Abstract
An investigation was made of the effect of dexamethasone (Dex) injection into the nucleus tractus solitarius (NTS) on the cardiovascular response to neuropeptide Y in rats.
Dex (39 pmol) injected into the NTS inhibited the hypotension and bradycardia caused by NPY (5 pmol) with a short latency (10 min) and a long duration of action (up to 4 h).
The rapid inhibition by Dex (39 pmol) of the cardiovascular response to NPY was not blocked by pretreatment with the glucocorticoid receptor blocker, RU38486 (47 or 117 pmol respectively), but was reversed by bicuculline (30 pmol).
Microiontophoresis of NPY (0.01 mM, pH 6.5) into the NTS increased the spontaneous firing of the majority (68.4%) of baroreflex-excited cells, but decreased the firing of most (73.7%) baroreflex-inhibited cells. In contrast, Dex (0.02 M, pH 6.5) decreased the spontaneous firing of the majority of baroreflex-excited cells (42.1% of normal response) and decreased the inhibition of baroreflex-inhibited cells (47.5% of normal response). The responses of the majority of baroreceptive cells to NPY were blocked by iontophoretic administration of Dex.
Dex (200 μM) increased the delayed rectifier outward K+ current by 31.4±1.1% (n=5), whereas NPY alone, at a concentration of 1.5 μM, inhibited the current by 28.6±0.8% (n=5). In the presence of Dex (200 μM), addition of NPY (1.5 μM) had no effect on the current.
In conclusion, NTS-administered-Dex attenuated the cardiovascular response to NPY injected into the same area via a rapid membrane effect, which was mediated by an action on GABAA receptors and on the delayed rectifier outward K+ channel.
Keywords: Dexamethasone, neuropeptide Y, nucleus tractus solitarius, cardiovascular response, GABAA receptor, baroreceptor reflex, delayed rectifier outward K+ channel
Introduction
It is well known that glucocorticoids participate in the regulation of blood pressure (Yagil et al., 1986; Tonolo et al., 1988; Grunfeld & Eloy, 1987). Glucocorticoid receptor (GR) immunoreactivity was found in adrenergic neurons and neuropeptide Y neurons in the nucleus tractus solitarius (NTS) (Fuxe et al., 1986; Harfstrand et al., 1986; 1989). Since NTS neurons are the termination points for all afferent baroreceptor fibres and have α2-adrenoceptors and neuropeptide Y (NPY) receptors (Yang et al., 1994), they play an important role in cardiovascular regulation (Kalia, 1981; Andresen & Kunze, 1994), and direct application of noradrenaline (NA) or NPY to the NTS has potent effects on the cardiovascular system (De Jong, 1974; Tseng et al., 1988). The depressor and bradycardia responses to direct application of noradrenaline to the NTS are abolished by prior administration of dexamethasone (Dex) (Ouyang et al., 1999). It is very likely that the cardiovascular responses to NPY application may also be influenced by glucocorticoids. Therefore, the present study was designed to test the effects of dexamethasone (Dex) on the cardiovascular response to NPY microinjected into the NTS and to analyse the underlying mechanism of its actions.
Methods
Cardiovascular experiments
Adult Wistar rats of either sex (250–300 g) were anaesthetized with a mixture of α-chloralose (35 mg kg−1, i.p.) and urethane (1 g kg−1, i.p.). The trachea was cannulated to prevent airway obstruction. A heparinized catheter (50 u ml−1 in 0.9% w v−1 saline) was inserted into the femoral artery and connected to a pressure transducer attached to a polygraph recorder for monitoring blood pressure and heart rate. The animal was placed on a heating pad to maintain rectal temperature at 37±0.5°C. The head of the animal was placed in a stereotaxic frame and adjusted to 45° from the horizontal plane. Neck muscles were electrocauterized to expose the posterior altano-occipital membrane and fine dissection was used to expose the caudal medulla in the region of the obex and calamus scriptorium.
