Abstract
The effects of SK&F 96365 on cationic current evoked either by activating muscarinic receptors with carbachol or by intracellularly applied GTPγS (in the absence of carbachol) were studied using patch-clamp recording techniques in single guinea-pig ileal smooth muscle cells.
SK&F 96365 reversibly inhibited the muscarinic receptor cationic current in a concentration-, time- and voltage-dependent manner producing concomitant alteration of the steady-state I-V relationship shape which could be explained by assuming that increasing membrane positivity increased the affinity of the blocker. The inhibition was similar for both carbachol- and GTPγS-evoked currents suggesting that the cationic channel rather than the muscarinic receptor was the primary site of the SK&F 96365 action.
Increased membrane positivity induced additional rapid inhibition of the cationic current by SK&F 96365 which was more slowly relieved during membrane repolarization. Both the inhibition and disinhibition time course could be well fitted by a single exponential function with the time constants decreasing with increasing positivity for the inhibition (e-fold per about 12 mV) and approximately linearly decreasing with increasing negativity for the disinhibition.
At a constant SK&F 96365 concentration, the degree of cationic current inhibition was a sigmoidal function of the membrane potential with a potential of half-maximal increase positive to about +30 mV and a slope factor of about −13 mV.
Increasing the duration of voltage steps at −80 or at 80 mV, increased the percentage inhibition; the degree of inhibition was almost identical at both potentials providing evidence that the same cationic channel was responsible for the cationic current both at negative and at positive potentials.
It is concluded that the distinctive and unique mode of SK&F 96365 action on the muscarinic receptor cationic channel is a valuable tool in future molecular biology studies of this channel.
Keywords: Gastrointestinal smooth muscle, carbachol, GTPγS, cationic current, SK&F 96365
Introduction
Acetylcholine, the major excitatory neurotransmitter in visceral smooth muscles, causes membrane depolarization and thus Ca2+ influx via voltage-gated Ca2+ channels. The depolarizing action is mediated primarily by M2 muscarinic receptor activation (Bolton & Zholos, 1997; Kang et al., 1997; Wang et al., 1997) which is linked to the voltage-dependent, [Ca2+]i-sensitive cationic channels via Gi/Go proteins (Wang et al., 1997). The pharmacological properties of these muscarinic receptor-gated cationic channels have been extensively studied in recent years though no specific blocker has yet been identified. However, a number of blockers which are generally considered to be more selective for other ion channels also effectively block the muscarinic receptor-linked cationic channels suggesting certain structural similarities.
Muscarinic receptor-gated cationic channels are also permeable to Ca2+ and thus may directly mediate Ca2+ influx during tonic rise in [Ca2+]i. The existence of such Ca2+ influx has been demonstrated with 110 mM Ca2+ in the bath (Bolton & Kitamura, 1983; Inoue & Isenberg, 1990; Fleischmann et al., 1997; Kim et al., 1998). Under these conditions a permeability ratio of Ca2+ over monovalent cations between 2 : 1 to 3.6 : 1 was extrapolated. Under a physiological Ca2+ gradient the fraction of the cationic current carried by Ca2+ at −60 mV was estimated at 1% in guinea-pig gastric myocytes (Kim et al., 1998) and at 14% in airway myocytes (Fleischmann et al., 1997). In guinea-pig ileal cells Ca2+ contribution to the cationic current when [Ca2+]o was raised from zero to 2.5–10 mM was also evident as some additional effects of Ca2+ which were not shared with Mg2+ were observed (Zholos & Bolton, 1995).
SK&F 96365 is a relatively novel inhibitor of receptor-stimulated Ca2+ entry (Merritt et al., 1990) and our aim in the present work was to test its possible effects on the cationic channels coupled to the muscarinic receptors in guinea-pig ileal cells. The rationale for such experiments was also provided by the recent detection of transcripts for Trp6 and Trp3 (genes related to the Drosophila transient receptor potential (trp) gene) in gastrointestinal smooth muscle cells. Trp6 may encode the channel underlying the muscarinic cationic current in colonic myocytes (Walker et al., 1999). Proteins encoded in these genes are likely to be involved in Ca2+ influx following receptor stimulation and InsP3 production to induce Ca2+ store depletion, so called store-operated Ca2+ entry (recently reviewed by Parekh & Penner, 1997). However, it is important to note that in other systems Trp6 mediates a muscarinic receptor-activated non-selective cationic conductance and Ca2+ entry not related to Ca2+ store depletion but instead directly stimulated by a G protein-coupled receptor and blocked by SK&F 96365 (Boulay et al., 1997). In a recent study Kim et al. (1998) have concluded that in guinea-pig gastric myocytes carbachol-activated cationic current was also not related to ICRAC (Ca2+ release-activated Ca2+ current).
Our results show that SK&F 96365 at micromolar concentrations inhibits cationic current in guinea-pig ileal cells in a distinctive voltage-dependent manner and thus may be a useful pharmacological tool in future studies.
Methods
Experimental procedures were generally the same as already described (Zholos & Bolton, 1995). Male adult guinea-pigs (300–400 g) were concussed by a sudden blow on the back of the head followed by immediate exsanguination. Experiments were performed at room temperature on single ileal smooth muscle cells from the longitudinal muscle layer obtained after collagenase treatment (1 mg ml−1) at 36°C for about 25 min.
