Abstract
This study examines the relationship between β3a- and β3b-adrenoceptor (AR) mRNA levels, β3-AR binding and changes in ileum responses in mice treated with the β3-AR agonist (R,R)-5-[2[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-dicarboxylate (CL316243), or the β3-AR antagonist 3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate (SR59230A), or dexamethasone or forskolin.
Levels of β3a- and β3b-AR mRNA and the maximum number of binding sites (Bmax) in ileum were unaffected following CL316243 treatment, although responses to CL316243 were reduced by 50% following 4 and 24 h treatment, indicating another desensitization mechanism not involving changes in receptor expression or number. β3a-AR mRNA levels were reduced in both brown (BAT) and white adipose tissue (WAT) but β3b-AR mRNA levels were significantly reduced only in WAT. Levels of β3a- and β3b-mRNA returned towards normal with continued treatment.
SR59230A treatment markedly increased β3-AR mRNA levels in ileum and BAT but not in WAT. The increase in β3-AR mRNA levels in ileum was associated with increased Bmax levels in binding analysis and increased responses to CL316243, suggesting these as the cause of sensitization.
Treatment with forskolin (4 h) or dexamethasone (4 h) significantly reduced β3a-AR mRNA levels in BAT and WAT but did not alter levels in ileum. Responses to CL316243 in ileum were unaffected by either treatment.
In summary, the β3-AR is differently regulated in adipose tissue and ileum: Treatment with SR59230A increased β3-AR number, mRNA and responsiveness in ileum, whereas treatment with CL316243 reduced responses without affecting β3-AR number or mRNA levels.
Keywords: β3-Adrenoceptors, ileum, adipose tissue, CL316243, SR59230A, regulation, mouse
Introduction
β3-ARs have been identified by functional, molecular and receptor binding techniques in white adipose tissue (WAT) where they mediate lipolysis (Harms et al., 1977; Emorine et al., 1989; Muzzin et al., 1991) and brown adipose tissue (BAT) where they mediate thermogenesis and increased oxygen consumption (Arch et al., 1984; Muzzin et al., 1991; Sillence et al., 1993). There is also evidence for their presence in gastrointestinal tissues as sympathetic nerve stimulation inhibits gastrointestinal motility and β3-ARs inhibit smooth muscle contraction in several gastrointestinal tissues from various species including guinea-pig, rat, rabbit and man (for review, see Manara et al., 1995). Molecular studies have demonstrated a similar widespread distribution in gastrointestinal tissues of mRNA coding for the β3-AR (Granneman et al., 1991; 1993; Evans et al., 1996; 1998; Bensaid et al., 1993; Krief et al., 1993; Summers et al., 1996; Roberts et al., 1997), while binding studies with (−)-[125I]-cyanopindolol (CYP) have demonstrated a site with the characteristic properties of the β3-AR (Roberts et al., 1995; 1997; Summers et al., 1996).
The β3-AR gene contains introns in all species including rat, mouse and human (Granneman et al., 1992) in contrast to the β1- and β2-AR genes which are intronless. In the mouse this leads to an additional level of complexity due to a recently discovered splice variant of the mouse β3-AR (β3b-AR) which has a C-terminus with 17 amino acids which differ from the 13 in the known receptor (β3a-AR) (Summers et al., 1998; Evans et al., 1999). Differences include an additional cysteine and two consecutive serine residues which could alter signalling or regulatory properties of the β3b-AR. β3b-AR mRNA is differentially expressed in mouse tissues, with the highest proportions in the hypothalamus and cortex >WAT= ileum>BAT (Summers et al., 1998; Evans et al., 1999).
