Abstract
The pharmacology of hSK1, a small conductance calcium-activated potassium channel, was studied in mammalian cell lines (HEK293 and COS-7). In these cell types, hSK1 forms an apamin-sensitive channel with an IC50 for apamin of 8 nM in HEK293 cells and 12 nM in COS-7 cells. The currents in HEK293 cells were also sensitive to tubocurarine (IC50=23 μM), dequalinium (IC50=0.4 μM), and the novel dequalinium analogue, UCL1848 (IC50=1 nM). These results are very different from the pharmacology of hSK1 channels expressed in Xenopus oocytes and suggest the properties of the channel may depend on the expression system. Our findings also raise questions about the role of SK1 channels in generating the apamin-insensitive slow afterhyperpolarization observed in central neurones.
Keywords: SK1, apamin, tubocurarine, dequalinium, UCL1848, HEK293 cells, COS-7 cells, afterhyperpolarization
Introduction
Small conductance calcium-activated potassium channels (SK channels) are thought to underlie the slow afterhyperpolarizations (AHP) that follow action potentials in many neurones (for reviews see Sah, 1996; Vergara et al., 1998). They are also found in many non-excitable cells. Subtypes of the SK channel have been postulated on the basis of pharmacological and biochemical evidence (Wadsworth et al., 1994; Dunn et al., 1996). A molecular basis for their diversity has recently been provided by the identification of three genes (SK1-3) coding for similar small conductance calcium-activated potassium channels (Kohler et al., 1996) which can form homo- or heteromeric channels when expressed in Xenopus oocytes (Ishii et al., 1997). These channels can be characterized by differences in their sensitivity to apamin, which is a high affinity blocker of the AHP in many neurones. However, in some central neurones such as hippocampal pyramidal cells, a very slow AHP (sAHP) that is insensitive to apamin follows a train of action potentials. When SK1-3 genes are expressed in Xenopus oocytes, the SK2 and SK3 homomeric channels are found to have a high affinity for apamin (IC50s of 60 pM and ∼1 nM respectively) whereas SK1 is relatively insensitive (IC50>100 nM) (Kohler et al., 1996; Vergara et al., 1998). It has been inferred from this that SK2 and SK3 channels contribute to the apamin-sensitive AHP currents and that SK1 channels underlie the apamin-insensitive sAHP current.
In this study, we have examined homomeric hSK1 expressed in mammalian cell lines (human embryonic kidney (HEK293) and monkey kidney (COS-7) cells) and report that in these expression systems the pharmacology differs from that reported in Xenopus oocytes. In addition to apamin, we have also studied the effects of tubocurarine, which has been shown, like apamin, to be less active on SK1 (IC50=350 μM) than on SK2 channels (IC50=5.4 μM) when expressed in Xenopus oocytes (Ishii et al., 1997). We have also tested dequalinium, a potent blocker of the apamin-sensitive potassium channels (Castle et al., 1993; Dunn, 1994), as well as a novel analogue of dequalinium, UCL1848, which is a high affinity blocker of the apamin-sensitive AHP but does not affect the apamin-insensitive sAHP in hippocampal neurones (Benton et al., 1999).
Methods
HEK293 cells and COS-7 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 2 mM L-glutamine, 50 μg ml−1 Penicillin-Streptomycin and 10% foetal bovine serum (HEK293 cells) or 10% new born calf serum (COS-7 cells) (all from Life Technologies, U.K.). For transfection purposes, the calcium phosphate method was used. Briefly, a total of 6 μg of DNA was added to 73 μl of 250 mM CaCl2. An equal volume of 2×HEPES buffered saline (HBS) solution (in mM: NaCl 280, HEPES 50, Na2HPO4 2.8, pH adjusted to 7.2 using 1M NaOH) was added dropwise to the CaCl2 solution. After 20 min incubation at room temperature, 75 μl of this solution was added dropwise to a dish of cells. The cells were then incubated for 3 h at 37°C. The medium was then removed from each dish and replaced with 15% glycerol in Hanks Balanced Salt Solution (HBSS; Life Technologies, U.K.) and left for 1 min at room temperature. The cells were then washed once with HBSS before addition of the growth medium. Cells were transfected with both hSK1 (a generous gift from Dr J.P. Adelman, Vollum Institute, U.S.A.) and CD8 DNAs. As a matter of routine, the pore region of hSK1 was re-sequenced using the BigDye Terminator Cycle sequencing kit (PE Applied Biosystems, U.K.) run on a AB1377 DNA sequencer. The sequence was identical to that of hSK1 deposited in GenBank (Accession no. HSU69883).
