Abstract
The effects of the dopamine D1-receptor agonist fenoldopam were compared with those of the D2-receptor agonist R(−)-propylnorapomorphine and vehicle on mean arterial pressure (MAP), mean circulatory filling pressure (MCFP, the driving force of venous return), arterial resistance (Ra), venous resistance (Rv), heart rate (HR) and cardiac output (CO) in groups of thiobutabarbitone-anaesthetized rats pre-treated with i.v. injection of mecamylamine (3.7 μmol kg−1) and continuously infused with noradrenaline (6.8 nmol kg−1 min−1).
The vehicle did not alter any haemodynamic variables. All doses of fenoldopam (0.5, 2 and 16 μg kg−1 min−1) reduced MAP, Ra and Rv, and increased CO. At the highest dose, fenoldopam also increased HR and reduced MCFP.
All doses of R(−)-propylnorapomorphine (0.5, 2 and 16 μg kg−1 min−1) increased MAP but did not significantly alter CO, Rv and MCFP. Both Ra and HR were increased by the highest dose of R(−)-propylnorapomorphine.
Our results indicate that fenoldopam reduces MAP and MCFP, and markedly increases CO through reductions of arterial and venous resistances. The effects of fenoldopam in dilating arterial resistance and capacitance vessels were similar. In contrast, R(−)-propylnorapomorphine elevates MAP through an increase in arterial resistance but has minimal effects on CO, MCFP and venous resistance. Both drugs have a small direct, positive chronotropic action at the highest dose.
Keywords: Fenoldopam, dopamine receptors, R(−)-propylnorapomorphine, vasodilator, capacitance vessels, vasodilatation, mean circulatory filling pressure, venous resistance
Introduction
Two types of peripheral dopamine receptors, D1 and D2 (previous names DA1 and DA2) were first identified by Goldberg (1972) and Langer (1974), respectively. Dopamine D1 receptors are primarily post-junctional and they mediate arterial dilatation, most notably in the renal, mesenteric, coronary and cerebral vascular beds (Goldberg, 1985; Cavero et al., 1982; 1987; van der Niepen et al., 1998). Dopamine D2 receptors are central as well as peripheral on the sympathetic ganglia and nerve terminals, and activations of these central and peripheral receptors increase sympathetic discharge (Damase-Michel et al., 1990) and inhibit the release of noradrenaline and dopamine (Cavero et al., 1982; Soares-da-Silva, 1990), respectively. The vascular effects of dopamine are however, not confined to the actions of D1 and D2 receptors. Whereas a low dose of dopamine activates primarily dopamine receptors leading to vasodilatation, a high dose activates α- and β-adrenoceptors. The activation of α- and β-adrenoceptors, in turn, increases total peripheral resistance and myocardial contractility, respectively. The multiple actions of dopamine are utilized in the management of cardiogenic shock.
Fenoldopam, a D1 receptor agonist, has been shown to preferentially dilate the renal vasculature in dogs (Kohli et al., 1988; Aronson et al., 1991), rats (Lefevre-Borg et al., 1988; Hedge et al., 1989) and hypertensive humans (Shusterman et al., 1993). In addition, it lowers arterial pressure and total peripheral resistance in various species of experimental animals (Hahn et al., 1982; Sengupta & Lokhandwala, 1985; Lappe et al., 1986; Szabo et al., 1986; Zhao et al., 1990) and humans (Ventura et al., 1984; Shusterman et al., 1993; Panacek et al., 1995; O'Connell et al., 1997). Unlike dopamine, fenoldopam lacks agonistic action at α- and β-adrenoceptors (Hahn et al., 1982; Ohlstein et al., 1985). Comparative clinical trials have demonstrated that the efficacy of i.v. infused fenoldopam is similar to that of sodium nitroprusside in reducing blood pressure in hypertensive emergencies (Shusterman et al., 1993; Panacek et al., 1995; Brogden & Markham, 1997). To our knowledge, there is no published information on the in vivo venous action of fenoldopam. The venous system plays a crucial role in regulating cardiac output through alterations of mean circulatory filling pressure and venous resistance. Mean circulatory filling pressure (MCFP) is the pressure that would exist throughout the vasculature following circulatory arrest and instantaneous equilibration of the circulatory pressure (Guyton et al., 1973), and is an indicator of the driving force of venous return (Tabrizchi & Pang, 1992; Rothe, 1993). Venous resistance, though lower than arterial resistance, is a major determinant of cardiac output due to low pressure in the venous circulation (Rothe, 1993; Pang, 1994). A reduction in venous resistance facilitates venous return and therefore increases cardiac output.
