Abstract
Protopine (Pro) from Corydalis tubers has been shown to have multiple actions on cardiovascular system, including anti-arrhythmic, anti-hypertensive and negative inotropic effects. Although it was thought that Pro exerts its actions through blocking Ca2+ currents, the electrophysiological profile of Pro is unclear. The aim of this study is to elucidate the ionic mechanisms of Pro effects in the heart.
In single isolated ventricular myocytes from guinea-pig, extracellular application of Pro markedly and reversibly abbreviates action potential duration, and decreases the rate of upstroke (dV/dt)max, amplitude and overshoot of action potential in a dose-dependent manner. Additionally, it produces a slight, but significant hyperpolarization of the resting membrane potential.
Pro at 25, 50 and 100 μM reduces L-type Ca2+ current (ICa,L) amplitude to 89.1, 61.9 and 45.8% of control, respectively, and significantly slows the decay kinetics of ICa,L at higher concentration. The steady state inactivation of ICa,L is shifted negatively by 5.9–7.0 mV (at 50–100 μM Pro), whereas the voltage-dependent activation of ICa,L remains unchanged. In contrast, Pro at 100 μM has no evident effects on T-type Ca2+ current (ICa,T).
In the presence of Pro, both the inward rectifier (IK1) and delayed rectifier (IK) potassium currents are variably inhibited, depending on Pro concentrations.
Sodium current (INa), recorded in low [Na+]o (40 mM) solution, is more potently suppressed by Pro. At 25 μM, Pro significantly attenuated INa at most of the test voltages (−60∼+40 mV, with a 53% reduction at −30 mV.
Thus, Pro is not a selective Ca2+ channel antagonist. Rather, it acts as a promiscuous inhibitor of cation channel currents including ICa,L, IK, IK1 as well as INa. These findings may provide some mechanistic explanations for the therapeutic actions of Pro in the heart.
Keywords: Protopine, herb medicine, antiarrhythmia, ionic currents, action potential, heart
Introduction
Protopine (Pro) is an isoquinoline alkaloid purified from the Chinese medicinal herb, Corydalis tubers. In folk medicine, the herb or its extract has been traditionally used to treat cardiovascular diseases such as hypertension, cardiac arrhythmia and thromboembolism. Recent experimental studies have shown that the active ingredient from this herb, Pro, significantly reduces blood pressure in dog (Wang et al., 1986) and the incidence of experimental arrhythmias in a variety of animal models (Burtsev et al., 1978; Lu et al., 1992). Moreover, Pro is able to relax smooth muscles of intestines and vessels (Huang et al., 1991), inhibit the spastic contraction of isolated guinea-pig ileum induced by acetylcholine and barium chloride, and antagonize the contractile effect of physostigmine-acetylcholine on cat ciliary muscle (Zhong et al., 1986). In isolated papillary muscle preparations, Pro regulates negatively cardiac contraction and shortens the action potential duration (Teng et al., 1989). More recently, Ko et al. (1992) suggested that Pro acts as a Ca2+ channel antagonist (see also CALBIOCHEM General Catalog, 1998), based on their observation that Pro inhibits the high-potassium induced, Ca2+-dependent contraction of rat aorta.
A direct electrophysiological characterization of Pro, however, has not yet been documented at the single cell level. To elucidate the ionic mechanisms underlying the Pro actions on cardiovascular system, we investigated systematically the effects of Pro on action potential, L-type Ca2+ current (ICa,L), T-type Ca2+ current (ICa,T), inward rectifier (IK1) and delayed rectifier (IK) potassium currents and sodium current (INa) in single ventricular myocytes from guinea-pigs, using whole-cell patch-clamp techniques.
Methods
Preparation of single ventricular myocytes
Young adult guinea-pigs (male, body weight 250∼350 g) were killed by a sudden blow on the neck. The heart was rapidly explanted and transferred to cold Ca2+-free solution (described below). The pericardium and part of the vasa were removed, the aorta was cannulated, and the cannula was attached to a Langendorf apparatus for retrograde intra-arterial perfusion of the heart with the aforementioned oxygenated (5% CO2 and 95% O2) Ca2+-free solution (37°C) for approximately 5 min. Then the heart was exposed to the collagenase-containing solution with the addition of 50 μM CaCl2, 0.33 g l−1 collagenase (type II, Sigma) and 1% bovine serum albumin (BSA, Sigma). After perfusion with 30 ml of the latter solution, the ventricles were dissected, minced, incubated and stirred mechanically in collagenase-free solution with 100 μM CaCl2 and 1% BSA for 10 min (37°C). Following sequential incubation with incremental Ca2+ concentrations (0.2∼1.0 mM), the cells (30–70% rod-shaped, striated ventricular myocytes) were harvested in 1.0 mM Ca2+ solution Tyrode solution (see below) and stored at room temperature (20–23°C) for up to 12 h until use.