A few experiments were performed with rSK2 transfected stably in HEK293 cells (kindly provided by Dr W. Joiner, Yale University, U.S.A.).
Transiently transfected cells were identified by adding CD8 antibody coated microspheres (Dynabeads M-450 CD8, Dynal, U.K.) to the cells 30 min before use (Jurman et al., 1994). HEK293 cells were used a minimum of 24 h after transfection whereas COS-7 cells were used at least 48 h after transfection. Cells were superfused at 5 ml min−1 with the following solution (in mM): NaCl 150, KCl 5, MgCl2 1, CaCl2 2, glucose 10, HEPES 10, pH adjusted to 7.4 with (1M) NaOH. Drugs were applied by switching to bathing fluid containing the drug using a multiway tap. The inlet tube was positioned such that the flow was directed onto the patched cell. Whole cell recordings from isolated cells were made using glass micropipettes (Clark Electromedical Instruments; GC15OTF-15) that were fire-polished and coated with Sylgard (Dow Corning, U.S.A.). They had resistances of 3–5 MΩ when filled with the following solution (in mM): KCl 130, HEDTA 5, HEPES 10, Na2ATP 2, MgCl2 3, CaCl2 0.67, pH adjusted to 7.2 using (1M) KOH. The calcium concentration was calculated to give 1 μM free calcium using the program ‘REACT' (G.L. Smith, University of Glasgow, U.K.). Cells were voltage-clamped at −80 mV using a List EPC-7 amplifier. One hundred ms voltage steps from −140 mV to +40 mV in 20 mV increments (Figure 1A) were applied every minute. Signals were filtered at 10 kHz and stored on a computer using pClamp6 (sampling frequency of 5 kHz; Axon Instruments) for further analysis.
Figure 1.
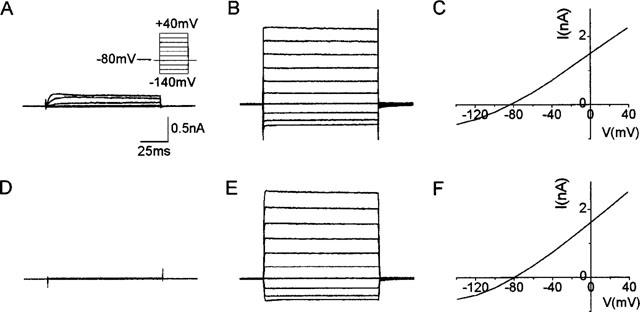
(A) Current recorded from untransfected HEK293 cells dialyzed with 1 μM free calcium and subjected to voltage steps from −140 mV to +40 mV (shown in inset). (B) Current obtained from HEK293 cells transiently transfected with hSK1 and dialyzed with 1 μM free calcium (same voltage protocol as in A). (C) The I-V curve for the cell in B. (D and E) Represent the currents in an untransfected COS-7 cell and a transfected COS-7 cell respectively when the cells were dialyzed with 1 μM free calcium and the voltage protocol shown in A applied. F is the I-V curve for the current shown in E. The calibration bars shown in A also apply to B, D and E.
Data were analysed using pClamp6 software. To minimize series resistance errors and the contribution of a significant amount of delayed rectifier current, the amplitude of the SK current was measured at a potential of −40 mV. The amplitude of the SK current in the presence of the drug was expressed as a percentage of the average amplitude before and after recovery from the drug. Results are expressed as mean±s.e.mean. The concentration-inhibition curves were fitted with the Hill equation:
where y is the percentage inhibition, [I] is the drug concentration, ymax is the maximum inhibition, n is the Hill coefficient and K is the IC50 value.
All chemicals were purchased from Sigma apart from apamin which was obtained from Peptides International and UCL1848 (1,1′-[1,5-pentan-diyl]-N,N′-[1,5-pentan-diyl]-bis-[4-aminoquinolinium] di-trifluoroacetate) which was synthesized by Professor C. R. Ganellin and colleagues at University College London, U.K.