The objectives of this study were to characterize the venous actions of fenoldopam through measurements of MCFP and venous resistance, and to contrast its vascular actions with those of R(−)-propylnorapomorphine, a D2 receptor agonist. There is evidence that apomorphine, though more recognized for its agonistic action at central dopamine receptors, also acts on peripheral presynaptic D2-receptors (Gessa & Corsini, 1981). Indeed, apomorphine dose-dependently reduced blood pressure when injected intravenously into anaesthetized dogs, but increased blood pressure when injected intravertebrally or intracisternally (Montastruc & Guiol, 1984). Moreover, the depressor response elicited by i.v. injection of apomorphine was inhibited by the peripheral D2-receptors antagonist, domperidone (Willems et al., 1981). It has also been shown that R(−)-propylnorapomorphine is more potent and longer-acting than apomorphine at D2-receptors in the rat brain (Neumeyer et al., 1973; Barnes et al., 1990). I.v. injections of apomorphine as well as propylnorapomorphine in rats increased blood pressure (van den Buuse, 1992). To disclose the venodilator action of the fenoldopam and avoid baroreflex-mediated alteration in venomotor tone when blood pressure is altered, the rats were pretreated with the ganglion blocker mecamylamine followed by continuous infusion of noradrenaline to elevate venous tone (Pang, 1994).
Methods
Animal preparation
Male Sprague-Dawley rats, weighing 400–500 g, were anaesthetized with thiobutabarbitone (100 mg kg−1 i.p.). Body temperature was maintained at 37±1°C with a rectal probe and a heat lamp attached to a Thermistemp Temperature Controller (Model 71; Yellow Spring Instrument Co. Inc., OH, U.S.A.). A polyethylene (PE50) catheter was inserted into the left iliac artery for the recording of mean arterial pressure (MAP) via a pressure transducer (P23DB, Gould Statham, CA, U.S.A.). Heart rate (HR) was derived electronically from the upstroke of the arterial pulse pressure by a Grass 7P4G tachograph. Additional catheters were inserted into the left ventricle via the right carotid artery and the right iliac artery for the injection of radioactively-labelled microspheres and the withdrawal of a reference blood sample, respectively, (Wang et al., 1995). The vehicle or drugs were administered through cannulae inserted into the right iliac vein and the left external jugular vein. A catheter was inserted into the inferior vena cava via the left iliac vein to measure central venous pressure (CVP) by another pressure transducer (P23DB, Gould Statham). A saline-filled, balloon-tipped catheter was advanced into the right atrium through the right external jugular vein for stopping the circulation when measuring mean circulatory filling pressure. The correct positioning of the balloon was tested by transiently inflating the balloon, which when correctly placed, caused a simultaneous decrease in MAP to 20–25 mmHg and an increase in CVP within 5 s of circulatory arrest. MAP, HR and CVP were continuously monitored and displayed on a Grass Polygraph (Model RPS 7C8). The rats were given 30 min to stabilize before taking baseline cardiovascular measurements.
The method for determining mean circulatory filling pressure has been described in detail elsewhere (Tabrizchi & Pang, 1992; Wang et al., 1995; Ng & Pang, 1998). Briefly, steady-state readings of MAP and CVP were noted at 4–5 s after inflation of the arterial balloon. To avoid rapid equilibration of arterial and venous pressures during circulatory arrest, the arterial pressure contributed by the small amount of trapped blood was mathematically corrected as follows: MCFP=VPP+1/60 (FAP-VPP), where FAP and VPP denote the final arterial pressure and venous plateau pressure, respectively, and 1/60 represents the ratio of arterial to venous compliance.
Measurement of cardiac output
A well-stirred suspension (100 μl) containing 20,000–25,000 microspheres (15 μm diameter) labelled with cobalt-57 (Du Pont Canada Inc., Ont., Canada) was injected and flushed over 10 s into the left ventricle at the end of the 30 min-equilibration period, and 8 min after the i.v. infusion of a drug or vehicle. At 10 s before the injection of each set of microspheres, a blood sample was withdrawn (Harvard infusion/withdrawal pump) from the right iliac arterial cannula into a heparinized saline-filled syringe at 0.35 ml min−1 for 45 s. The blood removed was slowly injected back to the rats immediately after the counting of radioactivity at 80–160 keV using a 1185 Series Dual Channel Automatic Gamma Counter (Nuclear-Chicago, IL, U.S.A.) with a 3 inch NaI crystal.