Electrophysiological experiments
The standard whole-cell current- and voltage-clamp techniques (Hamill et al., 1981) were employed for the recording of action potential and the ionic currents (ICa,L, ICa,T, IK1, IK and INa), respectively. All experiments were performed at room temperature (20–23°C). Several drops of cell suspension were placed in a custom-made recording chamber mounted on the stage of an inverted microscope (Olympus CK2, Japan). The chamber was perfused at a rate of 1.5–2.0 ml min−1 with bath solution (described below). The patch pipettes were pulled from soft glass capillaries (OD 1.3–1.5 mm, made in Shanghai Institute of Physiology) with a two-stage microelectrode puller (Narishige PB-7, Japan). The resistances of electrodes filled with pipette solution (described below) were in the range of 2∼3 MΩ. The junction potentials were compensated to nearly zero before gigasealing. After gigaseal formation, the whole-cell recording configuration was usually established by applying a brief suction to disrupt the patch membrane. The membrane capacitance and series resistance were compensated to minimize the capacitive transient and to improve the dynamic response. Cell membrane capacitance (134.5±4.8 pF, n=116) was recorded prior to the compensation. Currents or membrane potentials were acquired by a patch clamp amplifier CEZ2300 (Nihon Kohden, Japan) under control of pClamp5.51 software (Axon Instruments, U.S.A.).
Action potential was stimulated and recorded in current-clamp mode. After establishing whole-cell configuration in voltage-clamp mode, the amplifier was switched to current-clamp mode. Short depolarizing current pulses (3 ms square pulse, 1.5×threshold) was applied to initiate action potentials.
Solutions and drugs
The Ca2+-free solution for cell isolation was composed of (mM): NaCl 100, KCl 10, KH2PO4 1.2, MgSO4 5, Glucose 20, Taurine 10, MOPS (3-(N-Morpholino) propane sulphonic acid) 10, pH adjusted to 7.3 with KOH.
Action potentials were recorded in a Tyrode bath solution composed of (mM): NaCl 137, KCl 5.4, MgCl2 1.0, CaCl2 1.8, HEPES 10, Glucose 10 (pH adjusted to 7.4 with NaOH). The pipette solution for recording action potential contained (mM): KCl 140, MgCl2 2, HEPES 5, Na2ATP 5 (pH adjusted to 7.2 with KOH).
The external solution for ICa,L and ICa,T whole-cell recording contained (mM): TEA-Cl (tetraethylammonium chloride) 137, CsCl 5.4, MgCl2 1.0, CaCl2 1.8, HEPES 10, Glucose 10 (pH adjusted to 7.4 with 10% TEA-OH). During the recording of ICa,T, 20 μM TTX was added to the external solution to exclude any contamination of INa. The pipette solution consisted of (mM) CsCl 140, EGTA 10, HEPES 10, Mg-ATP 3, Tris-GTP 0.4 (pH adjusted to 7.2 with CsOH). The cells were perfused with the above bath solution for 5 min before current recording in order to completely wash out both Na+ and K+ in the recording bath solution.
Potassium currents, both IK1 and IK, were recorded in a modified Tyrode solution containing (mM): NaCl 137, KCl 5.4, MgCl2 1, CaCl2 1.8, HEPES 10, Glucose 10 (pH adjusted to 7.4 with NaOH) plus CdCl2 0.2 (to avoid the contamination of ICa,L). Holding potential (HP) was set at −40 or −30 mV to completely inactivate INa and ICa,T. The pipette filling solution for potassium currents recording was composed of (mM): K+ Aspartate 120, KCl 20, Na2ATP 5, MgATP 2, EGTA 5, HEPES 5 (pH adjusted to 7.2 with KOH).
INa was recorded in a low extracellular sodium solution (mM): Choline Chloride 110, NaCl 40, KCl 5.4, MgCl2 2, CaCl2 1.8, CdCl2 0.2, HEPES 5, Glucose 10 (pH adjusted to 7.4 with NaOH). The intracellular solution contained (in mM): KCl 140, MgCl2 0.5, EGTA 10, HEPES 10, K2ATP 2 (pH adjusted to 7.2 with KOH).
Pro, kindly provided by a pharmacy engineer Xiao-Chu Liu, is a white powder (acetate salt) with purity over 99%. We performed 1H-NMR analysis on Pro sample used in this study and found no peaks that related to the presence of impurity. Stock solution (10 mM) of Pro was prepared in distilled water, kept at 4.0°C. Mg-ATP, Tris-GTP, Na2ATP, CsCl, CsOH, TEA-Cl, TEA-OH, EGTA, HEPES, K+ Aspartate were all purchased from Sigma Chemical Co. All other chemicals were of reagent grade.
Data analysis
ICa,L amplitude was measured as the difference between the currents at peak and at the end of the 300-ms pulse. The Ca2+-conductance (g) as a function of membrane voltage (Vm) was calculated using
where I is the current amplitude of ICa,L at given Vm, Erev is the apparent reversal potential of ICa,L (+60 mV). The data were then fitted to a Boltzmann equation (Cohen et al., 1987),
where gmax refers to the maximal Ca2+ conductance, V0.5 is the half maximal conductance voltage, and κ is the slope factor.
When measuring the inactivation kinetics of ICa,L, we used two exponential fitting of the current traces to the equation
where τf and τs are the fast and slow inactivation time constants; Af and As are the amplitudes of these two components of ICa,L respectively; k and A0 are constants.
