Abstract
5-Hydroxytryptamine (5-HT) plays a role in the regulation of 3,4-dihydroxyphenylethylamine (dopamine) neurons in the brain, but the precise mechanism of regulation by 5-HT1A receptors of dopamine release has not been defined. The present study describes the effect of 5-{3-[[(2S)-1,4-benzodioxan-2ylmethyl]amino]propoxy}-1,3-benzodioxole HCl (MKC-242), a highly potent and selective 5-HT1A receptor agonist, on dopamine release in the prefrontal cortex using microdialysis in the freely moving rat.
Subcutaneous injection of MKC-242 (0.3–1.0 mg kg−1) increased extracellular levels of dopamine in the prefrontal cortex.
The effect of MKC-242 in the prefrontal cortex was antagonized by pretreatment with the selective 5-HT1A receptor antagonist, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide (WAY100635; 1 mg kg−1, i.p.).
Local application of WAY100635 (10 μM) via a microdialysis probe antagonized the effect of systemic MKC-242 in an increasing dopamine release, and locally infused 8-hydroxy-2-(di-n-propylamino)tetralin (10 μM) increased dopamine release in the prefrontal cortex.
MKC-242 increased cortical dopamine release in the rats pretreated with 5,7-dihydroxytryptamine (150 μg, i.c.v.) that caused an almost complete reduction in cortical 5-HT content.
The effect of MKC-242 to increase dopamine release was also observed in the hippocampus, but not in the striatum or nucleus accumbens.
Fluoxetine, a selective serotonin reuptake inhibitor, increased dopamine release in the prefrontal cortex, but not in the nucleus accumbens, while buspirone, a 5-HT1A receptor agonist, increased dopamine release in both brain regions.
The present results indicate that activation of postsynaptic 5-HT1A receptors increases dopamine release in a brain region-specific manner.
Keywords: 5-Hydroxytryptamine1A (5-HT1A) receptors, MKC-242, fluoxetine, dopamine release, antidepressant, microdialysis
Introduction
The 5-hydroxytryptamine (5-HT)1A-receptor subtype has been implicated in anxiolytic and antidepressant responses (Taylor, 1990; Lucki et al., 1994). 5-HT1A receptors are localized not only on the serotoninergic cell bodies of the raphe nuclei, but also on non-serotoninergic neurons in various brain regions including the hippocampus and prefrontal cortex (Pazos & Palacios, 1985). Agonist activation of the somatodendritic 5-HT1A autoreceptors reduces 5-HT cell firing, synthesis and release, while activation of the postsynaptic 5-HT1A receptors affects non-serotoninergic neurons (Fletcher et al., 1993). We showed that 5-{3-[((2S)-1,4-benzodioxan-2-ylmethyl)amino]propoxy}-1,3-benzodioxole (MKC-242), a potent and selective 5-HT1A receptor agonist with anxiolytic-like and antidepressant-like effects (Matsuda et al., 1995a,1995b; Abe et al., 1996; 1998a,1998b) facilitated in vivo release of noradrenaline (Suzuki et al., 1995) and acetylcholine (Somboothum et al., 1997) through activation of the postsynaptic and presynaptic 5-HT1A receptors, respectively. This effect on noradrenergic neurons (Chen & Reith, 1995; Hajos-Korcsok & Sharp, 1996; Hajos-Korcsok et al., 1999) may contribute to the antidepressant effect of 5-HT1A receptor agonists.
Previous studies also suggest a role for mesocortical dopaminergic pathways in mood regulation and antidepressant drug action (Willner, 1983; 1997; Knable & Weiberger, 1997) and their possible regulation by 5-HT1A receptors needs to be clarified. Arborelius et al. (1993), Rasmusson et al. (1994), Tanda et al. (1994) and Kuroki et al. (1996) reported that systemic administration of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), a typical 5-HT1A receptor agonist, increased 3,4-dihydroxyphenylethylamine (dopamine) release in rat prefrontal cortex. In those studies, however, the possibility was not excluded that an α2-adrenoceptor antagonistic action of 8-OH-DPAT may have contributed to the effect on dopamine release (Crist & Surprenant, 1987; Gobert et al., 1995). Furthermore, it is not known whether the 5-HT1A receptors modulating cortical dopamine release are localized presynaptically or postsynaptically. In this study, we examined the effect of the selective 5-HT1A receptor agonist, MKC-242, on dopamine release in rat prefrontal cortex using an in vivo microdialysis technique.
Methods
Animals and drugs
Male Wistar rats were maintained under controlled environmental conditions (22±1°C; 12 h–12 h light-dark cycle, lights on at 08:00, food and water ad libitum) for at least 1 week before being used for the experiments during which time they were accustomed to being handled. Procedures involving animals and their care were conducted according to Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society.
