Abstract
In addition to their traditional contractile function, vascular smooth muscle cells can be stimulated under inflammatory conditions to release a range of potent biological mediators. Indeed, we and others have shown that human vascular smooth muscle release the colony stimulating factors (CSF) granulocyte macrophage-CSF (GM-CSF) and granulocyte-CSF (G-CSF) as well as large amounts of prostaglandins following the induction of cyclo-oxygenase-2 (COX-2), when stimulated with cytokines. Here we demonstrate, for the first time, that co-induced COX-2 activity simultaneously suppresses GM-CSF release and potentiates G-CSF release by human vascular cells. Moreover, the differential regulation of GM-CSF and G-CSF release by COX-2 was mimicked by the prostacyclin (PGI2) mimetic, cicaprost. These observations suggest that PGI2, released following the induction of COX-2, differentially regulates the release of GM-CSF (suppresses) and G-CSF (potentiates) from human vascular cells.
Keywords: Cyclo-oxygenase-2, G-CSF, GM-CSF, smooth muscle cells, prostacyclin
Introduction
Colony stimulating factors (CSFs) such as granulocyte macrophage-colony stimulating factor (GM-CSF) are responsible for the proliferation and differentiation of cells in the bone marrow (Metcalf, 1986). However, these cytokines also modulate the function of mature leukocytes, including neutrophils, promoting their activation and survival (Lopez et al., 1986). In vascular diseases such as atherosclerosis damage to, or loss of, the endothelium results in exposure of the underlying vascular smooth muscle cells. These cells, representing the major cell type in both artery and vein, are potentially an important source of inflammatory mediators. Indeed we have recently shown that human arterial and venous smooth muscle cells can be induced to release GM-CSF and to express the inducible form of cyclo-oxygenase (COX), COX-2, when stimulated with inflammatory cytokines such as interleukin-1β (IL-1β) and tumour necrosis factor-α (TNFα) (Bishop-Bailey et al., 1998; Stanford et al., 2000). The constitutive form of COX, COX-1, as well as COX-2 is inhibited by the anti-inflammatory drugs which include indomethacin. However, indomethacin and other traditional NSAIDs inhibit COX-1 more readily than COX-2, a property that has been linked to the gastrointestinal side-effects associated with these drugs (Mitchell et al., 1993; Mitchell & Warner, 1999). More recently highly selective inhibitors of COX-2, such as 5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl) phenyl-2(5H)-furanone (DFU: Warner et al., 1999), have become available as experimental tools. In a limited number of previous studies using other cell types indomethacin has been shown to increase GM-CSF and decrease G-CSF levels (Hamilton et al., 1992; Lee et al., 1990). Thus the purpose of this study was (1) to investigate the relative roles of COX-1 versus COX-2 in GM-CSF and G-CSF release by human vascular smooth muscle cells and (2) to identify the importance of the major COX product of these cells (Bishop-Bailey et al., 1998), prostacyclin (PGI2), in the release of GM-CSF and G-CSF.
Methods
Cell culture
Arterial and venous smooth muscle cells were cultured as described previously (Stanford et al., 2000). In brief, samples of human internal mammary artery (IMA) and saphenous vein (SV) direct from surgery were dissected clean, cut into small pieces and placed in Dulbecco's modified Eagle's medium (DMEM) containing sodium pyruvate, phenol red and supplemented with 10% foetal calf serum, penicillin, streptomycin, glutamine, amphotericin B and MEM non-essential amino acids. Confluent cells (passage numbers 2–9 only) were plated onto 96 well plates for use in experiments. Serum was withdrawn from cells 24 h prior to treatment with inflammatory cytokines and drugs.
Cell treatment
At the beginning of each experiment new supplemented DMEM was added to cells. In some experiments cells were treated for 24 h with increasing concentrations of IL-1β (0.01–10 ng ml−1). A further set of experiments were carried out in which cells were pre-treated (approximately 5 min) with either the non-selective COX inhibitor, indomethacin (1×10−5 M), or the selective COX-2 compound, DFU (1×10−5 M), before the addition of IL-1β (1.0 ng ml−1). In the final part of this study cells were treated for 24 h with increasing concentrations of the prostacyclin (PGI2) mimetic, cicaprost (1×10−10–1×10−7 M), in the presence of both IL-1β (1.0 ng ml−1) and indomethacin (1×10−5 M). At the end of all experiments medium was removed from the cells and GM-CSF and G-CSF release were measured by ELISA. Cell viability was assessed by mitochondrial-dependent reduction of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to formazan. None of the treatments used affected viability of arterial or venous smooth muscle cells.