In experiments assessing the steady state inactivation of ICa,L (see Figure 4), the inactivation parameter (f∞) was determined by dividing peak current at given Vm with the maximum peak current (I/Imax). The f∞ data were then fitted to the following Boltzmann equation (Cohen et al., 1987)
where V0.5 is the half maximal inactivation voltage and κ the slope factor.
Figure 4.

Steady-state inactivation and voltage-dependent conductance of ICa,L. (A) Left panel: a double-pulse protocol, 1-s conditioning pulses to various voltages (−80∼0 mV in increments of 10 mV) from HP of −80 mV followed by a fixed test pulse to 0 mV for 300 ms, which was applied to examine the steady-state inactivation of ICa,L. Right panel: typical traces showing ICa,L at different steady inactivation states. (B) Effects of Pro on ICa,L steady-state inactivation and conductance-voltage relationship. Smooth curves are Boltzmann fittings for control (solid line) and Pro-treated (dashed line) groups. (See methods for details on calculation of I/Imax, g/gmax and Boltzmann fitting). n=7 for each data.
ICa,T, distinguished from ICa,L by its voltage dependence of activation and inactivation and pharmacological profiles, was measured by eliciting current at −40 mV from HP of −80 mV; by taking the difference between the currents elicited at −30 mV from HP of −80 mV and −40 mV. The ICa,T identity was further verified by its sensitivity to NiCl2 (50 μM).
All data of parameter measurements were expressed as mean±s.e. Student's test and paired t-test were used for statistical appraisal of drug effects when appropriate. A P value less than 0.05 was considered statistically significant.
Results
Effects of Pro on action potential
In single ventricular myocytes isolated from guinea-pig hearts, action potentials were recorded in current-clamp mode at 2-second intervals. The effects of Pro on action potential in a representative cell are shown in Figure 1. Pro induced a marked abbreviation of action potential duration (APD), a significant reduction in action potential amplitude (APA) and overshoot (OS), and a slowing of the maximal rate of upstroke ((dV/dt)max) (see Table 1), without altering the general shape of the action potential. These effects were dose-dependent and fully reversible upon washout of the drug. Table 1 summarizes the parametric measurements of action potentials in the absence and presence of Pro. At 25 μM Pro, the APD30, APD50 and APD90 were abridged by 30.5, 26.8 and 22.8%, respectively. The upstroke rate (dV/dt)max was depressed by 25%; APA and OS were reduced by 4.7 and 5.1 mV, respectively. In addition, Pro (25 μM) produced a slight (∼0.7 mV), but significant shift of the resting potential (RP) toward hyperpolarization. At a higher concentration (50 μM), Pro caused a further reduction in APA, OS, (dV/dt)max and APD, and hyperpolarized the RP by 3.1 mV. Thus, Pro alters multiple components of action potential configuration, indicating that Pro may affect more than single type of ionic currents that underlie the generation of action potential.
Figure 1.
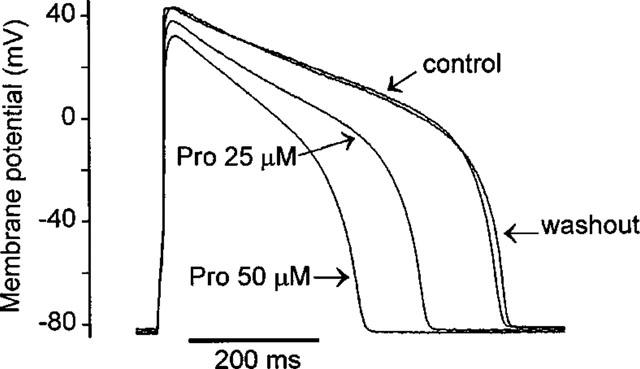
Original traces of action potentials recorded from a representative guinea-pig ventricular myocyte before (control), after exposure to Pro (25 or 50 μM) and upon washout.
Table 1.
Effects of protopine on parameters of action potential in guinea-pig ventricular myocytes

Pro modulates properties of ICa,L
L-type Ca2+ channels play a central role in cardiac electrical activity, excitation-contraction coupling, and under pathophysiological conditions, the Ca2+-dependent cardiac arrhythmia (January & Riddle, 1989; Berlin et al., 1989). To determine possible Pro action on ICa,L, we employed the whole-cell voltage-clamp technique to measure ICa,L amplitude and kinetics, and to characterize its voltage-dependent activation and inactivation properties.
