocardial reperfusion injury (Yang et al., 1997). Using the Ciba-Geigy inhibitor CGS 24592 these authors showed a reduction in infarct size which was similar to that resulting from ramiprilat administration. This protection was abolished by icatibant but unaffected by the ANF receptor antagonist HS-142-1. Their conclusion was that the infarct size reduction following NEP inhibition was mediated by kinins. Yang and colleagues (1997) did not examine arrhythmia severity during coronary occlusion although they did attempt to examine whether reperfusion arrhythmias were modified by CGS 24592. However, the model used (30 min coronary artery occlusion and then reperfusion) is inappropriate to examine these arrhythmias since they are inconsequential after this particular period of ischaemia (Kane et al., 1984). The fact that the ventricular premature complexes which did arise following reperfusion were reduced, albeit not significantly, by this NEP inhibitor is again suggestive of a role for kinins in cardioprotection. In contrast to the findings of Yang and colleagues (1997) and to our previous (Végh et al., 1991a; 1994) and also to our present work, bradykinin has been found to facilitate, rather than alleviate reperfusion arrhythmias in guinea-pig and human myocardial ischaemia models (Hatta et al., 1999). According to this study bradykinin released during myocardial ischaemia accumulates at sympathetic nerve endings and facilitates exocytotic and carrier mediated noradrenaline release which contribute to coronary vasoconstriction and to the generation of ventricular arrhythmias following reperfusion. This unfavourable effect of bradykinin was abolished by the bradykinin B2 receptor antagonist, icatibant (Hatta et al., 1999). However, icatibant was not able to inhibit noradrenaline release unless enalaprilate or a combined kininase I and kininase II inhibitor was present, indicating that under these conditions endogenous bradykinin levels at the nerve endings may not be high enough to facilitate ischaemic noradrenaline release. Although the explanation for this effect of bradykinin is still not known, most of the available evidence supports the idea that the potential beneficial (protective) effect of bradykinin depends on the site of the predominant bradykinin formation in the heart.
The cardioprotective effect of elevated levels of bradykinin resulting from inhibition of cardiac NEP activity has been recently demonstrated in isolated human cardiac membranes (Kokkonen et al., 1999). In these preparations, in which there is a low enzymatic activity of ACE, bradykinin metabolism is mediated mostly by NEP. These results suggest that inhibition of cardiac NEP activity could be cardioprotective by elevating the local concentration of bradykinin in the heart.
As with the Yang and colleagues (1997) study, albeit in a quite different model of ischaemia-reperfusion injury, the most likely explanation for the protective effects of Z13752A in the present experiments is potentiation of the cardioprotective effects of bradykinin by inhibition of its breakdown. Although ACE inhibition presumably plays a role, since Z13752A inhibits both enzymes (Figure 3b), the evidence from the IC50 values (0.0032 μM against ACE; 0.0018 μM against NEP; Morazzoni et al., 1998a) and from the present experiments showing a more marked potentiation of bradykinin vasodilator than of inhibition of angiotensin vasopressor responses in the presence of Z13752A (Figure 5), suggests a predominant effect on neutral endopeptidase 24.11. Indeed, responses to angiotensin II itself were potentiated by the drug (Figure 5), as in the human studies of Richards et al. (1992), an effect attributed by them to reduced angiotensin II clearance. The fact that the protection against arrhythmias was completely abolished by icatibant, a selective antagonist of bradykinin at B2 receptors, and that this drug also abrogated the changes in ST-segment elevation and in the degree of inhomogeneity of electrical activation within the ischaemic area, both indices of ischaemia severity, again supports the view that the cardioprotection observed is largely kinin-mediated. We do not know if this protection, like that afforded by bradykinin itself (Végh et al., 1993), is ultimately due to nitric oxide (NO) and prostacyclin generated and released as a result of an effect of bradykinin on endothelial B2 receptors (Parratt et al., 1997). However, it is known that NEP inhibition leads to an increase in NO production in canine isolated coronary microvessels, and that this is mediated by kinins (Zhang et al., 1998).