Unilateral microinjections were made stereotaxically into the NTS with a glass micropipette (tip diameter 50–60) connected to a microsyringe by polyethylene tubing. The tip of the pipette was positioned 0.5 mm rostral to the obex, 0.5 mm lateral to midline and 0.6 mm deep to the surface of brainstem. Dexamethasone-21-phosphate disodium, NPY and bicuculline (Bic) were dissolved in 0.9% saline (pH 7.2), whereas RU38486, a glucocorticoid receptor antagonist, was dissolved in sesame oil. Both Dex and RU38486 bind to the membrane-bound glucocorticoid receptor (Chen et al., 1993; Quelle et al., 1988) but the binding-affinity of RU38486 is greater than that of Dex (Chen et al., 1993). RU38486 also has a high affinity for the cytosolic glucocorticoid receptor and is a reliable competitive antagonist of this receptor (Agarwal et al., 1987). This compound has no agonist activity at either receptor. Each drug solution was delivered in a volume of 0.1 μl over approximately 20 s. Saline and sesame oil was used for control injections.
Experiment 1: NPY (5 pmol) was microinjected into the NTS, either alone or at various intervals (10 min, 0.5, 1, 2, 3 and 4 h) after Dex (39 pmol). Dex alone did not evoke any change in cardiovascular function.
Experiment 2: In order to determine whether the effect of Dex on the NPY response was mediated by glucocorticoid receptors, Dex was injected into the NTS 10 min after administration of the GR antagonist, RU38486 (47 or 117 pmol). Then, NPY (5 pmol) was locally applied to the same area, 30 min after injection of Dex. Microinjection of the GR antagonist alone had no effect on cardiovascular function.
Experiment 3: NPY (5 pmol) was administered into the NTS 10 min after coinjection of Dex (39 pmol) and Bic (30 pmol), a blocker of GABAA receptors. Microinjection of Bic alone had no effect on cardiovascular function (Barron et al., 1997).
Basal values of blood pressure were recorded for at least 15 min before each microinjection. At the conclusion of the experiment, 0.1 μl pontamine sky blue was injected for histological verification of injection sites. Data are expressed as mean±s.e.mean. Statistical significance between experimental and control groups was assessed with ANOVA, followed by Dunnett t-tests where appropriate.
Microiontophoresis studies
Methods for recording blood pressure and exposing the caudal medulla were the same as for the cardiovascular experiments. An additional catheter was inserted into the femoral vein for infusion of phenylephrine (2–4 kg−1, pH 7.4), in order to activate the baroreflex. Body temperature was maintained at 37±0.5°C via a heating blanket. Blood pressure and heart rate were monitored on a polygraph recorder.
Five-barreled glass microelectrodes with 2–5 μm tips were used to record extracellularly from single neuron in the NTS at the level of the area postrema, and to apply drugs by microiontophoresis at the site of recording. The central barrel was filled with a 2% solution (w v−1) of pontamine sky blue in 0.5 M sodium acetate and was used for recording cellular activity. Recording barrel resistance typically ranged from 7–12 MΩ. Three of the peripheral barrels were filled with the following drugs: NPY (0.01 mM, pH 6.5), anti-serum raised against NPY (dilution 1 : 500, pH 6.0), and Dex (0.02 M, pH 6.5). Automatic current balance was maintained through a fourth peripheral barrel containing 4 M NaCl. Positive and negative currents were passed through this barrel to control the possible current artifacts that might arise during drug application. NPY and NPY antiserum were iontophoresed as cations (using currents of up to 80 nA), whereas Dex was ionophoresed as anions (currents of up to 90 nA) or the otherwise was retained with backing currents of 15 nA.
A microdrive manipulator was used to advance or reverse the recording electrodes. When cells were encountered, their discharges were displayed on an oscilloscope (VC-10, Nihon Koden). Baseline firing rates were measured for 3 min of spontaneous activity. Baroreceptive cells were identified by infusion of phenylephrine through the femoral vein, and then drugs were applied by iontophoresis over 1 min. Neuronal discharges were recorded on videotape with a Sony videocassette recorder. After a cell was fully tested, the position of the recording tract was marked by passing 20 μA of anionic current through the recording barrel for 30 min, to deposit pontamine sky blue at the electrode tip. Animals were then perfused intracardially with 10% v v−1 formalin. The locations of each recording track and tested neurons were histologically verified in 50 frozen sections.
Neuronal discharges were analysed by IBM-compatible PC computer and histograms were constructed by HP-Laser 4LC Printer. Neurons were categorized as inhibited or excited, depending on whether iontophoretic application of drug produced a reproducible change in firing rate that was equal to or greater than 50% of baseline (measured for 30 s prior to drug application). Neurons, which failed to meet this criterion, were classified as unresponsive. The statistical significance between experimental groups was assessed by X2-analysis for unpaired data.