Electrical recordings
Whole-cell membrane current was recorded using low-resistance borosilicate patch pipettes (1–3 MΩ) and an Axopatch 200A (Axon Instruments Inc., Foster City, CA, U.S.A.) or EPC-5 (List Electronic, Darmstadt, Germany) voltage-clamp amplifier. Background current was measured before carbachol application or immediately after break-through when patch pipettes filled with GTPγS were used and was digitally subtracted off-line.
Solutions
Pipettes were filled with the following solution (in mM): CsCl 80, MgATP 1, creatine 5, NaGTP 1, glucose 20, HEPES 10, BAPTA 10, CaCl2 4.6 (calculated [Ca2+]i=100 nM), pH adjusted to 7.4 with CsOH (total Cs+ 124 mM). The presence of 1 mM GTP in this solution reduced desensitization to a minimum (Zholos & Bolton, 1996a). In experiments designed to activate cationic channels directly, without the activation of muscarinic receptors, GTP in the pipette solution was replaced with 200 μM GTPγS (Zholos & Bolton, 1994; 1996a).
The basic external solution in which cationic current was recorded consisted of (in mM): CsCl 120, glucose 12, HEPES 10, pH adjusted to 7.4 with CsOH (total Cs+ 124 mM). The cells prior to experiment were kept in the following solution (mM): NaCl 120, KCl 6, CaCl2 2.5, MgCl2 1.2, glucose 12, HEPES 10, pH adjusted to 7.4 with NaOH.
Complete exchange of the external solution was achieved within about 1 s as described previously (Zholos & Bolton, 1995).
Data analysis
Concentration-effect curves were fitted by a logistic function in the following form:
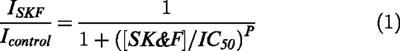 |
where IControl and ISK&F are respectively the cationic current amplitudes before in the presence of various SK&F 96365 concentrations, [SK&F]; IC50 equals the SK&F 96365 concentration at which current amplitude was reduced by 50% and p is the slope factor of the inhibition curve.
The data was analysed and plotted using MicroCal Origin software (MicroCal Software, Inc., Northampton, MA, U.S.A.). Values are given as the means±s.e.mean.
Chemicals used
Collagenase (type 1A), adenosine 5′ triphosphate (ATP, magnesium salt), guanosine 5′-triphosphate (GTP, sodium salt), guanosine 5′-O-(3-thiotriphosphate) (GTP-γS, tetralithium salt), creatine, N-2-hydroxyethylpiperazine-N′-2-ethanesulphoic acid (HEPES), 1,2-bis(2-aminophenoxy) ethane- N,N,N′,N′-tetraacetic acid (BAPTA), and carbamylcholine chloride (carbachol) were obtained from Sigma Chemical Co. (Poole, Dorset, U.K.) 1-[β-[3-(4-Methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole·HCl (SK&F 96365) was from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA, U.S.A.).
Results
It was observed in contraction studies that SK&F 96365 at 30 μM reduced the size of contractions to 50 μM carbachol strips of strips of isolated longitudinal muscle by more than 50%. However, in view of its ability to inhibit several channels types (see Discussion) this effect may result from binding at a number of channels or sites in the smooth muscle cell.
Effects of SK&F 96365 on carbachol-activated current
In these experiments inward cationic current was evoked by carbachol applied in the bathing solution to cells voltage-clamped at −40 mV at least 3 min after break-through. Carbachol was applied in the bath at 50 μM, a concentration close to the maximally effective in these cells (Bolton & Zholos, 1997). When current became steady, the current-voltage relationship was measured with a slow 6 s duration voltage ramp from 80 mV to −120 mV (Figure 1A, control trace). SK&F 96365 added to the external solution cumulatively at ascending concentrations produced cationic current inhibition with a characteristic change in the I-V relationship shape. Thus, current in the negative voltage range progressively lost its typical U-shaped dependence on the membrane potential whereas in the positive range the inhibition at all concentrations used was more substantial (Figure 1A). As an example, at 30 μM SK&F 96365 inhibited cationic current at 80 mV by 96% compared to 63% inhibition at −120 mV. Plotting relative current amplitude measured at two different potentials against SK&F 96365 concentration revealed an increase in the apparent dissociation constant from 6.5 to 14.5 μM with hyperpolarization from 80 to −80 mV (Figure 1B). Such progressive increase of the IC50 value was observed over the entire range of potentials (Figure 1C).
Figure 1.
Effects of SK&F 96365 on carbachol-activated cationic current in single guinea-pig ileal smooth muscle cells. (A) Steady-state current-voltage relationships measured by 6 s duration voltage ramps in the presence of 50 μM carbachol and SK&F 96365 at the indicated concentrations. (B) Relative amplitude of the muscarinic cationic current in a single experiment at 80 and −80 mV plotted against SK&F 96365 concentration on a semilogarithmic scale and fitted according to equation 1. Dotted lines indicate the IC50 values. (C) Voltage dependence of the IC50 values.