MKC-242 and N-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide (WAY100635) were gifts from Mitsubishi Chemical Co. (Yokohama, Japan). Fluoxetine was a gift from Eli Lilly and Co. (Indianapolis, U.S.A.). All other chemicals used were of the highest commercially available purity. All drugs were freshly prepared. MKC-242 for s.c. injection (1 ml kg−1) was suspended in 0.5% w v−1 carboxymethylcellulose (CMC). 5,7-Dihydroxytryptamine (5,7-DHT) was dissolved in 0.9% w v−1 NaCl containing 0.2% ascorbic acid. Other drugs were dissolved in 0.9% w v−1 NaCl. For direct administration into the prefrontal cortex via the dialysis probe, drugs were dissolved in a salt solution (composition, mM: NaCl 147, KCl 4, CaCl2 2.3).
Microdialysis procedure
Rats (250–350 g) were anaesthetized with pentobarbitone sodium (40 mg kg−1, i.p.) and stereotaxically implanted with guide cannulae (one site per animal) for the dialysis probes (Eicom, Japan) at the prefrontal cortex (A +3.2, L−1.2, V 5.2, from bregma and dura), hippocampus (A−5.3, L−4.4, V 6.5), striatum (A +0.7, L−2.6, V 6.5) and nucleus accumbens (A +1.7, L−1.2, V 7.6) (Paxinos & Watson, 1986) as reported previously (Suzuki et al., 1995; Somboothum et al., 1997). The active probe membrane for the prefrontal cortex, hippocampus and striatum was 3 mm in length and that for the nucleus accumbens was 2 mm. The day after surgery the probes were perfused at a constant flow rate of 2 μl min−1 with Ringer's solution. A 4 h stabilization period was allowed before 20 min microdialysis samples (40 μl) were taken and immediately injected onto a h.p.l.c. column for subsequent assay of dopamine. The probe recovery for dopamine was 7.9±0.2% (n=6).
Analysis of dialysates
Dialysates were assayed by h.p.l.c. with electrochemical detection: Eicompak CA-5ODS column (2.1 mm i.d.×150 mm: Eicom, Kyoto, Japan) and graphite electrode (Eicom) set at +450 mV against an Ag/AgCl reference electrode were used as described previously (Suzuki et al., 1995). The mobile phase contained 80 mM sodium phosphate buffer (pH 6.0), 500 mg L−1 sodium octanesulphonic acid, 150 μM EDTA and 20% v v−1 methanol.
Lesions of serotoninergic neurons with 5,7-DHT
Rats (220–250 g) were anaesthetized with pentobarbitone (40 mg kg−1, i.p.). Lesions of 5-HT neurons were carried out by injection of 5,7-DHT (150 μg as free base, 20 μl per rat, over a period of 2 min) into the lateral ventricles (A−0.8, L−1.5 and V 4.5 from skull surface) as reported previously (Suzuki et al., 1995; Somboothum et al., 1997). Desipramine at 25 mg kg−1, i.p. was injected 30 min before 5,7-DHT to protect noradrenergic neurons. The animals were used 2 weeks after the i.c.v. injection.
Determination of brain amines and their metabolites
The rats were killed by decapitation, and the samples for HPLC were prepared from the frontal cortex as previously reported (Matsuda et al., 1989). Eicompak CA-5ODS (4.6 mm i.d.×150 mm) (Eicom) was used for 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and noradrenaline assays. The mobile phase contained 40 mM sodium acetate-citrate buffer (pH 3.5), 170 mg L−1 sodium octanesulphonic acid, 15 μM EDTA and 13% v v−1 methanol. Other conditions were reported previously (Matsuda et al., 1995b).
Data analysis
The average of three fractions before drug administration was defined as 100% (control), and the subsequent perfusate levels were expressed as a percentage of the control. Statistical analyses were conducted by two-way ANOVA followed by Tukey's test or Student's t-test. P values of 5% or less were considered statistically significant.
Results
Basal level of dopamine, corrected with probe recovery, in the prefrontal cortex was 44±6 pg per fraction (mean±s.e.mean of eight determinations). Subcutaneous injection of MKC-242 at 0.3 and 1.0 mg kg−1 increased extracellular dopamine levels by about 50% in the prefrontal cortex (Figure 1). The maximal effect of MKC-242 to increase dopamine release was observed at 60 min after injection, and duration of the effect seemed to be longer in the higher dose than in the lower dose. MKC-242 at 0.1 mg kg−1 was ineffective (data not shown). Pretreatment with the 5-HT1A receptor antagonist, WAY100635, blocked the effect of MKC-242 on dopamine release in the prefrontal cortex, although the antagonist alone did not affect the basal release of dopamine (Figure 2). Two-way ANOVA analysis showed that the effect of WAY100635 on MKC-242-induced dopamine release was significant (saline/MKC-242 vs WAY100635/MKC-242: F(11.127)=2.252, P=0.015). Local perfusion of WAY100635 via a dialysis probe during the experiment also attenuated the effect of MKC-242 in increasing cortical dopamine release (Figure 3). Two-way ANOVA analysis showed that the effect of WAY100635 on MKC-242-induced dopamine release was significant (saline/MKC-242 vs WAY100635/MKC-242: F(11.132)=2.344, P=0.011). The effect of 8-OH-DPAT administered locally via a dialysis probe depended on the period of perfusion with the agonist: 8-OH-DPAT at 10 μM for 60 min increased dopamine release, but perfusion for 20 min did not (Figure 4). For local application of a 5-HT1A agonist, we used 8-OH-DPAT instead of MKC-242 because of its hydrophobicity.
