Abstract
The β-carboline, harmane (0.1–1.0 nmol) produces dose dependent hypotension when microinjected unilaterally into the rostral ventrolateral medulla (RVLM) of the anaesthetized rat. The potency of harmane on blood pressure is similar to that of the imidazoline, clonidine. The hypotensive effects of both clonidine and harmane are reversed by microinjection of the relatively I1-receptor selective antagonist efaroxan (20 nmol). These results are consistent with harmane acting at an I1-receptor in the RVLM. This is the first report of an endogenous ligand for I1-receptors that has central effects on blood pressure.
Keywords: Imidazoline receptors, β-carbolines, harmane, clonidine, RVLM, blood pressure
Introduction
Imidazoline receptors have a high affinity for drugs with an imidazoline, oxazoline or guanidium structure (Regunathan & Reis, 1996; Bousquet et al., 1998). Binding studies show that they can be divided into at least two subtypes, I1-receptors and I2-receptors (Regunathan & Reis, 1996; Bousquet et al., 1998). The functional roles of these receptors are poorly understood (Regunathan & Reis, 1996; Eglen et al., 1998; Bousquet et al., 1998). However, drugs with high affinity for imidazoline I1-receptors have been shown to produce falls in blood pressure when injected into the rostral ventrolateral medulla (RVLM) of rats (Ernsberger et al., 1990), cats (Bousquet et al., 1984) and rabbits (Head et al., 1997a). The role of I1-receptors in the falls in blood pressure associated with peripheral administration of these compounds is more controversial (Eglen et al., 1998). However, careful experiments using dose response curves in the presence of relatively selective antagonists suggest that they do play a role (Head & Burke, 1998).
The presence of imidazoline receptors in the RVLM lead to a search for an endogenous ligand. To date, three compounds have been isolated from the brain that bind imidazoline receptors (Li et al., 1994; Grigg et al., 1998; Hudson et al., 1999). Only two have been fully structurally identified (Li et al., 1994; Hudson et al., 1999). Agmatine was the first endogenous compound to be shown to have high affinity for imidazoline receptors (Li et al., 1994). However, its functional significance is questionable as it has no effect when injected into the RVLM (Head et al., 1997b). Harmane, β-carboline, was recently reported to have nanomolar affinity for I1-imidazoline receptors, similar to that reported for clonidine (Hudson et al., 1999), and is present endogenously in the brain (Moncrieff, 1989; Rommelspacher et al., 1991). The effect of this compound on blood pressure has not been investigated to date. Thus the aims of the present study were to determine (1) whether the β-carboline harmane could modulate blood pressure when injected into the RVLM; (2) compare its potency to that of clonidine and (3) determine whether the response was mediated by imidazoline receptors.
Methods
Outbred male Sprague-Dawley rats (220–300 g) were used. The experimental protocol was approved by the Monash Medical Centre and Monash University Animal Ethics committees and conform to the guidelines set out by the National Health and Medical Research Council and all government regulations. The rats were anaesthetized with urethane (1.5 g kg−1 i.p.) and the right femoral artery was cannulated to enable blood pressure monitoring. The head of the rat was placed into a Kopf stereotaxic apparatus so that lambda and bregma were positioned on the same horizontal plane. A 4 mm diameter burr hole was made in the skull and the pressor region of the left RVLM was located with microinjection of L-glutamate (100 nL, 0.1 M in Ringers) using a glass micro-pipette (maximum pressor response 17.6±1.7 mmHg, n=24). Typical co-ordinates were 2.5–3.5 mm caudal to the lambdoid suture, 1.8–2.2 mm lateral of the lambda-bregma midline and 8.5–8.9 mm ventral of the dorsal surface of the cerebellum (Badoer & Merolli, 1998). After locating the pressor region, the pipette was withdrawn, filled with the appropriate solution and then reinserted into the RVLM. There were four groups (n=6) of animals that received either harmane (0.01–1.0 nmol, the highest dose possible due to low solubility), its vehicle (0.1% DMSO in Ringers), clonidine (0.01–10 nmol) or its vehicle (Ringers). Increasing doses of each drug (or vehicle, 100 nL) were administered into the RVLM at 15 min intervals. Fifteen minutes after the final injection of drug or its respective vehicle, efaroxan (20 nmol given as two 100 nL injections separated by 1 min) was injected into the RVLM. The blood pressure and heart rate responses were recorded continuously on a MacLab data acquisition unit (ADInstruments, Castle Hill, Australia). At the end of the experiment pontamine sky blue was injected (100 nL) via the micropipette to mark the injection site in the RVLM which was confirmed histologically.