Heterologous regulation of the β3-AR is complex and controlled at the level of the gene. In BAT cell cultures noradrenaline, adrenaline and BRL37344 all rapidly but transiently reduce β3-AR mRNA levels, and the effects are mimicked by forskolin (Bengtsson et al., 1996). In rats, cold exposure, noradrenaline, isoprenaline, glucagon and BRL37344 all reduce β3-AR mRNA levels in WAT and BAT (Granneman & Lahners, 1992). In cultured adipocytes this effect is mimicked by 8-bromo-cyclic AMP (Granneman & Lahners, 1994). In murine 3T3-F442A adipocytes, insulin, dexamethasone and BRL37344 all decrease β3-AR mRNA levels (Feve et al., 1992; 1994). Recent studies indicate that the β3-AR is regulated differently in gut and adipose tissue. Genetically obese C57BL/6J (ob/ob) mice express low levels of β3-AR mRNA in WAT and BAT unlike their lean counterparts, and show decreased catecholamine-stimulated cyclic AMP production in WAT (Collins et al., 1994). However, in ileum and colon, β3-AR mRNA and β3-AR mediated responses are indistinguishable in obese and lean mice (Evans et al., 1998). Tachyphylaxis has been reported in several gastrointestinal tissues including rat distal colon, gastric fundus and guinea-pig gastric fundus (Kelly & Houston, 1996; McLaughlin & MacDonald, 1990; 1991; Coleman et al., 1987) where exposure to a high concentration of several BRL compounds between consecutive concentration/response curves to isoprenaline caused tachyphylaxis.
This study uses reverse transcription-polymerase chain reaction (RT–PCR), functional and receptor binding techniques to determine if procedures known to affect β3-AR mRNA and β3-AR mediated responses in adipose tissue are capable of affecting β3-AR mRNA or receptor levels and β3-AR mediated responses in ileum. The study demonstrates that in mouse ileum treatment with the β3-AR antagonist SR59230A increased β3-AR mRNA, receptor number and responsiveness, whereas treatment with the β3-AR agonist CL316243 reduced responses without affecting β3-AR number or mRNA levels.
Methods
Treatment of animals
Eight-week-old male Swiss mice were injected i.p. with drugs or vehicle (0.9% NaCl), except in the forskolin experiments where the vehicle was 30% dimethylsulfoxide (DMSO) / 70% saline. CL316243 or SR59230A (1 mg kg−1) were injected i.p. and the mice killed 1, 4 or 24 h later. For the 24 h treatments, injections were made at both 0 and 12 h and the animals killed at 24 h. Forskolin (3 mg kg−1), dexamethasone (1 mg kg−1) or vehicle was injected i.p. at 0 h and mice killed at 4 h. Experiments were also conducted with forskolin administered at both 0 and 1 h and mice killed at 2 h.
Organ bath studies
Mice were anaesthetized with 80% CO2/20% O2 and decapitated. A 10–15 cm segment of the small intestine finishing 2–3 cm above the ileocaecal junction was removed and its contents flushed out with cold Krebs-Henseleit solution (composition mM: NaCl 118.4, KCl 4.7, MgSO4·7H2O 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11, CaCl2 2.5) containing EDTA (0.04 mM) and ascorbic acid (0.1 mM) pH 7.4. Longitudinal segments (approximately 2 cm) were mounted on tissue hooks and suspended in jacketed organ baths containing Krebs-Henseleit solution maintained at 37°C under 5 mN resting tension and the buffer bubbled with 95% O2/5% CO2. Recordings were made using a Ugo Basile isotonic transducer (model 7006) and a MacLab system connected to a Macintosh IIci computer. Tissues were contracted twice with carbachol (1 μM) and then washed every 5 min for 1 h. Following the washing steps, tissues were contracted with carbachol (1 μM) (approximately 80% maximum; data not shown) for an approximate 10–15 min equilibration period before cumulative concentration-response (c-r) curves to CL316243 (0.3 nM–10 μM) were constructed with increments of 0.5 log units until a stable state was observed. At the end of each c-r curve, tissues were maximally relaxed with papaverine (10 μM) and responses are expressed as a percentage of the papaverine response. Four tissues were used simultaneously, with one c-r curve performed in each tissue. Non-linear regression was used to fit sigmoid c-r curves to the data (GraphPad Prism version 2.0) and to determine pEC50 values. Values are expressed as mean±s.e.mean of n observations. All n values refer to the number of tissues used with 2–4 tissues obtained from each animal. 2-way ANOVA tests were used to determine significance of variations between curves. Probability values less than or equal to 0.05 were considered significant.
