Abstract
The circadian activity of the hypothalamic-pituitary-adrenal (HPA) axis is regulated by caloric flow in rats. During the dark cycle, it has been shown that, in fasted rats, the time-course profile of plasma concentrations of adrenocorticotropin (ACTH) and corticosterone parallels the profile of food intake in ad libitum fed animals.
Cholecystokinin (CCK) is involved in regulating food intake in rodents. CCK-8 reduces food intake by acting on CCK-A receptors subtype.
This work aims at establishing an eventual relationship between the modulatory role of CCK on food intake and its effect on HPA axis activity during fasting.
We studied the effect of CCK-A and CCK-B receptor antagonists on food intake during the first period of the dark cycle. Under these conditions we observed that the CCK-A receptor antagonist, SR-27897 (0.3 mg kg−1), but not the CCK-B receptor antagonist, L-365260 (1 mg kg−1), increases food-intake.
In a second series of experiments we observed that the increase of both ACTH and corticosterone plasma level elicited by fasting, was prevented by SR-27897, but not by L-365260.
These results indicate that CCK-A receptor blockade during fasting prevents the activation of the HPA axis.
Keywords: Cholecystokinin, CCK-A receptor, SR-27897, L-365260, HPA axis, feeding, fasting
Introduction
Feeding behaviour is known to be regulated by both glucose utilization and lipid expenditure. The involvement of lipid deposits has been stressed by the recent discovery of leptin. This hormone originates in adipose tissue and acts on hypothalamic centres. As an additional factor of feeding regulation, the role of some neuropeptides has been established. On this context, neuropeptide Y (NPY) is known to stimulate feeding, whereas cholecystokinin (CCK) is considered as one of the major satiety signals (Barrachina et al., 1997 and references cited therein)
In the hypothalamus, both CCK-A and CCK-B receptor gene expression has been detected in the paraventricular nucleus (PVN) (Meister et al., 1994). In addition, intraperitoneal administration of CCK-8 activates catecholaminergic neurons in the caudal medulla projecting to the PVN (Rinaman et al., 1995). These anatomical considerations seem to be endowed with functional relevance since systemically administered CCK-8 induces corticotropin-releasing hormone (CRH) release (Kamilaris et al., 1992; Calogero et al., 1993), and activates c-fos gene transcription in PVN neurons containing CRH (Verbalis et al., 1991). Moreover CCK, in synergy with leptin, increase c-Fos synthesis in the PVN (Barrachina et al., 1997).
The role of CCK in hypothalamus seems to be linked to the control of feeding behaviour. CCK appears to be released as a satiety signal. Accordingly, administration of CCK-8 into the PVN or into the ventromedial hypothalamus (VMH) inhibits feeding, and lesions of the VMH or PVN suppress CCK-8 induced satiety (Dourish et al., 1989 and references cited therein). Akana et al. (1994) have demonstrated that food intake rhythm drives the activity of the hypothalamic-pituitary-adrenal axis (HPA) axis. These authors have shown that food deprivation during the dark phase of the circadian rhythm, i.e., the period of maximal HPA activity and food intake in rodents, activates the HPA axis. These results, taken together with the involvement of CCK in satiety, led us to speculate that CCK could play a role in the relationship between the rhythm of food intake and the activity of HPA axis.
The goal of this work has been to investigate the role of CCK on the modulation of the HPA axis activity both in fasted and fed rats. With this aim we have studied the effect of selective CCK-A and CCK-B receptor antagonists on ACTH and corticosterone plasma levels both in fed and in food deprived rats during the initial period of the dark cycle, which corresponds to the period of maximal food intake in rodents.
Methods
Animals
Male Wistar rats (Charles River, Spain) weighing 150–180 g at the time of the experiment were housed under inversed controlled dark/light cycle (8–20 h/20–8 h) for a week before the experiment. Water and food were available ad libitum. Animals were pair housed, even in food intake experiments, in order to avoid stress by isolation. Red light was on full time.