Materials
Human recombinant IL-1β and G-CSF were bought from R&D Systems. Matched ELISA reagents to develop immunoassays for human GM-CSF were bought from Pharmingen. Matched G-CSF antibody pairs for human G-CSF ELISA were bought from R&D Systems. Indomethacin was from Sigma, DFU was a gift from Merck and cicaprost from Dr F. McDonald at Schering, Berlin, Germany.
Results
In the absence of cytokines arterial and venous smooth muscle cells released low or undetectable levels of both GM-CSF and G-CSF. IL-1β stimulated the release of GM-CSF and G-CSF from both cell types in a concentration-dependent manner producing maximum release at a concentration of 1.0 ng ml−1. IL-1β-stimulated arterial and venous cells both released higher levels of G-CSF than GM-CSF (G-CSF vs GM-CSF: Molecular weights 21 vs 14–35 kDa: IMA 7308±908 vs 294±14 pg ml−1, n=9: SV 11354±715 vs 167±7 pg ml−1, n=9).
Indomethacin and DFU significantly inhibited the release of G-CSF from stimulated arterial (Figure 1a) and venous (4375±919 vs indomethacin 1271±205, DFU 1518±338; pg ml−1, n=8) smooth muscle cells. In contrast, indomethacin and DFU significantly potentiated the release of GM-CSF from both cell types (IMA: Figure 1b: SV: 86±11 vs indomethacin 408±23, DFU 421±31; pg ml−1, n=10) in the presence of IL-1β. No significant difference was observed in CSF release from stimulated arterial or venous cells treated with indomethacin vs DFU. In separate experiments, indomethacin or DFU at 1×10−5 M completely blocked COX activity (measured as prostaglandin E2 production by radioimmunoassay; Mitchell et al., 1993) by either arterial or venous smooth muscle cells (data not shown).
Figure 1.
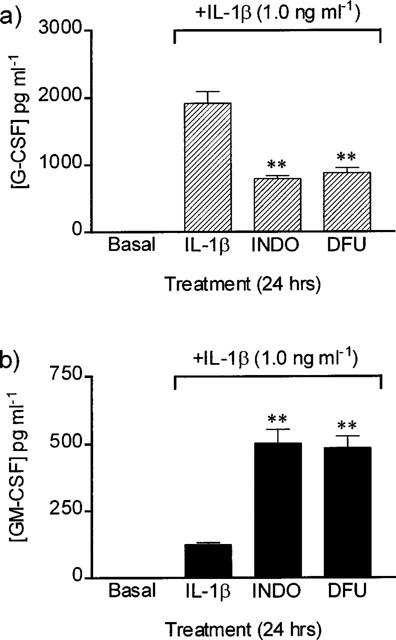
Release of (a) G-CSF and (b) GM-CSF from human arterial smooth muscle cells stimulated with IL-1β (1.0 ng ml−1) in the presence of indomethacin (INDO: 1×10−5 M) or DFU (1×10−5 M). One way ANOVA vs IL-1β, post-test Dunnett: **P<0.01, n=12 experiments using cells cultured from four patients.
In arterial cells cicaprost reversed, in a concentration-dependent fashion, the decrease in G-CSF and increase in GM-CSF release stimulated by indomethacin (Figure 2). Similarly when venous cells were treated with IL-1β and indomethacin the increase in GM-CSF release (1016±25 pg ml−1) was reversed by cicaprost (EC50, 7.1×10−10 M) with a maximum effect being seen at 1×10−9 M cicaprost (158±30 pg ml−1; n=9). Again the decreased production of G-CSF seen in venous cells treated IL-1β and indomethacin (3822±274 pg ml−1) was reversed by cicaprost (EC50, 3.7×10−10 M) with a maximum effect seen with 1×10−7 M cicaprost (11406±896 pg ml−1; n=9).
Figure 2.
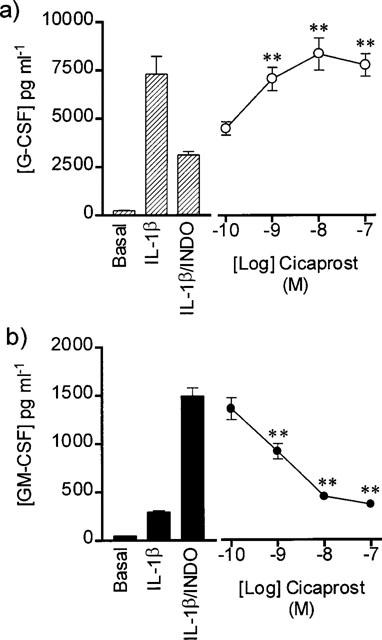
Effect of cicaprost on (a) G-CSF and (b) GM-CSF release by human cultured arterial smooth muscle cells pre-treated with indomethacin (INDO: 1×10−5 M) and stimulated for 24 h with IL-1β (1.0 ng ml−1). Figure represents n=9 using cells cultured from three patients. One way ANOVA vs IL-1β/INDO, post-test Dunnett: **P<0.01.