Tissue collection and RNA extractions
All tissues were obtained from mice anaesthetized and killed as above and tissue prepared as previously described (Roberts et al., 1999). Frozen tissue was crushed in 10 ml polypropylene Falcon tubes using a stainless steel pestle and mortar pre-cooled in liquid N2. Total RNA was extracted by homogenizing crushed tissue samples in Trizol reagent (Gibco BRL) using a PRO200 homogenizer. The homogenizer was dismantled and washed between each sample to avoid cross-contamination. The yield and quality of RNA was assessed by measuring absorbance at 260 and 280 nm and by electrophoresis on 1.3% agarose gels.
Reverse transcription/Polymerase chain reaction (RT/PCR)
cDNAs were synthesized by reverse transcription of 1 μg of each total RNA using oligo (dT)15 as a primer as previously described (Roberts et al., 1999). PCR amplification was carried out on cDNA equivalent to 100 ng of starting RNA using oligonucleotide primers specific for the mouse β3-AR (forward, 5′-TCTAGTTCCCAGCGGAGTTTTCATCG-3′ and reverse, 5′-CGCGCACCTTCATAGCCATCAAACC-3′) or the internal standard β-actin (forward, 5′-ATCCTGCGTCTGGACCTGGCTG-3′ and reverse 5′-CCTGCTTGCTGATCCACATCTGCTG-3′) as previously described (Roberts et al., 1999). The actin reverse primer was labelled prior to PCR with [γ-33P]-ATP (Pharmacia) (Evans et al., 1998). The β3-AR and actin primers are intron-spanning to reveal any contaminating genomic DNA (Evans et al., 1999). For each set of primers, the log (PCR product) versus cycle number was plotted and a cycle number chosen within the linear portion of the graph (Evans et al., 1998). Cycle numbers were 16 for actin, 24 for β3-AR (WAT and BAT samples) and 30 for β3-AR (ileum samples). Following amplification, PCR products were electrophoresed on 1.3% agarose gels and transferred onto Hybond N+ membranes by Southern blotting in 0.4 M NaOH/1M NaCl. For detection of β3-AR products, membranes were hybridized with a probe specific for the β3-AR (5′-TGCCAACTCCGCCTTCAACCCGGTC-3′, end labelled with 50 μCi [γ-33P]-ATP and T4 polynucleotide kinase (Pharmacia)) as previously described (Roberts et al., 1999). Radioactivity was detected with a Molecular Dynamics SI phosphorimager, and bands quantitated using ImageQuantNT Software (Molecular Dynamics).
Analysis of molecular biology experiments
β3-AR levels were normalized for actin levels (β3-AR/actin) and expressed as a percentage of the mean±s.e.mean value of n animals for control tissues from vehicle treated animals. Treated and control samples were compared only when they had been processed within a single PCR experiment and run on the same gel, ensuring valid comparisons between treated and controls in a given tissue. Student's unpaired t-test (two tailed) was used to determine significance of differences. Probability values less than or equal to 0.05 were considered significant.
Receptor binding assay
Following the specified treatments, mice were killed as described above and ileum removed, rinsed in saline and the mucosa gently removed by scraping with a sterile microscope slide. The tissue was weighed and homogenized in 20–25 ml of ice cold buffer (10 mM Tris-HCl, pH 7.4 4°C, containing 10 μM phenylmethylsulphonylfluoride, 250 mM sucrose) using a PRO200 homogenizer. The homogenate was centrifuged at 39×g for 10 min at 4°C, the supernatant kept and centrifuged again at 39,000×g for 15 min at 4°C. The pellet was resuspended in binding buffer (50 mM Tris-HCl pH 7.4 room temperature, 5 mM MgCl2, 1 mM EDTA, 10 mg ml−1 bacitracin, 10 mg ml−1 leupeptin, 10 mg ml−1 pepstatin A, 0.5 mg ml−1 aprotinin) and stored on ice until use. Protein was determined by the method of Lowry et al. (1951) with BSA used as a standard.