Harmane HCl was kindly synthesized by Dr Colin Barrow and Matt Grigg of the Department of Chemistry, University of Melbourne. Efaroxan and clonidine were obtained from Sigma (St Louis, U.S.A.). Sterile Ringers (in mM): NaCl 144; KCl, 4; CaCl2, 2, was obtained from Baxter (Poongabbie, Australia). All other chemicals were of analytical grade and obtained from commercial suppliers. Statistics were performed using GBstat (Dynamic Microsystems, Silver Spring, U.S.A.) and either one way analysis of variance with repeated measures and Dunnett's test, or two way analysis of variance and Scheffe's test as appropriate. The probability of a type I error of less than 5% (P<0.05) was taken to indicate statistical significance. Values are given as mean±s.e.mean.
Results
Basal values for MAP and HR in each experimental group are given in Table 1. There were no significant differences in basal MAP and HR between the drug groups or their respective controls. Cumulative injection of clonidine (0.1–10 nmol) produced a significant dose dependent fall in MAP and HR (Figure 1A,C) (P<0.05, one way ANOVA). Injection of clonidine vehicle into the RVLM had no effect on MAP and HR over the same time frame (Figure 1A,C).
Table 1.
Basal MAP and HR values
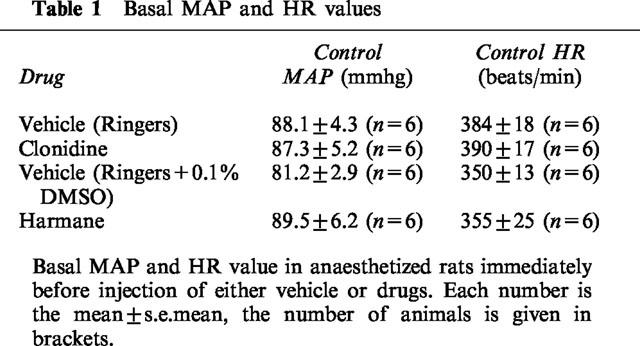
Figure 1.
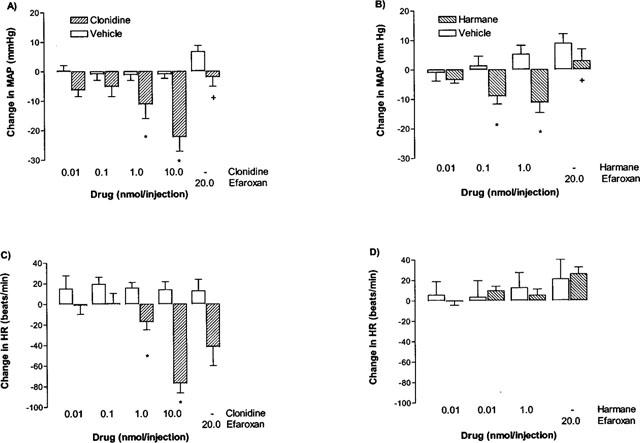
Effect of drugs or vehicle when injected into the RVLM. 100 nL of each drug or vehicle was injected cumulatively at 15 min intervals. (A) Effect of vehicle, followed by efaroxan or clonidine, followed by efaroxan on MAP. (B) Effect of vehicle, followed by efaroxan or harmane, followed by efaroxan on MAP. (C) Effect of vehicle, followed by efaroxan or clonidine, followed by efaroxan on HR. (D) Effect of vehicle, followed by efaroxan or harmane, followed by efaroxan on HR. Basal values are given in Table 1. *Represents a significant difference from the matched control (P<0.05, two way ANOVA). +Represents a significant interaction between efaroxan and drug (P<0.05, two way ANOVA).
Cumulative injection of harmane (0.01–1.0 nmol) also produced a significant dose dependent fall in MAP (Figure 1B, P<0.05, one way ANOVA), in contrast to the cumulative injection of harmane vehicle which produced a small, non-significant increase in MAP. Neither harmane nor its vehicle produced a significant change in HR (Figure 1D).