Results
hSK1 expression in HEK293 cells
Transfected cells usually had five or more CD8 antibody coated microspheres attached to them. Transfected HEK293 cells when patched with pipettes containing 1 μM free calcium gave currents up to 5 nA (at +40 mV), generally showing outward rectification at negative potentials (Figure 1B,C). The SK1 currents ran down initially but often stabilized within 5 min. These cells had membrane potentials of −80 mV and the current also reversed at approximately −80 mV (Figure 1C). The input resistance was usually less than 100 MΩ. In contrast, untransfected HEK293 cells, when patched onto with pipettes containing 1 μM free calcium as before, displayed only delayed rectifier currents as has been previously reported (Yu & Kerchner, 1998; Figure 1A). These cells had membrane potentials between 0 mV and −20 mV with input resistances of more than 1 GΩ.
Apamin (30 nM) blocked the hSK1 current rapidly (within 2 min) in HEK293 cells (Figure 2A). The block reversed within 5 min. The IC50 value was 7.7±1.7 nM (Hill coefficient of unity, Figure 2C). However, in 15% (3/20) of the cells tested the current was found to be only partially blocked by 30 nM apamin (37.2±2.0%, n=3). The results from these cells were excluded from the data presented above.
Figure 2.
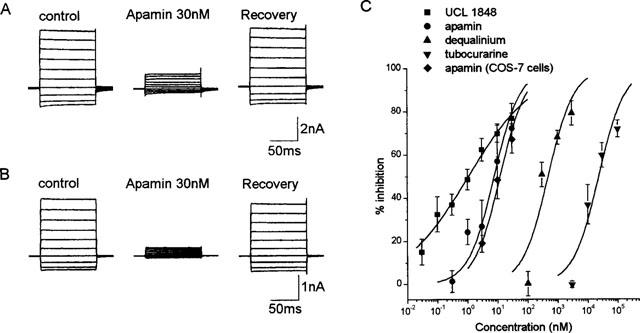
Examples of the block by apamin of the current produced by hSK1 transfected in HEK293 cells (A) and COS-7 cells (B). For both examples, the trace in the presence of 30 nM apamin was obtained 2 min after bath-application of the drug. The recovery trace was obtained 4 min after washout. (C) Concentration-inhibition curves for apamin, UCL1848, tubocurarine and dequalinium. All the curves were fitted using the Hill equation with ymax constrained to 100%. For the dose-response curves for apamin, tubocurarine and dequalinium, the Hill slopes have been constrained to one. Each data point is the mean and standard error of 3–7 observations.
Tubocurarine and dequalinium also reduced the hSK1 current significantly (Figure 2C). The IC50 values for the block by tubocurarine and dequalinium were found to be 23.5±5.3 μM (Figure 2C) and 0.48±0.1 μM (Figure 2C) respectively. UCL1848 was also found to potently block the hSK1 current (IC50=1.1±0.4 nM). The concentration-response curve produced by UCL1848, however, had a shallow slope of 0.4±0.1 when fitted with the Hill equation. Like apamin, the block produced by all these compounds was rapid with recovery occurring within 5 min of washout.
hSK1 expression in COS-7 cells
As the pharmacology of hSK1 expressed in HEK293 cells is very different from that in Xenopus oocytes, we decided to express hSK1 in a second mammalian cell line (COS-7 cells). Untransfected COS-7 cells possess only a very small delayed rectifier type current when patched with pipettes containing 1 μM free calcium (n=5; Figure 1D). The membrane potential of these cells was around 0 mV and the input resistance was more than 1 GΩ. In contrast, transfected COS-7 cells had an input resistance of less than 100 MΩ and membrane potentials between −70 mV and −80 mV. Currents of up to 4 nA (at +40 mV) could be recorded (Figure 1E). These currents were generally stable, showing little rundown. The current reversed at −80 mV (Figure 1F), suggesting that it was carried by potassium ions. Apamin also rapidly blocked the hSK1 current in COS-7 cells (Figure 2B) with an IC50 value of 12.2±1.3 nM (Hill coefficient constrained to 1; Figure 2C). This IC50 value is comparable to that observed in HEK293 cells (IC50 of 8 nM). The effect was reversible within 5 min (Figure 2B).
rSK2 in HEK293 cells
To determine whether the properties of other SK channels also vary when expressed in mammalian cell lines, we tested the effects of apamin on HEK293 cells stably transfected with the rSK2 gene. In HEK293 cells expression of rSK2 generated calcium-activated currents of up to 5 nA (at +40 mV). As found in Xenopus oocytes, apamin is an extremely potent blocker, with 100 pM apamin giving 71±8% (n=4) inhibition within 5 min. The effects were reversible within 10 min of washout.