Saturation experiments were performed at room temperature in a total volume of 100 μl in 96-well microtiter plates. Homogenate (approximately 20–40 μg protein) was incubated with (−)-[125I]-cyanopindolol (CYP) (100–2000 pM) for 60 min in the absence or presence of (−)-alprenolol (1 mM) to define non-specific binding. The reaction was terminated by rapid filtration through GF/C filters pre-soaked in 0.5% polyethyleneimine using a Packard Cell Harvester. Filters were washed four times with ice cold buffer (50 mM Tris-HCl pH 7.4 4°C), dried, 30 μl Microscint-O (Packard) added and radioactivity measured using the Packard Top-Count. Experiments were performed in duplicate with tissues from n animals. Results are expressed as mean±s.e.mean of n. Data was analysed using non-linear curve fitting (GraphPad PRISM version 2.0) using a one site fit to obtain KD and maximum binding sites (Bmax) values. 2-way ANOVA tests were used to determine significance of variations between curves. Probability values less than or equal to 0.05 were considered significant.
Drugs and reagents
CL316243 (Dr T. Nash) and SR59230A (Dr L. Manara) were gifts from Wyeth-Ayerst and Sanofi-Midi respectively. The drugs and reagents used were as follows: [γ-33P]-ATP (2000 Ci mmol−1, Bresatec, Adelaide, SA, Australia); (−)-[125I]-CYP (2200 Ci mmol−1, NEN Life Science Products, Boston, MA, U.S.A.); forskolin, (−) alprenolol, aprotinin, bacitracin, dexamethasone-21-phosphate, DMSO, carbachol (carbamylcholine chloride), polyethyleneimine, phenylmethylsulphonylfluoride, papaverine hydrochloride (Sigma Chemical Company, St. Louis, MO, U.S.A.); leupeptin, pepstatin A, TRIZOL reagent, oligo(dT)15 Taq polymerase, 10×PCR buffer, salmon sperm DNA, oligonucleotide primers and probe (Gibco BRL, Life Technologies, Gaithersburg, MD, U.S.A.); RNasin, 10×RT buffer, AMV reverse transcriptase (Promega, Madison, WI, U.S.A.); dNTPs, One-Phor-All Plus buffer, T4 polynucleotide kinase (Amersham Pharmacia Biotech, Uppsala, Sweden); L(+)-ascorbic acid (Merck, Frankfurt, Germany); EDTA (AJAX Chemicals, Melbourne, Australia). All other chemicals were of analytical grade.
Stock solutions of SR59230A were prepared in 50% distilled water, 25% ethanol and 25% DMSO, forskolin and pepstatin A in DMSO, dexamethasone in saline, and the remaining drugs in distilled water.
Results
Evaluation of the experimental protocol
In order to examine the relaxant effects of CL316243, ileum was precontracted with carbachol (1 μM) using a concentration determined from a c-r curve to give a response ∼80% maximum that was sustained throughout the experiment (data not shown).
In experiments where animals were pretreated with CL316243, it was important to show that any remaining CL316243 could be effectively removed from the tissue before the organ bath experiments commenced. In order to assess whether CL316243 could be effectively removed, ileum was contracted with carbachol (1 μM) for 15–20 min followed by relaxation to CL316243 (100 nM) or isoprenaline (1 μM). After 1 h of washing every 5 min, subsequent contractions to carbachol were not significantly different (P=0.69 and 0.76 respectively) (data not shown). Tissues were then re-exposed to CL316243 or isoprenaline. Relaxations to the first and second exposure of CL316243 or isoprenaline were not significantly different (P=0.97 and 0.22 respectively). These experiments demonstrated that the protocol effectively removed any remaining CL316243 from the tissue. Ileum was therefore washed for 1 h every 5 min before use.
Evaluation of the RT/PCR method
In molecular studies, PCR was carried out using a forward primer corresponding to a section of the coding region located in exon 1 of the β3-AR, and a reverse primer coding to a section of exon 2 immediately 3′ to intron 1. In the β3a-AR, PCR fragments derived from mRNA would be 234 bp while fragments derived from contaminating genomic DNA would have been 697 bp due to inclusion of the first intron (Van Spronsen et al., 1993). The major PCR bands observed from WAT, BAT and ileum in this study are consistent with a predicted size of 234 bp from mRNA. The 2nd band of 330 bp observed in all three tissues is the product from the β3b-AR mRNA produced by alternate splicing at a novel acceptor site 100 bp upstream from the previously characterized start of exon 2 (Summers et al., 1998; Evans et al., 1999). For each set of primers, the log (PCR product) versus cycle number was plotted and a cycle number chosen within the linear portion of the graph (Evans et al., 1998).