Efaroxan (20 nmol) alone had no significant effect on either MAP or HR when injected after the appropriate vehicle (Figure 1). Efaroxan injected after the last clonidine dose (10 nmol) produced a significant reversal of the hypotensive effect of clonidine on MAP by 15 min post-injection (P<0.05, interaction term, two way ANOVA), but did not significantly attenuate the fall in HR (Figure 1A,C). Similarly, efaroxan injected after the last harmane dose (1 nmol) significantly reversed the harmane-induced fall in MAP within 5–15 min (P<0.05, interaction term, two way ANOVA; Figure 1B).
Discussion
The most important finding of this study was that unilateral microinjection of harmane into the RVLM produced a significant dose dependent fall in MAP. The fall in MAP produced by 1 nmol harmane was qualitatively similar to that of 1 nmol clonidine, suggesting that they are of similar potency, and is in agreement with their reported affinity for I1-receptors (IC50 for harmane 30 nM, Hudson et al., 1999; Ki for clonidine 35 nM; Musgrave & Hughes, 1998). Higher doses of harmane could not be used due to its limited solubility. Harmane, a β-carboline that is found endogenously within the brain (Moncrieff, 1989; Rommelspacher et al., 1991) has been reported to have nearly a 1000 fold selectivity for I1-receptors over α2-adrenoceptors (IC50 for harmane 18 μM; Hudson pers comm). This suggests that harmane is producing hypotension via I1-receptors. Our finding that the relatively I1-receptor selective antagonist efaroxan (Haxhiu et al., 1994) significantly antagonized the hypotensive effect of harmane is consistent with an action at I1-receptors. Physiological antagonism cannot account for this effect as efaroxan by itself had no significant effect on MAP or HR in matched time controls.
Clonidine injected unilaterally into the RVLM produced a significant, dose dependent fall in MAP. This finding is consistent with previous reports of clonidine producing falls in MAP when injected into the RVLM. Similarly, the hypotensive effect of clonidine was blocked by efaroxan, consistent with previous reports (Ernsberger, 1990). While there are drugs such as rilmenidine and moxonidine which are somewhat more selective than clonidine for I1-receptors, clonidine is the best characterized in terms of its effects in the RVLM.
The effect of clonidine in the RVLM has been interpreted as being due to an action on imidazoline I1-receptors rather than α2-adrenoceptors (Regunathan & Reis 1996; Bousquet et al., 1998). Firstly, drugs with high affinity for α2-adrenoceptors but poor affinity at I1-receptors have little effect on blood pressure when injected into the RVLM, while those with high affinity for I1-receptors have substantial effects on blood pressure independent of their affinity at α2-adrenoceptors (Bousquet et al., 1984; Ernsberger et al., 1990). Secondly, the MAP depressor effect of clonidine is reversed by drugs such as efaroxan which is relatively more selective for I1-receptors over α2-adrenoceptors (Haxhiu et al., 1994), but not by the selective α2-adrenoceptor antagonists SKF 86466 or yohimbine (Ernsberger et al., 1990; 1997). This suggests that α2-adrenoceptors play little role in the hypotensive effects of drugs injected into the RVLM, re-enforcing the view that harmane is acting via I1-receptors.
In contrast to the results on MAP, the effect of clonidine on HR was not blocked by efaroxan. This, combined with the lack of effect of harmane on HR, suggests that I1-receptors are not involved in the HR response to clonidine. Our study would appear to be the first reporting the effect of antagonists on the HR effects of clonidine after microinjection into the RVLM.
In conclusion, this is the first demonstration that an endogenous compound with high affinity for I1-receptors has an effect on blood pressure when injected into the RVLM. The ability of the relatively selective I1-receptor antagonist efaroxan to reverse this effect suggests that it is due to an action at I1-receptors rather than some other site. These findings suggest that one or more β-carbolines may be endogenous ligands for I1-receptors and may represent a new structural class of compounds for the production of therapeutic substances.
Acknowledgments
I.F. Musgrave is an Australian National Health and Medical Research Council Senior Research Officer (Grant ID 981123). E. Badoer is supported by the Australian National Heart Foundation.