In experiments shown in Figure 2, the ICa,L was elicited by depolarization from −40 mV to 0 mV for 300 ms and continuously followed at 5-s intervals to monitor drug effects (Figure 2A, inset). In these experiments, we used Na+-free and K+-free external and internal solutions (see Methods), to avoid contamination from sodium and potassium currents, and applied a 1-s preconditioning pulse from HP of −80 mV, to inactivate sodium and T-type Ca2+ channels. Under these conditions, the baseline peak ICa,L density was 10.7±1.0 nA/nF (n=22), similar to that reported previously (Main et al., 1998). After exposure to Pro (25 μM) for 2 min, ICa,L amplitude was reduced by 18% relative to control (c), but was largely recovered subsequent to a 2-min washout (w) (Figure 2A). Figure 2B shows the time course of Pro inhibition of ICa,L and the washout of the drug in a representative experiment. The half time of Pro inhibition is about 25 s and the half time of washout of Pro effect is about 35 s. Figure 2C shows the dose-dependent inhibition of Pro on ICa,L amplitude: ICa,L was reversibly declined to 85.6, 62.1 and 48.9% of control, at 25, 50 and 100 μM of Pro, respectively. At the higher concentrations (50–100 μM), Pro selectively prolonged the fast inactivating component of ICa,L, increasing τf from 11.35±0.91 to 16.60±2.12 ms (n=6, Pro=100 μM, P<0.05), with no significant effect on the slow inactivating component (τs, 168.74±11.9 ms in control vs 203.1±24.7 in Pro, n=6, P>0.05). The relative amplitude ratio of the two components was not altered, either (Af/As, 1.15±0.18 and 0.96±0.09, prior to and after Pro application, n=6, P>0.05).
Figure 2.
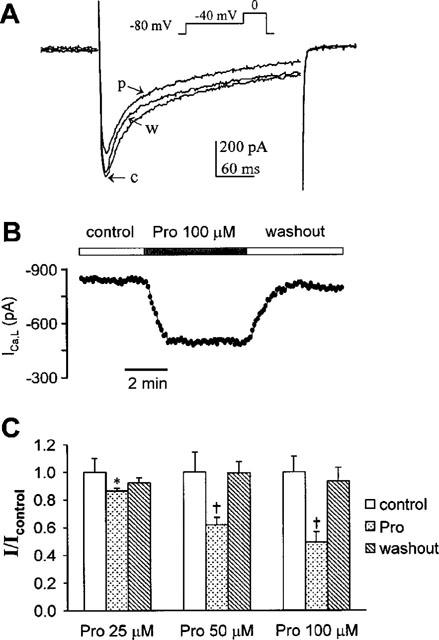
Effects of Pro on ICa,L. (A) The superimposed ICa,L traces were from a typical myocyte. Inset: A 1-s preconditioning pulse of −40 mV from HP of −80 mV was followed by a depolarizing pulse to 0 mV for 300 ms to elicit ICa,L. After exposure to 25 μM Pro for 2 min (p), ICa,L amplitude was reduced by 18% relative to control (c). It was partially recovered subsequent to a 2-min washout (w). (B) A typical example of time course of Pro (100 μM) action on ICa,L and subsequent washout of the drug effect. (C) Dose-dependent inhibition of Pro on ICa,L amplitude. The data were calculated as the ratio to the control currents (n=5∼7 for each group). *P<0.05, †P<0.01 vs controls.
To examine the voltage-dependent activation of ICa,L, families of ICa,L traces were obtained by depolarizing the cells to voltages ranging from −30 to +60 mV in 10 mV increments and at 0.2 Hz. Figure 3A shows, from left to right, original traces of ICa,L in control condition, 2 min after perfusion with 50 μM Pro, and 2 min after washout, respectively. Figure 3B plots the average current-voltage relationships from these experiments. Both the representative example and the average data indicate that in the presence of the drug, there is a near proportional reduction of ICa,L at all voltages tested. Hence, while diminishing ICa,L, Pro does not affect the activation gating mechanism of the L-type channel.
Figure 3.
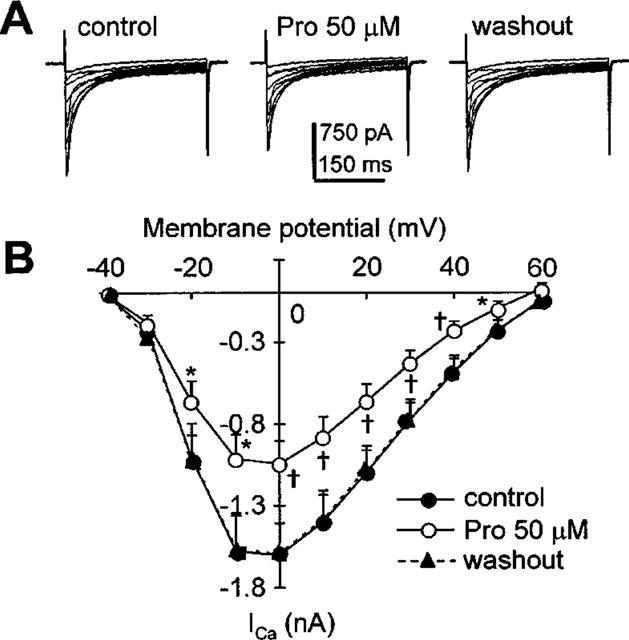
Current-voltage relationship of ICa,L in the absence and presence of 50 μM Pro. A 1-s preconditioning pulse of −40 mV from HP of −80 mV was followed by various depolarizing pulses (−30∼+60 mV, voltage increment 10 mV, duration 300 ms) to elicit ICa,L at 0.2 Hz. (A) Original recordings of ICa,L at different voltages under control condition (left), after exposure to 50 μM Pro, and upon 2-min washout. (B) Average data on effects of Pro on ICa,L over the entire voltage range. *P<0.05, †P<0.01 vs controls, n=7.