Figure 1.

Effect of s.c. injection of MKC-242 on extracellular dopamine levels in the prefrontal cortex. Rats were treated with CMC and MKC-242 at zero time. Points are means±s.e.mean of 3–5 rats. aP<0.05, bP<0.01, compared with the corresponding value of vehicle (two-way ANOVA followed by Tukey's test).
Figure 2.
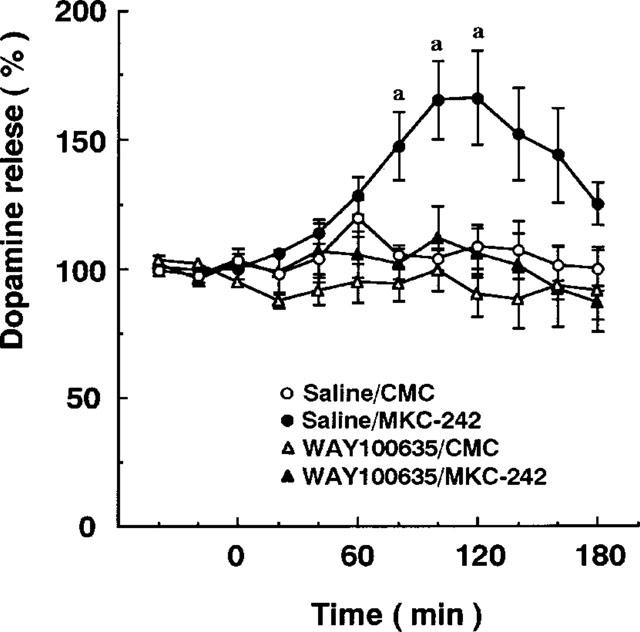
Effect of WAY100635 on MKC-242-induced increase in extracellular dopamine levels in the prefrontal cortex. MKC-242 at 1.0 mg kg−1 was injected s.c. at 40 min. WAY100635 at 1.0 mg kg−1 was administered s.c. 30 min before the agonist. Points are means±s.e.mean of 5–7 rats. aP<0.05, compared with the corresponding value of saline/CMC treatment (two-way ANOVA followed by Tukey's test).
Figure 3.

Effect of local application of WAY100635 on MKC-242-induced increase in extracellular dopamine levels in the prefrontal cortex. MKC-242 at 1.0 mg kg−1 was injected s.c. at 60 min. WAY100635 at 10 μM was perfused into the cortex via the dialysis probe for the time indicated by the horizontal bars. Points are means±s.e.mean of 4–7 rats. aP<0.05, bP<0.01, cP<0.001, compared with the corresponding value of saline/CMC treatment (two-way ANOVA followed by Tukey's test).
Figure 4.
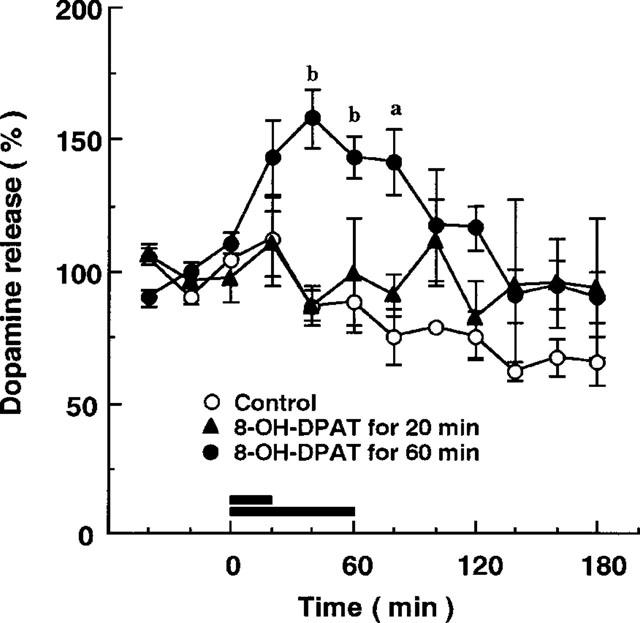
Effect of local application of 8-OH-DPAT on extracellular dopamine levels in the frontal cortex. 8-OH-DPAT at 10 μM was perfused into the cortex via the dialysis probe for the time indicated by the horizontal bars. Points are means±s.e.mean of 3–5 rats. aP<0.05, bP<0.01, compared with the corresponding value of control (two-way ANOVA followed by Tukey's test).