Whole-cell recordings
Wistar rats (12–15 day-old) were killed by cervical dislocation. The brainstem was rapidly removed and placed in cold (4°C) buffer (in mM): NaCl 110, sodium succinate 10, KCl 5, CaCl2 0.2, MgCl2 5, D-glucose 15, HEPES 15, pH 7.4, and equilibrated with 100% O2. Using a vibratome, a horizontal medullary slice (700 μm) was prepared from the area containing the medial and dorsal solitary nuclei, 0.5 mm rostral and 2 mm caudal to the obex. The slices were equilibrated in HEPES buffer with 100% O2 at 32°C for 2 h. They were then placed in buffer containing pronase E (1 mg ml−1) for 40 min at 32°C. The neurons were isolated by gentle trituration in DMEM culture medium, using fire-polished Pasteur pipettes of decreasing diameter. The whole solution was then transferred to a recording dish. The neurons were allowed to settle on the bottom and the DMEM replaced with an extracellular solution comprising (in mM): NaCl 137, KCl 5.4, MgCl2 1, D-glucose 10, TTX 0.01, 4-aminopyridine 5. Whole cell recordings were obtained from neurons that were characteristically bipolar in shape, and typically 15.0×10.5 3.03 m in size. This type of neuron has been previously shown to receive baroreceptor terminals (Mendelowitz et al., 1992).
Methods for making patch pipettes and whole cell recording were similar to those described by Hamill et al. (1981). The patch pipettes had a resistance of 3 MΩ when tested in extracellular solution. Pipettes were filled with a solution containing (in mM) KCl 140, MgCl2 2, Na2ATP 2, EGTA 10, HEPES 10, and titrated to pH 7.3. The indifferent electrode was an Ag-AgCl plug connected to the bath via a 150 mM KCl agar bridge. Voltage-activated currents were evoked using the 0.3203 Ap CLAMP 0.3203 A program (Version 6.0, Axon Instruments), run on an IBM compatible microcomputer DT 125 kHz interface (Lab Master). To obtain outward potassium currents, the cell was clamped at a holding potential of −80 mV, and serially depolarized in +10 mV steps from −80 mV to +30 mV, each sustained 300 ms in duration. Voltage-activated currents were also analysed and plotted using CLAMP program. Data are expressed as mean±s.e.mean. The statistical significance between experimental groups was assessed by Student t-test for paired data. All recordings were made at 22.5°C.
Drugs
All of the following substances were obtained from Sigma: dexamethasone-21-phosphate disodium (Dex), neuropeptide Y (NPY), neuropeptide Y antiserum, bicuculline (Bic), phenylephrine, pontamine sky blue, pronase E, HEPES, ethyleneglycolbis- (b-aminoethyl-ether)-N,N,N N tetraacetic acid (EGTA), tetraethyl-ammonium chloride (TEA), 4-aminopyridine (4-AP), tetrodotoxin (TTX), Na2ATP (adenosine-5 triphosphate disodium salt), and DMEM (Dulbecco Modified Eagle Medium). RU38486 [7β-hydroxy-11β-(4-dimethylamino-phenyl) 17α-(1-propymyl) estra-4,9-diene-3-one] was kindly donated by Roussel-UCLAF (Romainville, France).
Results
Effect of Dex on the depressor response induced by NPY
As seen in Table 1, NPY (5 pmol) alone injected into the NTS significantly decreased both blood pressure and heart rate (−29.0±2.7 mmHg, −35±11 beats min−1, n=12). Microinjection of Dex (39 pmol) into the NTS did not significantly alter the hypotensive and bradycardic responses to simultaneous administration of NPY (5 pmol). However, if Dex was injected 10 min before NPY, it markedly attenuated the depressor and bradycardic responses to NPY, which effect remained for 4 h (Figures 1 and 2).
Table 1.
Effect of microinjection of Dex into the NTS on cardiovascular response to NTS-administered-NPY

Figure 1.