Chemicals
SR-27897 (1-[[2-(4-(2-chlorophenyl)thiazol-2yl)aminocarbonyl]-indolyl]-acetic acid) was kindly provided by Sanofi Recherche (France). L-365260 [(3R)-(+)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1, 4-benzodiazepin-3-yl)-3-methylphenylurea) was a gift of Merck Sharp & Dohme Research Laboratories (U.S.A.). Human ACTH was supplied by Bachem (Switzerland). Corticosterone hydrochloride was obtained from Sigma (U.S.A.). [3H]-Corticosterone and [125I]-ACTH were obtained from Amersham (U.K.). ACTH antiserum (IgG-ACTH-1) and second goat antirabbit serum (IgG-GARGG) were purchased from IgG-Corporation (U.S.A.). Corticosterone antiserum was supplied by Bioclin (U.K.). Trypsin and trypsin inhibitor were supplied by Boehringer Mannheim (Germany). Other reagents and solvents were from Sigma (U.S.A.).
Injection procedure
Either SR-27897 or L-365260 were administered s.c. in ethanol: polyethyleneglycol 200 (5 : 95) in a volume of 1 ml kg−1, 120 min before lights off. At this time food was weighed (feeding experiments) or retired (hormone experiments). Doses of these drugs (0.3 mg kg−1 for SR-27897 and 1 mg kg−1 for L-365260) have been shown to elicit long-lasting CCK-A (Poncelet et al., 1993; Gully et al., 1993) and CCK-B selective responses (Hernando et al., 1994; 1996) after i.p. administration. In our model we have administered all drugs s.c., which ensure a blunter and wider plasma level time course profile for this administration route than i.p.
Feeding experiments
Food was weighed 120 min before the lights were off. Drugs were administered at the same time. Food was weighed 90 min after lights off. Food intake was expressed in g rat−1. Food intake was determined for two animals during a period of 210 min.
Hormone studies
Animals had food and water available ad libitum until the beginning of the experiment. Rats were distributed in four experimental groups. The variables of the study were food availability and pharmacological treatment. In the food deprived groups, food was removed 120 min before the lights were off. Non-deprived animals had food and water ad libitum during the experiment. Either drugs or vehicle were administered 120 min before lights off. Ninety minutes after the lights were off (and 210 min after drugs administration and/or food deprivation) rats were killed by decapitation. Animals belonging to the same cage were killed with a delay less than 30 s. Trunk blood was collected in chilled EDTA-coated tubes and then centrifuged at 2000 r.p.m. during 10 min at 4°C. Plasma aliquots were kept at −70°C until RIA.
Measurement of ACTH and corticosterone plasma level
ACTH was measured by double antibody precipitation RIA, as described by Woods et al. (1992). Rabbit IgG-ACTH-1 was used as the primary antiserum. Separation of the bound fraction was carried out by precipitation with a second antiserum (IgG-GARGG) and PEG 6000, 6%. Synthetic human ACTH was used as a reference standard and [125I]-ACTH as a tracer. Under the conditions employed, the assay detects 0.5 fmol/tube of human ACTH, the intra assay coefficient of variation being 10%. Dilutions curves of the sample were parallel with those of the standard.
Corticosterone was determined according to the method described by Armario & Castellanos (1984). Briefly, plasma samples were incubated for 1 h with trypsin, then the digestion was stopped with a trypsin inhibitor (30 min). Specific corticosterone antiserum and tracer were then added, and the mixture incubated at 4°C during 24 h. Free tracer was precipitated with charcoal 1%. Corticosterone was used as reference standard and [3H]-corticosterone as tracer. The sensitivity was 12 pg tube−1. The intra-assay coefficient of variation was 7%. As in the case of ACTH, dilutions curves of the sample were parallel with those of the standard.
Analysis of data
Comparisons were made using a two-way ANOVA. The factors of variation were drug treatment and food availability. Individual comparisons within a given group (fasted or food-deprived) were analysed by the Student's t-test (Snedecor & Cochran, 1980).
Results
Hormone studies
The effect of SR-27897 (0.3 mg kg−1, s.c., 120 min before lights off) on both ACTH and corticosterone plasma level was determined 90 min after lights were off. As indicated in Table 1, two-way ANOVA revealed significant drug treatment (F(1,24)=7.382; P<0.05) and food deprivation (F(1,24)=4.701; P<0.05) effects with significant interaction between treatment and fasting (F(1,24)=9.618; P<0.01). In food deprived animals, SR-27897 significantly reduced the increase of ACTH plasma level (Student's t-test; P<0.01) whereas in non-fasted animals, SR-27897 was without effect. In the case of corticosterone, two-way ANOVA indicated a significant effect of fasting (F(1,40)=26.692; P<001) and a non-significant effect for treatment (F(1,40)=2.591). The interaction between treatment and fasting was significant (F(1,40)=7.221; P<0.05). In food deprived animals, SR-27897 significantly reduced the increase of corticosterone plasma levels (Student's t-test; P<0.05) whereas in non-fasted animals, SR-27897 was without effect.
