Abstract
This study investigates the effect of partially metabolic controlled long-term (34 weeks) streptozotocin (STZ)-induced diabetes on relaxation and contractile responses of isolated coronary arteries to seven different vasoactive agents.
The average fasting and non-fasting blood glucose concentrations (mM) were significantly elevated in STZ-induced diabetic rats (P<0.0001; 10.4±0.4 and 16.6±1.1, n=15) compared to those (4.3±0.03 and 4.7±0.18, n=11) in age-matched controls. The level of glycated haemoglobin (HbA1) was also significantly (P<0.0001) increased in STZ-induced diabetic rats. In STZ-induced diabetic rats, the HbA1 levels were significantly correlated with the non-fasting blood glucose concentrations (r=0.76; P=0.003; n=13). In both groups, there was no significant correlation between the HbA1 levels and maximal responses or sensitivities to the vasoactive agents.
The maximal relaxation induced by rat-αcalcitonin gene-related peptide (rat-αCGRP) was significantly attenuated in the coronary arteries of STZ-induced diabetic rats (P<0.05; 40±7%, n=15) compared to that in age-matched controls (63±3%, n=11). However, there was no significant difference in the sensitivity to rat-αCGRP between the two groups.
There was no significant difference in either maximal response or sensitivity to any of the six other vasoactive agents between STZ- induced diabetic rats (n=15) and age-matched controls (n=11).
Our results show that partially metabolic controlled long-term (34 weeks) STZ-induced diabetes causes a selective depression of rat-αCGRP-induced relaxation in the intramural coronary arteries of Wistar rats.
Keywords: Calcitonin gene-related peptide receptor, CGRP, streptozotocin-induced diabetes, vasoactive agents, neurotransmitters, coronary artery
Introduction
Vascular dysfunction, e.g. alterations in the reactivity of blood vessels to neurotransmitters and hormones, are a well-established complication of diabetes mellitus. It is suggested that failure in the adaptive coronary flow response to cardiac hyperactivity in diabetic subjects may, in part, be responsible for the higher incidence of ischemic heart disease in the diabetic population (Durante et al., 1989). A number of studies using streptozotocin(STZ)-induced diabetic animals have shown that the severity and duration of diabetes and insulin treatment are important factors affecting both endothelium-dependent and -independent vascular responses to various vasoactive agents (Orie & Aloamaka, 1993; Taylor et al., 1994a; Savage et al., 1995; Pieper, 1997; Rodríguez-Mañas et al., 1998; Van-Buren et al., 1998; Kobayashi & Kamata, 1999).
Diabetes is normally induced at a relative young age in rats by giving 35–65 mg kg−1 STZ, and the effect of long-term STZ-induced diabetes generally covers an observation period of 8–20 weeks, with the exception of three studies (MacLeod & McNeill, 1985; Chang & Stevens, 1992; Van-Buren et al., 1998) with observation periods of 40 or 52 weeks. In these studies the rats were treated with a relative low (40 mg kg−1) or moderate dose (50 or 55 mg kg−1) of STZ.
The purpose of the present study is to investigate the effect of long-term diabetes (34 weeks), induced at an early age (10 weeks of age), on relaxation and contractile responses of rat intramural coronary arteries by treating female Wistar rats with 65 mg kg−1 STZ. Because of the severity and the long observation period we were forced to partially control the STZ-induced diabetes with slow releasing insulin implants due to animal welfare regulations in Denmark.
Methods
Experimental animals
Diabetes was induced in 10 week-old female Wistar rats (197–211 g) by injection of STZ (65 mg kg−1, Sigma-Aldrich, U.S.A.), dissolved in 0.1 M trisodium citrate buffer (pH=4.5), in the tail vein. Onset of diabetes was confirmed by the presence of glycosuria 48 h after injection. In order to check the diabetic state of the rats, the glucose concentrations in tail blood samples were measured with a glucometer (Reflolux®, Boehringer Mannheim, Mannheim, Germany). A group of age- and sex-matched control rats were injected with the vehicle and kept under identical housing conditions with free access to water and food ad libitum. After induction of diabetes, both groups of rats were kept under observation for 34 weeks before the experimentation. During this period body weight, blood glucose (fasting or non-fasting) and severity of diabetic-related symptoms were monitored once a week. The rats were deprived of food 10 h prior to fasting blood glucose measurements.
Insulin treatment
In order to reduce the rate of death among diabetic rats during the observation period, these animals were treated with insulin implants (Linplant®, LinShin Canada, Inc.). The insulin treatment was given approximately three times during the observation period, when blood glucose values exceeded 20 mM in 2 consecutive weeks. The implants were sterilized in 2% povidone-iodine solution and inserted by a 12 gauge hypodermic needle under the dorsal skin of the neck. This procedure was carried out under a short acting local anaesthetic (4 mg s.c. Xylocaine 20 mg ml−1, Astra Södertälje, Sweden). Every implant contained palmitic acid as excipient and gradually released the insulin by erosion, at a dose of approximately 1 unit per day.
Measurement of glycated haemoglobin (HbA1)
The haemoglobin variants in heparinized full blood samples were separated on a cation-exchange resin column, and the percentage of glycated haemoglobin (HbA1) was determined by a spectrophotometric assay (Glycated Hemoglobin Kit, Sigma Diagnostics, U.S.A.).
Measurement of force-development in coronary arteries
The animals were anaesthetized with ether and killed by exsanguination. Then, the heart was removed and kept in ice-cold (4°C) oxygenated physiological salt solution (PSS). Afterwards, distal intramural segments (1–2 mm long) of the left anterior descending coronary artery were dissected from the hearts of STZ-induced diabetic female Wistar rats and age-matched controls, as previously described (Nyborg & Mikkelsen, 1985). The arteries were mounted as rings on two 40 μm stainless steel wires connected to a force transducer and a micrometer, respectively, in the organ bath of a small vessel myograph (J/P Trading I/S, Aarhus, Denmark), which allowed direct determination of the isometric wall tension while the internal circumference of the vessels was controlled (Mulvany & Nyborg, 1980).