Besides kinin breakdown, NEP is also concerned with the breakdown of other peptides such as endothelin (Sokolovsky et al., 1990) and atrial natriuretic peptide (ANP). ANP when infused intravenously in the model we have used in the present study also reduces arrhythmia severity during occlusion and reperfusion (Végh et al., 1998) and could conceivably play a role in the cardioprotective effects of Z13752A. Although selective ANP receptor antagonists are available we have no means of examining such a role for ANP in this particular large animal model. The finding that icatibant abolishes the cardioprotection resulting from Z13752A administration however would argue against this possibility, as does the study of Yang and colleagues (1977) showing that the protective effects of the NEP inhibitor CGS 24592 are unaffected by ANP receptor blockade.
We believe that these results add weight to the hypothesis (Parratt & Végh, 1996) that bradykinin acts as an endogenous myocardial protective substance (Parratt, 1994) and that it plays a role in the protection of the myocardium afforded by ischaemic preconditioning. This hypothesis, the evidence for which has been recently reviewed (Parratt et al., 1997), suggests that brief (preconditioning) periods of ischaemia result, like clinical coronary angioplasty, in the enhanced release of bradykinin from the heart. This then acts on endothelial B2 receptors and stimulates the generation and release of other mediators which, like bradykinin itself, are able to protect the heart against the consequences of prolonged ischaemia. That NO is a particularly important mediator is borne out by the marked attenuation of the cardioprotective effects of bradykinin, given by intracoronary administration, by inhibitors of the L-arginine-NO pathway (Végh et al., 1993). It would be interesting to determine if NEP inhibition potentiates and/or prolongs the antiarrhythmic effects of ischaemic preconditioning. Like similar previous studies involving adenosine potentiation this would require using a sub-threshold preconditioning stimulus and an analysis of how long the cardioprotective effects of Z13752A are maintained. The present study does not attempt to examine these possibilities.
In summary then, this particular ACE/NEP inhibitor protects the heart from the consequences of ischaemia and evidence is adduced to suggest that this protection is mediated by bradykinin.
Acknowledgments
We are grateful to Professor Claudio Semeraro for his interest in this project. We are also grateful to the British Council for continued support of the collaboration between the Glasgow and Szeged departments and to the Hungarian Scientific Research Foundation (OTKA), the Hungarian Ministry of Culture and Education (MKM-FKFP) and the National Healthcare Scientific Committee (ETT) for continued financial support for the programme on the mechanisms of early ischaemia-induced ventricular arrhythmias.
Abbreviations
- A1
angiotensin I
- A2
Angiotensin II
- ACE
angiotensin converting enzyme
- B2
Bradykinin 2 receptor
- DABP
diastolic arterial blood pressure
- LAD
left anterior descending coronary artery
- LCX
left circumflex coronary artery
- LV
left ventricle
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic pressure
- MABP
mean arterial blood pressure
- NEP
neutral endopeptidase