Discussion
The surprising and important finding of this study is that when hSK1 is transiently expressed in two different mammalian cell lines, the current produced is predominantly apamin-sensitive. These results are very different from those obtained when hSK1 is expressed in Xenopus oocytes (Kohler et al., 1996; Ishii et al., 1997). To find out whether this effect was unique to apamin, other blockers of the apamin-sensitive channels were also tested. The hSK1 current in HEK293 cells was moderately sensitive to tubocurarine (IC50 of 23 μM) whereas the current obtained when hSK1 is expressed in Xenopus oocytes is relatively insensitive (IC50 of 350 μM; Ishii et al., 1997).
hSK1 channels in mammalian cell lines were also blocked by dequalinium and UCL1848, which are known inhibitors of apamin-sensitive potassium channels. UCL1848 was particularly potent (Figure 2C). Interestingly, the dose-response curve observed for this compound was shallow (Hill slope=0.4), suggesting that binding sites with different affinities for UCL1848 may be present.
In contrast to our findings with SK1, the pharmacology of SK2 and SK3 expressed in mammalian cell lines does not seem to differ from that in Xenopus oocytes. With rSK2, our own findings give an IC50 for apamin of just below 100 pM, which is comparable to the IC50 of 60 pM in oocytes (Kohler et al., 1996). For rSK3 expressed in HEK293 cells, the IC50 for apamin is approximately 2 nM (Hosseini et al., 1999), close to the value of 1 nM observed in oocytes (Vergara et al., 1998). Therefore, of the SK channels so far cloned, only the pharmacology of SK1 significantly differs in different expression systems.
These results show that the hSK1 protein can form an apamin-sensitive channel when expressed in mammalian cell lines. One possible explanation is that there is a change in the folding or assembly of the channels as, for example, occurs with nicotinic receptors (Lewis et al., 1997). Interestingly, in this case, a small proportion of the channels in the mammalian cell line behaved as in oocytes whilst the properties of the majority of the channels corresponded to the native channel phenotype. We have also found that in 3/20 of the cells tested, the current was only partially blocked by apamin, suggesting that some apamin-insensitive hSK1 channels, possibly similar to those in oocytes, may assemble in the mammalian cell lines we have examined.
An alternative explanation for our findings is that additional accessory subunits expressed in either some mammalian cell lines or Xenopus oocytes may be able to combine with hSK1 and change the properties of the channel that is formed. Indeed, Wadsworth et al. (1997) have purified a number of polypeptides with different molecular weights, which can be photolabelled by apamin. These may represent SK (α) subunits and possible ‘β subunits' alone or in combination. The presence or absence of these additional subunits may determine the apamin-sensitivity of hSK1.
It is worth noting that SK1 channels in these mammalian cell lines are still less sensitive to apamin than SK2 and SK3 channels. Therefore, the amino acid residues that Ishii et al. (1997) suggested were important for apamin sensitivity in oocytes, are probably also important for apamin binding in mammalian cell lines. However, these residues may also influence folding or the binding of auxiliary subunits to SK1 subunits as suggested by the result that mutated SK1 subunits form channels that are less susceptible to rundown in oocytes (Khawaled et al., 1999).
Because of its apamin-insensitivity and distribution in the brain, hSK1 has been suggested to underlie the sAHP in central neurons (Vergara et al., 1998). The present work, however, shows that hSK1, under certain conditions, can form apamin-sensitive channels. The identity of the channel subunits underlying the sAHP is, therefore, still uncertain and further work needs to be done to establish whether SK1 is solely responsible for the apamin-insensitive sAHP or might indeed contribute to the apamin-sensitive AHP in central neurones.