Lesions of serotoninergic neurons with 5,7-DHT caused a marked reduction in 5-HT (by 97%) and 5-HIAA (by 96%) levels, while it did not affect significantly noradrenaline or dopamine levels in the prefrontal cortex (Table 1). There was no difference in basal dopamine release between control and 5,7-DHT-treated groups: the dopamine levels (mean±s.e.mean, n=9–11) in the prefrontal cortex of vehicle- and 5,7-DHT-treated rats were 46±10 and 39±5 pg per fraction, respectively. MKC-242 increased extracellular dopamine levels in the prefrontal cortex of 5,7-DHT-treated rats: there was no difference in MKC-242-induced increase in dopamine release between saline and 5,7-DHT-pretreated rats (Figure 5).
Table 1.
Effect of serotoninergic neuronal lesions with 5,7-DHT on noradrenaline (NA), dopamine (DA), 5-HT and their metabolite levels (ng g−1 tissue) in rat frontal cortex

Figure 5.

Effect of pretreatment with 5,7-DHT on MKC-242-induced increase in extracellular dopamine levels in the prefrontal cortex. MKC-242 at 1.0 mg kg−1 was injected s.c. 14 days after rats were injected i.c.v. with saline and 5,7-DHT at 150 μg. Points are means±s.e.mean of 4–8 rats. aP<0.05, bP<0.01, cP<0.001, compared with 5,7-DHT/CMC (two-way ANOVA followed by Tukey's test).
The effect of MKC-242 on dopamine release was also studied in other brain regions. Systemic administration of MKC-242 at 1.0 mg kg−1 increased dopamine release in the hippocampus by about 100%, but not in the striatum or nucleus accumbens (Figure 6). The maximal effect of MKC-242 to increase dopamine release in the hippocampus was observed at 80 min and continued until 140 min.
Figure 6.
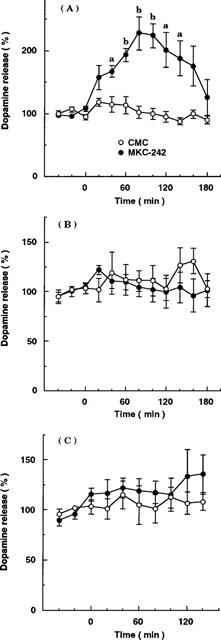
Effect of s.c. injection of MKC-242 on extracellular dopamine levels in the hippocampus (A), striatum (B) and nucleus accumbens (C). Rats were treated with saline and MKC-242 at 1.0 mg kg−1 at zero time. Points are means±s.e.mean of 4–6 rats. aP<0.05, bP<0.01, compared with the corresponding value of vehicle (two-way ANOVA followed by Tukey's test).
Figure 7 shows the effects of buspirone and fluoxetine on dopamine release in the prefrontal cortex. Subcutaneous injection of these drugs at 3 and 10 mg kg−1 caused a dose-dependent increase in extracellular dopamine levels in the prefrontal cortex. Buspirone and fluoxetine at 10 mg kg−1 caused 3 fold and 2 fold increases in cortical dopamine release, respectively. The maximal effects by buspirone and fluoxetine were observed at 60–120 and 40 min after injection, respectively. Fluoxetine as well as MKC-242 did not affect dopamine release in the striatum or nucleus accumbens, while buspirone increased dopamine release by about 50% in the nucleus accumbens (Figure 8).
Figure 7.
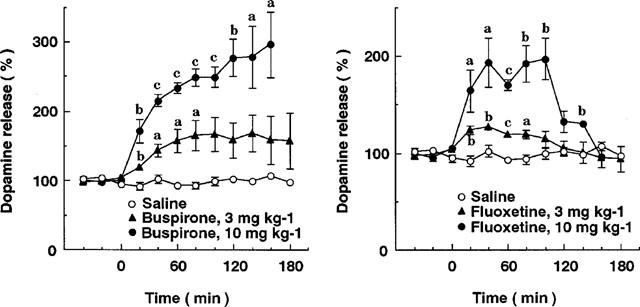
Effects of buspirone and fluoxetine on extracellular dopamine levels in the prefrontal cortex. Rats were treated with saline, buspirone and fluoxetine at zero time. Points are means±s.e.mean of 3–5 rats. aP<0.05, bP<0.01, cP<0.001, compared with the corresponding value of saline treatment (two-way ANOVA followed by Tukey's test).
Figure 8.

Effects of buspirone and fluoxetine on extracellular dopamine levels in the striatum (A) and nucleus accumbens (B). Rats were treated with saline, buspirone and fluoxetine at zero time. Points are means±s.e.mean of 5–7 rats. aP<0.05, compared with the corresponding value of saline treatment (two-way ANOVA followed by Tukey's test).