Experimental protocol
Rats were randomly divided into three groups (n=6 each). Immediately after baseline measurements of haemodynamic variables, all groups were given i.v. bolus injections of mecamylamine (3.7 μmol kg−1) followed by i.v. infusion of noradrenaline (6.8 nmol kg−1 min−1) at 10 min later. The dose of mecamylamine used was found to abolish ganglionic transmission for more than 2 h (Wang & Pang, 1991). After another 10 min, each group of rats was infused with either fenoldopam (0.5, 2 and 16 μg kg−1 min−1), R(−)-propylnorapomorphine (0.5, 2 and 16 μg kg−1 min−1) or an equal volume of vehicle (0.9% NaCl) for 10 min each dose. In preliminary studies, we found that a higher dose (32 μg kg−1 min−1) of neither fenoldopam nor propylnorapomorphine caused a larger change in MAP. CO followed by mean circulatory filling pressure measurements were taken at 8 min after the infusion of a drug or vehicle, at the plateau phase of response to each drug. A recovery period of 5 min, during which infusion was stopped, was allowed between doses.
Drugs
Fenoldopam was a gift from Neurex Inc. (CA, U.S.A.). R(−)-propylnorapomorphine hydrochloride (PNAM) and thiobutabarbitone (Inactin) were from Research Biochemicals International (MA, U.S.A.). All drugs were dissolved in normal saline (0.9% NaCl) and prepared fresh daily.
Calculations and data analysis
Cardiac output (CO, ml min−1), arterial resistance (Ra, mmHg min ml−1) and venous resistance (Rv, mmHg min ml−1) were calculated according to the following equations:
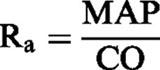 |
 |
Due to the technical difficulty in monitoring right atrial pressure in small animals, CVP rather than right atrial pressure was used to estimate the pressure gradient to venous return (mean circulatory filling pressure minus right atrial pressure). This is legitimate as mean CVP is nearly identical to mean right atrial pressure (Rothe, 1993).
All values are presented as mean±s.e.mean. Comparisons were made with analysis of variance (ANOVA) followed by Duncan's multiple range test, with P<0.05 as the criterion for statistical significance.
Results
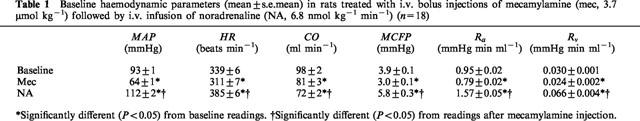
Baseline group values of MAP, HR, CO, MCFP, Ra and Rv were not significantly different from each other among the three groups of rats, and the group values were pooled (Table 1). Mecamylamine significantly decreased MAP, HR, CO, MCFP, Ra and Rv. The subsequent infusion of noradrenaline significantly increased MAP, HR, MCFP, Ra, Rv and decreased CO. The combination of ganglionic blockade and noradrenaline increased MAP, HR, MCFP, Ra and Rv but reduced CO from the respective pre-treatment baselines.
Table 1.
Baseline haemodynamic parameters (mean±s.e.mean) in rats treated with i.v. bolus injections of mecamylamine (mec, 3.7 μmol kg−1) followed by i.v. infusion of noradrenaline (NA, 6.8 nmol kg−1 min−1) (n=18)
The vehicle (time-control) did not significantly alter any of the haemodynamic variables. Relative to readings in the vehicle group, all three doses of fenoldopam increased CO and reduced MAP, Ra as well as Rv (Figure 1). At the highest dose, HR was increased while MCFP was reduced. R(−)-propylnorapomorphine, on the other hand, increased MAP at all doses, but did not significantly alter CO, MCFP and Rv. Ra and HR were elevated only at the highest dose.
Figure 1.