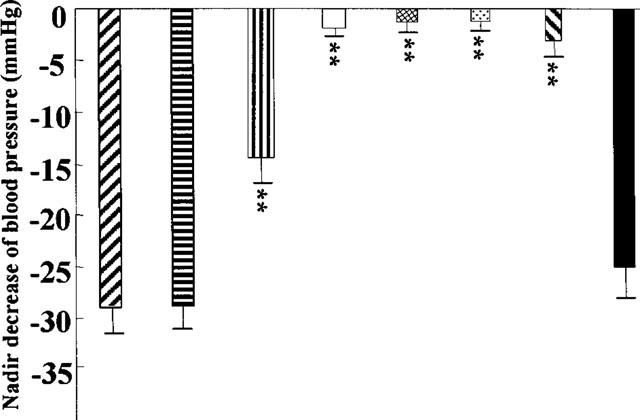
Effect of Dex on changes of blood pressure induced by microinjection of NPY into the NTS. [right to left hatched box] NPY (5 pmol) alone microinjected into the NTS; [vertical lined box] NPY (5 pmol) and Dex (20 ng) coinjected into the NTS; [horizontal lined box] NPY (5 pmol) microinjected into the NTS 10 min after pretreatment with Dex (20 ng); [open box] NPY (5 pmol) microinjected into the NTS 30 min after pretreatment with Dex (20 ng); [cross-hatched box] NPY (5 pmol) microinjected into the NTS 60 min after pretreatment with Dex (20 ng); [dotted box]NPY (5 pmol) microinjected into the NTS 120 min after pretreatment with Dex (20 ng); [left to right hatched box] NPY (5 pmol) microinjected into the NTS 180 min after pretreatment with Dex (20 ng); [filled box] NPY (5 pmol) microinjected into the NTS 240 min after pretreatment with Dex (20 ng).
Figure 2.

Effect of Dex on changes of heart rate induced by microinjection of NPY into the NTS. [right to left hatched box] NPY (5 pmol) alone injected into the NTS; [vertical lined box] NPY (5 pmol) and Dex (20 ng) coinjected into the NTS; A: NPY (5 pmol) microinjected into the NTS 10 min after Dex (20 ng) administered into the same area; [horizontal lined box] NPY (5 pmol) microinjected into the NTS 30 min after Dex (20 ng) administered into the same area; B: NPY (5 pmol) microinjected into the NTS 60 min after Dex (20 ng) administered into the same area; [cross-hatched box] NPY (5 pmol) microinjected into the NTS 120 min after Dex (20 ng) administered into the same area; [left to right hatched box] NPY (5 pmol) microinjected into the NTS 180 min after Dex (20 ng) administered into the same area; [filled box] NPY (5 pmol) microinjected into the NTS 240 min after Dex (20 ng) administered into the same area.
In vivo electrophysiological data
Seventy-five neurons located in the NTS were examined for their responses to transient elevations of arterial blood pressure. A neuron was classified as baroreceptive if it displayed either an increase or decrease of at least 30% from baseline spike activity in response to transient elevation of blood pressure. Figure 3 illustrates such responses observed in 38 of the 75 cells (50.7%) after infusion of phenylephrine. Of these 38 baroreceptive cells, 19 displayed decreases in firing rates, whereas the remaining 19 showed the increases. All of the baroreceptive cells were tested for their responses to iontophoretic administration of Dex, NPY and NPY antiserum. Iontophoresis of Dex made the spontaneous firing of some baroreflex-excited cells decrease (42.1%) and those of some baroreflex-inhibited cells increase (47.5%). In contrast, application of NPY (n=34) increased the spontaneous firing of the majority of baroreflex-excited cells (68.4%) and decreased the firing of baroreflex-inhibited cells (73.7%) (Figure 4). And all the responses to NPY were blocked by local administration of NPY antiserum (data not shown). If the treatment of Dex before the administration of NPY would attenuate both of these responses in the majority of baroreceptive cells (73.5% cells, P<0.01) (Figure 4).
Figure 3.

Baroreceptive cells in the NTS response to arterial blood pressure elevated. (A) Arterial blood pressure changed after injection of phenylephrine; (B) Baroreflex-excited cell; (C) Baroreflex-inhibited cell.
Figure 4.
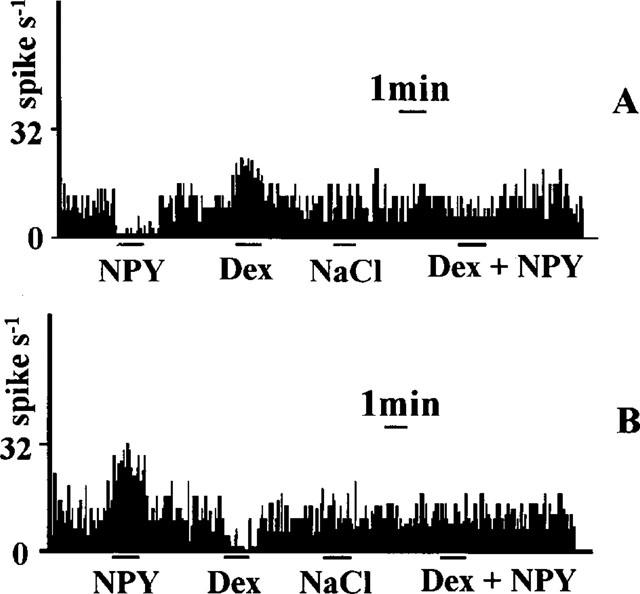
Effects of NPY and Dex on the activities of baroreceptive cells in the NTS. (A) Baroreflex-inhibited cell; (B) Baroreflex-excited cell.