Discussion
Previous studies suggest that serotoninergic regulation of the meso-cortical dopaminergic pathway projecting from the ventral tegmental area to the prefrontal cortex plays a role in the effects of antidepressants (Willner, 1983; Tanda et al., 1994; Carlson et al., 1996) and atypical antipsychotic drugs (White & Wang, 1983; Meltzer, 1996). For example, activation of 5-HT1B (Iyer & Bradberry, 1996), 5-HT2A (Gobert & Millan, 1999) or 5-HT3 (Chen et al., 1992) receptors enhances directly or indirectly dopamine release in the prefrontal cortex, whereas activation of 5-HT2C receptors inhibits cortical dopamine release (Millan et al., 1998). The present study aimed to clarify the regulation by 5-HT1A receptors of cortical dopamine release using the selective 5-HT1A receptor agonist, MKC-242, and the selective 5-HT1A receptor antagonist, WAY100635. MKC-242 has high affinity for 5-HT1A receptors (Ki: 0.35 nM), and it is about 500 fold and more than 1000 fold more active at the 5-HT1A site than at the 5-HT2A site and at the 5-HT1B, 5-HT2C, 5-HT3, α2-adrenergic and dopamine D1 sites, respectively (Matsuda et al., 1995b). The present study suggests that activation of the 5-HT1A receptors causes cortical dopamine release and the degree of the effect is similar to that of the 5-HT2A receptor agonist-induced cortical dopamine release (Gobert & Millan, 1999).
The present study demonstrates that treatment with 5,7-DHT that destroys presynaptic serotoninergic nerve fibres does not alter the effect of MKC-242 in increasing cortical dopamine release. This finding suggests that dopamine release is modulated in part by postsynaptic 5-HT1A receptors in the prefrontal cortex. Since exogenous 5-HT facilitated dopamine release in the prefrontal cortex (Iyer & Bradberry, 1996), it is possible that 5,7-DHT lesions could eliminate this action of endogenous 5-HT on dopamine release. However, the recent (Matsumoto et al., 1998) and present studies showed that there was no difference in basal dopamine release between vehicle- and 5,7-DHT-treated rats. This may be explained by 5,7-DHT-induced supersensitivity of 5-HT1B or 5-HT2A receptors (Van de Kar et al., 1989; Butler et al., 1990; Sijbesma et al., 1991), since these receptors can exert a stimulatory influence on dopamine release (Iyer & Bradberry, 1996; Gobert & Millan, 1999). The involvement of the postsynaptic 5-HT1A receptors in MKC-242-induced cortical dopamine release is further supported by the experiments of local application of a 5-HT1A receptor agonist and antagonist. The effect of MKC-242 in increasing cortical dopamine release was blocked by local application of WAY100635, and local application of 8-OH-DPAT increased dopamine release in the cortex. It should be noted that a long duration of WAY100635 or 8-OH-DPAT perfusion was required. This implies that the site of action of these drugs is away from the position of the probe: the postsynaptic 5-HT1A receptors modulating cortical dopamine release appear to be localized on sites other than dopaminergic nerve terminals.
Tanda et al. (1994) reported that 8-OH-DPAT increased dopamine release in the prefrontal cortex, but not in the nucleus accumbens. In line with this finding, MKC-242 increased dopamine release in the prefrontal cortex, but not in the striatum or nucleus accumbens. Furthermore, the 5-HT1A receptor agonist-induced increase in dopamine release was greater in the hippocampus than in the prefrontal cortex. The region-specific effect may be explained by the idea that dopamine release is modulated by postsynaptic 5-HT1A receptors. The 5-HT1A receptors are dense in the hippocampus, while they are sparse in the striatum and nucleus accumbens (Pazos & Palacios, 1985). In this regard, we have found that locally perfused 5-HT, but not 8-OH-DPAT, at 10 μM increased dopamine release in the striatum and nucleus accumbens in a WAY100635-insensitive manner (data not shown). This suggests that 5-HT receptor subtypes other than 5-HT1A receptors are involved in dopamine release in these brain regions. In contrast to MKC-242 and 8-OH-DPAT, systemic buspirone increased dopamine release in the nucleus accumbens. This observation is also reported by Tanda et al. (1994). Furthermore, the previous studies showed that systemic administration of buspirone and intrastriatal administration of ipsapirone enhanced dopamine release in the striatum (Algeri et al., 1988; Golemibiowska & Wedzony, 1993). These azapirone compounds act not only as 5-TH1A receptor agonists but also α2 adrenoceptor and dopamine D2 receptor antagonists. Since α2 adrenoceptor and dopamine D2 receptor antagonists increase dopamine release (Gobert et al., 1998; Matsumoto et al., 1998), it is likely that the effects of azapirones on these receptors contribute at least partly to the increase in dopamine release in the 5-HT1A receptor-poor regions.