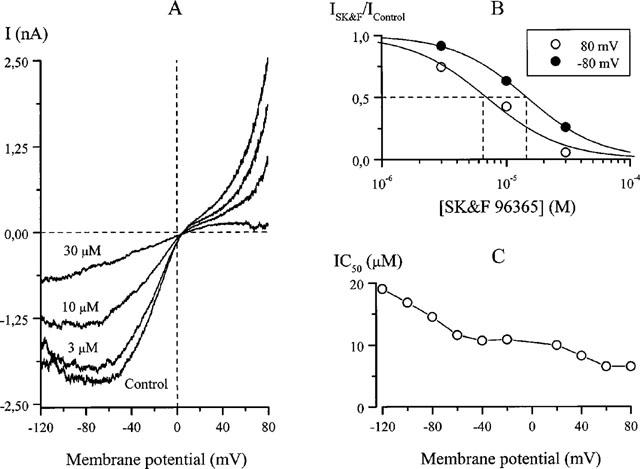
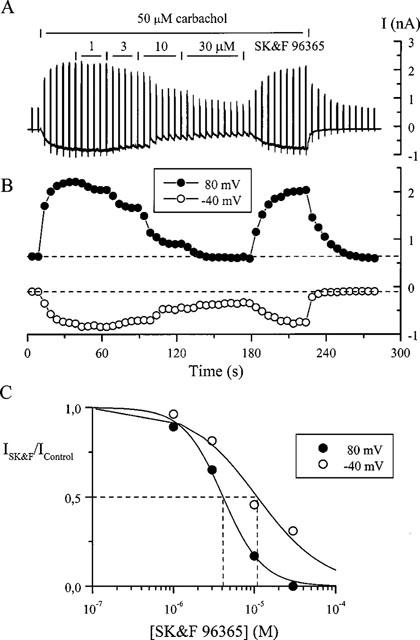
The above observations suggested that the inhibitory potency was a function of the membrane potential. In the experiment illustrated in Figure 2A voltage pulses from −40 to 80 mV (600 ms duration) were applied every 5 s while SK&F 96365 was added at ascending concentrations in the presence of 50 μM carbachol. At 1 μM the inhibition was apparent only at 80 but not at −40 mV; at higher concentrations the current at 80 mV was blocked more rapidly and to a larger extent than at −40 mV (Figure 2C). Nearly full recovery from the inhibition was observed within about 1 min after SK&F 96365 removal. It also appeared that the block accumulated at positive potentials and was somewhat relieved after stepping back to −40 mV as inward current relaxations at this potential were observed. To study this phenomenon we used longer voltage steps as illustrated in Figure 3. After obtaining a control steady-state response to carbachol at −40 mV and stepping to 80 mV for 20 s, SK&F 96365 was applied which resulted in a small slowly developing inhibition at −40 mV. However, stepping to 80 mV in the presence of the blocker resulted in a rapid additional inhibition which developed with a time constant of 0.6 s. Returning the potential to the holding level evoked an inward tail current with a time constant of 5.6 s which could be attributed to the voltage-dependent disinhibition of the muscarinic receptor cationic channels.
Figure 2.
Voltage dependence of the inhibitory effect of SK&F 96365 on carbachol-activated cationic current. (A,B) time- and concentration dependence of the inhibition at two test potentials. SK&F 96365 was applied at ascending concentrations in the presence of 50 μM carbachol while rectangular voltage steps to 80 mV from the holding potential of −40 mV (0.6 duration) were applied every 5 s. Dotted lines in B indicate background current amplitude at corresponding potentials measured before carbachol application. (C) concentration-effect curves at 80 mV (IC50=4.1 μM, P=1.8) and −40 mV (IC50=10.9 μM, P=1.0).
Figure 3.
Membrane depolarization induced rapid additional inhibition of the cationic current by SK&F 96365. Carbachol was applied at the moment indicated and was maintained at 50 μM throughout the experiment. SK&F 96365 applied at −40 mV partially inhibited cationic current with a time constant of 9.4 s. Upon voltage step to 80 mV additional strong inhibition with a time constant of 0.6 s occurred which was relieved after repolarization to −40 mV with a time constant of 5.6 s (superimposed dotted lines show single exponential fittings). Note the 7 min gap in the record to wash out SK&F 96365.
From the initial and final steady-state current amplitudes during current relaxation at −40 mV one could estimate the amount of block during a preceding step to a wide range of test potentials, even at potentials close to the reversal potential where current could not be measured directly (e.g. 0 mV under our experimental conditions). In the experiment illustrated in Figure 4A a series of long steps was applied (20 s duration, note that only the last 5 s segment at each potential is plotted) in the presence of 10 μM SK&F 96365. Percentage of the depolarization-induced inhibition (calculated as shown for the fourth trace) is plotted against the preceding test potential in B. At a constant concentration of SK&F 96365 the inhibition was a sigmoidal function of the membrane potential characterized by the potential of half-maximal increase of 18 mV and the slope factor of −18 mV (continuous line in Figure 4B).
Figure 4.