Table 1.
Effect of SR-27897 (0.3 mg kg−1) on plasma ACTH (fmol ml−1) and corticosterone (ng ml−1) levels 210 min after food deprivation
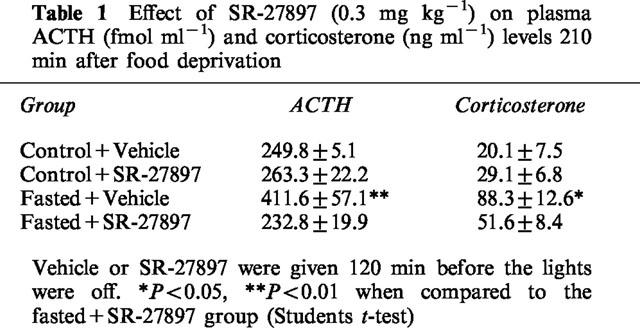
The effect of L-365260 (1 mg kg−1, s.c., 120 min before lights off), a CCK-B receptor antagonist, was tested under identical experimental conditions. No effect for L-365260 was detected either on ACTH (440.6±83.1 fmol ml−1 in fasted+ vehicle group vs 475.3±42.7 fmol ml−1 in fasted+L-365260 group) or on corticosterone (103.2±6.4 ng ml−1 in fasted+ vehicle group vs 90±10.4 ng ml−1 in fasted+L-365260 group) plasma concentrations.
Feeding studies
As illustrated in Figure 1, SR-27897 (0.3 mg kg−1, s.c., 120 min before lights off) significantly increased food intake (55.5% over the control group; Student's t-test *P<0.05). In contrast, L-365260 (1 mg kg−1, s.c., 120 min before lights off) did not modify food intake. Animals in the control group fed 1.8±0.15 g rat−1.
Figure 1.
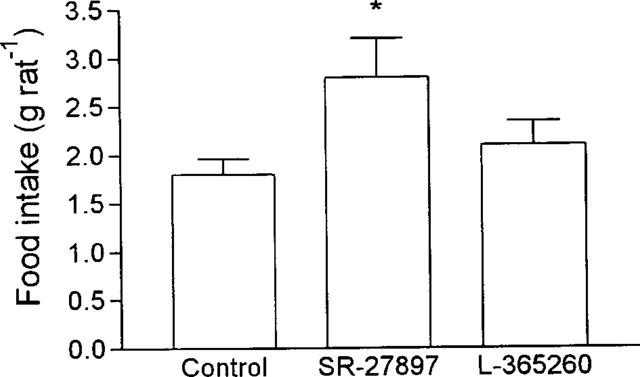
Effect of SR-27897 (0.3 mg kg−1, s.c., 120 min before lights off) and L-365260 (1 mg kg−1, s.c., 120 min before lights off) on food intake. Food intake was expressed in g rat−1. SR-27897 significantly enhanced food intake (Student's t-test *P<0.01).
Discussion
In this work we describe the effect of both CCK-A and CCK-B receptor antagonists on the activity of the HPA axis both in fasted and in fed rats. Under our conditions, the CCK-A receptor antagonist, SR-27897 prevented the increase of both ACTH and corticosterone plasma level induced by food deprivation during the dark period of the circadian rhythm. Under the same conditions, no effect of the CCK-B receptor antagonist, L-365260, was detected. Both SR-27897 and L-365260 are highly selective drugs with KD values about 1 nM and are endowed with selectivity factors ranging between 500-1000 (Gully et al., 1993; Chang et al., 1989).
The dose of SR-27897 used in this study has been shown to elicit long-lasting effects in other experimental paradigms (Gully et al., 1993) and to antagonize the hypophagic effect of CCK in rats (Poncelet et al., 1993). Under our experimental conditions, 0.3 mg kg−1 SR-27897 significantly increased food intake. The involvement of CCK-A receptors in the satiating effect of CCK is a controversial matter. Although most data in this field suggest the involvement of CCK-A receptors (Crawley et al., 1991), some authors have indicated, in turn, that CCK-B receptors, rather than CCK-A receptors, are involved (Dourish et al., 1989). Most of the experiments have been carried out during the light period of the circadian rhythm and in previously fasted animals. On the basis that rodents specially feed during the first phase of the dark cycle, we have performed our experiments in satiated animals, during the dark period. In our opinion, if CCK is physiologically involved in satiety phenomena, as it is generally considered, these conditions should evidence more accurately the effect of CCK antagonists than those used in previous studies.