Assessment of coronary vascular function
After mounting, the arteries were equilibrated at 37°C for 30 min in oxygenated (95% O2 and 5% CO2) PSS with the following composition (in mM). NaCl 119, NaHCO3 25, KCl 4.7, KH2PO4 1.18, MgSO4·7H2O 1.17, CaCl2·2H2O 1.5, ethylene diamine tetraacetic acid (EDTA) 0.027 and glucose 5.5 with pH adjusted to 7.4. The vessels were then stretched to their optimal lumen diameter 11=0.9×1100, where 1100 is an estimate of the diameter the vessel would have under a passive transmural pressure of 100 mmHg (13.3 kPa (N m−2)), in order to obtain optimal condition for active tension development (Nyborg et al., 1987). Each experiment was initiated by contracting the vessels repeatedly with KPSS (similar composition to PSS except that NaCl was exchanged with KCl on an equimolar basis) until reproducible wall tensions were recorded. At the end of each experiment, the maximal contractile response of the vessels (ΔTmax) was determined by measuring the differences in vessel wall tension (newton per meter of vessel wall, N m−1), when the vessels were maximally contracted with KPSS to which 10 μM serotonin and 10 μM prostaglandin F2α (PGF2α) were added, and when maximally relaxed in calcium-free PSS to which ·10−4 M papaverine was added (Nyborg, 1991). Calcium-free PSS was similar in composition to PSS except that the CaCl2 was replaced with 0.01 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA). The intrinsic tone (spontaneous myogenic tone), determined as the difference in wall tension of the vessels when kept in PSS and calcium-free PSS, was similar in both groups of rats throughout the experiment. Vessels were accepted only if the maximal active pressure (calculated according to the Laplace relation: ΔPmax=2×ΔTmax/11) exceeded 13.3 kPa.
Experimental protocol
In these series of experiments, the effect of partially metabolic controlled long-term (34 week) STZ-induced diabetes on coronary arteries was investigated by constructing consecutive cumulative concentration-response curves in half log molar increments. We used seven different vasoactive agents on each arterial segment in the following order: rat-αcalcitonin gene-related peptide (rat-αCGRP) (10 pM–100 nM), acetylcholine (0.1 nM–100 μM), serotonin (0.1 nM–100 μM), noradrenaline (1 nM–100 μM), isoprenaline (1 nM–10 μM), phenylephrine (1 nM–100 μM) and sodium nitroprusside (10 pM–100 μM). Relaxation curves were constructed in coronary arteries precontracted with 10 μM PGF2α. The coronary arteries were activated once for 3 min with KPSS (125 mM K+), with a 15 min washout period between each concentration-response curve.
Drugs
Drugs used were rat-αCGRP, 5-hydroxytryptamine HCl, noradrenaline HCl, acetylcholine chloride, sodium nitroprusside, isoprenaline HCl, phenylephrine HCl (Sigma-Aldrich, St Louis, MO, U.S.A.), prostaglandin F2α (Dinoprost®, UpJohn, Belgium) and papaverine 30 mg ml−1 (Nycomed DAK A/S, Denmark). All compounds (except papaverine) were dissolved in distilled water. Stock solutions (100, 10 or 0.1 mM) were stored at −20°C and dilutions were made just before experimentation.
Data analysis and statistics
Relaxations are expressed as a percentage of the PGF2α-induced tensions (precontraction tension) and contractions are expressed as a percentage of ΔTmax. Sensitivity to agonists is expressed as pD2-value, where pD2=−log(EC50 [M]), and EC50 [M] is the molar concentration of agonist required to produce half-maximum relaxation or contraction. All concentration-response curves were analysed by iterative nonlinear regression analysis using GraphPAD Prism programme (GraphPAD Corp, SanDiego, CA, U.S.A.). Each regression line was fitted to a sigmoid equation: R/Rmax=A[M]n/(A[M]n+EC50[M]n), where Rmax is the maximum response developed to the agonist, A[M] is the concentration of agonist and n is a curve-fitting parameter, the Hill coefficient (Kenakin 1986). Results are given as mean±s.e.mean (n=number of rats). Differences between mean values were analysed using either a two-tailed Student's t-test (parametric) or non-parametric test for paired or unpaired observations where appropriate. The level of significance was for all tests set to P-values less than 0.05.
Results
Animals injected with STZ gained significantly less weight compared to age-matched controls (mean weight: diabetic 233.79±4.16 g, n=15 vs control 273.16±4.16 g, n=11, P<0.0001). However, we found no significant correlation between the body weight and maximal responses or sensitivities to the vasoactive agents in either group of rats. The average blood glucose concentrations (fasting and non-fasting) were significantly elevated in STZ-induced diabetic rats (P<0.0001; 10.4±0.4 and 16.6±1.1 mM, n=15) compared to those (4.3±0.03 and 4.7±0.18 mM, n=11) in age-matched controls. There were no significant correlations between the blood glucose concentrations and maximal responses or sensitivities to the vasoactive agents in either group of rats.
Effect of the level of HbA1 on coronary vascular response
The average estimated HbA1 level in blood samples was also significantly increased in STZ-induced diabetic rats (P<0.0001; 3.9±0.2%, n=13) compared to that (2.5±0.1%, n=11) in age-matched controls. In both controls and STZ-induced diabetic rats, there were no significant correlations between the HbA1 levels and maximal responses or sensitivities to the vasoactive agents. However, we found a significant correlation (r=0.76; P=0.003; n=13) between the HbA1 values and blood glucose concentrations (non-fasting) only in STZ-induced diabetic rats (Figure 1).
Figure 1.