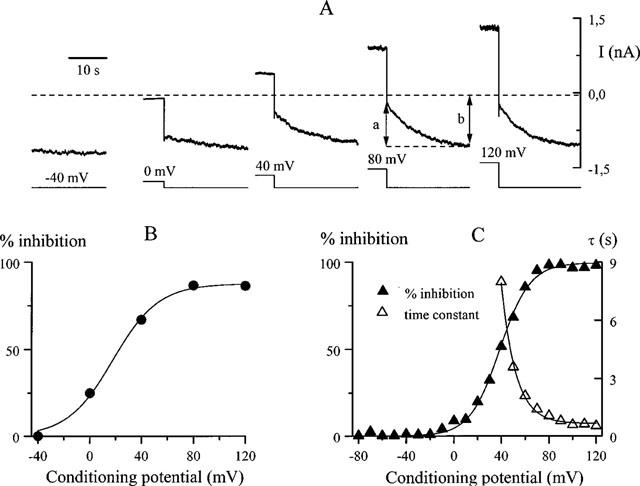
Voltage dependence and kinetics of the inhibition at fixed SK&F 96365 concentration. (A) cationic current evoked by 50 μM carbachol at −40 mV in control (left) and following 20 s duration voltage steps to different test potentials (note that only the last 5 s segment is shown for each trace during the test pulse). All traces were recorded in the presence of 10 μM SK&F 96365. Dotted line indicates background current amplitude at −40 mV before agonist application. (B) per cent of depolarization-induced inhibition of the cationic current plotted against test potential for the data shown in A. The values were calculated as a/b×100% with a and b measured as shown in A. Data points were fitted by a Boltzmann distribution with the potential of half-maximal inhibition of 18 mV and slope factor of −18 mV. (C) per cent inhibition of the cationic current evoked by intracellular 200 μM GTPγS application (no carbachol in the bath) depending on test potential in the presence of 30 μM SK&F 96365. Voltage protocol was similar to that illustrated in A. Best-fit values for the potential of half-maximal inhibition and slope factor were +38 mV and −13 mV, respectively. In the same cell the time constant of the inhibition during test depolarization decreased e-fold per 11.9 mV.
Effects of SK&F 96365 on GTPγS-activated current
Since inhibition and disinhibition of the carbachol-activated cationic current by SK&F 96365 developed more slowly than activation and deactivation of the current upon carbachol application and removal (Figure 2A,B), it was possible that the blocker affected agonist interaction with the muscarinic receptor. Competitive inhibition of agonist binding to the muscarinic receptors by SK&F 96365 has been reported (Lin & Wang, 1996). To test this possibility in the next series of experiments cationic conductance was activated by GTPγS applied intracellularly in order to activate G-proteins directly, so bypassing the muscarinic receptors. It has been shown previously that GTPγS in the guinea-pig ileal cells activates the same cationic conductance (Komori & Bolton, 1990; Zholos & Bolton, 1996a). Following break-through with patch pipettes containing 200 μM GTPγS (no carbachol in the bath) cationic current measured at −40 mV developed slowly to reach a steady-state level within approximately 3–5 min and then was sustained for many tens of minutes (Zholos & Bolton, 1996a). This GTPγS-induced current was inhibited by SK&F 96365 in a similar voltage-dependent manner suggesting that the cationic channel rather than the muscarinic receptor was the binding site for SK&F 96365.
As the GTPγS-evoked current was very stable (Zholos & Bolton, 1996a) the voltage dependence and kinetics of the inhibitory action of SK&F 96365 could be studied in more detail. Using the voltage protocol illustrated in Figure 4A, cationic current relaxations at −40 mV following voltage steps to various test potentials with 10 mV increments were analysed. In the example illustrated in Figure 4C, SK&F 96365 at 30 μM produced voltage-dependent inhibition with the potential of half-maximal increase of 38 mV and the slope factor of −13 mV (continuous line connecting closed triangles in Figure 4C). In different cells these parameters varied to some extent with an average values of 50.7±8.6 mV for the V½ of the inhibitory effect and −17.3±1.5 mV for the slope factor (n=6). In some cells there was no inhibition at potentials negative to about −20 mV (e.g. Figure 4C), or even a slight increase of the current after SK&F 96365 application. Over the same voltage range membrane depolarization accelerated the rate of the inhibition approximately with the same voltage dependence (continuous line connecting open triangles in Figure 4C, e-fold decrease of the time constant per 11.9 mV).
Since membrane depolarization accelerated and potentiated the inhibitory effect of SK&F 96365 on cationic channel membrane hyperpolarization was expected to accelerate the kinetics of the disinhibition process. We employed the voltage protocol shown in Figure 5A, bottom, to study this process. A voltage step to 80 mV was followed by a step to potentials ranging from −10 to −120 mV with 10 mV increments. In the control the previously described voltage-dependent deactivation of the cationic current with a time constant of several tens of milliseconds was seen at potentials negative to about −50 mV (Figure 5A, top) (Inoue & Isenberg, 1990; Zholos & Bolton, 1994). Relaxation of the current in the opposite direction was seen in the presence of SK&F 96365 (Figure 5A, middle; compare to Figures 3 and 4A). The rate of this process was accelerated with membrane hyperpolarization so that the time constant decreased from about 4 s at −10 mV to 1.4 s at −120 mV (Figure 5B). The inset in Figure 5B shows that both development of the inhibition and disinhibition could be well described by a single exponential function. In this example as in Figure 4C there was no effect at −40 mV and inhibition was first established at 80 mV before examining recovery at various potentials.