Mechanism of the Dex effect on NPY responses
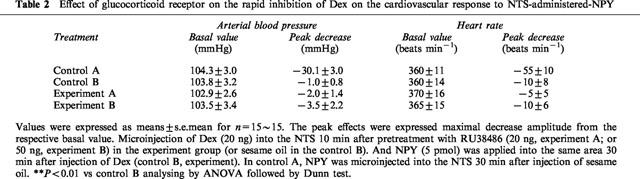
The results described above show that Dex has a rapid effect on the hypotensive responses to NPY. In order to test whether this effect involves the GR, the GR antagonist RU38486 was used. Local application of RU38486 (47 or 117 pmol) to the NTS 10 min before Dex did not block the rapid inhibitory effect of Dex on the hypotensive response to NTS-administered-NPY (Table 2), suggesting that the effects of Dex are not mediated by the GR.
Table 2.
Effect of glucocorticoid receptor on the rapid inhibition of Dex on the cardiovascular response to NTS-administered-NPY
Because corticosterone, the natural glucocorticoid, produces rapid effects in the nervous system by modulating GABAA receptors (Gee, 1988; Turner et al., 1989), we then examined this possibility in the NTS. Bic (a GABAA receptor antagonist; 30 pmol) was administered simultaneously with Dex (39 pmol), 30 min before administration of NPY (5 pmol). The inhibitory effect of Dex on NPY responses was abolished by Bic (Table 3).
Table 3.
Effect of GABAA receptor on the rapid inhibition of Dex on the cardiovascular response to NTS-administered-NPY

In vitro electrophysiological data
With 0.01 mM TTX and 5 mM 4-AP present in the extracellular solution, and nominally Ca2+-free internal and external solutions, a delayed rectifier outward K+ current was evident when the membrane potential was held at −80 mV and serially depolarized in +10 mV steps from −80 mV to +30 mV (Figure 4). This current was incompletely blocked by 10 mM TEA. The current was activated slowly and the time to reach half of the peak current (τ1/2) was voltage dependent (25.6±2.6 ms at 0 mV, 20.8±2.2 ms at +10 mV, 15.2±1.2 ms at +20 mV, 10.5±1.0 at +30 mV; n=4). The delayed rectifier outward K+ current was not activated if the potential was less than −30 mV (Figure 5).
Figure 5.
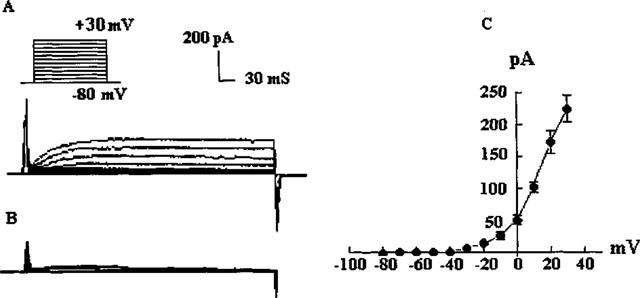
Delayed rectifier outward potassium current. (A) Current records in response to depolarization in steps from −80 to +30 mV; (B) Current records under addition of TEA (10 mM); (C) Current-voltage relation of steady state delayed rectifier outward potassium current for five cells.
Application of Dex (200 μM) increased the delayed rectifier outward K+ current by 31.4±1.1% (n=5). NPY alone (1.5 μM) inhibited the current by 28.6±0.8% (n=5), but in the presence of Dex (200 μM), NPY had no effect (Figure 6).
Figure 6.

Effects of Dex and NPY on the delayed rectifier outward potassium currents of acutely dissociated neuron in the NTS. (A) Current records in response to depolarization in steps from −80 to +30 mV under normal extracellular solution; (B) Current response to addition of Dex (200 μM) into the extracellular solution; (C) Current response to addition of NPY (1.5 μM) into the extracellular solution; (D) Current response to addition of NPY (1.5 μM) into the extracellular solution after pretreatment with Dex (200 μM).