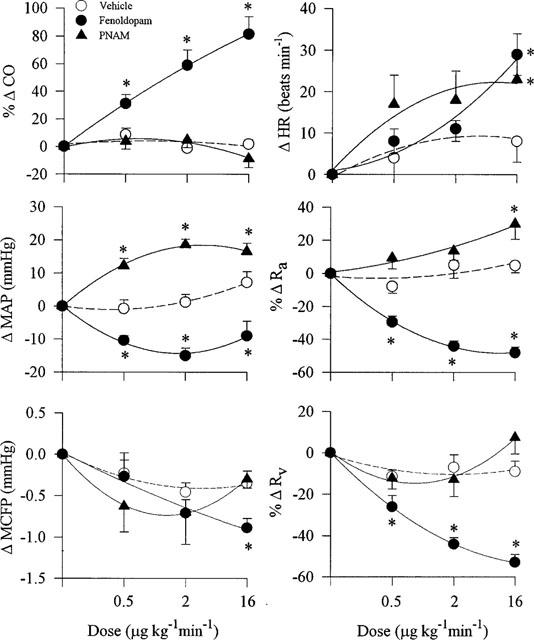
Effects (mean±s.e.mean) of i.v. infusions of fenoldopam, R(−)-propylnorapomorphine or equivalent volumes of vehicle (0.9% NaCl) on cardiac output (CO), mean arterial pressure (MAP), mean circulatory filling pressure (MCFP), heart rate (HR), arterial resistance (Ra) and venous resistance (Rv) in three groups of rats (n=6 each) pre-treated with i.v. mecamylamine (3.7 μmol kg−1) and noradrenaline (6.8 nmol kg−1 min−1). All measurements were obtained at 8 min after the start of an infusion of a drug or vehicle. *Significantly different (P<0.05) from the corresponding values in the vehicle group.
Discussion
Our results show that i.v. infusion of fenoldopam elicited dose-dependent reductions in MAP and Ra, as well as increases in CO. Therefore, fenoldopam decreased blood pressure by decreasing arteriolar resistance. HR was increased only at the highest dose. Our results are in accord with those of Szabo et al. (1986) which showed that i.v. infusion of fenoldopam (1–30 μg kg−1 min−1) into pithed rabbits dose-dependently decreased MAP, and increased HR at the highest dose. Fenoldopam has been shown to possess antagonistic activity at α1-adrenoceptors in perfused rat kidneys in situ (Martin & Broadley, 1995). Moreover, fenoldopam at 5 but not 2 μg kg−1 min−1 inhibited by 14% the renal arterial constrictor effect of the α1-adrenoceptor agonist phenylephrine (Kohli et al., 1988). Hence, α-adrenoceptor blockade may have contributed to the vasodilator effect of a high dose of fenoldopam.
All doses of R(−)-propylnorapomorphine elevated MAP, but did not significantly alter CO. Ra and HR were increased significantly only at the highest dose. Our results are consistent with those of van den Buuse et al. (1996) which show that i.v. injection of R(−)-propylnorapomorphine or quinpirole (D2-receptor agonist) into conscious rats caused a pressor response. Quinpirole injected i.v. also increased MAP in conscious rats in a dose-dependent manner due to the activation of central D2-receptors leading to increased sympathetic outflow and vasopressin release (Nagahama et al., 1986a). As well, continuous i.v. infusion of quinpirole into conscious rats caused a pressor response (Igarashi et al., 1987). In contrast, apomorphine has been shown to reduce blood pressure in anaesthetized rats (Ramirez & Enero, 1980) and dogs (Montastruc et al., 1985; 1989); the latter was due to the activation of peripheral presynaptic D2-receptors leading to the inhibition of noradrenaline release from sympathetic nerve terminals and the adrenal medulla. Quinpirole injected i.v. into anaesthetized rats also caused a depressor response (Sengupta & Lokhandwala, 1985; Nagahama et al., 1986b; Cavero et al., 1987). It has also been shown by Damase-Michel et al. (1990) that i.v. injection of quinpirole into conscious dogs reduced blood pressure and increased HR. However, quinpirole elicited a pressor response after pretreatment with i.v. injection of domperidone which does not readily cross the blood-brain barrier. Furthermore, intracisterna magna injection of quinpirole increased blood pressure as well as HR. These results show that quinpirole has two mechanisms of action: a peripheral depressor component, and a central pressor and chronotropic component. Interestingly, in our preliminary studies, i.v. bolus injections (rather than infusion) of R(−)-propylnorapomorphine produced a decrease in blood pressure (data not shown). Therefore, dopamine D2 receptor agonists may elicit a centrally-mediated pressor or peripherally-mediated depressor response depending on the lipophilicity of the drug, mode of drug administration, plasma concentration and the experimental condition.
Since the rats were ganglion-blocked with mecamylamine, the tachycardic response elicited by the highest dose of either fenoldopam or R(−)-propylnorapomorphine was likely due to a direct positive chronotropic action. In this respect, a direct positive chronotropic action of quinpirole (Damase-Michel et al., 1990) and fenoldopam (Cavero et al., 1987) has been reported.