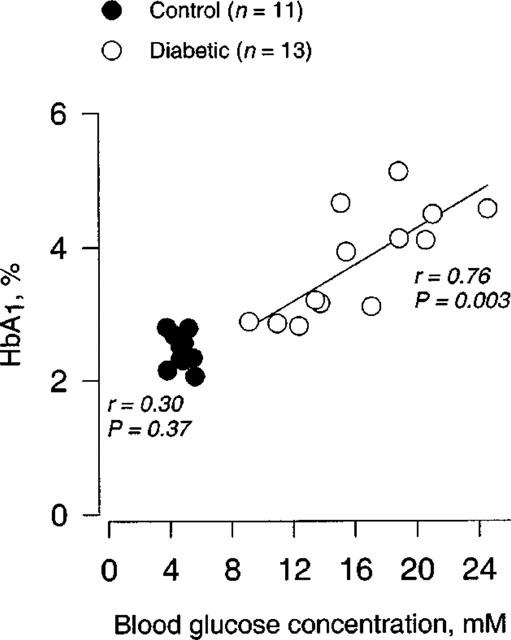
Relationship between the levels of HbA1 and blood glucose concentrations (non-fasting) in STZ-induced diabetic Wistar rats (n=13) and age-matched controls (n=11).
Effect of 34-week STZ-induced diabetes on relaxation responses of coronary arteries
Throughout the experiment, there were no significant differences either in the levels of precontraction tone (per cent of ΔTmax) induced by PGF2α or in the ΔTmax-values (N m−1) between STZ-induced diabetic rats and age-matched controls (Figure 2). The ΔTmax-values being 4.47±0.27 N·m−1 (n=15) vs 3.87±0.34 N·m−1 (n=11), in STZ-induced diabetic rats and age-matched controls, respectively. Mean lumen diameters (l1) of the coronary arteries were 219±9 μm (n=15) vs 218±10 μm (n=11), in STZ-induced diabetic rats and age-matched controls, respectively.
Figure 2.
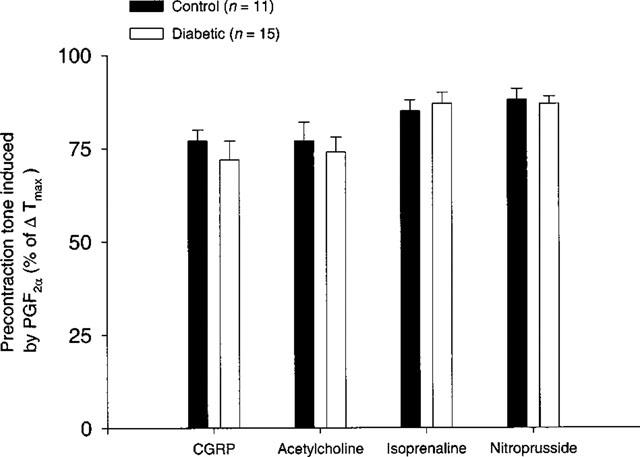
The level of precontraction tone induced by 10 μM PGF2α in concentration-response curves for rat-αCGRP, acetylcholine, isoprenaline and sodium nitroprusside during the experiment with coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Values are given as mean±s.e.mean. The level of PGF2α-induced precontraction tone is given as percentage fraction of ΔTmax.
rat-αCGRP-induced relaxations
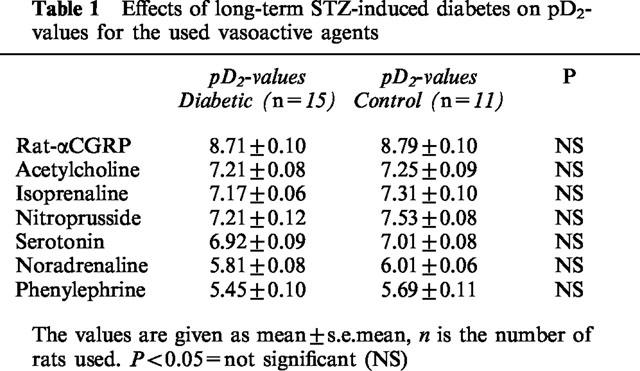
There was a significant (P<0.05) depression of rat-αCGRP (10 pM–100 nM) induced maximal relaxation in STZ-induced diabetic rats compared to age-matched controls (Figure 3). However, we found no significant difference in sensitivity to rat-αCGRP between these groups of rats (Table 1).
Figure 3.
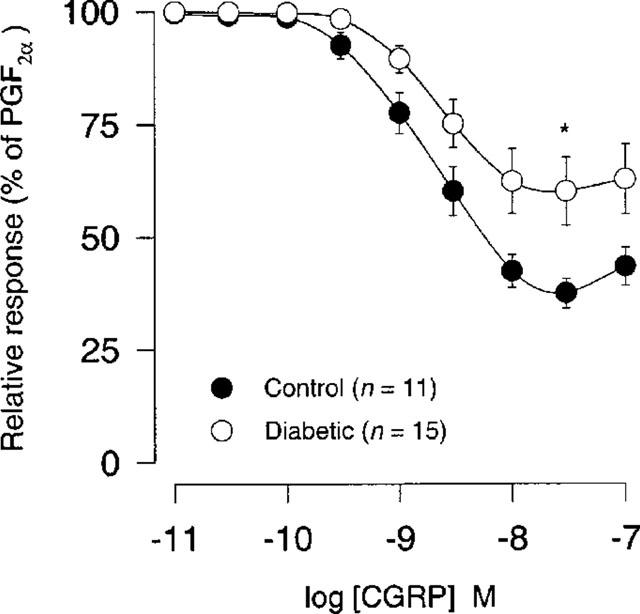
Rat-αCGRP concentration-response curves (10 pM–100 nM) after precontraction with 10 μM PGF2α in coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Points represent mean values and vertical bars indicate±s.e.mean where this value exceeds the size of symbol. Relative responses are given as percentage fraction of the initial vessel response to PGF2α (10 μM) just before they were challenged with rat-αCGRP. *Significantly different from control with P<0.05.
Table 1.
Effects of long-term STZ-induced diabetes on pD2-values for the used vasoactive agents
Acetylcholine-induced relaxations
There was no significant difference either in sensitivity or in maximal response (per cent of PGF2α) to acetylcholine (0.1 nM–100 μM) between STZ-induced diabetic rats and age-matched controls (Figure 4; Table 1).
Figure 4.