Figure 5.
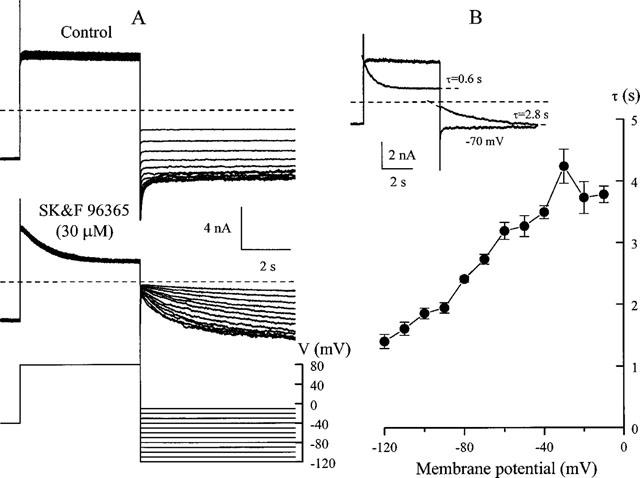
Voltage dependence of the disinhibition kinetics. (A) GTPγS-evoked cationic current relaxations at various test potentials following voltage step to 80 mV in control and in the presence of 30 μM SK&F 96365. (B) mean values for the disinhibition time constant plotted against test potential (n=5). Superimposed current traces shown in the inset were obtained using GTPγS to activate the cationic conductance before and after SK&F 96365 application. Inhibition by 30 μM SK&F 96365 at 80 mV and disinhibition at −70 mV developed with the time constants indicated. The horizontal dotted lines indicate zero current level.
Using voltage steps of variable duration we also made a quantitative comparison between fractions of current inhibited at positive potentials and recovering from the inhibition at negative potentials. In the experiment illustrated in Figure 6A, cationic current was activated by intracellular GTPγS and, in the presence of 30 μM SK&F 96365, the duration of a voltage pulse from −80 to 80 mV was incremented from 0.5 to 12 s to induce a gradually increasing inhibition. Following repolarization to −80 mV disinhibition was seen. Figure 6B compares per cent inhibition of the current at both potentials for this (circles) and another cell (squares) studied with this protocol. Nearly identical values were obtained for the inhibition measured at the end of the pulse to 80 mV and immediately after repolarization to −80 mV thus indicating that the same channels were involved in the cationic current generation at both potentials.
Figure 6.
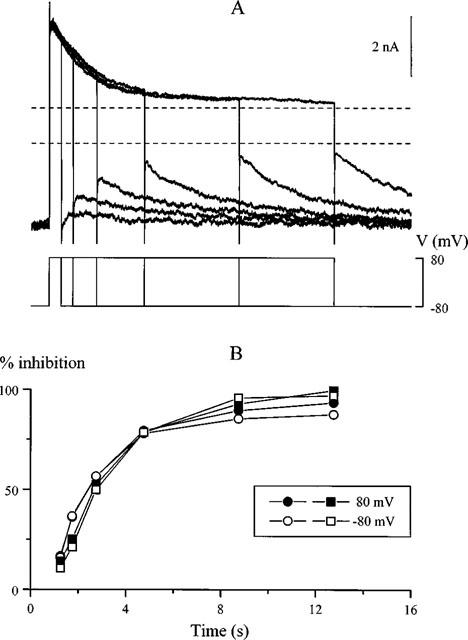
Comparison of fractional current inhibition of GTPγS-evoked cationic current by SK&F 96365 at 80 and at −80 mV. (A) superimposed current traces and voltage protocol used in this experiment to study GTPγS-induced cationic current in the presence of 30 μM SK&F 96365. Dotted lines show background current amplitude at 80 mV (upper line) and −80 mV (lower line) measured immediately after break-through before the GTPγS effect had developed. (B) per cent inhibition of the current at 80 mV and −80 mV calculated from the traces shown in A (circles) and for another cell studied with the same protocol (squares). Note the same time scale in both panels.
Discussion
In the present study we characterized the inhibitory action of the imidazole compound SK&F 96365, generally considered as a selective inhibitor of receptor-mediated Ca2+ entry, in particular of store-operated Ca2+ influx, which is the dominant Ca2+ entry mechanism in nonexcitable cells (for a review see Parekh & Penner, 1997). In previous studies this drug was found to inhibit receptor-stimulated Ca2+ entry following store depletion in various cell types such as human platelets, neutrophils and endothelial cells (Merritt et al., 1990), lymphocytes (Mason et al., 1993), HL-60 cells (Koch et al., 1994), Jurkat T cells (Sei et al., 1995) and in several different smooth muscles (Li et al., 1997; Wayman et al., 1997; Takemoto et al., 1998; Wassdal et al., 1998; Yang, 1998; Lagaud et al., 1999). However, SK&F 96365 is neither very potent (the IC50 in most cases around 10 μM) nor selective as it can also inhibit voltage-gated Ca2+ channels (Merritt et al., 1990), K+ channels (Schwarz et al., 1994), the SR Ca2+ ATPase (Mason et al., 1993), stimulate phosphoinositide hydrolysis and cause intracellular Ca2+ release (Arias-Montano et al., 1998) and facilitate nicotinic receptor desensitization (Hong & Chang, 1994).