Since the venodilator activity of a drug is best revealed in animals with suppression of the sympathetic nervous activity and/or elevation of venomotor tone (Tabrizchi & Pang, 1992; Pang, 1994), the rats in the current study were given mecamylamine to obliterate autonomic reflex and infused with noradrenaline to elevate venous tone. Under these conditions, fenoldopam reduced Rv in a dose-dependent fashion and decreased MCFP at the highest dose. R(−)-propylnorapomorphine, on the other hand, did not significantly alter MCFP or Rv. Therefore, fenoldopam, but not R(−)-propylnorapomorphine, has a venodilator action.
A comparison of Ra and Rv indicates that the highest dose of fenoldopam (16 μg kg−1 min−1) caused similar degree of arterial (−48±3%) and venous (−53±4%) dilatation. CO was dose-dependently and markedly increased (+81±12%) by fenoldopam due to the reductions in flow resistances, Ra and Rv. MAP, however, was only slightly reduced (−9±5 mmHg by the highest dose of fenoldopam). The profile of vasodilator action of fenoldopam was similar to that of a supramaximal dose of nitroglycerin (6.4 μg kg−1 min−1) which, under similar experimental conditions, also elicited a small reduction of MAP (−9±3 mmHg), but a marked elevation of CO (62±7%) due to equivalent reductions of arterial (−43±3%) and venous (−44±6%) resistances (Ng & Pang, 1998). By contrast, a supramaximal dose of sodium nitroprusside (128 μg kg−1 min−1) elicited a large reduction in MAP (−55±4 mmHg), a smaller increase in CO (51±11%), and a larger reduction in arterial resistance (−64±3%) than venous resistance (−49±5%). We have also shown that diethylamine/nitric oxide (DEA/NO) complex and S-nitroso-N-acetylpenicillamine (SNAP), two novel nitrovasodilators, have a profile of arterial to venous selectivity similar to that of sodium nitroprusside, but not that of nitroglycerin (Ng & Pang, 1998).
It is of interest that unlike sodium nitroprusside, i.v. infusion of either fenoldopam or nitroglycerin into normotensive rats caused a modest reduction in MAP. Fenoldopam is, however, as efficacious as sodium nitroprusside in severe hypertension (see Introduction). Likewise, nitroglycerin is efficacious in the emergency management of hypertension (Abdelwahab et al., 1995; Murphy, 1995). It is widely accepted that organic nitrovasodilators such as nitroglycerin is useful for the management of ischaemic heart disease due to their relative vasodilator selectivity for capacitance vessels and large arteries relative to arterioles, and modest hypotensive action when orally administered. Since fenoldopam shares the same arteriolar to venous dilator profile as nitroglycerin, it would be of interest to examine its cardiovascular action in animals with coronary artery diseases.
To summarize, all doses of fenoldopam reduced MAP, Ra and Rv, and increased CO. The highest dose of fenoldopam also increased HR and lowered MCFP. In contrast to fenoldopam, R(−)-propylnorapomorphine increased MAP at all doses and increased HR and Ra at the highest dose, but did not alter CO, MCFP and Rv at any dose. Our findings indicate that fenoldopam is a venodilator with a similar efficacy in reducing arterial and venous resistance.
Acknowledgments
This work was supported by the Heart and Stroke Foundation of B.C. & Yukon, Canada. S.S.W. Ng was a recipient of the University of British Columbia Graduate Fellowship award.