The present study showed that MKC-242 as well as fluoxetine, a selective 5-HT reuptake inhibitor, preferentially increased dopamine release in the prefrontal cortex. The region-specific activation of the dopamine system by MKC-242 and fluoxetine may contribute to the antidepressant-like effect of these drugs. Furthermore, the present finding implies that the activation of this receptor may have a beneficial effect for treatment of schizophrenia, since activation of cortical dopaminergic system may be important for the ameliorating effect of atypical antipsychotic drugs on negative symptoms in schizophrenia (Meltzer, 1989; Andersson et al., 1995). In contrast, it is unlikely that the activation of cortical dopaminergic system contribute to the anxiolytic-like effect of 5-HT1A receptor agonists, since anxiogenic agents and stress cause dopamine release in the prefrontal cortex (Bradberry et al., 1991; Yoshioka et al., 1996; Matsumoto et al., 1998).
In conclusion, the present study demonstrates that the 5-HT1A receptor agonist MKC-242 preferentially increased extracellular dopamine level in the prefrontal cortex and hippocampus via activation of postsynaptic 5-HT1A receptors. The exact anatomical site of these receptors remains to be determined.
Acknowledgments
This research was supported by grants from the Ministry of Education, Science and Culture of Japan and from Mitsubishi Chemical Co., Yokohama, Japan. We acknowledge the donations of MKC-242 by Dr M. Egawa (Mitsubishi Chemical Co.), WAY100635 by Dr M. Egawa, and fluoxetine by Dr T. Okimura (Eli Lilly Japan K.K.).
Abbreviations
- CMC
carboxymethylcellulose
- 5,7-DHT
5,7-dihydroxytryptamine
- MKC-242
5-{3-[((2S)-1,4-benzodioxan-2-ylmethyl)amino]propoxy}-1,3-benzodioxole
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- WAY100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide
References
- ABE M., NAKAI H., TABATA R., SAITO K., EGAWA M. Effect of 5-[3-[((2S)-1,4-benzodioxan-2-ylmethyl)amino]propoxy]-1,3-benzodioxole HCl (MKC-242), a novel 5-HT1A-receptor agonist, on aggressive behavior and marble burying behavior in mice. Jpn. J. Pharmacol. 1998a;76:297–304. doi: 10.1254/jjp.76.297. [DOI] [PubMed] [Google Scholar]
- ABE M., SAITO K. Reduction of wrap restraint stress-induced defecation by MKC-242, a novel benzodioxan derivative, via 5-HT1A-receptor agonist action in rats. Jpn. J. Pharmacol. 1998b;77:211–217. doi: 10.1254/jjp.77.211. [DOI] [PubMed] [Google Scholar]
- ABE M., TABATA R., SAITO K., MATSUDA T., BABA A., EGAWA M. Novel benzodioxan derivative, 5-[3-[((2S)-1,4-benzodioxan-2-ylmethyl) amino]propoxy]-1,3-benzodioxole HCl (MKC-242), with anxiolytic-like and antidepressant-like effects in animal models. J. Pharmacol. Exp. Ther. 1996;278:898–905. [PubMed] [Google Scholar]
- ALGERI S., DE LUIGI A., DE SIMONI M.G., IMERI L., MARCONI M., NAVA S., PEREGO C., SACCHETTI G. Multiple and complex effects of buspirone on central dopaminergic system. Pharmacol. Biochem. Behav. 1988;29:823–826. doi: 10.1016/0091-3057(88)90217-1. [DOI] [PubMed] [Google Scholar]
- ANDERSSON J.L., NOMIKOS G.G., MARCUS M., HERTEL P., MATHE J.M., SVENSSONTH T.H. Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn Schmiedebergs Arch. Pharmacol. 1995;352:374–385. doi: 10.1007/BF00172774. [DOI] [PubMed] [Google Scholar]
- ARBORELIUS L., NOMIKOS G.G., HACKSELL U., SVENSSON T.H. (R)-8-OH-DPAT preferentially increases dopamine release in rat medial prefrontal cortex. Acta Physiol. Scand. 1993;148:465–466. doi: 10.1111/j.1748-1716.1993.tb09584.x. [DOI] [PubMed] [Google Scholar]
- BRADBERRY C.W., LORY J.D., ROTH R.H. The anxiogenic β-carboline FG 7142 selectively increases dopamine release in rat prefrontal cortex as measured by microdialysis. J. Neurochem. 1991;56:748–752. doi: 10.1111/j.1471-4159.1991.tb01987.x. [DOI] [PubMed] [Google Scholar]
- BUTLER P.D., PRANZATELLI M.R., BARKAI A.I. Regional central serotonin-2 receptor binding and phosphoinositide turnover in rats with 5,7-dihydroxytryptamine lesions. Brain Res. Bull. 1990;24:125–129. doi: 10.1016/0361-9230(90)90296-c. [DOI] [PubMed] [Google Scholar]