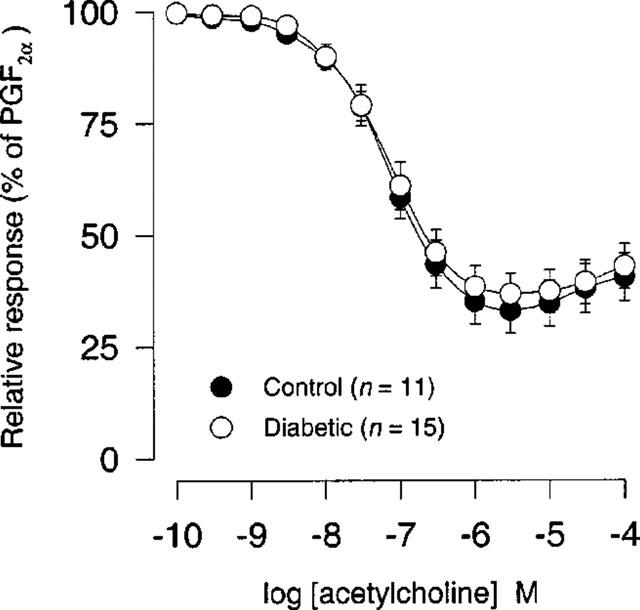
Acetycholine concentration-response curves (0.1 nM–100 μM) after precontraction with 10 μM PGF2α in coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Points represent mean values and vertical bars indicate±s.e.mean where this value exceeds the size of symbol. Relative responses are given as percentage fraction of the initial vessel response to PGF2α (10 μM) just before they were challenged with acetylcholine.
Isoprenaline-induced relaxations
We found no significant difference either in sensitivity or in maximal response (per cent of PGF2α) to isoprenaline (1 nM–10 μM) between STZ-induced diabetic rats and age-matched controls (Figure 5; Table 1).
Figure 5.
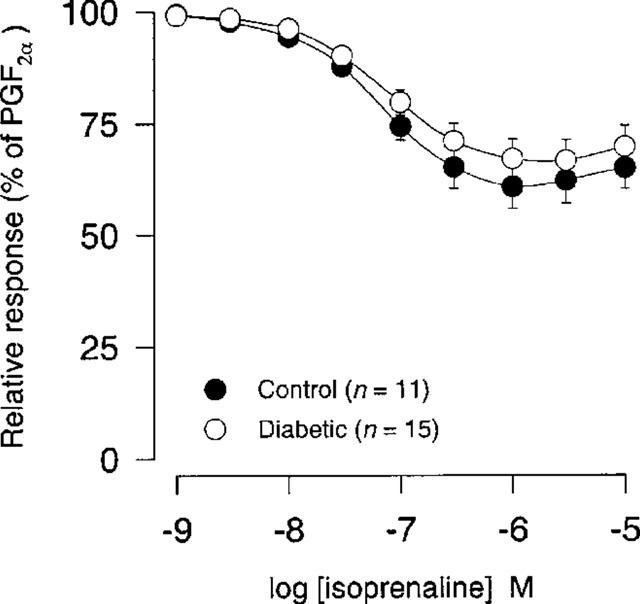
Isoprenaline concentration-response curves (1 nM–10 μM) after precontraction with 10 μM PGF2α in coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Points represent mean values and vertical bars indicate±s.e.mean where this value exceeds the size of symbol. Relative responses are given as percentage fraction of the initial vessel response to PGF2α (10 μM) just before they were challenged with isoprenaline.
Sodium nitroprusside-induced relaxations
There was no significant difference either in sensitivity or in maximal response (per cent of PGF2α) to sodium nitroprusside (10 pM–100 μM) between STZ-induced diabetic rats and age-matched controls (Figure 6; Table 1).
Figure 6.
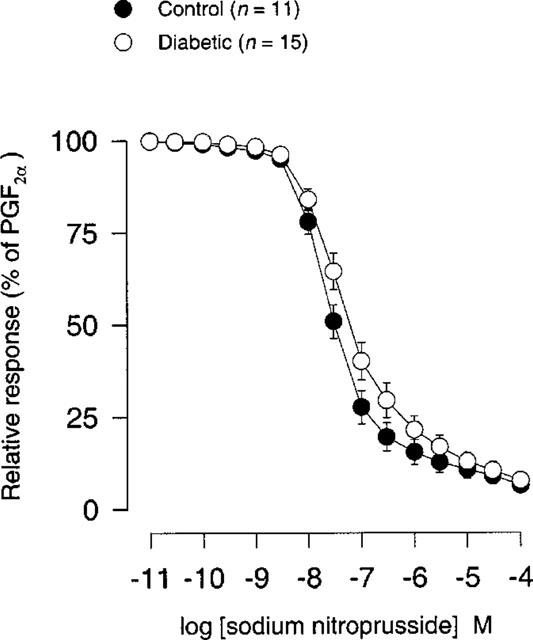
Sodium nitroprusside concentration-response curves (10 pM–100 μM) after precontraction with 10 μM PGF2α in coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Points represent mean values and vertical bars indicate±s.e.mean where this value exceeds the size of symbol. Relative responses are given as percentage fraction of the initial vessel response to PGF2α (10 μM) just before they were challenged with sodium nitroprusside.
Effect of 34-week STZ-induced diabetes on contractile responses of coronary arteries
Serotonin-induced contractions
There was no significant difference either in sensitivity or in maximal contraction (per cent of ΔTmax) to serotonin (0.1 nM–100 μM) between STZ-induced diabetic rats and age-matched controls (Figure 7; Table 1).
Figure 7.