Cationic channels opening causes membrane depolarization during muscarinic cholinergic excitation of visceral smooth muscles. Some pharmacological properties of these channels have been recently studied in detail. Many well-known blockers of other channels were found to be effective in inhibiting the muscarinic receptor cationic current, generally within typical concentration ranges. These include inorganic Ca2+ channel blockers Zn2+, Cd2+, Ni2+, Co2+, Mn2+ (Inoue, 1991; Inoue & Chen, 1993; Lee et al., 1993; Fleischmann et al., 1997) in which case the block was practically voltage-independent with little change in the reversal potential or sensitivity to the agonist. K+ channel organic blockers inhibited the cationic current in a voltage-dependent manner with quinidine being the most potent (IC50=0.25 μM) followed by quinine (1 μM), 4-aminopyridine (3.3 mM), TEA+ (4.1 mM), procaine (1–5 mM) (which also blocks many other voltage-gated channels and the Ca2+ release process) (Chen et al., 1993; Lee et al., 1993; Kim et al., 1995; 1998). Caffeine, a Ca2+-releasing agent, also blocks cationic current (∼10 mM) (Chen et al., 1993). Diphenylamine-2-carboxylate (DPC) derivatives (DCDPC and flufenamic acid), which strongly inhibit Ca2+-activated cationic channels and Cl− channels, also block muscarinic receptor cationic current with IC50 values of about 30 μM (Chen et al., 1993). Among these blockers only quinidine appears to be a relatively selective for the muscarinic conductance.
Many blockers behave in a voltage-dependent manner and several mechanisms have been proposed to explain this though in most cases it is very difficult to distinguish between them (for a review see Hille, 1992). For the cationic current, inhibition by TEA, 4-AP, procaine, quinine, quinidine and caffeine was strongly attenuated by membrane depolarization, an effect explained by the location of the binding site some way down the channel pore, partway across the voltage field, or, for positively charged blockers like TEA, by the effect of driving force (Chen et al., 1993; Kim et al., 1995). An alternative, or additional, possibility is that the channel macromolecule is influenced by the membrane potential such that the binding site changes its availability or affinity towards the blocking molecule (Hille, 1992). Considering such a mechanism of block it is interesting to note that cationic channel gating occurs over a considerably more negative voltage range and depends on the amount of activated G proteins; generally the V1/2 values for the Boltzmann-type curves are negative to about −80 mV (Zholos & Bolton, 1994) as compared to the V1/2 value for the blocking action of SK&F 96365 which was positive to about 20 mV. Open-channel block (exemplified by the effects of internal TEA and quaternary ammonium ions or local anaesthetics on other channels) thus can be excluded. The direction of current flow also seems irrelevant because percentage of the inhibition by SK&F 96365 was a smooth sigmoidal function of the membrane potential in the voltage range where cationic current reversed or was only outward (e.g. Figure 4B,C).
Kinetics of the inhibition and disinhibition during voltage steps as well as recovery from the inhibition after wash-out imply that SK&F 96365 falls into the category of ‘slow' blockers which have long residency time (Hille, 1992). Acceleration of the inhibition with increasing membrane positivity had the same voltage dependence as the relative potency of the blocker (Figure 4C) and together with the acceleration of the disinhibition with membrane hyperpolarization (Figure 5) was in complete agreement with the effects of the membrane potential on the apparent dissociation constant.
From our previous work a number of significant differences in the muscarinic receptor cationic current behaviour at positive and negative potentials has emerged. Notably, (i) the Boltzmann-type relation describes the cationic conductance only at negative potentials whereas in the positive range considerable deviation is seen (compare also to Inoue & Isenberg, 1990); (ii) desensitization was very slow at positive potentials such that cationic current measured at 80 mV changed very little whereas at the same time and in the same cell current at −120 mV could be nearly completely lost (Zholos & Bolton, 1994; 1996a); (iii) flash-release within the cell of GTP or GDPβS were almost without effect at positive potentials but strongly increased or inhibited, respectively, cationic current at negative potentials (Zholos & Bolton, 1994); (iv) external divalent cations strongly inhibited cationic current at negative potentials but the effect was negligible at positive potentials (e.g. Figure 11A,B in Zholos & Bolton, 1995); (v) sensitivity to the agonist and the rate of current development were higher the more positive the potential (Zholos & Bolton, 1996b). We have suggested that the same cationic channel is gated differently at different potentials depending on the amount of activated G-proteins in the cell providing that several activated G-proteins may bind to the same channel thus prolonging its opening at any potential; with hyperpolarization more bound G-proteins are required to open the channel. Similar allosteric regulation of gating by binding of variable numbers of Gβγ subunits to muscarinic K+ channel has recently been suggested to explain G protein-mediated shifts in modal preference in atrial cells (Ivanova-Nikolova & Breitwieser, 1997). Moreover, our recent single channel studies have shown that, though in ileal smooth muscle cells two different cationic channels are present with single-channel conductances of 8–9 and 40–50 pS, the major contribution both at negative and positive potentials is made by the channel with larger conductance (Zholos & Bolton, 1998).