Discussion
These results demonstrate that microinjection of Dex into the NTS attenuates the hypotension and bradycardia induced by NPY administered into the same area. The genomic effect of steroid hormones takes longer than 30 min to occur (typically hours to days). However, the inhibitory effect of Dex in the NTS occurred rapidly, within 10 min. Our microiontophoretic data provides further evidence for the rapid effect of Dex in the NTS. Within just 1 min Dex not only attenuated the effects of NPY on baroreceptive cells, but also in many cases abolished the NPY effects.
There are several ways in which steroids can have rapid actions on membranes (Golden et al., 1998; Orchinik et al., 1991; Passaquin et al., 1998; Quelle et al., 1998; Sandi et al., 1996; Turner et al., 1989). One mechanism is the allosteric modulation of the GABAA receptor (Gee, 1988; Turner et al., 1989). In the present study, the glucocorticoid receptor antagonist, RU38486, in concentrations sufficient to block the effect of Dex on glucocorticoid receptors (Zhu et al., 1995), did not block the rapid inhibition by Dex of the cardiovascular response to NTS-administered-NPY. However, injection of Bic into the NTS, which does not alter blood pressure and heart rate by itself (Barron et al., 1997), prevented the Dex effect. NPY neurons in the NTS not only express glucocorticoid receptors (Harfstrand et al., 1989), but also receive inputs from GABAergic terminals (Pickel et al., 1989). Our results suggest that Dex may influence these neurons by modulating their GABAA receptor activity. Activation of GABAA receptors in the NTS tonically elevates blood pressure and decreases the depressor reflex (Kubo & Kihara, 1988; Barron et al., 1997). This is consistent with the effect of Dex on the activity of baroreceptive NTS cells in our study and indicates that the decreases in depressor reflex are likely to be mediated by GABAA receptors rather than the glucocorticoid receptor. Whether the mechanism of this effect on the GABAA receptor is the same as endogenous corticosterone is yet to be determined.
There is also evidence that glucocorticoids modulate various plasma membrane ion channels to reduce responses of excitable cells (Joels & de Kloet, 1994; Wang, 1997). For example, Dex modulates the release of ACTH through changes in activity of Na+-channels, Ca2+-activated K+-channels and the delayed rectifier outward K+-channel (Lim et al., 1998; Shipston et al., 1996). In the present study, we recorded a delayed rectifier outward K+ current in NTS neurons, using Ca2+-free internal and external solutions with the whole cell recording configuration. Dex augmented this current in the NTS and decreased membrane excitability. In contrast, NPY attenuated the delayed rectifier current, which increased membrane excitability. This may be an important mechanism underlying the rapid inhibitory effect of Dex on the cardiovascular response to NPY.
In conclusion, microinjection of Dex into the NTS attenuates the cardiovascular response to NTS-administered NPY through rapid actions on membrane. The rapid inhibitory effect of Dex in the NTS was probably due to a combined effect on modulation of the GABAA receptor and the delayed rectifier outward K+ channel, rather than via the glucocorticoid receptor. Dex also has a rapid inhibitory effect on the cardiovascular response to NA microinjected into the NTS (Ouyang et al., 1999), and this may occur through a similar mechanism.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No 39670275).