This point is more clearly shown in Figure 4B, where Ca2+ conductance (g/gmax) is plotted as a function of voltage. The Boltzmann fittings (smooth lines, see Methods) yielded nearly identical values for either the half maximal conductance voltage V0.5 (−18.67±0.48 mV in control vs −19.16±0.72 mV in Pro, n=7, P>0.05) or for the slope factor κ (5.41±0.44 in control vs 5.53±0.67 mV in Pro, n=7, P>0.05). In fact, the entire curves in the presence and absence of Pro virtually overlap each other. These observations substantiate the idea that Pro has little effect on the voltage-dependence of L-type channel activation.
To examine the Pro-mediated change on the steady-state inactivation of ICa,L, we used a double-pulse protocol (Figure 4A): a 1-s conditioning pulse to various voltages (−80∼0 mV in increments of 10 mV) followed by a fixed test pulse to 0 mV to elicit ICa,L. The inactivation parameter (f∞), the half maximal inactivation voltage (V0.5) and the slope factor κ were determined according to Equation (4) in Methods. As shown in Figure 4B, Pro (50 μM) shifted leftward the inactivation curve by 5.9 mV (V0.5: −31.40±0.17 vs −25.51±0.17 mV in control, n=7, P<0.01), whereas it did not alter the κ value (4.95±0.16 vs 5.61±0.14 mV in control, n=7, P>0.05). A 7.0 mV negative shift in V0.5, with no change of κ, was observed at 100 μM Pro (data not shown). These indicate that Pro enhances the voltage-dependent inactivation of ICa,L, which contributes, in part, to its inhibitory effects on ICa,L described above.
Pro does not affect ICa,T
Next we measured ICa,T in the absence and presence of Pro at depolarizing voltages of −40 and −30 mV, from a HP of −80 mV. The current elicited at −30 mV consisted of two components: ICa,L and ICa,T. The ICa,T was separated from the total current by subtracting the ICa,L recorded at a HP of −40 mV, where ICa,T was inactivated (Figure 5A). ICa,T was also measured as the total current at depolarizing voltage of −40 mV, when the high threshold ICa,L was not yet activated (Figure 5B). The identity of ICa,T was further verified by its sensitivity to 50 μM Ni2+ (Figure 5B). As shown in Figure 5, Pro, at the highest concentration, did not inhibit ICa,T. On average, the peak ICa,T elicited at −40 mV were −27.6±5.6 pA (n=4) and −28.2±6.2 pA (n=4), in the absence and presence of 100 μM Pro, respectively.
Figure 5.

Pro does not affect properties of ICa,T. (A) Pro has no effect on ICa,T recorded at −30 mV. The currents elicited from HP of −80 mV consist of ICa,T and ICa,L (upper traces), while only ICa,L is activated from a HP of −40 mV (middle traces). The subtraction of currents at a HP of −40 mV from those at −80 mV measures the ICa,T at −30 mV (lower traces). (B) Pro has no effect on ICa,T at −40 mV. ICa,T is the predominant component at this voltage, as the total current is completely blocked by NiCl2 (50 μM).
Inhibition of IK1 by Pro
We investigated the possible effect of Pro on IK1, an important current for maintaining the RP near the K+ equilibrium potential (Katz, 1993). Figure 6A shows the original IK1 traces elicited by 300-ms test pulses ranging from −120 to +30 mV from HP of −30 mV prior to (left panel), during drug application (middle) and after washout of 50 μM Pro (right). Regardless of Pro, an inward current was observed over voltages ranging from −120 to −70 mV and a minor outward current at voltages beyond −70 mV, properties characteristic of IK1. During exposure to 50 μM Pro, both the inward and outward IK1 decreased progressively and reached a steady state within ∼3 min (Figure 6A). Figure 6A, right panel, shows that this effect is largely reversible. Figure 6B shows continuous recording of IK1 in a representative cell during sequential application and washout of 25, 50 and 100 μM Pro, the on- and off-rate of Pro action on IK1 were similar to those for Pro effect on ICa,L shown in Figure 2B. The voltage- and dose-dependence of the inhibitory effects of Pro on IK1 were shown in Figure 6C. At all concentrations used (25–100 μM), Pro significantly suppressed IK1, with an average of 55% reduction observed at 100 μM and −120 mV.
Figure 6.
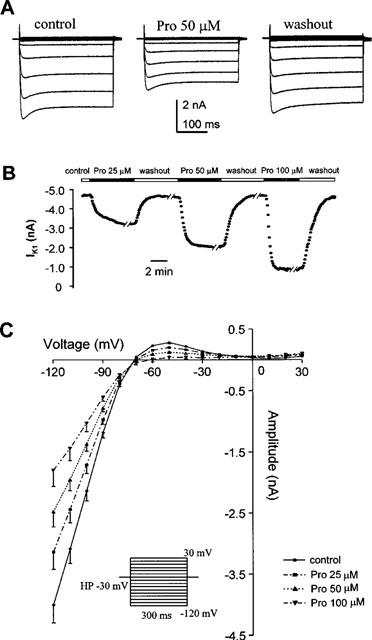
Effects of Pro on current-voltage relationship of IK1. (A) From left to right, representative traces of IK1 at different voltages under control, with Pro 50 μM and after washout, respectively. (B) Continuous recordings of IK1 during repetitive application and washout of Pro at 25, 50, and 100 μM. (C) Dose-dependent inhibition of IK1 by Pro (25–100 μM, n=7). The inset shows the voltage protocol.