- SABP
systolic arterial blood pressure
- VBP
ventricular premature beats
- VF
ventricular fibrillation
- VT
ventricular tachycardia
References
- BOUCHARD J.-F., CHOUINARD J., LAMONTAGNE D. Role of kinins in the endothelial protective effect of ischaemic preconditioning. Br. J. Pharmacol. 1998;123:413–420. doi: 10.1038/sj.bjp.0701619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIELL H.B., CARSON R.R., BALLARD K.D., THOMAS G.R., PRIVITERA P.J. Effects of captopril on limiting infarct size in conscious dogs. J. Cardiovasc. Pharmacol. 1984;6:1043–1047. [PubMed] [Google Scholar]
- EHRING T., BAUMGART D., KRAJCAR M., HÜMMELGEN M., KOMPA S., HEUSCH G. Attenuation of myocardial stunning by the ACE inhibitor ramiprilat through a signal cascade of bradykinin and prostaglandins but not nitric oxide. Circulation. 1994;90:1368–1384. doi: 10.1161/01.cir.90.3.1368. [DOI] [PubMed] [Google Scholar]
- ERDõS E.G., SKIDGEL R.A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3:145–151. [PubMed] [Google Scholar]
- GOTO M., LIU Y.G., YANG X.M., ARDELL J.L., COHEN M.V., DOWNEY J.M. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ. Res. 1995;77:611–621. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- GRAF K., KOEHNE P., GRÄFE M., ZHANG M., AUCH-SCHWELK W., FLECK E. Regulation and differential expression of neutral endopeptidase 24.11 in human endothelial cells. Hypertension. 1995;26:230–235. doi: 10.1161/01.hyp.26.2.230. [DOI] [PubMed] [Google Scholar]
- GROCOTT-MASON R.M., ANNING P.B., LEWIS M.J., SHAH A.M. Captopril modifies ventricular relaxation in intact hearts via endogenous bradykinin. Endothelium. 1993;1 supplement:S62. [Google Scholar]
- HATTA E., MARUYAMA R., MARSHALL S.J., IMAMURA M., LEVI R. Bradykinin promotes ischemic norepinephrine release in guinea pig and human hearts. J. Pharmacol. Exp. Ther. 1999;288:919–927. [PubMed] [Google Scholar]
- IKRAM H. The renin-angiotensin-aldosterone system and cardiac ischaemia. Heart. 1996;76 Supp 3:60–67. doi: 10.1136/hrt.76.3_suppl_3.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANE K.A., PARRATT J.R., WILLIAMS F.M. An investigation into the characteristics of reperfusion-induced arrhythmias in the anaesthetised rat and their susceptibility to antiarrhythmic agents. Br. J. Pharmacol. 1984;82:349–357. doi: 10.1111/j.1476-5381.1984.tb10769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASZALA K., VÉGH Á., PAPP J.G., PARRATT J.R. Modification by bradykinin B2 receptor blockade of protection by pacing against ischaemia-induced arrhythmias. Eur. J. Pharmacol. 1997;328:51–60. doi: 10.1016/s0014-2999(97)83027-0. [DOI] [PubMed] [Google Scholar]
- KOKKONEN J.O., KUOPPALA A., SAARINEN J., LINDSTEDT K.A., KOVANEN P.T. Kallidin- and bradykinin-degrading pathways in human heart. Degradation of kallidin by aminopeptidase M-like activity and bradykinin by neutral endopeptidase. Circulation. 1999;99:1984–1990. doi: 10.1161/01.cir.99.15.1984. [DOI] [PubMed] [Google Scholar]
- LINZ W., MARTORANA P.A., GRÖTSCH H., BEI-YIN Q., SCHÖLKENS B.A. Antagonizing bradykinin (BK) obliterates the cardioprotective effects of bradykinin and angiotensin-converting enzyme (ACE) inhibitors in ischemic hearts. Drug Devel. Res. 1990;19:393–408. [Google Scholar]