Effect of β3-AR agonist treatment on receptor binding levels and relaxation responses in mouse ileum and levels of β3-AR mRNA in WAT, BAT and ileum
In order to confirm the effect of β3-AR agonists in adipose tissues and examine the effects in gut tissue, CL316243 (1 mg kg−1 i.p.) was administered for 1 h (Figure 1), 4 h (Figure 2), or 24 h (Figure 3). β3a- and β3b-AR mRNA levels in WAT were markedly reduced following 4 h (87 and 90% respectively) and 24 h (67 and 60% respectively) CL316243 treatment, but not following 1 h treatment. β3a-AR mRNA levels in BAT were halved following 1 h and reduced by more than 70% following 4 h treatment with CL316243, although levels were returning back to control levels after 24 h treatment. There was no significant change in β3b- AR mRNA levels in BAT at any time point examined, although levels were reduced by 40% following 4 h treatment. β3a- and β3b-AR mRNA levels in ileum were unchanged at all time points examined.
Figure 1.
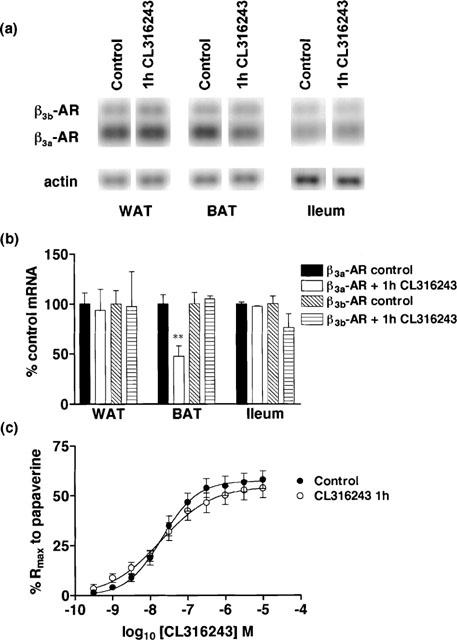
Effect of in vivo CL316243 (1 h) treatment (1 mg kg−1) on β3a-AR and β3b-AR mRNA levels in WAT, BAT and ileum and on responses to CL316243 in ileum. (a,b) β3a-AR mRNA levels in WAT and ileum were not altered but levels in BAT (**P<0.01) were significantly reduced following 1 h CL316243 treatment. β3b-AR mRNA levels were unchanged in WAT, BAT and ileum. Bars show mean±s.e.mean (n=6). (c) β3-AR mediated responses in ileum were unaffected following 1 h CL316243 treatment (2-way ANOVA ns). Points show mean and vertical lines indicate s.e.mean (n=8).
Figure 2.
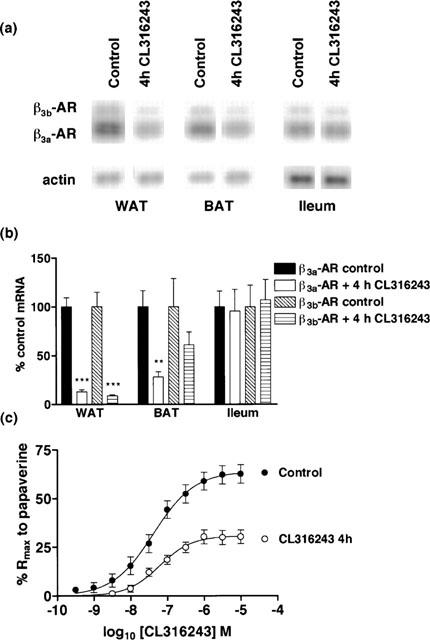
Effect of in vivo CL316243 (4 h) treatment (1 mg kg−1) on β3a-AR and β3b-AR mRNA levels in WAT, BAT and ileum and on responses to CL316243 in ileum. (a,b) β3a-AR mRNA levels were significantly reduced in WAT (***P<0.001) and BAT (***P<0.001) but not ileum following treatment. β3b-AR mRNA levels were significantly reduced in WAT (***P<0.001) following treatment. Bars show mean±s.e.mean (n=5–6). (c) 4 h CL316243 treatment desensitized β3-AR mediated responses in mouse ileum with a significant reduction in the Rmax response of the tissue with no change in pEC50 (2-way ANOVA ***P<0.0001). Points show mean and vertical lines indicate s.e.mean (n=12–13).