Discussion
Here we have confirmed previous studies showing that human vascular smooth muscle cells are capable of releasing GM-CSF (Stanford et al., 2000) and G-CSF (Zoellner et al., 1992) when stimulated with cytokines. Under the same conditions we have previously shown that these cells express COX-2 and release large quantities of prostaglandins (particularly prostacyclin; Bishop-Bailey et al., 1998). Moreover in the current study we have shown that inhibition of prostaglandin production by either indomethacin or DFU differentially modulates GM-CSF and G-CSF release by human vascular smooth muscle cells.
Two isoforms of COX have been identified to date. COX-1 is expressed constitutively and thought to regulate physiological processes. COX-2 is expressed after stimulation with cytokines and predominates at the site of inflammation (Mitchell & Warner, 1999). Thus, COX-2 is thought to be the active isoform involved in inflammatory events. Indomethacin effects both forms of COX, but is a more potent inhibitor of COX-1 than of COX-2 (Mitchell et al., 1993; Warner et al., 1999; Mitchell & Warner, 1999). Limited studies, in other cell types, have shown that indomethacin increases GM-CSF, and decreases G-CSF, production after cytokine treatment (Hamilton et al., 1992; Lee et al., 1990). Using human vascular smooth muscle cells, we found that the ability of indomethacin to increase GM-CSF and decrease G-CSF occurs simultaneously. In our study, as was the case for others in the literature, cytokine stimulation of cells was required in order to see any effects of indomethacin on CSF release. Under these conditions, we might expect that COX-2 and not COX-1 predominates in cells or tissue. Indeed, we found that when the highly selective COX-2 inhibitor, DFU was added to cytokine-stimulated cells at concentrations that block COX-2 but have no effect on COX-1 (Warner et al., 1999), GM-CSF was increased and G-CSF was decreased. In fact, the effects of DFU on CSF release were indistinguishable to those of indomethacin. This observation shows that under experimental inflammatory conditions, COX-2 activity differentially regulates GM-CSF and G-CSF production by human vascular cells (this study) and suggests that a similar phenomenon occurs in other cell types where only indomethacin was used (Hamilton et al., 1992; Lee et al., 1990).
In our study we found that the effects of COX inhibition on CSF release were dramatically reversed in parallel by cicaprost. In these studies, cicaprost was very potent with maximal effects seen at concentrations as low as 1×10−9 M. This suggests, but is not definitive proof, that the effects of COX activity on CSF release occur at the level of prostacyclin-IP receptors. In support of this, in a recent study addressing the effects of COX-2 on GM-CSF only, we found prostaglandin E2 (PGE2) reversed the effects of indomethacin only at very high concentrations (Stanford et al., 2000). These observations are in line with one other using human blood mononuclear cells (Luttmann et al., 1996) where cicaprost reversed fully the effects of indomethacin on GM-CSF release (effects on G-CSF were not addressed). In contrast to observations using vascular smooth muscle cells (this study) or mononuclear cells (Luttmann et al., 1996), the effects of indomethacin on GM-CSF production by human synovial fibroblasts were reversed by PGE2 and not by a prostacyclin mimetics (iloprost: Agro et al., 1996). Thus, it seems that where COX-2 is expressed the release of GM-CSF and G-CSF will be differentially modulated by either IP or EP receptor activation, depending upon the cell type studied.
GM-CSF and G-CSF preferentially activate different populations of leukocytes. Indeed, GM-CSF is thought to act on a wider range of leukocytes including neutrophils, eosinophils and monocytes (Lopez et al., 1986; Eischen et al., 1991; Erickson-Miller et al., 1990), whereas G-CSF is thought to be active mainly on neutrophils (Colotta et al., 1992). Thus, pathways that increase the release of one and inhibit the release of another will have profound effects on the populations of leukocytes present at the site of inflammation. We have identified COX-2 as such a pathway and suggest that in this capacity it has a central role in the regulation of inflammatory and immunological events.
Acknowledgments
This work was funded by grants from the British Heart Foundation and The Wellcome Trust.