Our experimental protocol was based on that reported by Akana et al. (1994). These authors showed that food deprivation during the dark period of the circadian rhythm leads to the activation of the HPA axis. Our study was aimed at characterizing an eventual role for CCK in this activation of the HPA during fasting. In fact, Akana et al. (1994) have suggested that during fasting, the normal drive to food intake is switched to drive the HPA axis. This mechanism is probably responsible for the increase in corticosterone-induced gluconeogenesis (Baxter & Forsham, 1972). Our hypothesis is that CCK may be a candidate to regulate this phenomena. Interestingly, it has been proposed that CCK, through CCK-A receptors, may be a regulatory factor of glucose homeostasis, since exogenous CCK increases glucose elimination at high carbohydrate levels, and elicits a glucagon-like effect at low carbohydrate levels (Verspohl et al., 1992). Thus, under fasting conditions, endogenous CCK, through CCK-A receptors, may activate the HPA axis to stimulate glucose production.
From our data, we cannot indicate the anatomical substrate for the effect observed. SR-27897 could act peripherally on adenohypophysis to stimulate ACTH release. However, it has been demonstrated that CCK-induced release of ACTH is secondary to hypothalamic activation (Kamilaris et al., 1992). SR-27897 could also act on vagal afferents leading to the activation of catecholaminergic inputs of the PVN (Rinaman et al., 1995). On the other hand, the hypothalamus could be an alternative site for a central action of the CCK-A antagonist. In any case, our results show that under fasting conditions, but not in satiated rats, CCK regulates both ACTH and corticosterone release. Interestingly, we have previously demonstrated that devazepide, another CCK-A receptor antagonist, does not modify plasmatic concentrations of these hormones in other situations leading to the activation of the HPA axis, such as forced-swim stress (Hernando et al., 1996).
In summary our data show that CCK-A receptor blocking prevents the activation of the HPA axis that occurs during fasting in rats. Under the same conditions, CCK-B receptor blocking has no effect either on ACTH or on corticosterone plasma levels. These data suggest that CCK could be a regulatory factor of HPA axis activity under conditions of food deprivation, i.e. low glucose sources intake.
Acknowledgments
This work was supported by a Concerted Action of the European Union (BMH 1-CT-94-1108), a grant from CICYT (SAF 95-027), and a grant from DGICYT (UE 95-0017). M.M. Garrido is a predoctoral fellow supported by the Universidad Complutense de Madrid. M. Ruiz-Gayo is a senior postdoctoral fellow supported by Ministerio de Educación y Cultura (Spain). We are grateful to Prof M. Dallman for helpful discussion of experimental design.
Abbreviations
- ACTH
adrenocorticotropin
- CCK
cholecystokinin
- CRH
corticotropin-releasing hormone
- HPA
hypothalamic-pituitary-adrenal
- NPY
neuropeptide Y
- PVN
paraventricular nucleus
- VMH
ventromedial hypothalamus
References
- AKANA S.F., STRACK A.M., HANSON E.S., DALLMAN M. Regulation of activity of the hypothalamic-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135:1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- ARMARIO A., CASTELLANOS J.M. A simple procedure for direct corticosterone radioimmunoassay in the rat. Rev. Esp. Fisiol. 1984;40:437–442. [PubMed] [Google Scholar]
- BARRACHINA M.D., MARTINEZ V., WANG L., WEI J.Y., TACHÉ Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER J.D., FORSHAM P.H. Tissue effects of glucocorticoids. Am. J. Med. 1972;52:573–589. doi: 10.1016/0002-9343(72)90154-4. [DOI] [PubMed] [Google Scholar]