Acknowledgments
We thank Dr R. Hosseini for sequencing the pore region of hSK1. We are also grateful to Professor D. H. Jenkinson and Drs G.W.J. Moss and D.C.H. Benton for their helpful comments and discussions. This work was funded by the Wellcome Trust. M. Shah is an MRC scholar.
Abbreviations
- hSK1
rSK2 and rSK3, human (h) or rat (r) small conductance calcium-activated potassium channels
- AHP
afterhyperpolarization
- HEK293
human embryonic kidney cell line
- COS-7
monkey kidney cell line
References
- BENTON D.C.H., DUNN P.M., CHEN J.Q., GALANAKIS D., GANELLIN C.R., MALIK-HALL M., SHAH M., HAYLETT D.G., JENKINSON D.H. UCL1848: a novel bis-quinolinium cyclophane which blocks apamin-sensitive K+ channels with nanomolar affinity. Br. J. Pharmacol. 1999;128:39P. [Google Scholar]
- CASTLE N.A., HAYLETT D.G., MORGAN J.M., JENKINSON D.H. Dequalinium: a potent inhibitor of apamin-sensitive K+ channels in hepatocytes and of nicotinic responses in skeletal muscle. Eur. J. Pharmacol. 1993;236:201–207. doi: 10.1016/0014-2999(93)90590-e. [DOI] [PubMed] [Google Scholar]
- DUNN P.M. Dequalinium, a selective blocker of the slow afterhyperpolarization in rat sympathetic neurons in culture. Eur. J. Pharmacol. 1994;252:189–194. doi: 10.1016/0014-2999(94)90596-7. [DOI] [PubMed] [Google Scholar]
- DUNN P.M., BENTON D.C.H., CAMPOS ROSA J., GANELLIN C.R., JENKINSON D.H. Discrimination between subtypes of apamin-sensitive Ca2+-activated K+ channels by gallamine and a novel bis-quaternary quinolinium cyclophane, UCL 1530. Br. J. Pharmacol. 1996;117:35–42. doi: 10.1111/j.1476-5381.1996.tb15151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSSEINI R., BENTON D.C.H., HAYLETT D.G., MOSS G.W.J. Cloning of an SK channel from rat sympathetic neurones. J. Physiol. 1999;518:122P. [Google Scholar]
- ISHII T.M., MAYLIE J., ADELMAN J.P. Determinants of apamin and d-tubocurarine block in SK potassium channels. J. Biol. Chem. 1997;272:23195–23200. doi: 10.1074/jbc.272.37.23195. [DOI] [PubMed] [Google Scholar]
- JURMAN M.E., BOLAND L.M., LIU Y., YELLEN G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- KHAWALED R., BRUENING-WRIGHT A., ADELMAN J.P., MAYLIE J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Arch.-Eur. J. Physiol. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- KOHLER M., HIRSCHBERG B., BOND C.T., KINZIE J.M., MARRION N.V., MAYLIE J., ADELMAN J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- LEWIS T.M., HARKNESS P.C., SIVILOTTI L.G., COLQUHOUN D., MILLAR N.S. The ion channel properties of a rat recombinant neuronal nicotinic receptor are dependent on the host cell type. J. Physiol. 1997;505:299–306. doi: 10.1111/j.1469-7793.1997.299bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAH P. Ca2+-activated K+ currents in neurones: Types, physiological roles and modulation. Trends in Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- VERGARA C., LATORRE R., MARRION N.V., ADELMAN J.P. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- WADSWORTH J.D.F., DOORTY K.B., STRONG P.N. Comparable 30 kDa apamin binding polypeptides may fulfil equivalent roles within putative subtypes of small-conductance Ca2+-activated K+ channels. J. Biol. Chem. 1994;269:18053–18061. [PubMed] [Google Scholar]
- WADSWORTH J.D.F., TORELLI S., DOORTY K.B., STRONG P.N. Structural diversity among subtypes of small-conductance Ca2+- activated potassium channels. Arch. Biochem. Biophys. 1997;346:151–160. doi: 10.1006/abbi.1997.0280. [DOI] [PubMed] [Google Scholar]
- YU S.P., KERCHNER G.A. Endogenous voltage-gated potassium channels in human embryonic kidney (HEK293) cells. J. Neurosci. Res. 1998;52:612–617. doi: 10.1002/(SICI)1097-4547(19980601)52:5<612::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]