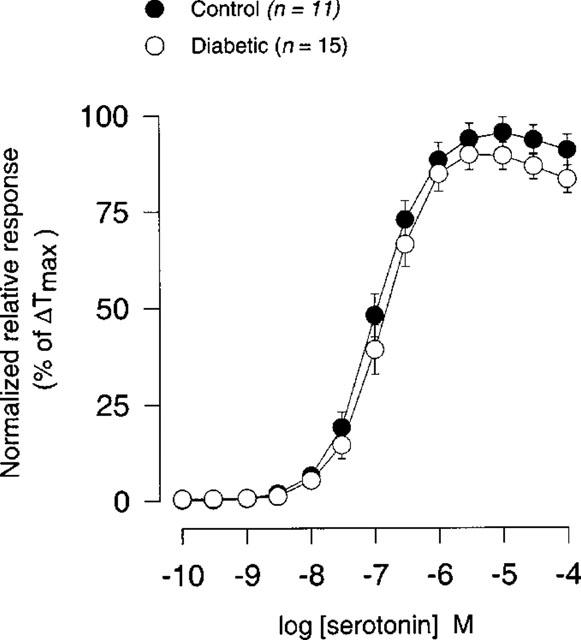
Concentration-response curves for serotonin (0.1 nM–100 μM) in coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Points represent mean values and vertical bars indicate±s.e.mean where this value exceeds the size of symbol. Normalized relative responses are given as percentage fraction of ΔTmax.
Noradrenaline-induced contractions
There was no significant difference either in sensitivity or in maximal contraction (per cent of ΔTmax) to noradrenaline (1 nM–100 μM) between STZ-induced diabetic rats and age-matched controls (Figure 8; Table 1).
Figure 8.
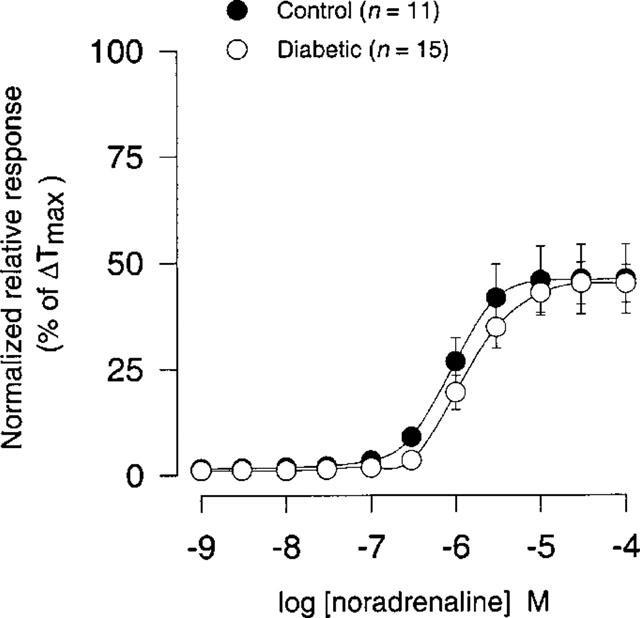
Concentration-response curves for noradrenaline (1 nM–100 μM) in coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Points represent mean values and vertical bars indicate±s.e.mean where this value exceeds the size of symbol. Normalized relative responses are given as percentage fraction of ΔTmax.
Phenylephrine-induced contractions
There was no significant difference either in sensitivity or in maximal contraction (per cent of ΔTmax) to phenylephrine (1 nM–100 μM) between STZ-induced diabetic rats and age-matched controls (Figure 9; Table 1).
Figure 9.
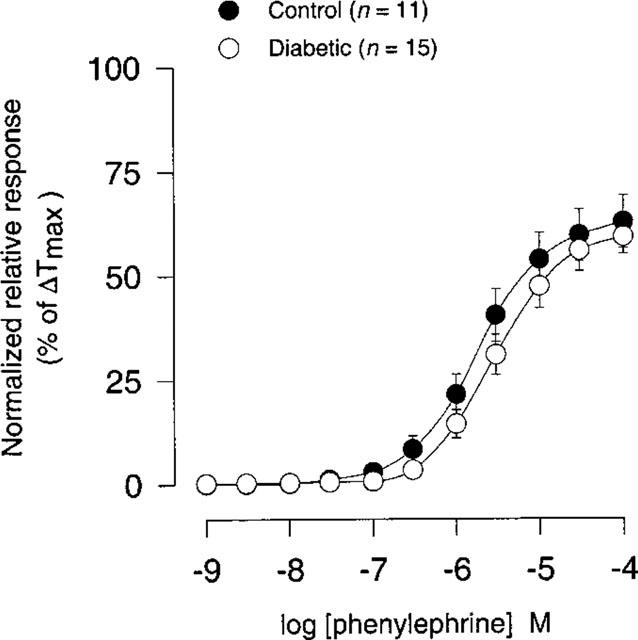
Concentration-response curves for phenylephrine (1 nM–100 μM) in coronary arteries from STZ-induced diabetic Wistar rats (n=15) and age-matched controls (n=11). Points represent mean values and vertical bars indicate±s.e.mean where this value exceeds the size of symbol. Normalized relative responses are given as percentage fraction of ΔTmax.
Discussion
Our study showed that the partially metabolic controlled long-term (34 weeks) STZ-induced diabetic state, caused a selective impairment of rat-αCGRP-induced relaxations in rat intramural coronary arteries. However, the sensitivity of the coronary arteries to rat-αCGRP was not altered by the diabetic state.
A recent study investigating the neurogenic cutaneous vasodilatation in STZ-induced diabetic rats, has shown that release of calcitonin gene-related peptide (CGRP) is diminished in diabetes and that treatment with either insulin or nerve growth factor normalized the microvascular responses to neurogenic released CGRP (Bennett et al., 1998). Another study has shown that STZ-induced diabetes causes a selective damage of CGRP-like immunoreactive enteric nerve fibres in rats (Belai & Burnstock, 1987). Rittenhouse et al. (1995) showed a significant reduction of CGRP-encoding mRNA in sensory neurons in the lumbar 4–6 dorsal root ganglia of STZ-induced diabetic rats.
Furthermore, our study showed that the endothelium-dependent acetylcholine-induced relaxations as well as endothelium-independent relaxations induced by sodium nitroprusside were preserved in the coronary arteries of STZ-induced diabetic rats, indicating a normal NO-cGMP pathway. A number of studies have also shown that the vascular responses to sodium nitroprusside are neither affected by severity of STZ-induced diabetic state (Durante et al., 1989; Kamata et al., 1989; Taylor et al., 1992; 1995; Endo et al., 1995; Kamata & Kondoh, 1996) nor by the duration of diabetes (Furman & Sneddon, 1993; Van-Buren et al., 1998). Our results concerning endothelium-dependent acetylcholine-induced relaxations are consistent with the studies where isolated aortic rings (Pieper, 1997) or mesenteric resistance arteries (Taylor et al., 1994a) were obtained from insulin-treated STZ-induced diabetic rats, which were completely normalized regarding the blood glucose level.