Abbreviations
- RVLM
rostral ventrolateral medulla
References
- BADOER E., MEROLLI J. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are activated by haemorrhage. Brain Res. 1998;791:317–320. doi: 10.1016/s0006-8993(98)00140-1. [DOI] [PubMed] [Google Scholar]
- BOUSQUET P., DONTENWILL M., GRENEY H., FELDMAN J. I1-imidazoline receptors: an update. J. Hypertens. 1998;16 Suppl:S1–5. [PubMed] [Google Scholar]
- BOUSQUET P., FELDMAN J., SCHWARTZ J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J. Pharmacol. Exp. Ther. 1984;230:232–236. [PubMed] [Google Scholar]
- EGLEN R.M., HUDSON A.L., KENDALL D.A., NUTT D.J., MORGAN N.G., WILSON V.G., DILLON M. Seeing through a glass darkly - casting light on imidazoline I sites. Trends Pharmacol. Sci. 1998;19:381–390. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- ERNSBERGER P., FRIEDMAN J.E., KOLETSKY R.J. The I1-imidazoline receptor: from binding site to therapeutic target in cardiovascular disease. J. Hypertens. 1997;15 Suppl:S9–23. doi: 10.1097/00004872-199715011-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNSBERGER P., GIULIANO R., WILLETTE R.N., REIS D.J. Role of imidazole receptors in the vasodepressor response to clonidine analogs in the rostral ventrolateral medulla. J. Pharmacol. Exp. Ther. 1990;253:408–418. [PubMed] [Google Scholar]
- GRIGG M., MUSGRAVE I.F., BARROW C.J. Isolation and partial structure determination of a clonidine-displacing substance from bovine lung and brain. J. Autonom. Nerv. Sys. 1998;72:86–93. doi: 10.1016/s0165-1838(98)00092-7. [DOI] [PubMed] [Google Scholar]
- HAXHIU M.A., DRESHAJ I., SCHAFER S.G., ERNSBERGER P. Selective antihypertensive action of moxonidine is mediated by I1-imidazoline receptors in the rostral ventrolateral medulla. J. Cardiovasc. Pharmacol. 1994;24 Suppl 1:S1–S8. doi: 10.1097/00005344-199424001-00002. [DOI] [PubMed] [Google Scholar]
- HEAD G.A., BURKE S.L., CHAN C.K. Central imidazoline receptors and centrally acting anti-hypertensive agents. Clin. Exp. Hypertens. 1997a;19:591–605. doi: 10.3109/10641969709083172. [DOI] [PubMed] [Google Scholar]
- HEAD G.A., CHAN C.K., GODWIN S.J. Central cardiovascular actions of agmatine, a putative clonidine-displacing substance, in conscious rabbits. Neurochem. Int. 1997b;30:37–45. doi: 10.1016/s0197-0186(96)00044-7. [DOI] [PubMed] [Google Scholar]
- HEAD G.A., BURKE S.L. Relative importance of medullary brain nuclei for the sympatho-inhibitory actions of rilmenidine in the anaesthetized rabbit. J. Hypertens. 1998;16:503–517. doi: 10.1097/00004872-199816040-00012. [DOI] [PubMed] [Google Scholar]
- HUDSON A.L., PRICE R., TYACKE R.J., LALIES M.D., PARKER C.A., NUTT D.J. Harmane, norharmane and tetrahydro β-carboline have high affinity for rat imidazoline binding sites. Br. J. Pharmacol. 1999;126:2P. [Google Scholar]
- LI G., REGUNATHAN S., BARROW C.J., ESHRAGHI J., COOPER R., REIS D.J. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- MONCRIEFF J. Determination of pharmacological levels of harmane, harmine and harmaline in mammalian brain tissue, cerebrospinal fluid and plasma by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. 1989;496:269–278. doi: 10.1016/s0378-4347(00)82576-1. [DOI] [PubMed] [Google Scholar]
- MUSGRAVE I.F., HUGHES R.A. Investigation of I1-imidazoline receptors using microphysiometry and molecular modelling. J. Auton. Nerv. Syst. 1998;72:137–146. doi: 10.1016/s0165-1838(98)00098-8. [DOI] [PubMed] [Google Scholar]
- REGUNATHAN S., REIS D.J. Imidazoline receptors and their endogenous ligands. Ann. Rev. Pharmacol. Toxicol. 1996;36:511–544. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- ROMMELSPACHER H., MAY T., SUSILO R. Beta-carbolines and tetrahydroisoquinolines: detection and function in mammals. Planta Med. 1991;57:S85–S92. [PubMed] [Google Scholar]