Effects of Pro on IK
Another type of potassium channels present abundantly in cardiac myocytes is the delayed rectifier potassium channels, whose current (IK) plays an important role in determining action potential repolarization (Katz, 1993). We thus studied whether Pro can also affect this type of potassium current. From HP of −40 mV, various depolarizing pulses of 6 s duration (−30 mV to +50 mV) were applied, and IK was activated in a time- and voltage-dependent manner (Figure 7). Pro at 50 μM reduced the amplitude of IK by ∼40% at voltages higher than −10 mV. Similarly, Pro considerably depressed the tail IK that was evoked upon repolarization and deactivated at the HP of −40 mV. As was the case with IK1, this inhibitory effect on IK was reversible as well as dose-dependent (data not shown).
Figure 7.

Effects of Pro on I-V relationship of IK. (A) Families of IK traces were obtained by depolarization to different voltages (−30 mV∼+50 mV) for 6 s from a HP of −40 mV in cadmium-containing bath solution (CdCl2 0.2 mM), prior to and after application of 50 μM Pro. (B) The averaged data on effects of Pro (50 μM) on IK-voltage relationship. It significantly suppressed IK at voltages higher than −10 mV. *P<0.05, †P<0.01 vs controls, n=8.
Effect of Pro on INa
The aforementioned data show that Pro induces profound changes in the early phase of action potential, suggesting that sodium channels are also likely a target of the drug. To test this possibility, we recorded INa in cells bathed in 40 mM [Na+]o (A low [Na+]o is conventionally used to reduce the INa magnitude to ensure the proper voltage control). As shown in Figure 8, INa under control conditions exhibited a bell-shaped voltage-dependence over the range of −60 mV to +40 mV, with the maximal INa (−5.3±0.49 nA, n=6) occurring at −30 mV. Exposure of the cells to 25 μM Pro resulted in a marked reduction (53% at −30 mV) of INa; the reduced INa had a similar voltage-dependence and a maximal INa of −2.48±0.25 nA (n=6, P<0.001) at −30 mV. This suggests that INa is most susceptible to the Pro blockade among the ionic currents tested. Again, the effects of Pro on INa were reversible (data not shown). Taken together, we conclude that in addition to ICa,L, Pro inhibits both sodium and potassium currents, rendering a markedly altered action potential in cardiac myocytes.
Figure 8.
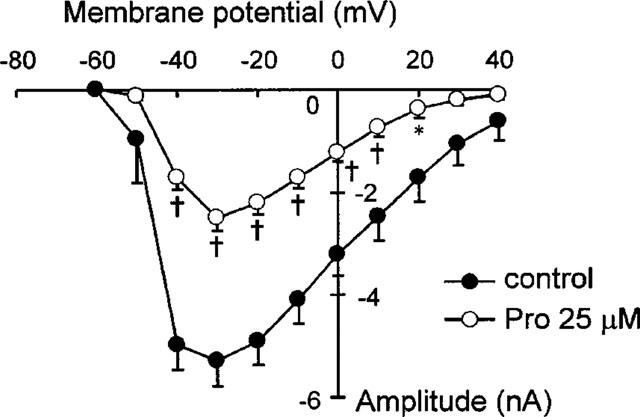
Suppression of INa by Pro. INa was recorded with large pipette (<1.25 MΩ) in low [Na+]o (40 mM), cadmium-containing (CdCl2 0.2 mM) bath solution. Various depolarizing pulses to −60∼+40 mV from HP of −100 mV were applied to measure the whole profile of INa. *P<0.05, †P<0.01 vs controls (n=6).
Discussion
In the present study, we investigated for the first time the electrophysiological effects of Pro at the single cell level using patch-clamp techniques. The present results show that Pro is a multi-channel blocker, suppressing not only L-type Ca2+ channel, but also inward rectifier potassium channel, delayed rectifier potassium channel and sodium channel. However, a limited selectivity is observed as Pro has no significant effect on T-type Ca2+ channel. Collectively, it is clear that ‘Ca2+ channel antagonist' is a misnomer to characterize the effects as well as the mechanism of action of Pro on the cardiovascular system (Ko et al., 1992; see also technical information in Calbiochem General Catalog, 1998). Hence, we suggest the tentative use of ‘promiscuous cation channel inhibitor' to better reflect the electrophysiological profile of Pro. The specific mechanisms involved in Pro actions, however, are presently unknown. While its selectivity between L- and T-type channels supports the idea that the drug interacts directly with the channels, it can not be ruled out that the drug may modify the environment of the channels (e.g., fluidity of the membrane) to exert a more general, less specific effect on multiple channels. The observation that a relatively high Pro concentration is required to elicit a significant effect in cardiac myocytes is consistent with previous studies in smooth muscle preparations (Huang et al., 1991) and in human or rabbit platelets (Ko et al., 1989; Saeed et al., 1997; Shen et al., 1999).