- LINZ W., WIEMER G., GOHLKE P., UNGER T., SCHÖLKENS B.A. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol. Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- LIU Y.-H., YANG X.-P., SHAROV V.G., SIGMON D.H., SABBAH H.N., CARRETERO O.A. Paracrine systems in the cardioprotective effect of angiotensin-converting enzyme inhibitors on myocardial ischemia/reperfusion injury in rats. Hypertension. 1996;27:7–13. doi: 10.1161/01.hyp.27.1.7. [DOI] [PubMed] [Google Scholar]
- LONN E.M., YUSUF S., JHA P., MONTAGUE T.J., TEO K.K., BENEDICT C.R., PITT B. Emerging role of angiotensin-converting enzyme inhibitors in cardiac and vascular protection. Circulation. 1994;90:2056–2068. doi: 10.1161/01.cir.90.4.2056. [DOI] [PubMed] [Google Scholar]
- MARTORANA P.A., KETTENBACH B., BREIPOHL G., LINZ W., SCHÖLKENS B.A. Reduction of infarct size by local angiotensin-converting enzyme inhibition is abolished by a bradykinin antagonist. Eur. J. Pharmacol. 1990;182:395–396. doi: 10.1016/0014-2999(90)90301-l. [DOI] [PubMed] [Google Scholar]
- MARTORANA P.A., LINZ W., SCHÖLKENS B.A. Does bradykinin play a role in the cardiac antiischemic effect of the ACE-inhibitors. Basic Res. Cardiol. 1991;86:293–296. doi: 10.1007/BF02191526. [DOI] [PubMed] [Google Scholar]
- MAXWELL A.J., HUSSEINI W.K., PIEDIMONTE G., HOFFMAN J.I.E. Effects of inhibiting neutral endopeptidase and kininase II on coronary and systemic hemodynamics in rats. Am. J. Physiol. 1995;269:H1016–H1029. doi: 10.1152/ajpheart.1995.269.3.H1016. [DOI] [PubMed] [Google Scholar]
- MORAZZONI G., ALLIEVI L., BRANCA E., DA ROS B., FERLENGA P., LEGNANI G., MARCHINI F., POCCHIARI F., SEMERARO C.In vitro and ex vivo characterization of Z13752A, a new dual-acting ACE/NEP inhibitor Fourth European Congress of Pharmaceutical Sciences 1998a6Suppl 1S33Milan, September 11–13, 1998. EPSCED(abstract 139) [Google Scholar]
- MORAZZONI G., ALLIEVI L., PAUSELLI F., POCCHIARI F., SEMERARO C.Dual inhibition of ACE and NEP activities induced by i.v. and oral administration of Z13752A in spontaneously hypertensive rats Fourth European Congress of Pharmaceutical Sciences 1998b6Suppl 1S33Milan, September 11–13, 1998. EPSCED(abstract 140) [Google Scholar]
- NODA K., SASAGURI M., IDEISHI M., IKEDA M., ARAKAWA K. Role of locally formed angiotensin II and bradykinin in the reduction of myocardial infarct size in dogs. Cardiovasc. Res. 1993;27:334–340. doi: 10.1093/cvr/27.2.334. [DOI] [PubMed] [Google Scholar]
- ORLOWSKY M., WILK S. Purification and specificity of a membrane bound metalloendopeptidase from bovine pituitary. Biochemistry. 1981;20:4924–4950. doi: 10.1021/bi00520a021. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R. Cardioprotection by angiotensin converting enzyme inhibitors–the experimental evidence. Cardiovasc. Res. 1994;28:183–189. doi: 10.1093/cvr/28.2.183. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., VÉGH Á. Pronounced antiarrhythmic effects of ischemic preconditioning. Cardioscience. 1994;5:9–18. [PubMed] [Google Scholar]
- PARRATT J.R., VÉGH Á. Endothelial cells, nitric oxide and ischaemic preconditioning. Basic Res. Cardiol. 1996;91:27–30. doi: 10.1007/BF00788857. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., VÉGH Á.Preconditioning induces both immediate and delayed protection against arrhythmias resulting from ischaemia and reperfusion Advances in Organ Biology. Myocardial Preservation and Cellular Adaptation 1998JAI Press Inc. Stamford, Connecticut; 1–20.In: (eds) Bittar, E.E. & Das, D.K. [Google Scholar]