Abbreviations
- CO
cardiac output
- CVP
central venous pressure
- DEA/NO
diethylamine/nitric oxide complex
- FAP
final arterial pressure
- HR
heart rate
- MAP
mean arterial pressure
- MCFP
mean circulatory filling pressure
- Ra
arterial resistance
- Rv
venous resistance
- SNAP
S-nitroso-N-acetylpenicillamine
- VPP
venous plateau pressure
References
- ABDELWAHAB W., FRISHMAN W., LANDAU A. Management of hypertensive urgencies and emergencies. J. Clin. Pharmacol. 1995;35:747–762. doi: 10.1002/j.1552-4604.1995.tb04116.x. [DOI] [PubMed] [Google Scholar]
- ARONSON S., GOLDBERG L.I., GLOCK D., ROTH S., MOSS J., ROIZEN M.F. Effects of fenoldopam on renal blood flow and systemic hemodynamics during isoflurane anesthesia. J. Cardiothoracic Vasc. Anes. 1991;5:29–32. doi: 10.1016/1053-0770(91)90089-c. [DOI] [PubMed] [Google Scholar]
- BARNES J.M., BARNES N.M., COSTALL B., NAYLOR R.J. The actions of (−)N-n-propylnorapomorphine and selective dopamine D1 and D2 receptor agonists to modify release of [3H]dopamine from the rat nucleus accumbens. Neuropharmacology. 1990;29:327–336. doi: 10.1016/0028-3908(90)90090-e. [DOI] [PubMed] [Google Scholar]
- BROGDEN R.N., MARKHAM A. Fenoldopam: a review of its pharmacodynamic and pharmacokinetic properties and intravenous clinical potential in the management of hypertensive urgencies and emergencies. Drugs. 1997;54:634–650. doi: 10.2165/00003495-199754040-00008. [DOI] [PubMed] [Google Scholar]
- CAVERO I., MASSINGHAM R., LEFEVERE-BORG F. Peripheral dopamine receptors, potential targets for a new class of antihypertensive agents. Part II: Sites and mechanisms of action of dopamine receptor agonists. Life Sci. 1982;31:1059–1069. doi: 10.1016/0024-3205(82)90078-9. [DOI] [PubMed] [Google Scholar]
- CAVERO I., THIRY C., PRATZ J., LAWSON K. Cardiovascular characterization of DA-1 and DA-2 dopamine receptor agonists in anesthetized rats. Clin. Exp. Hypertension - Part A, Theory & Practice. 1987;9:931–952. doi: 10.3109/10641968709161458. [DOI] [PubMed] [Google Scholar]
- DAMASE-MICHEL C., MONTASTRUC J.L., GHARIB C., GEELEN G., DE SAINT-BLANQUAT G., TRAN M.A. Effect of quinpirole, a specific dopamine DA2 receptor agonist on the sympathoadrenal system in dogs. J. Pharmacol. Exp. Ther. 1990;252:770–777. [PubMed] [Google Scholar]
- GESSA G.L., CORSINI G.U. Apomorphines and Other Dopaminomimetics. I. New York: Raven Press; 1981. [Google Scholar]
- GOLDBERG L.I. Cardiovascular and renal actions of dopamine: potential clinical application. Pharmacol. Rev. 1972;24:1–29. [PubMed] [Google Scholar]
- GOLDBERG L.I. Dopamine: receptors and clinical applications. Clin. Physiol. Biochem. 1985;3:120–126. [PubMed] [Google Scholar]
- GUYTON A.C., JONES C.E., COLEMAN T.G. Circulatory Physiology: Cardiac output and its regulation. Saunders: Philadelphia; 1973. Mean circulatory pressure, mean systemic pressure, and mean pulmonary pressure and their effect on venous return; pp. 205–221. [Google Scholar]
- HAHN R.A., WARDELL J.R., SARAU H.M., RIDLEY P.T. Characterization of the peripheral and central effects of SK&F 82526, a novel dopamine receptor agonist. J. Pharmacol. Exp. Ther. 1982;223:305–313. [PubMed] [Google Scholar]
- HEDGE S.S., RICCI A., AMENTA F., LOKHANDWALA M.F. Evidence from functional and autoradiographic studies for the presence of tubular dopamin-1 receptors and their involvement in the renal effects of fenoldopam. J. Pharmacol. Exp. Ther. 1989;251:1237–1245. [PubMed] [Google Scholar]
- IGARASHI Y., CHEN Y.F., WYSS M., LINDHEIMER M.D., OPARIL S. Continuous intravenous infusion of LY171555, a potent selective D2 receptor agonist, lowers blood pressure in the conscious rat. Pharmacology. 1987;35:194–202. doi: 10.1159/000138311. [DOI] [PubMed] [Google Scholar]