In the study carried out by Rodríguez-Mañas et al. (1998), it was shown that vasorelaxant responses to acetylcholine in both aortic segments and mesenteric microvessels after 8 weeks of STZ-induced diabetes were only normalized in diabetic rats with a good metabolic control (HbA1c: 5.5–7.4%; blood glucose ≈16 mM), thus indicating a limit for appearance of endothelial damage associated with the metabolic sequelae of diabetes. This is in good agreement with our results from STZ-induced diabetic rats, which received low-dose insulin treatment (release rate 1 U day−1) during the observation period, causing a relatively low glucose concentration and HbA1 level (HbA1 ≈4%; blood glucose ≈17 mM).
The general impression is that the endothelial dysfunction is observed in STZ-induced diabetic animals with a poor metabolic control having blood glucose concentrations higher than 20 mM. There are, however, some exceptions regarding the functional integrity of vascular endothelium in STZ-induced diabetic rats not treated with insulin.
Furman & Sneddon (1993) showed that the endothelium-dependent vasodilator responses to acetylcholine were preserved in rat mesenteric arteries after 15–17 weeks of STZ-induced diabetes. In this study, the diabetic animals had an average blood glucose level of 39 mM. Another study comparing isolated perfused mesenteric artery and aorta from STZ-induced diabetic rats (average blood glucose concentration ≈22 mM), demonstrated that the acetylcholine-induced relaxations after 2–10 weeks of STZ-induced diabetes were preserved in aorta but not in mesenteric arteries, leading to the conclusion that endothelial cell layers in resistance flow-regulating arteries were more sensitive to the diabetes related damages compared to conduit arteries (Taylor et al., 1994b).
In our study, α-adrenoceptor (phenylephrine) as well as β- adrenoceptor (isoprenaline) and the combined α- and β- adrenoceptor (noradrenaline) mediated responses in rat coronary arteries were not affected at all by the state of diabetes. A previous study using isolated arteries from mesenteric and hindlimb circulation showed no significant difference in constrictor response to phenylephrine between STZ-induced diabetic rats (average blood glucose concentration ≈36 mM) and controls after 3 weeks of STZ-induced diabetes (Taylor et al., 1995). Studies investigating the effect of noradrenaline on vessels from STZ-induced diabetic rats, have come up with conflicting results. Some studies have shown an increase in sensitivity to noradrenaline with no change in maximal response (Taylor et al., 1992; 1994b; Savage et al., 1995; Van-Buren et al., 1998), while others have shown unaltered sensitivity to noradrenaline with an elevated (Taylor et al., 1994a; Kamata et al., 1988) or unaltered maximal response (Furman & Sneddon, 1993; Taylor et al., 1994b; Sjogren & Edvinsson, 1988). Myers & Messina (1996) showed that vasoconstrictor responses to noradrenaline in rat cremaster third order arterioles were depressed after 4 weeks of STZ-induced diabetes, and to an even greater extent after 8 weeks of STZ-induced diabetes, indicating that the extent of the depression in noradrenaline responsiveness in STZ-induced diabetic rats was dependent on the duration of the hyperglycaemic state. Another study using isolated aortic ring segments from STZ-induced diabetic rats has shown that the maximal contractile responses to noradrenaline and serotonin were significantly increased after 1 and 4 weeks but not after 12 weeks of diabetes, indicating duration-dependent changes in vascular responses in the course of STZ-induced diabetes (Orie & Aloamaka, 1993). The explanation for these discrepant findings is not obvious, but variation in the duration and severity of diabetes, type of vessel used in experiments, experimental conditions and strain differences, could be contributory to the conflicting results.
In our experiments, we can not rule out the possibility that chronic low-dose insulin treatment might be involved in restoration of coronary artery response to vasoactive agents in the STZ-induced diabetic rats. Two studies have clearly demonstrated that insulin might be directly responsible for some of the changes in vascular reactivity in the STZ-model of diabetes. Savage et al. (1995) investigated the contractile responses of mesenteric arteries to noradrenaline with and without neuropeptide Y using four groups of rats, namely non-diabetic rats and rats with 4-week STZ-induced diabetes that were either untreated or treated with insulin or food restricted to restore near-normoglycaemia. The authors concluded that increased reactivity to noradrenaline in untreated diabetic and diet-restricted vessels was probably due to insulin lack (hypoinsulinaemia) and not to diabetes per se. A recent study performed by Kobayashi & Kamata (1999) on rats with 10-week STZ-induced diabetes, showed that high-dose insulin treatment (hyperinsulinaemia) enhanced noradrenaline-induced contractility in rat aortae.
In our study, the response to CGRP is not normalized under the partial metabolic control, indicating that the CGRP receptor system could be very sensitive to STZ-induced diabetes. It is well-known that CGRP can cause vasodilatation via a number of mechanisms. Previous studies have shown that STZ-induced diabetes can have a disrupting effect on some of these mechanisms such as endothelial function (Kamata et al., 1989; Taylor et al., 1992; 1994a,1994b; 1995) activity of smooth muscle ATP-sensitive potassium channels (KATP) (Bouchard et al., 1997; Zimmermann et al., 1997) and the levels of intracellular second messenger systems (Kamata et al., 1989). However, rat-αCGRP induces endothelium-independent relaxations in rat coronary resistance arteries (Prieto et al., 1991; Sheykhzade & Nyborg, 1998), and glibenclamide (a selective inhibitor of KATP) has no effect on relaxations induced by rat-αCGRP in these vessels (Prieto et al., 1991). Our results then indicate that impairment of the CGRP-induced response is related to the vascular smooth muscle CGRP receptors and their transduction pathway.