- PARRATT J.R., VÉGH Á., PAPP J.G. Bradykinin as an endogenous myocardial protective substance with particular reference to ischemic preconditioning–a brief review of the evidence. Can. J. Physiol. Pharmacol. 1995;73:837–842. doi: 10.1139/y95-114. [DOI] [PubMed] [Google Scholar]
- PARRATT J.R., VÉGH Á., ZEITLIN I.J., AHMAD M., OLDROYD K., KASZALA K., PAPP J.G. Bradykinin and endothelial-cardiac myocyte interactions in ischemic preconditioning. Am. J. Cardiol. 1997;80 3A:124A–131A. doi: 10.1016/s0002-9149(97)00467-0. [DOI] [PubMed] [Google Scholar]
- PIEDIMONTE G., NADEL J.A., LONG C.S., HOFFMAN J.I.E. Neutral endopeptidase in the heart: Neutral endopeptidase inhibition prevents isoproterenol-induced myocardial hypoperfusion in rats by reducing bradykinin degradation. Circ. Res. 1994;75:770–779. doi: 10.1161/01.res.75.4.770. [DOI] [PubMed] [Google Scholar]
- PRADELLA L., BRAMBILLA N., VEZZOLA M., PALMA S., MORAZZONI G., ALLIEVI L., MARCHINI F., PAUSELLI M., POCCHIARI F., SEMERARO C.Z13752A, a new potent dual angiotensin converting enzyme and neutral endopeptidase inhibitor produces antihypertensive effect in SHR rats and DOCA salt hypertensive rats Fourth European Congress of Pharmaceutical Sciences 19986Suppl 1S36Milan, September 11–13, 1998. EPSCED(abstract 141) [Google Scholar]
- RASTEGAR M.A., VÉGH Á., PAPP J.G.Y., PARRATT J.R.Does inhibition of bradykinin catabolism modify the severity of arrhythmias in myocardial ischaemia J. Mol. Cell. Cardiol. 199830A6P15 (abstract) [Google Scholar]
- RICHARD V., GHALEH B., BERDEAUX A., GIUDICELLI J.-F. Comparison of the effects of EXP2174, an angiotensin II antagonist and enalaprilate on myocardial infarct size in anaesthetised dogs. Br. J. Pharmacol. 1993;110:969–974. doi: 10.1111/j.1476-5381.1993.tb13908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS A.M., WITTERT G.A., ESPINER E.A., YANDLE T.G., IKRAM H., FRAMPTON C. Effect of inhibition of endopeptidase 24.11 on responses to angiotensin II in human volunteers. Circ. Res. 1992;71:1501–1507. doi: 10.1161/01.res.71.6.1501. [DOI] [PubMed] [Google Scholar]
- RYAN J.W., CHUNG A., AMMONS C., CARLTON M.K. A simple radioassay for angiotensin-converting enzyme. Biochem. J. 1977;167:501–504. doi: 10.1042/bj1670501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHÖLKENS B.A., LINZ W., KÖNIG W. Effects of the angiotensin converting enzyme inhibitor, ramipril, in isolated ischaemic rat heart are abolished by a bradykinin antagonist. J. Hypertension. 1989;6:S25–S28. doi: 10.1097/00004872-198812040-00004. [DOI] [PubMed] [Google Scholar]
- SHIMADA Y., AVKIRAN M. Attenuation of reperfusion arrhythmias by selective inhibition of angiotensin-converting enzyme/kininase II in the ischemic zone: Mediated by endogenous bradykinin. J. Cardiovasc. Pharmacol. 1996;27:428–438. doi: 10.1097/00005344-199603000-00017. [DOI] [PubMed] [Google Scholar]