- CARLSON J.N., VISKER K.E., NIELSEN D.M., JKELLER R.W., JR, GLICK S.D. Chronic antidepressant drug treatment reduces turning behavior and increases dopamine levels in the medial prefrontal cortex. Brain Res. 1996;707:122–126. doi: 10.1016/0006-8993(95)01341-5. [DOI] [PubMed] [Google Scholar]
- CHEN J., PAREDES W., VAN PRAAG H.M., LOWINSON J.H., GARDNER E.L. Presynaptic dopamine release is enhanced by 5-HT3 receptor activation in medial prefrontal cortex of freely moving rats. Synapse. 1992;10:264–266. doi: 10.1002/syn.890100308. [DOI] [PubMed] [Google Scholar]
- CHEN N.H., REITH M.E. Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin. J. Neurochem. 1995;64:1585–1597. doi: 10.1046/j.1471-4159.1995.64041585.x. [DOI] [PubMed] [Google Scholar]
- CRIST J., SURPRENANT A. Evidence that 8-hydroxy-2-(n-dipropylamino)tetralin (8-OH-DPAT) is a selective α2-adrenoceptor antagonist on guinea-pig submucous membranes. Br. J. Pharmacol. 1987;92:341–347. doi: 10.1111/j.1476-5381.1987.tb11329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLETCHER A., CLIFFE I.A., DOURISH C.T. Silent 5-HT1A receptor antagonists: utility as research tools and therapeutic agents. Trends Pharmacol Sci. 1993;14:441–448. doi: 10.1016/0165-6147(93)90185-m. [DOI] [PubMed] [Google Scholar]
- GOBERT A., LEJEUNE F., RIVET J.M., AUDINOT V., NEWMAN TANCREDI A., MILLAN M.J. Modulation of the activity of central serotoninergic neurons by novel serotonin1A receptor agonists and antagonists: a comparison to adrenergic and dopaminergic neurons in rats. J. Pharmacol. Exp. Ther. 1995;273:1032–1046. [PubMed] [Google Scholar]
- GOBERT A., MILLAN M.J. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- GOBERT A., RIVET J.M., AUDINOT V., NEWMAN-TANCREDI A., CISTARELLI L., MILLAN M.J. Simultaneous quantification of serotonin, dopamine and noradrenaline levels in single frontal cortex dialysates of freely-moving rats reveals a complex pattern of reciprocal auto- and heteroreceptor-mediated control of release. Neuroscience. 1998;84:413–429. doi: 10.1016/s0306-4522(97)00565-4. [DOI] [PubMed] [Google Scholar]
- GOLEMBIOWSKA K., WEDZONY K. Enhancement by ipsapirone of dopamine release in the rat striatum. Pol. J. Pharmacol. 1993;45:299–308. [PubMed] [Google Scholar]
- HAJOS KORCSOK E., MCQUADE R., SHARP T. Influence of 5-HT1A receptors on central noradrenergic activity: microdialysis studies using (±)-MDL 73005EF and its enantiomers. Neuropharmacology. 1999;38:299–306. doi: 10.1016/s0028-3908(98)00175-0. [DOI] [PubMed] [Google Scholar]
- HAJOS KORCSOK E., SHARP T. 8-OH-DPAT-induced release of hippocampal noradrenaline in vivo: evidence for a role of both 5-HT1A and dopamine D1 receptors. Eur. J. Pharmacol. 1996;314:285–291. doi: 10.1016/s0014-2999(96)00560-2. [DOI] [PubMed] [Google Scholar]
- IYER R.N., BRADBERRY C.W. Serotonin-mediated increase in prefrontal cortex dopamine release: pharmacological characterization. J. Pharmacol. Exp. Ther. 1996;277:40–47. [PubMed] [Google Scholar]
- KNABLE M.B., WEINBERGER D.R. Dopamine, the prefrontal cortex and schizophrenia. J. Psychopharmacol. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- KUROKI T., ICHIKAWA J., DAI J., MELTZER H.Y. R(+)-8-OH-DPAT, a 5-HT1A receptor inhibits amphetamine-induced serotonin and dopamine release in rat medial prefrontal cortex. Brain Res. 1996;743:357–361. doi: 10.1016/s0006-8993(96)01111-0. [DOI] [PubMed] [Google Scholar]
- LUCKI I., SINGH A., KREISS D.S. Antidepressant-like behavioral effects of serotonin receptor agonists. Neurosci. Biobehav. Rev. 1994;18:85–95. doi: 10.1016/0149-7634(94)90039-6. [DOI] [PubMed] [Google Scholar]
- MATSUDA T., SEONG Y.H., AONO H., KANDA T., BABA A., SAITO K., TOBE A., IWATA H. Agonist activity of a novel compound, 1-[3-(3,4-methylenedioxyphenyl)propyl]-4-phenyl piperazine (BP-554), at central 5-HT1A receptors. Eur. J. Pharmacol. 1989;170:75–82. doi: 10.1016/0014-2999(89)90136-2. [DOI] [PubMed] [Google Scholar]
- MATSUDA T., SOMBOONTHUM P., SUZUKI M., ASANO S., BABA A. Antidepressant-like effect by postsynaptic 5-HT1A receptor activation in mice. Eur. J. Pharmacol. 1995a;280:235–238. doi: 10.1016/0014-2999(95)00254-i. [DOI] [PubMed] [Google Scholar]