Comparing CGRP concentration-response relations in STZ-induced diabetic rats and controls, the attenuated CGRP response in STZ-induced diabetic rats resembles the response-pattern usually seen after irreversible receptor antagonism in a system with little or no receptor reserve, where the maximal response (Emax) is depressed without a parallel rightward shift in the log concentration-response curve.
STZ-induced diabetes could perhaps bring about glycosylation and/or conformational changes of the CGRP receptor. Genetic studies have indicated that potential sites for glycosylation in the CGRP receptor are located extracellularly in the first transmembrane segment (Njuki et al., 1993). Alterations in the molecular structure of CGRP receptors and a following inability (or reduced ability) of CGRP receptors to transduce the exogenous CGRP signal may explain the desensitization-pattern we have observed in our study. This assumption is further supported by the unchanged response to isoprenaline in the STZ-induced diabetic rats, indicating that the intracellular cyclic AMP second messenger system is unaffected in these rats.
In conclusion, our study clearly demonstrates a selective attenuation of rat-αCGRP-induced relaxations caused by long-term (34 weeks) STZ-induced diabetes, under partial metabolic control, in rat coronary arteries. The exact mechanism behind the attenuation of CGRP responses by STZ-induced diabetes remains to be elucidated in the future.
Acknowledgments
This work is supported by the Danish Heart Foundation, Grant No. 97-1-1-15-22503/98-2-2-19-22636 and Novo Nordisk Research Foundation. We wish to thank Bidda Rolin (M.S.) and Peter Oturai (M.D.) Novo Nordisk A/S for technical advice. We also wish to extend our gratitude to Dr Kyoung S.K. Chang and Prof Wendel C. Stevens, Oregon Health Sciences University, for sharing their experiences with the STZ-model of long-term diabetes with us.
Abbreviations
- Calcium-free PSS
substitution of CaCl2 with 0.01 mM ethylene glycolbis(β-aminoethyl ether)-N,N,N′, N′- tetraacetic acid in physiological salt solution
- CGRP
calcitonin gene-related peptide
- EDTA
ethylene diamine tetraacetic acid
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′, N′- tetraacetic acid
- HbA1
glycated haemoglobin
- KATP
ATP-sensitive potassium channel
- KPSS
equimolar substitution of NaCl with KCl in physiological salt solution
- l1
optimal lumen diameter of vessel
- l100
an estimate of vessel-diameter under a passive transmural pressure of 100 mmHg
- PGF2α
prostaglandin F2α
- PSS
physiological salt solution
- Rat-αCGRP
rat-αcalcitonin gene-related peptide
- STZ
streptozotocin
- ΔTmax
maximal contractile response of vessel
References
- BELAI A., BURNSTOCK G. Selective damage of intrinsic calcitonin gene-related peptide-like immunoreactive enteric nerve fibers in streptozotocin-induced diabetic rats. Gastroenterology. 1987;92:730–734. doi: 10.1016/0016-5085(87)90025-4. [DOI] [PubMed] [Google Scholar]
- BENNETT G.S., GARRETT N.E., DIEMEL L.T., BRAIN S.D., TOMLINSON D.R. Neurogenic cutaneous vasodilatation and plasma extravasation in diabetic rats: effect of insulin and nerve growth factor. Br. J. Pharmacol. 1998;124:1573–1579. doi: 10.1038/sj.bjp.0701986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHARD J.F., DUMONT E.C., LAMONTAGNE D. Decreased of vascular response to iloprost in diabetic rats. Arch. Mal. Coeur. Vaiss. 1997;90:1071–1074. [PubMed] [Google Scholar]
- CHANG K.S.K., STEVENS W.C. Endothelium-dependent increase in vascular sensitivity to phenylephrine in long-term streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1992;107:983–990. doi: 10.1111/j.1476-5381.1992.tb13395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURANTE W., SUNAHARA F.A., SEN A.K. Effect of diabetes on metabolic coronary dilatation in the rat. Cardiovasc. Res. 1989;23:40–45. doi: 10.1093/cvr/23.1.40. [DOI] [PubMed] [Google Scholar]
- ENDO K., ABIRU T., MACHIDA H., KASUYA Y., KAMATA K. Endothelium-derived hyperpolarizing factor does not contribute to the decrease in endothelium-dependent relaxation in the aorta of streptozotocin-induced diabetic rats. Gen. Pharmacol. 1995;26:149–153. doi: 10.1016/0306-3623(94)00159-k. [DOI] [PubMed] [Google Scholar]
- FURMAN B.L., SNEDDON P. Endothelium-dependent vasodilator responses of the isolated mesenteric bed are preserved in long-term streptozotocin diabetic rats. Eur. J. Pharmacol. 1993;232:29–34. doi: 10.1016/0014-2999(93)90724-v. [DOI] [PubMed] [Google Scholar]
- KAMATA K., KONDOH H. Impairment of endothelium-dependent relaxation of the isolated basilar artery from streptozotocin-induced diabetic rats. Res. Commun. Mol. Pathol. Pharmacol. 1996;94:239–249. [PubMed] [Google Scholar]
- KAMATA K., MIYATA N., KASUYA Y. Mechanisms of increased responses of the aorta to α-adrenoceptor agonists in streptozotocin-induced diabetic rats. J. Pharmacobio-Dyn. 1988;11:707–713. doi: 10.1248/bpb1978.11.707. [DOI] [PubMed] [Google Scholar]
- KAMATA K., MIYATA N., KASUYA Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1989;97:614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P.Analysis of dose-response data Pharmacological Analysis of Drug-Receptor Interaction 1986New York: Raven Press. pp; 31–51.In: Kenakin T.P. (ed.) [Google Scholar]