However, given so many important differences, the additional evidence on SK&F 96365 for the same channel mediating cationic current generation at negative and positive potentials is very important. Our quantitative comparison between fractions of current inhibited at positive potentials and relieved from the inhibition at negative potentials gave almost identical values (Figure 6) thus providing a strong evidence for the same type of cationic channel generating current in the whole voltage range.
In conclusion, the inhibitory action of SK&F 96365 on the muscarinic receptor cationic channels in ileal smooth muscle cells is consistent with the recent suggestion that Trp6 may encode the channel (Walker et al., 1999). However, this is not strong evidence as many non-specific effects have been reported for this drug. Nevertheless, this blocker has a unique voltage-dependent mode of action for this particular channel likely to be very useful in identifying expressed channel proteins in future molecular biology studies.
Acknowledgments
Supported by grant number 051162/Z from The Wellcome Trust.
Abbreviations
- ATP
adenosine 5′ triphosphate
- BAPTA
1,2-bis(2-aminophenoxy) ethane- N,N,N′,N′-tetraacetic acid
- GTP
guanosine 5′-triphosphate
- GTP-γS
guanosine 5′-O-(3-thiotriphosphate)
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- ICRAC
Ca2+ release-activated Ca2+ current
- SK&F 96365
1-[β-[3-(4-Methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole·HCl
References
- ARIAS-MONTANO J.A., GIBSON W.J., YOUNG J.M. SK&F 96365 (1-[beta-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenylethyl]-1H-imidazole hydrochloride) stimulates phosphoinositide hydrolysis in human U373 MG astrocytoma cells. Biochem. Pharmacol. 1998;56:1023–1027. doi: 10.1016/s0006-2952(98)00125-7. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B., KITAMURA K. Evidence that ionic channels associated with the muscarinic receptor of smooth muscle may admit calcium. Br. J. Pharmacol. 1983;78:405–417. doi: 10.1111/j.1476-5381.1983.tb09405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B., ZHOLOS A.V. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- BOULAY G., ZHU X., PEYTON M., JIANG M., HURST R., STEFANI E., BIRNBAUMER L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- CHEN S., INOUE R., ITO Y. Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br. J. Pharmacol. 1993;109:793–801. doi: 10.1111/j.1476-5381.1993.tb13644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMANN B.K., WANG Y.X., KOTLIKOFF M.I. Muscarinic activation and calcium permeation of nonselective cation currents in airway myocytes. Am. J. Physiol. 1997;272:C341–C349. doi: 10.1152/ajpcell.1997.272.1.C341. [DOI] [PubMed] [Google Scholar]
- HILLE B. Ionic Channels of Excitable Membranes. Sunderland, Massachusetts, USA: Sinauer Associates, Inc; 1992. [Google Scholar]
- HONG S.J., CHANG C.C. Facilitation of nicotinic receptor desensitization at mouse motor endplate by a receptor-operated Ca2+ channel blocker, SK&F 96365. Eur. J. Pharmacol. 1994;265:35–42. doi: 10.1016/0014-2999(94)90220-8. [DOI] [PubMed] [Google Scholar]
- INOUE R. Effect of external Cd2+ and other divalent cations on carbachol-activated non-selective cation channels in guinea-pig ileum. J. Physiol. 1991;442:447–463. doi: 10.1113/jphysiol.1991.sp018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., CHEN S.Physiology of muscarinic receptor-operated nonselective cation channels in guinea-pig ileal smooth muscle ‘Nonselective Cation Channels: Pharmacology, Physiology, and Biophysics' 1993Birkhauser: Basel; 261–268.In: Siemen, D. & Hescheler, J. (eds.) [DOI] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 1990;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOVA-NIKOLOVA T.T., BREITWIESER G.E. Effector contributions to Gβγ-mediated signalling as revealed by muscarinic potassium channel gating. J. Gen. Physiol. 1997;109:245–253. doi: 10.1085/jgp.109.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG T.M., KIM S.J., RHEE P.L., RHEE J.C., KIM K.W. Carbachol activates a nonselective cation current through M2 muscarinic receptor subtype in guinea pig gastric smooth muscle. J. Auton. Nervous Sys. 1997;65:146. [Google Scholar]