Figure 3.
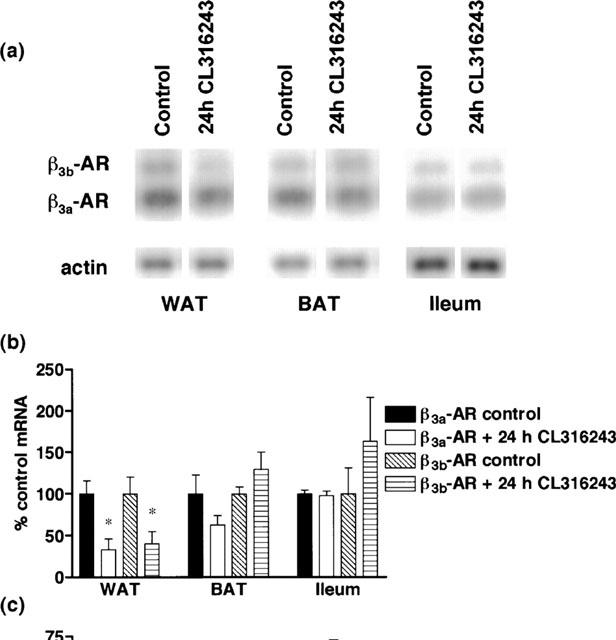
Effect of in vivo CL316243 (24 h) treatment (1 mg kg−1) on β3a-AR and β3b-AR mRNA levels in WAT, BAT and ileum and on responses to CL316243 in ileum. (a,b) β3a-AR and β3b-AR mRNA levels in WAT were significantly reduced (*P<0.05) following 24 h CL316243 treatment but levels were unaffected in BAT or ileum. Bars show mean±s.e.mean (n=5–6). (c) β3-AR mediated responses in ileum were desensitized following treatment, with the Rmax responses to CL316243 significantly reduced with no change in pEC50 values (2-way ANOVA ***P<0.0001). Points show mean and vertical lines indicate s.e.mean (n=6).
In ileum, (−)-[125I]-CYP binding occurred in a concentration-dependent manner and bound to a single population of sites (Figure 4). Following CL316243 treatment, there was a significant (*P<0.05) increase in the specific binding curves following 4 h, but not 24 h CL316243 treatment (4 h control: Bmax 63.0±10.2 fmol mg−1 protein, KD 781.7±275.2 pM, n=6; 4 h treated: Bmax 79.4±11.0 fmol mg−1 protein, KD 903.8±262.4 pM, n=6; 24 h control: Bmax 77.2±8.5 fmol mg−1 protein, KD 593.7±159.8 pM, n=6; 24 h treated; Bmax 76.3±10.1 fmol mg−1 protein, KD 541.5±182.8 pM, n=6).
Figure 4.
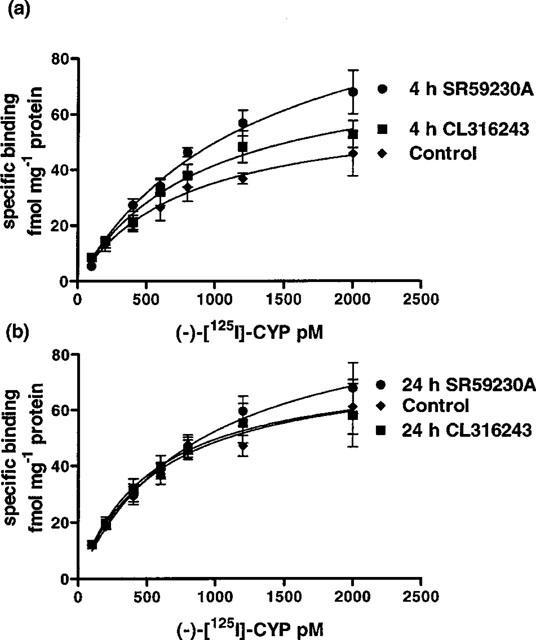
Saturation binding curve of (−)-[125I]-CYP to mouse ileal membrane preparations. Graphs show specific binding; (a) shows the significant increase in (−)-[125I]-CYP binding following SR59230A (4 h) (2-way ANOVA ***P<0.0001) and CL316243 (4 h) treatment (2-way ANOVA *P<0.05); (b) shows that 24 h SR59230A or CL316243 treatment had no significant effect on (−)-[125I]-CYP binding (2-way ANOVA ns). Points show mean±s.e.mean (n=5–6).