- CALOGERO A.E., NICOLISIS A.M.G., MONCADA M.L., CONIGLIONE F., VICARI E., POLOSA P., D'AGATA R. Effects of cholecystokinin octapeptide on the hypothalamic-pituitary-adrenal axis function and on vasopressin, prolactin and growth hormone release in humans. Neuroendocrinology. 1993;58:71–76. doi: 10.1159/000126514. [DOI] [PubMed] [Google Scholar]
- CHANG R.S., CHEN T.B., BOCK M.G., FREIDINGER R.M., CHEN R., ROSEGAY A., LOTTI V.J. Characterization of the binding of [3H]L-365260: a new potent and selective brain cholecystokinin (CCK-B) and gastrin receptor antagonist radioligand. Mol. Pharmacol. 1989;35:803–808. [PubMed] [Google Scholar]
- CRAWLEY J.N., FISKE S.M., DURIEUX C., DERRIEN M., ROQUES B.P. Centrally administered cholecystokinin suppresses feeding through a peripheral-type receptor mechanism. J. Pharmacol. Exp. Ther. 1991;257:1076–1080. [PubMed] [Google Scholar]
- DOURISH J., RYCROFT W., IVERSEN S.D. Postponement of satiety by blockade of brain cholecystokinin (CCK-B) receptors. Science. 1989;245:1509–1511. doi: 10.1126/science.2781294. [DOI] [PubMed] [Google Scholar]
- GULLY D., FREHEL D., MARCY C., SPINAZZI A., LESPY L., NELIAT G., MAFFRAND J.P., LE FUR G. Peripheral biological activity of SR 27897: a new potent non-peptide antagonist of CCK-A receptors. Eur. J. Pharmacol. 1993;232:13–19. doi: 10.1016/0014-2999(93)90722-t. [DOI] [PubMed] [Google Scholar]
- HERNANDO F., FUENTES J.A., ROQUES B.P., RUIZ-GAYO M. The CCK-B receptor antagonist, L-365260, elicits antidepressant-type effects in the forced-swimming test in mice. Eur. J. Pharmacol. 1994;261:257–263. doi: 10.1016/0014-2999(94)90115-5. [DOI] [PubMed] [Google Scholar]
- HERNANDO F., FUENTES J.A., RUIZ-GAYO M. Impairment of stress adaptative behaviours in rats by the CCK-A receptor antagonist, devazepide. Br. J. Pharmacol. 1996;118:400–406. doi: 10.1111/j.1476-5381.1996.tb15416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMILARIS T.C., JOHNSON E.O., CALOGERO A.E., KALOGERAS K.T., BERNARDINI R., CHROUSOS G.P., GOLD P.W. Cholecystokinin-octapeptide stimulates hypothalamic-pituitary-adrenal function in rats: Role of corticotropin-releasing hormone. Endocrinology. 1992;130:1764–1774. doi: 10.1210/endo.130.4.1312423. [DOI] [PubMed] [Google Scholar]
- MEISTER B., BROBERGER C., VILLAR M.J., HOKFELT T. Cholecystokinin B receptor gene expression in hypothalamic neurosecretory neurons after experimental manipulations. Neuroendocrinology. 1994;60:458–469. doi: 10.1159/000126782. [DOI] [PubMed] [Google Scholar]
- PONCELET M., ARNONE M., HEAULME M., GONALONS N., GUEDET C., SANTUCCI V., THURNEYSSEN O., KEANO P., GULLY D., LE FUR G., SOUBRIÉ P. Neurobehavioural effects of SR 27897, a selective cholecystokinin type A (CCK-A) receptor antagonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:102–107. doi: 10.1007/BF00168544. [DOI] [PubMed] [Google Scholar]
- RINAMAN L., HOFFMAN G.E., DOHANICS J., LE W.W., STRICKER E.M., VERBALIS J.G. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus in rats. J. Comp. Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- SNEDECOR G.W., COCHRAN W.G. Statistical Methods 1980Iowa: The Iowa State University Press; 7th Edition [Google Scholar]
- VERBALIS J.G., STRICKER E.M., ROBINSON A.G., HOFFMAN G.E. Cholecystokinin activates c-fos expression in hypothalamic oxytocin and corticotropin-releasing hormone neurons. J. Neuroendocrinol. 1991;3:205–213. doi: 10.1111/j.1365-2826.1991.tb00264.x. [DOI] [PubMed] [Google Scholar]
- VERSPOHL E.J., ZOLL C., WAHL M.A., AMMO H.P.T. The role of cholecystokinin (CCK8) on glucose production and elimination, and on plasma insulin and glucose in rats. Peptides. 1992;13:1091–1095. doi: 10.1016/0196-9781(92)90012-r. [DOI] [PubMed] [Google Scholar]
- WOODS M.D., SHIPSTON J., MULLENS E.L., ANTONI F.A. Pituitary corticotrope tumor (AtT20) cells as a model system for the study of early inhibition by glucocorticoids. Endocrinology. 1992;131:2873–2880. doi: 10.1210/endo.131.6.1332850. [DOI] [PubMed] [Google Scholar]