The observation that Pro affects various types of currents are in general agreement with the multifaceted changes in action potential and resting potential. During an action potential, the rapid depolarization (Phase 0) is mainly due to a fast increase in membrane conductance to sodium, causing a large inward, depolarizing INa. Consistent with its potent blocking effects on INa, Pro suppressed the amplitude, the overshoot as well as the upstroke rate of action potential. Since the action potential duration depends on the delicate balance of inward and outward currents in the late phase (Phase 2) of action potential, the depressions on IK (outward) and ICa,L (inward) would have opposing effects on action potential duration. However, it is difficult to predict the net outcome from the concomitant changes of IK and ICa,L. To this end, the observed shortening of APD by Pro may suggest that the suppression of ICa,L (which tends to abbreviate APD) outweighs the suppression of IK (which tends to prolong APD). Alternatively, it is possible that Pro may have some unidentified actions on other ionic channels or even electrogenic transporters (e.g., Na+/K+ pump, Na+/Ca2+ exchanger) to shift the balance toward a briefer APD. A secondary change in the inward Na+/Ca2+ exchange current due to reduced intracellular Ca2+ transient (see below) may provide additional explanation for the shortening of APD (Grantham & Cannell, 1996; Janvier et al., 1997). The mild hyperpolarization of membrane potential by Pro, despite inhibition of IK1, also points to the possibility that Pro may act through additional ionic or electrogenic mechanisms.
Electrophysiological characterization of Pro provides a framework to understanding of the major Pro actions in the heart, namely, the negative inotropic effect, the anti-hypertensive effect and the anti-arrhythmic effect. It has been well established that cardiac excitation-contraction coupling is initiated by the sarcolemmal Ca2+ influx through L-type Ca2+ channels activated during an action potential. In addition to directly contributing to cytosolic Ca2+, ICa,L dictates the sarcoplasmic reticulum (SR) Ca2+ release via the Ca2+-induced Ca2+ release mechanism (Fabiato, 1983), and affects the SR Ca2+ load in a more complex manner (Eisner et al., 1998). The attenuation of ICa,L, which is further aggravated by the shortening of APD, would almost inevitably lead to a reduction in Ca2+ transients elicited by action potential and consequently, a weakened cardiac contraction (which may or may not be beneficial depending on the circumstances). Indeed, confocal microscopic imaging of voltage-clamped cells exposed to Pro revealed a simultaneous reduction in the whole-cell ICa,L, the fluorescence signal from the Ca2+ indicator fluo-3, and the cellular contractility (Song & Cheng, unpublished data). Similar mechanisms may underlie the relaxation effect of Pro in smooth muscles (Huang et al., 1991). Both the negative inotropy in heart and the relaxation effect in vascular smooth muscles (Teng et al., 1989; Ko et al., 1992) may afford the basis for the therapeutic use of Pro in the treatment of hypertensive diseases.
In animal experimental model, it was shown, early in 1970s, that Pro is two to three times more potent than quinidine and novocainamide in preventing aconitic and Ca2+ induced arrhythmia (Burtsev et al., 1978). Later, Lu et al. (1992) explored the anti-arrhythmic effects of Pro on 8 models of experimental arrhythmias and found that Pro has protective effects on Ca2+, aconite, phenylephrine-induced rat arrhythmias, chloroform-induced mouse ventricular fibrillation, and electric stimulus-induced rabbit ventricular fibrillation. The present study offers direct electrophysiological evidence to base these observations on mechanistic grounds. First, hyperpolarization of the resting membrane potential may help slow spontaneous discharge frequency of an ectopic automatic pacemaker, suppress the abnormal automaticity. Second, the inhibition of INa tends to further reduce the membrane excitability and slow the conduction rate. The latter favours to transform unidirectional block to bi-directional block and thus terminates reentry or prevents it from occurring by creating an area of complete block in the re-entry pathway (Zipes, 1997). Moreover, the antagonistic effect on Ca2+ channel of Pro mimics the action of Type IV anti-arrhythmic drugs (i.e., Ca2+ channel blockers), reducing the likelihood of Ca2+-dependent arrhythmias (DAD, EAD) (Zipes, 1997) by relieving the cells from Ca2+ stresses (e.g., smaller Ca2+ influx, possibly lower resting Ca2+ and SR Ca2+ load). Finally, the effect on ICa,L may be also relevant to cardiac pacemaker activity, as the role of ICa,L is increasingly recognized in the primary pacemaker cells (Verheijck et al., 1999). Future studies are warranted for a full understanding of Pro actions in cardiac system.
Acknowledgments
This work was supported by a Grant-in-aid (No. 95-02-24) from Yunnan Provincial Committee of Scientific and Technological Foundation, P.R. China. Protopine used in the present study was a generous gift from a pharmacy engineer Xiao-Chu Liu. The authors also thank Dr. Qian-Sheng Yu for performing the 1H-NMR analysis of protopine purity.