- KOHLI J.D., GLOCK D., GOLDBERG L.I. Relative DA1-dopamine-receptor agonist and α-adrenoceptor antagonist activity of fenoldopam in the anesthetized dog. J. Cardiovasc. Pharmacol. 1988;11:123–126. doi: 10.1097/00005344-198801000-00018. [DOI] [PubMed] [Google Scholar]
- LANGER S.Z. Presynaptic regulation of catecholamine release. Biochem. Pharmacol. 1974;23:1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- LAPPE R.W., TODT J.A., WENDT R.L. Effects of fenoldopam on regional vascular resistance in conscious spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1986;236:187–191. [PubMed] [Google Scholar]
- LEFEVRE-BORG F., LORRAIN J., LECHAIRE J., THIRY C., HICKS P.E., CAVERO I. Studies on the mechanisms of the development of tolerance to the hypotensive effects of fenoldopam in rats. J. Cardiovasc. Pharmacol. 1988;11:444–455. doi: 10.1097/00005344-198804000-00010. [DOI] [PubMed] [Google Scholar]
- MARTIN S.W., BROADLEY K.J. Renal vasodilatation by dopexamine and fenoldopam due to α1-adrenoceptor blockade. Br. J. Pharmacol. 1995;115:349–355. doi: 10.1111/j.1476-5381.1995.tb15884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTASTRUC J.L., GAILLARD G., RASCOL O., TRAN M.A., MONTASTRUC P. Effect of apomorphine on adrenal medullary catecholamine levels. Fundamental Clin. Pharmacol. 1989;3:665–670. doi: 10.1111/j.1472-8206.1989.tb00467.x. [DOI] [PubMed] [Google Scholar]
- MONTASTRUC J.L., GUIOL C. Experimental study of the hypotensive effect of apomorphine. Arch. des Maladies Coeur Vaisseaux. 1984;77:1176–1180. [PubMed] [Google Scholar]
- MONTASTRUC J.L., GUIOL C., TRAN M.A., LHOSTE F., MONTASTRUC P. Studies on the cardiovascular actions of apomorphine in dogs: central versus peripheral mechanisms and role of the adrenal medulla. Arch. Int. Pharmacol. Ther. 1985;277:92–103. [PubMed] [Google Scholar]
- MURPHY C. Hypertensive emergencies. Emergency Med. Clin. N. Am. 1995;13:973–1007. [PubMed] [Google Scholar]
- NAGAHAMA S., CHEN Y.F., LINDHEIMER M.D., OPARIL S. Mechanism of the pressor action of LY171555, a selective dopamine D-2 receptor agonist, in the conscious rat. J. Pharmacol. Exp. Ther. 1986a;236:735–742. [PubMed] [Google Scholar]
- NAGAHAMA S., CHEN Y.F., LINDHEIMER M.D., OPARIL S. Mechanism of the depressor action of LY171555, a selective dopamine D-2 receptor agonist, in the anesthetized rat. J. Pharmacol. Exp. Ther. 1986b;239:426–432. [PubMed] [Google Scholar]
- NEUMEYER J.L., NEUSTADT B.R., OH K.Y., WEINHARDT K.K., BOYCE C.B., ROSENBERG F.J., TEIGER D.G. Apomorphine. 8. Total synthesis and pharmacological evaluation of (plus or minus)-apomorphine, (plus or minus)-apocodeine, (plus or minus)-N-n-propylnorapomorphine, and (plus or minus)-N-n-propylnorapocodeine. J. Med. Chem. 1973;16:1223–1228. doi: 10.1021/jm00269a601. [DOI] [PubMed] [Google Scholar]
- NG S.S.W., PANG C.C.Y. Venous versus arterial actions of diethylamine/NO complex and S-nitroso-N-acetylpenicillamine (SNAP) in vivo. Br. J. Pharmacol. 1998;125:1247–1251. doi: 10.1038/sj.bjp.0702175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONNELL D.P., RAGSDALE N.V., BOYD D.G., FELDER R.A., CAREY R.M. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension. 1997;29:115–122. doi: 10.1161/01.hyp.29.1.115. [DOI] [PubMed] [Google Scholar]
- OHLSTEIN E.H., ZABKO-POTAPOVICH B., BERKOWITZ B.A. The DA1 receptor agonist fenoldopam (SK&F 82526) is also an α2-adrenoceptor antagonist. Eur. J. Pharmacol. 1985;118:321–329. doi: 10.1016/0014-2999(85)90143-8. [DOI] [PubMed] [Google Scholar]
- PANACEK E.A., BEDNARCZYK E.M., DUNBAR L.M., FOULKE G.E., HOLCSLAW T.L. Randomized, prospective trial of fenoldopam vs sodium nitroprusside in the treatment of acute severe hypertension. Fenoldopam Study Group. Academic Emergency Med. 1995;2:959–965. doi: 10.1111/j.1553-2712.1995.tb03122.x. [DOI] [PubMed] [Google Scholar]