Abbreviations
- 4-AP
4-Aminopyridine
- Bic
Bicuculline
- CaCl2
Calcium chloride
- Dex
Dexamethasone
- DMEM
Dulbecco Modified Eagle Medium
- EGTA
Ethyleneglycolbis-[b-aminoethyl-ether]-N,N,N N tetraacetic acid
- GABAA
γ-amino-n-butyric acid A subtype
- HEPES
N-[2-hydroxyethyl]peperazine-N [2-ethanesulphonic acid]
- KCl
potassium chloride
- Mg2Cl2
magnesium chloride
- NA
noradrenaline
- Na2ATP
Adenosine 5 Triphosphate disodium salt
- NaCl
sodium chloride
- NPY
neuropeptide Y
- NTS
nucleus tractus solitarius
- RU38486
7 β-hydroxy-11 β-[4-dimethylamino-phenyl]17 α-[1-propymyl]estra-4,9-diene-3-one
- TEA
tetraethyl-ammonium chloride
- TTX
tetradotoxin
References
- AGARWAL M.K., HAINQUE R., MOUSTAID N., LAZER G. Glucocorticoid antagonists. FEBS Lett. 1987;217:221–225. doi: 10.1016/0014-5793(87)80667-1. [DOI] [PubMed] [Google Scholar]
- ANDRESEN M.C., KUNZE D.L. Nucleus tractus solitarius gateway to neural circulatory control. Ann. Rev. Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- BARRON K.W., PAVELKA S.M., GARRETT K.M. Diazepan-sensitive GABAA receptors in the NTS participate in cardiovascular control. Brain Res. 1997;773:53–60. doi: 10.1016/s0006-8993(97)00882-2. [DOI] [PubMed] [Google Scholar]
- CHEN Y.Z., FU H., GUO Z.Membrane receptor for glucocorticoids in mammalian Neurons Methods in Neurosciences 199311Academic Press, San Diego; 16–28.Conn, M. (ed) [Google Scholar]
- DE JONG W. Noradrenaline: central inhibitory control of blood pressure and heart rate. Eur. J. Pharmacol. 1974;29:179–181. doi: 10.1016/0014-2999(74)90188-5. [DOI] [PubMed] [Google Scholar]
- FUXE K., AGNATI L.F., HARFSTRAND A., JASON A.M., NEUMEYER A., NDESSON K., RUGGERI M., ZOLI M., GOLDSTEIN M. Morphofunctional studies on the neuropeptide Y/adrenaline costoring nerve terminal systems in the dorsal cardiovascular region of the medulla oblongata. Focus on receptor-receptor interactions in cotransmission. Prog. Brain Res. 1986;68:303–320. doi: 10.1016/s0079-6123(08)60246-0. [DOI] [PubMed] [Google Scholar]
- GEE K. Steroid modulation of the GABA/benzodiazene receptor-linked chloride ionophore. Mol. Neurobiol. 1988;2:201–217. doi: 10.1007/BF02935636. [DOI] [PubMed] [Google Scholar]
- GOLDEN G.A., RUBIN R.T., MASON R.P. Steroid hormones partition to distinct sites in a model membrane bilayer: direct demonstration by small-angle X-Ray diffraction. Biochimica et Biophysica Acta. 1998;1368:161–166. doi: 10.1016/s0005-2736(97)00227-7. [DOI] [PubMed] [Google Scholar]
- GRUNFELD J.P., ELOY L. Role des glucocorticoids dans la regulation de la pression arterielle. Press Med. 1987;16:1365–1367. [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;329:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARFSTRAND A., CINTRA A., FUXE K., ARONSSON M., WIKSTROM A., OKRET S. Regional differences in glucocorticoid receptor immunoreactivity among neuropeptide Y immunoreactive neurons of the rat brain. Acta Physiol. Scand. 1989;135:3–9. doi: 10.1111/j.1748-1716.1989.tb08544.x. [DOI] [PubMed] [Google Scholar]
- HARFSTRAND A., FUXE K., CINTRA A., AGNATI L.F., ZINI I., WIKSTROM A.-C., OKRET S., YU Z.-Y., GOLDSTEIN M., STEINBUSCH H., VERHOFSTRAD A., GUSTAFSSON J.-A. Glucocorticoid receptor immunoreactivity in monaminergic neurons of rat brain. Proc. Natl. Acad. Sci. U.S.A. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOELS S.M., DE KLOET E.R. Mineralocorticoid and glucocorticoid receptors in the brain–implications for ion permeability and transmitter systems. Prog. Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- KALIA M.P.Localization of aortic and carotid baroreceptor and chemoreceptor primary afferents in the brain stem Central Nervous System Mechanism in Hypertension 1981Raven Press: New York; 9–24.Ferrario, C.M. (ed.) [Google Scholar]
- KUBO T., KIHARA M. Evidence for γ-aminobutyric acid receptor-mediated modulation of the aortic baroreceptor reflex in the nucleus tractus solitarii of the rat. Neurosci. Lett. 1988;89:156–160. doi: 10.1016/0304-3940(88)90373-4. [DOI] [PubMed] [Google Scholar]