Abbreviations
- AP
action potential
- APA
AP amplitude
- APD
AP duration
- BSA
bovine serum albumin
- (dV/dt)max
maximal rate of upstroke of AP
- ICa,L
L-type Ca2+ current
- ICa,T
T-type Ca2+ current
- IK
delayed rectifier potassium current
- IK1
inward rectifier potassium current
- INa
sodium current
- HP
holding potential
- κ
slope factor
- OS
overshoot of AP
- Pro
protopine
- RP
resting membrane potential
- SR
sarcoplasmic reticulum
- V0.5
voltage at half maximal activation or inactivation
References
- BERLIN J.R., CANNELL M.B., LEDERER W.J. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ. Res. 1989;65:115–126. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- BURTSEV V.N., DORMIDONTOV E.N., SALIAEV V.N. Prevention of ventricular fibrillation with the aid of protopine in animal experiments. Kardiologiia. 1978;18:76–79. [PubMed] [Google Scholar]
- COHEN N.M., LEDERER W.J. Calcium current in isolated neonatal rat ventricular myocytes. J. Physiol. 1987;391:169–191. doi: 10.1113/jphysiol.1987.sp016732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISNER D.A., TRAFFORD A.W., DIAZ M.E., OVEREND C.L., O'NEILL S.C. The control of Ca release from the cardiac sacroplasmic reticulum: regulation versus autoregulation. J. Physiol. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- FABIATO A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- GRANTHAM C.J., CANNELL M.B. Ca2+ influx during the cardiac action potential in guinea pig ventricular myocytes. Circ. Res. 1996;79:194–200. doi: 10.1161/01.res.79.2.194. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflgers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HUANG Y.H., ZHANG Z.Z., JIANG J.X. Relaxant effects of protopine on smooth muscles. Acta. Pharmacol. Sinica. 1991;12:16–19. [PubMed] [Google Scholar]
- JANUARY C.T., RIDDLE J.M. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ. Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- JANVIER N.C., HARRISON S.M., BOYETT M.R. The role of inward Na+-Ca+ exchange current in the ferret ventricular action potential. J. Physiol. (London) 1997;498:611–625. doi: 10.1113/jphysiol.1997.sp021887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ A.M. Cardiac ion channels. N. Eng. J. Med. 1993;328:1244–1251. doi: 10.1056/NEJM199304293281707. [DOI] [PubMed] [Google Scholar]
- KO F.N., WU T.S., LU S.T., WU Y.C., HUANG T.F., TENG C.M. Antiplatelet effects of protopine isolated from Corydalis Tubers. Thromb. Res. 1989;56:289–298. doi: 10.1016/0049-3848(89)90170-9. [DOI] [PubMed] [Google Scholar]
- KO F.N., WU T.S., LU S.T., WU Y.C., HUANG T.F., TENG C.M. Ca2+-channel blockade in rat thoracic aorta by protopine isolated from corydalis tubers. Japan. J. Pharmacol. 1992;58:1–9. doi: 10.1254/jjp.58.1. [DOI] [PubMed] [Google Scholar]
- LU Z.A., WAN D.C., CHEN Z.H., WANG X.H. Effects of protopine on experimental arrhythmias. Chin. Pharmaceu. J. 1992;30:81–84. [Google Scholar]
- MAIN M.C., BRYANT S.M., HART G. Regional difference in action potential characteristics and membrane currents of guinea-pig left ventricular myocytes. Exp. Physiol. 1998;83:747–761. doi: 10.1113/expphysiol.1998.sp004156. [DOI] [PubMed] [Google Scholar]
- SAEED S.A., GILANI A.H., MAJOO R.U., SHAH B.H. Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacol. Res. 1997;36:1–7. doi: 10.1006/phrs.1997.0195. [DOI] [PubMed] [Google Scholar]
- SHEN Z.Q., CHEN Z.H., DUAN L. Effect of protopine on cytosolic Ca2+ in rabbit platelets. Acta Pharmacol. Sinica. 1999;20:338–340. [PubMed] [Google Scholar]
- TENG C.X., CHEN Z.H., ZHAO G.S. Effects of protopine on action potential and contractility of isolated papillary muscles from the guinea pig. Acad. J. Kunming Med. Coll. 1989;10:44–46. [Google Scholar]
- VERHEIJCK E.E., VAN GINNEKEN A.C., WILDERS R., BOUMAN L.N. Contribution of L-type Ca2+ current to electrical activity in sinoatrial nodal myocytes of rabbits. Am. J. Physiol. 1999;276:H1064–H1077. doi: 10.1152/ajpheart.1999.276.3.H1064. [DOI] [PubMed] [Google Scholar]
- WANG D.Y., CHENG M.Z., WANG C.G., ZHANG S.E., CHEN T.F., DAI P.X. Effects of the alkaloids of Corydalis decumbens on cerebral and peripheral circulation in dogs. Chin. J. Integr. Trad. Wes. Med. 1986;6:477–479. [PubMed] [Google Scholar]
- ZHONG R.X., SHI R.R., HUANG L.X., LIU H., TAO J.Y., JIANG M.H., YANG X.Y., DENG S.S. Spasmolytic effect of protopine and Corydalis decumbens on isolated cat ciliary muscle and guinea pig ileum. Chin. Trad. Herb. Drugs. 1986;17:303–306. [Google Scholar]
- ZIPES D.P.Management of cardiac arrhythmias: Pharmacological, electrical, and surgical techniques Heart Disease 1997Philadelphia: W.B. Saunders; 593–639.In Braunwald, E. ed5th edn, pp [Google Scholar]