- PANG C.C.Y. The effects of Drugs on the Venous System 1994Austin, Texas; 1–139.Landers, R.G. (ed) [Google Scholar]
- RAMIREZ A.J., ENERO M.A. Blood pressure and heart rate response to apomorphine in urethane anesthetized rats. Acta Physiol. Latinoamericana. 1980;30:199–203. [PubMed] [Google Scholar]
- ROTHE C.F. Mean circulatory filling pressure: its meaning and measurements. J. Appl. Physiol. 1993;74:499–509. doi: 10.1152/jappl.1993.74.2.499. [DOI] [PubMed] [Google Scholar]
- SENGUPTA S., LOKHANDWALA M.F. Characterization of the hypotensive action of dopamine receptor agonists fenoldopam and quinpirole in anaesthetised rats. J. Autonomic Pharmacol. 1985;5:289–294. doi: 10.1111/j.1474-8673.1985.tb00552.x. [DOI] [PubMed] [Google Scholar]
- SHUSTERMAN N.H., ELLIOTT W.J., WHITE W.B. Fenoldopam, but not nitroprusside, improves renal function in severely hypertensive patients with impaired renal function. Am. J. Med. 1993;95:161–168. doi: 10.1016/0002-9343(93)90256-o. [DOI] [PubMed] [Google Scholar]
- SOARES-DA-SILVA P. The effects of quinpirole and fenoldopam on the potassium-evoked overflow of endogenous dopamine and noradrenaline in dog mesenteric arteries. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;341:37–42. doi: 10.1007/BF00195055. [DOI] [PubMed] [Google Scholar]
- SZABO B., HEDLER L., STARKE K. Dopamine1 receptor agonist and alpha-2 adrenoceptor antagonist effects of fenoldopam in rabbits. J. Pharmacol. Exp. Ther. 1986;239:881–886. [PubMed] [Google Scholar]
- TABRIZCHI R., PANG C.C.Y. Effects of drugs on body venous tone, as reflected by mean circulatory filling pressure. Cardiovasc. Res. 1992;26:443–448. doi: 10.1093/cvr/26.5.443. [DOI] [PubMed] [Google Scholar]
- VAN DEN BUUSE M. Central effects of quinpirole on blood pressure of spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1992;262:303–311. [PubMed] [Google Scholar]
- VAN DEN BUUSE M., MORTON S.J., CORNISH J.L., HEAD G.A. Prolonged central effects of quinpirole on cardiovascular regulation. J. Pharmacol. Exp. Ther. 1996;277:473–483. [PubMed] [Google Scholar]
- VAN DER NIEPEN P., DUPONT A.G., FINNE E., SIX R.O. Hypotensive and regional haemodynamic effects of fenoldopam and quinpirole in the anaesthetised rat. J. Hypertension. 1988;6 Suppl.:S687–S689. doi: 10.1097/00004872-198812040-00216. [DOI] [PubMed] [Google Scholar]
- VENTURA H.O., MESSERLI F.H., FROHLICH E.D., KOBRIN I., OIGMAN W., DUNN F.G., CAREY R.M. Immediate hemodynamic effects of a dopamine-receptor agonist (fenoldopam) in patients with essential hypertension. Circulation. 1984;69:1142–1145. doi: 10.1161/01.cir.69.6.1142. [DOI] [PubMed] [Google Scholar]
- WANG Y.X., LIM S.L., PANG C.C.Y. Increase by NG-nitro-L-arginine methyl ester (L-NAME) of resistance to venous return in rats. Br. J. Pharmacol. 1995;114:1454–1458. doi: 10.1111/j.1476-5381.1995.tb13369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y.X., PANG C.C.Y. Possible dependence of pressor and heart effects of NG-nitro-L-arginine on autonomic nerve activity. Br. J. Pharmacol. 1991;103:2004–2008. doi: 10.1111/j.1476-5381.1991.tb12367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLEMS J.L., BOGAERT M.G., BUYLAERT W. Preliminary observations on the interaction of domperidone with peripheral dopamine receptors. Jap. J. Pharmacol. 1981;31:131–133. doi: 10.1254/jjp.31.131. [DOI] [PubMed] [Google Scholar]
- ZHAO R.R., FENNELL W.H., ABEL F.L. Effects of dopamine D1 and dopamine D2 receptor agonists on coronary and peripheral hemodynamics. Eur. J. Pharmacol. 1990;190:193–202. doi: 10.1016/0014-2999(90)94126-i. [DOI] [PubMed] [Google Scholar]