- KIM S.J., AHN S.C., SO I., KIM K.W. Quinidine blockade of the carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Br. J. Pharmacol. 1995;115:1407–1414. doi: 10.1111/j.1476-5381.1995.tb16631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S.J., KOH E.M., KANG T.M., KIM Y.C., SO I., ISENBERG G., KIM K.W. Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes. J. Physiol. 1998;513:749–760. doi: 10.1111/j.1469-7793.1998.749ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH B.D., FAUROT G.F., KOPANITSA M.V., SWINNEY D.C. Pharmacology of a Ca2+ influx pathway activated by emptying the intracellular Ca2+ stores in HL-60 cells: evidence that a cytochrome P-450 is not involved. Biochem. J. 1994;302:187–190. doi: 10.1042/bj3020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., BOLTON T.B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J. Physiol. 1990;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGAUD G.J.L., RANDRIAMBOAVONJY V., ROUL G., STOCLET J.C., ANDRIANTSITOHAINA R. Mechanism of Ca2+ release and entry during contraction elicited by norepinephrine in rat resistance arteries. Am. J. Physiol. 1999;276:H300–H308. doi: 10.1152/ajpheart.1999.276.1.H300. [DOI] [PubMed] [Google Scholar]
- LEE H.K., BAYGUINOV O., SANDERS K.M. Role of nonselective cation current in muscarinic responses of canine colonic muscle. Am. J. Physiol. 1993;265:C1463–C1471. doi: 10.1152/ajpcell.1993.265.6.C1463. [DOI] [PubMed] [Google Scholar]
- LI L., KANKAANRANTA H., VAALI K., PAAKKARI I., VAPAATALO H. Econazole, miconazole and SK&F 96365 inhibit depolarization-induced and receptor-operated contraction of guinea-pig isolated trachea in vitro. Eur. J. Pharmacol. 1997;331:221–225. doi: 10.1016/s0014-2999(97)01068-6. [DOI] [PubMed] [Google Scholar]
- LIN W.W., WANG C.W. SK&F 96365 inhibits carbachol-induced phosphoinositide turnover in human neuroblastoma SH-SY5Y and rat cerebellar granule cells. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:53–58. doi: 10.1007/BF00168706. [DOI] [PubMed] [Google Scholar]
- MASON M.J., MAYER B., HYMEL L.J. Inhibition of Ca2+ transport pathways in thymic lymphocytes by econazole, miconazole, and SKF 96365. Am. J. Physiol. 1993;264:C654–C662. doi: 10.1152/ajpcell.1993.264.3.C654. [DOI] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.A., MOORES K.E., RINK T.J. SK&F96365, a novel inhibitor of receptor-stimulated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- SCHWARZ G., DROOGMANS G., NILIUS B. Multiple effects of SK&F 96365 on ionic currents and intracellular calcium in human endothelial cells. Cell Calcium. 1994;15:45–54. doi: 10.1016/0143-4160(94)90103-1. [DOI] [PubMed] [Google Scholar]
- SEI Y., TAKEMURA M., GUSOVSKY F., SKOLNICK P., BASILE A. Distinct mechanisms for Ca2+ entry induced by OKT3 and Ca2+ depletion in Jurkat T cells. Exp. Cell Res. 1995;216:222–231. doi: 10.1006/excr.1995.1028. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO M., TAKAGI K., OGINO K., TOMITA T. Comparison of contractions produced by carbachol, thapsigargin and cyclopiazonic acid in the guinea-pig tracheal muscle. Br. J. Pharmacol. 1998;124:1449–1454. doi: 10.1038/sj.bjp.0701993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER R.L., MASON H.S., KENYON J.L., HOROWITZ B. Cloning and transcriptional expression of nonselective cation channels from gastrointestinal smooth muscles. Biophys. J. 1999;76:A292. [Google Scholar]
- WANG Y.X., FLEISCHMANN B.K., KOTLIKOFF M.I. M2 receptor activation of nonselective cation channels in smooth muscle cells: Calcium and Gi/Go requirements. Am. J. Physiol. 1997;273:C500–C508. doi: 10.1152/ajpcell.1997.273.2.C500. [DOI] [PubMed] [Google Scholar]
- WASSDAL I., NICOLAYSEN G., IVERSEN J.G. Bradykinin causes contraction in rat uterus through the same signal pathway as oxytocin. Acta Physiol. Scand. 1998;164:47–52. doi: 10.1046/j.1365-201X.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- WAYMAN C.P., McFADZEAN I., GIBSON A., TUCKER J.F. Cellular mechanisms underlying carbachol-induced oscillations of calcium-dependent membrane current in smooth muscle cells from mouse anococcygeus. Br. J. Pharmacol. 1997;121:1301–1308. doi: 10.1038/sj.bjp.0701279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG C.M. Dissociation of intracellular Ca2+ release and Ca2+ entry response to 5-hydroxytryptamine in cultured canine tracheal smooth muscle cells. Cell Signal. 1998;10:735–742. doi: 10.1016/s0898-6568(98)00020-5. [DOI] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. G-protein control of voltage dependence as well as gating of muscarinic metabotropic channels in guinea-pig ileum. J. Physiol. 1994;478:195–202. doi: 10.1113/jphysiol.1994.sp020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. J. Physiol. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. A novel GTP-dependent mechanism of ileal muscarinic metabotropic channel desensitization. Br. J. Pharmacol. 1996a;119:997–1012. doi: 10.1111/j.1476-5381.1996.tb15770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B.Properties of the muscarinic cationic channel in longitudinal smooth muscle of guinea pig small intestine ‘Smooth Muscle Excitation' 1996bAcademic Press Ltd: London; 155–173.In: Bolton, T.B. & Tomita T (eds.) [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic control of cationic channel opening in smooth muscle. J. Physiol. 1998;511:3S–4S. [Google Scholar]