Abbreviations
- COX
cyclo-oxygenase
- CSF
colony stimulating factor
- DFU
5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl) phenyl-2(5H)-furanone
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte macrophage-colony stimulating factor
- IL-1β
interleukin-1β
- IMA
internal mammary artery
- INDO
indomethacin
- PGE2
prostaglandin E2
- PGI2
prostacyclin
- SV
saphenous vein
- TNFα
tumour necrosis factor-α
References
- AGRO A., LANGDON C., SMITH F., RICHARDS C.D. Prostaglandin E2 enhances interleukin-8 (IL-8) and IL-6 but inhibits GM-CSF production by IL-1 stimulated human synovial fibroblasts in vitro. J. Rheumatol. 1996;23:862–868. [PubMed] [Google Scholar]
- BISHOP-BAILEY D., PEPPER J.R., LARKIN S.W., MITCHELL J.A. Differential induction of cyclo-oxygenase-2 in human arterial and venous smooth muscle: role of endogenous prostanoids. Arterioslcer. Thromb. Vasc. Biol. 1998;18:1655–1661. doi: 10.1161/01.atv.18.10.1655. [DOI] [PubMed] [Google Scholar]
- COLOTTA F., RE F., POLENTARUTTI N., SOZZANI S., MANTOVANI A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- EISCHEN A., VINCENT F., BERGERAT J.P., LOUIS B., FARADJI A., BOHBOT A., OBERLING F. Long term cultures of human monocytes in vitro. Impact of GM-CSF on survival and differentiation. J. Immunol. Methods. 1991;143:209–221. doi: 10.1016/0022-1759(91)90046-i. [DOI] [PubMed] [Google Scholar]
- ERICKSON-MILLER C.L., BRENNAN J.K., ABBOUD C.N. Examination of survival, proliferation and cell surface antigen expression of human monocytes exposed to macrophage colony-stimulating factor (M-CSF) Int. J. Cell Cloning. 1990;8:346–356. doi: 10.1002/stem.5530080503. [DOI] [PubMed] [Google Scholar]
- HAMILTON J.A., PICCOLI D.S., CEBON J., LAYTON J.E., RATHANASWANI P., MCCOLL S.R., LEIZER T. Cytokine regulation of colony-stimulating factor (CSF) production in cultured human synovial fibroblasts. II. Similarities and differences in the control of interleukin-1 induction of granulocyte-macrophage CSF and granulocyte-CSF production. Blood. 1992;79:1413–1419. [PubMed] [Google Scholar]
- LEE M.T., KAUSHANSKI K., RALPH P., LADNER M.B. Differential expression of M-CSF, G-CSF and GM-CSF by human monocytes. J. Leuk. Biol. 1990;47:275–282. doi: 10.1002/jlb.47.3.275. [DOI] [PubMed] [Google Scholar]
- LOPEZ A.F., WILLIAMSON D.J., GAMBLE J.R., BEGLEY C.G., HARLAN J.M., KLEBANOFF S.J., WALTERSDORPH A., WONG G., CLARK S.C., VADAS M.A. Recombinant human granulocyte macrophage-colony stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression and survival. J. Clin. Invest. 1986;78:1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTMANN W., HERZOG V., VIRCHOW J.C., MATTHYS H., THIERAUCH K.H., KROEGEL C. Prostacyclin modulates granulocyte/macrophage colony-stimulating factor release by human blood mononuclear cells. Pulm. Pharmacol. 1996;9:43–48. doi: 10.1006/pulp.1996.0005. [DOI] [PubMed] [Google Scholar]
- METCALF D. The molecular biology and functions of the granulocyte-macrophage colony stimulating factors. Blood. 1986;67:257–267. [PubMed] [Google Scholar]
- MITCHELL J.A., AKARASEREENONT P., THIEMERMANN C., FLOWER R.J., VANE J.R. Selectivity of nonsteroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclo-oxygenase. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.A., WARNER T.D. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 1999;128:1121–1132. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANFORD S.J., PEPPER J.R., MITCHELL J.A.Cyclo-oxygenase-2 regulates Granulocyte macrophage-colony stimulating factor, but not interleukin-8, production by human vascular cells: role of cyclic AMP Arterioscler. Thromb. Vasc. Biol. 2000. In press [DOI] [PubMed]
- WARNER T.D., GIULIANO F., VOJNOVIC I., BUKASA A., MITCHELL J.A., VANE J.R. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOELLNER H., FILONZI E.L., STANTON H.R., LAYTON J.E., HAMILTON J.A. Human arterial smooth muscle cells synthesise granulocyte colony-stimulating factor in response to interleukin-1 alpha and tumour necrosis factor-alpha. Blood. 1992;80:2805–2810. [PubMed] [Google Scholar]