- MATSUDA T., YOSHIKAWA T., SUZUKI M., ASANO S., SOMBOONTHUM P., TAKUMA K., NAKANO Y., MORITA T., NAKASU Y., KIM H.S., EGAWA M., TOBE A., BABA A. Novel benzodioxanderivative, 5-(3-[((2S)-1,4-benzidioxan-2-ylmethyl)amino]propoxy)-1,3-benzodioxole HCl (MKC-242), with a highly potent and selective agonist activity at rat central serotonin1A receptors. Jpn. J. Pharmacol. 1995b;69:357–366. doi: 10.1254/jjp.69.357. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO M., YOSHIOKA M., TOGASHI H., MORI K., UENO K., SAITO H. Effects of idazoxan on dopamine release in the prefrontal cortex of freely moving rats. Eur. J. Pharmacol. 1998;343:165–170. doi: 10.1016/s0014-2999(97)01544-6. [DOI] [PubMed] [Google Scholar]
- MELTZER H.Y. Clinical studies on mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology. 1989;99 Suppl.:S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- MELTZER H.Y. Pre-clinical pharmacology of atypical antipsychotic drugs: a selective review. Br. J. Psychiatry. 1996;29 Suppl.:23–31. [PubMed] [Google Scholar]
- MILLAN M.J., DEKEYNE A., GOBERT A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the front cortex in vivo. Neuropharmacology. 1998;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1986San Diego: Academic Press; Second edition [Google Scholar]
- PAZOS A., PALACIOS J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- RASMUSSON A.M., GOLDSTEIN L.E., DEUTCH A.Y., BUNNEY B.S., ROTH R.H. 5-HT1a agonist±8-OH-DPAT modulates basal and stress-induced changes in medial prefrontal cortical dopamine. Synapse. 1994;18:218–224. doi: 10.1002/syn.890180307. [DOI] [PubMed] [Google Scholar]
- SIJBESMA H., SCHIPPER J., DE KLOET E.R., MOS J., VAN AKEN H., OLIVIER B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol. Biochem. Behav. 1991;38:447–458. doi: 10.1016/0091-3057(91)90305-l. [DOI] [PubMed] [Google Scholar]
- SOMBOONTHUM P., MATSUDA T., ASANO S., SAKAUE M., BABA A. MKC-242, a novel 5-HT1A receptor agonist, facilitates cortical acetylcholine release by a mechanism different from that of 8-OH-DPAT in awake rats. Neuropharmacology. 1997;36:1733–1739. doi: 10.1016/s0028-3908(97)00174-3. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., MATSUDA T., ASANO S., SOMBOONTHUM P., TAKUMA K., BABA A. Increase of noradrenaline release in the hypothalamus of freely moving rat by postsynaptic 5-hydroxytryptamine1A receptor activation. Br. J. Pharmacol. 1995;115:703–711. doi: 10.1111/j.1476-5381.1995.tb14990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANDA G., CARBONI E., FRAU R., DI CHIARA G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential. Psychopharmacology. 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- TAYLOR D.P. Serotonergic agents in anxiety. Ann. NY Acad. Sci. 1990;600:545–557. doi: 10.1111/j.1749-6632.1990.tb16909.x. [DOI] [PubMed] [Google Scholar]
- VAN DE KAR L.D., CARNES M., MASLOWSKI R.J., BONADONNA A.M., RITTENHOUSE P.A., KUNIMOTO K., PIECHOWSKI R.A., BETHEA C.L. Neuroendocrine evidence for denervation supersensitivity of serotonin receptors: effects of the 5-HT agonist RU 24969 on corticotropin, corticosterone, prolactin and renin secretion. J. Pharmacol. Exp. Ther. 1989;251:428–434. [PubMed] [Google Scholar]
- WHITE F.J., WANG R.Y. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science. 1983;221:1054–1057. doi: 10.1126/science.6136093. [DOI] [PubMed] [Google Scholar]
- WILLNER P. Dopamine and depression: a review of recent evidence. III. The effects of antidepressant treatments. Brain Res. 1983;287:237–246. doi: 10.1016/0165-0173(83)90007-3. [DOI] [PubMed] [Google Scholar]
- WILLNER P. The mesolimbic dopamine system as a target for rapid antidepressant action. Int. Clin. Psychopharmacol. 1997;12 Suppl. 3:S7–S14. doi: 10.1097/00004850-199707003-00002. [DOI] [PubMed] [Google Scholar]
- YOSHIOKA M., MATSUMOTO M., TOGASHI H., SAITO H. Effect of conditioned fear stress on dopamine release in the rat prefrontal cortex. Neurosci. Lett. 1996;209:201–203. doi: 10.1016/0304-3940(96)12631-8. [DOI] [PubMed] [Google Scholar]