- KOBAYASHI T., KAMATA K. Effect of insulin treatment on smooth muscle contractility and endothelium-dependent relaxation in rat aortae from established STZ-induced diabetic rats. Br. J. Pharmacol. 1999;127:835–842. doi: 10.1038/sj.bjp.0702554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD K.M., MCNEILL W.C. The influence of chronic experimental diabetes on contractile responses of rat isolated blood vessels. Can. J. Physiol. Pharmacol. 1985;63:52–57. doi: 10.1139/y85-009. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., NYBORG N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br. J. Pharmacol. 1980;71:585–596. doi: 10.1111/j.1476-5381.1980.tb10977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS T.O., MESSINA E.J. Depressed arteriolar responsiveness to norepinephrine in streptozotocin-induced diabetes in the rat. Prostaglandins. 1996;52:415–430. doi: 10.1016/s0090-6980(96)00100-1. [DOI] [PubMed] [Google Scholar]
- NJUKI F., NICHOLL C.G., HOWARD A., MAK J.C., BARNES P.J., GIRGIS S.I. A new calcitonin-receptor-like sequence in rat pulmonary blood vessels. Clin. Sci. 1993;85:385–388. doi: 10.1042/cs0850385. [DOI] [PubMed] [Google Scholar]
- NYBORG N.C.B. Ageing is associated with increased 5-HT2-receptor affinity and decreased receptor reserve in rat isolated coronary arteries. Br. J. Pharmacol. 1991;102:282–286. doi: 10.1111/j.1476-5381.1991.tb12167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYBORG N.C.B., BAANDRUP U., MIKKELSEN E.O., MULVANY M.J. Active, passive and myogenic characteristics of isolated rat intramural coronary resistance arteries. Pflügers Arch./Eur. J. Physiol. 1987;410:664–670. doi: 10.1007/BF00581329. [DOI] [PubMed] [Google Scholar]
- NYBORG N.C.B., MIKKELSEN E.O. Characterization of β-adrenoceptors subtype in isolated ring preparations of intramural rat coronary small arteries. J. Cardiovasc. Pharmacol. 1985;7:1113–1117. doi: 10.1097/00005344-198511000-00016. [DOI] [PubMed] [Google Scholar]
- ORIE N.N., ALOAMAKA C.P. Duration-dependent variability in the response of diabetic rat aorta to noradrenaline and 5-hydroxytryptamine. Gen. Pharmacol. 1993;24:243–246. doi: 10.1016/0306-3623(93)90042-v. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M. Divergent actions of chronic insulin treatment in vivo versus acute treatment ex vivo on diabetic-induced endothelial dysfunction. Life Sci. 1997;60:PL371–PL376. doi: 10.1016/s0024-3205(97)00292-0. [DOI] [PubMed] [Google Scholar]
- PRIETO D., BENEDITO S., NYBORG N.C.B. Heterogeneous involvement of endothelium in calcitonin gene-related peptide-induced relaxation in coronary arteries from rat. Br. J. Pharmacol. 1991;103:1764–1768. doi: 10.1111/j.1476-5381.1991.tb09860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITTENHOUSE P.A., MARCHAND J.E., CHEN J., KREAM R.M., LEEMAN S.E. Streptozotocin-induced diabetes is associated with altered expression of peptide-encoding mRNA in rat sensory neurons. Peptides. 1995;17:1017–1022. doi: 10.1016/0196-9781(96)00129-5. [DOI] [PubMed] [Google Scholar]
- RODRÍGUEZ-MAÑAS L., ANGULO J., PEIRÓ C., LIERGO J.L., SÁNCHEZ-FERRER A., LÓPEZ-DÓRIGA P., SÁNCHEZ-FERRER C.F. Endothelial dysfunction and metabolic control in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1998;123:1495–1502. doi: 10.1038/sj.bjp.0701749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVAGE M.W., BODMER C.V., WALKER A.B., BUCHAN I.E., MASSON E.A., WILLIAMS G. Vascular reactivity to noradrenaline and neuropeptide Y in the streptozotocin-induced diabetic rats. Eur. J. Clin. Invest. 1995;25:974–979. doi: 10.1111/j.1365-2362.1995.tb01976.x. [DOI] [PubMed] [Google Scholar]
- SHEYKHZADE M., NYBORG N.C.B. Characterization of calcitonin gene-related peptide (CGRP) receptors in intramural rat coronary arteries from male and female Sprague Dawley rats. Br. J. Pharmacol. 1998;123:1464–1470. doi: 10.1038/sj.bjp.0701760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOGREN A., EDVINSSON L. Vasomotor changes in isolated coronary arteries from diabetic rats. Acta. Physiol. Scand. 1988;134:429–436. doi: 10.1111/j.1748-1716.1988.tb08511.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR P.D., GRAVES J.E., POSTON L. Selective impairment of acetylcholine-mediated endothelium-dependent relaxation in isolated resistance arteries of the streptozotocin-induced diabetic rat. Clin. Sci. Colch. 1995;88:519–524. doi: 10.1042/cs0880519. [DOI] [PubMed] [Google Scholar]
- TAYLOR P.D., MCCARTHY A.L., TOMAS C.R., POSTON L. Endothelium-dependent relaxation and noradrenaline sensitivity in mesenteric resistance arteries of the streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1992;107:393–399. doi: 10.1111/j.1476-5381.1992.tb12757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR P.D., OON B.B., THOMAS C.R., POSTON L. Prevention by insulin treatment of endothelial dysfunction but not enhanced noradrenaline-induced contractility in mesenteric resistance arteries from streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1994a;111:35–41. doi: 10.1111/j.1476-5381.1994.tb14020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR P.D., WICKENDEN A.D., MIRRLEES D.J., POSTON L. Endothelial function in isolated perfused mesentery and aortae of rats with streptozotocin-induced diabetes: effect of treatment with the aldose reductase inhibitor, ponalrestat. Br. J. Pharmacol. 1994b;111:42–48. doi: 10.1111/j.1476-5381.1994.tb14021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN-BUREN T., VLEEMING W., KRUTZEN M.M., VAN DE KUIL T., GISPEN W.H., DE WILDT D.J. vascular responses of isolated mesenteric resistance and basilar arteries from short- and long-term diabetic rats. Naunyn. Schmiedebergs Arch. Pharmacol. 1998;358:663–670. doi: 10.1007/pl00005309. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN P.A., KNOT H.J., STEVENSON A.S., NELSON M.T. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ. Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]