- SOKOLOVSKY M., GALRON R., KLOOG Y., BDOLAH A., INDIG F.E., BLUMBERG S., FLEMINGER G. Endothelins are more sensitive than sarafotoxins to neutral endopeptidase: Possible physiological significance. Proc. Natl., Acad. Sci. U.S.A. 1990;87:4702–4706. doi: 10.1073/pnas.87.12.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOBÉ T.J.M., LANGEN C.D.J., DE TIO R.A., BEL K.J., MOOK P.H., WESSELING H. Effects of bradykinin on inducible sustained ventricular tachycardia two weeks after myocardial infarction in pigs. J. Cardiovasc. Pharmacol. 1991;17:701–706. doi: 10.1097/00005344-199105000-00003. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., KOMORI S., SZEKERES L., PARRATT J.R. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc. Res. 1992;26:487–495. doi: 10.1093/cvr/26.5.487. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., PAPP J.G., PARRATT J.R. Attenuation of the antiarrhythmic effects of ischaemic preconditioning by blockade of bradykinin B2 receptors. Br. J. Pharmacol. 1994;113:1167–1172. doi: 10.1111/j.1476-5381.1994.tb17120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VÉGH Á., PAPP J.G., SZEKERES L., PARRATT J.R. Prevention by an inhibitor of the L-arginine-nitric oxide pathway of the antiarrhythmic effects of bradykinin in anaesthetized dogs. Br. J. Pharmacol. 1993;110:18–19. doi: 10.1111/j.1476-5381.1993.tb13764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VÉGH Á., RASTEGAR M.A., PAPP J.G.Y., PARRATT J.R.The antiarrhythmic effects of ANP in a canine model of ischaemia-reperfusion J. Mol. Cell. Cardiol. 199830A7P20 (abstract) [Google Scholar]
- VÉGH Á., SZEKERES L., PARRATT J.R. Protective effects of preconditioning of the ischaemic myocardium involve cyclo-oxygenase products. Cardiovasc. Res. 1990;24:1020–1022. doi: 10.1093/cvr/24.12.1020. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., SZEKERES L., PARRATT J.R. Local coronary infusions of bradykinin profoundly reduce the severity of ischaemia-induced arrhythmias in anaesthetised dogs. Br. J. Pharmacol. 1991a;104:294–295. doi: 10.1111/j.1476-5381.1991.tb12424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VÉGH Á., SZEKERES L., PARRATT J.R. Transient ischaemia induced by rapid cardiac pacing results in myocardial preconditioning. Cardiovasc. Res. 1991b;25:1051–1053. doi: 10.1093/cvr/25.12.1051. [DOI] [PubMed] [Google Scholar]
- VÉGH Á., SZEKERES L., UDVARY E. Effect of the blood supply to the normal noninfarcted myocardium on the incidence and severity of early post-occlusion arrhythmias in dogs. Basic Res. Cardiol. 1987;82:159–171. doi: 10.1007/BF01907063. [DOI] [PubMed] [Google Scholar]
- WALL T.M., SHEEHY R., HARTMAN J.C. Role of bradykinin in myocardial preconditioning. J. Pharmacol. Exp. Ther. 1994;270:681–689. [PubMed] [Google Scholar]
- WILLIAMS D.O., SHERLAG B.J., HOPE R.R., EL-SHERIF N., LAZZARA R. The pathophysiology of malignant ventricular arrhythmias during acute myocardial ischemia. Circulation. 1974;50:1163–1172. doi: 10.1161/01.cir.50.6.1163. [DOI] [PubMed] [Google Scholar]
- YANG X.-P., LIU Y.-H., PETERSON E., CARRETERO O.A. Effect of neutral endopeptidase 24.11 inhibition on myocardial ischemia/reperfusion injury: The role of kinins. J. Cardiovasc. Pharmacol. 1997;29:250–256. doi: 10.1097/00005344-199702000-00014. [DOI] [PubMed] [Google Scholar]
- ZHANG X., NASJLETTI A., XU X., HINTZE T.H. Neutral endopeptidase and angiotensin-converting enzyme inhibitors increase nitric oxide production in isolated canine coronary microvessels by a kinin-dependent mechanism. J. Cardiovasc. Pharmacol. 1998;31:623–629. doi: 10.1097/00005344-199804000-00023. [DOI] [PubMed] [Google Scholar]