- LIM M.C., SHIPSTON M.J., ANTONI F.A. Depolarization counteracts glucocorticoid inhibition of adenohypophysical corticotroph cells. Br. J. Pharmacol. 1998;124:1735–1743. doi: 10.1038/sj.bjp.0702024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELOWITZ D., YANG M., ANDRESEN M.C., KUNZE D.L. Localization and retention in vitro of fluorescently aortic baroreceptor terminals on neurons from nucleus tractus solitarius. Brain Res. 1992;581:339–343. doi: 10.1016/0006-8993(92)90729-s. [DOI] [PubMed] [Google Scholar]
- ORCHINIK M., MURRAY T.F., MOORE F.L. A cortisosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- OUYANG M., GE G.H., WANG S. Effect of dexamethasone on cardiovascular response induced by noradrenaline in the nucleus tractus solitarius. Acta Pharmacol. Sin. 1999;20:167–170. [PubMed] [Google Scholar]
- OUYANG M., WANG S. Rapid vasodepression induced by large dose of dexamethasone injected into the nucleus tractus solitarius. Acta Zool. Sin. 1999;45:311–316. [Google Scholar]
- PASSAQUIN A.-C., LHOTE P., RUEGG U.T. Calcium influx inhibition by steroids and analogs in C2C12 skeletal muscle cells. Br. J. Pharmacol. 1998;124:1751–1759. doi: 10.1038/sj.bjp.0702036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKEL V.M., CHAN J., MASSARI V.J. Neuropeptide Y-like immunoreactivity in neurons of the solitary tract nuclei: vesicular localization and synaptic input from GABAergic terminals. Brain Res. 1989;476:265–278. doi: 10.1016/0006-8993(89)91247-x. [DOI] [PubMed] [Google Scholar]
- QUELLE F.W., SMITH R.V., HRYCYNA C.A., KALIBAN T.D., CROOKS J.A., O RIEN J.M. [3H]Dexamethasone binding to plasma membrane-enriched fractions from liver of nonadrenalectomized rats. Endocrinology. 1988;123:1642–1651. doi: 10.1210/endo-123-3-1642. [DOI] [PubMed] [Google Scholar]
- SANDI C., VENERO C., GUAZA C. Nitric Oxide synthesis inhibitors prevent Rapid behavioral effects of corcoticosterone in rats. Neuroendocrinology. 1996;63:446–453. doi: 10.1159/000127070. [DOI] [PubMed] [Google Scholar]
- SHIPSTON M.J., KELLY J.S., ANTONI F.A. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J. Biol. Chem. 1996;271:9197–9200. doi: 10.1074/jbc.271.16.9197. [DOI] [PubMed] [Google Scholar]
- TONOLO G., FRASER R., CONNEL J.H.C., KENYON C.J. Chronic low-dose infusion of dexamethasone in rats: Effects on blood pressure, body weight and plasma atrial netriuretic peptide. J. Hypertens. 1988;6:25–31. [PubMed] [Google Scholar]
- TSENG C.H., MOSQUEDA-GRACIA R., APPALSAMY M., ROBERTSON D. Cardiovascular effects of neuropeptide Y in rat brain stem nuclei. Circ. Res. 1988;64:55–61. doi: 10.1161/01.res.64.1.55. [DOI] [PubMed] [Google Scholar]
- TURNER D.M., RANSON R.W., YANG J.S., OLSEN R.W. Steroid anesthetic and naturally occurring analogs modulate the GABAA receptor complex at a site distinct from barbiturates. J. Pharmacol. Exp. Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- WANG W. The ionic mechanism of hyperpolarization induced by glucocorticoid and its modulatory effects in mammalian neurons. Prog. Physiol. Sci. 1997;28:229–231. [PubMed] [Google Scholar]
- YAGIL Y., KOREEN R., KRAKOFF L.R. Role of mineralocorticoids and glucocorticoids in blood pressure regulation in normotensive rats. Am. J. Physiol. 1986;251:H1354–H1360. doi: 10.1152/ajpheart.1986.251.6.H1354. [DOI] [PubMed] [Google Scholar]
- YANG S.-N., FIOR D.F., HEDLUND P.B., AGNATI L.F., FUXE K. Antagonistic regulation of α2-adrenoceptors by neuropeptide Y receptor subtypes in the nuclear tractus solitarii. Eur. J. Pharmacol. 1994;271:201–212. doi: 10.1016/0014-2999(94)90281-x. [DOI] [PubMed] [Google Scholar]
- ZHU B.G., CHEN Y.Z. Rapid facilitation of glucocorticoids on the high affinity uptake of L-glutamine by cerebral synapses in rats. Chin. J. Neurosci. 1995. p. P156.