Although no significant changes occurred in β3-AR mRNA or in (−)-[125I]-CYP Bmax levels in ileum (except following 4 h CL316243 treatment), β3-AR responses to CL316243 were clearly desensitized following 4 and 24 h treatment (Figures 2c,3c). Rmax responses to CL316243 in ileum were significantly reduced from 63.9±3.3% (n=13) to 30.8±1.8% (n=12) following 4 h treatment, with no changes in pEC50 values observed between control (7.38±0.12; n=13) and treated mice (7.22±0.12; n=12) (2-way ANOVA ***P<0.0001). Following 24 h treatment, Rmax responses were still significantly reduced by more than 50% (69.0±4.4%; n=6 to 30.1±3.5%; n=6) while pEC50 values were unaffected (control: 7.50±0.15; n=6; treated: 7.37±0.26; n=6) (2-way ANOVA ***P<0.0001). Following 1 h treatment, c-r curves to CL316243 were not significantly different in either Rmax or pEC50 values between control (Rmax 57.4±2.4%; pEC50 7.69±0.10; n=8) and treated (Rmax 54.7±3.2%; pEC50 7.72±0.15; n=8) mice (2-way ANOVA ns) (Figure 1c).
Effect of β3-AR blockade by SR59230A on receptor binding levels and relaxation responses in ileum and on levels of β3-AR mRNA in WAT, BAT and ileum
Mice were treated with the β3-AR antagonist SR59230A (1 mg kg−1 i.p. 4 h) to test if β3-ARs in gut and adipose tissue could be up-regulated by receptor blockade. After SR 59230A treatment, β3a- and β3b-AR mRNA levels increased by more than 80% in BAT whereas in ileum, β3a-AR mRNA levels were doubled and β3b-AR mRNA levels increased by more than four times control levels. No change in β3-AR mRNA levels was observed in WAT (Figure 5).
Figure 5.
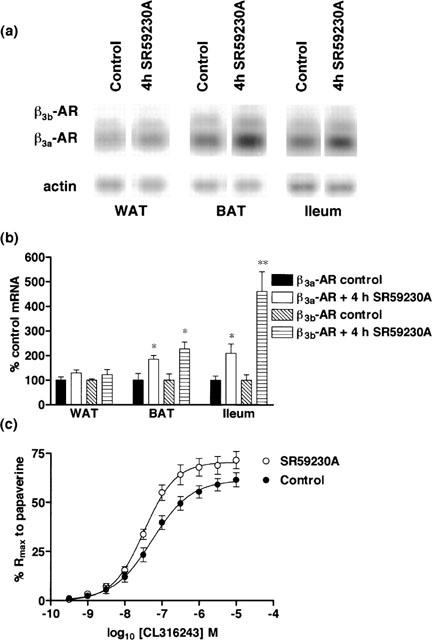
Effect of in vivo SR59230A (4 h) treatment (1 mg kg−1) on β3a-AR and β3b-AR mRNA levels in WAT, BAT and ileum and on responses to CL316243 in ileum. (a,b) SR59230A treatment significantly increased β3a-AR mRNA levels in BAT (*P<0.05) and ileum (*P<0.05) but not in WAT. β3b-AR mRNA levels were significantly increased in BAT (*P<0.05) and ileum (**P<0.01) but not in WAT following treatment. Bars show mean±s.e.mean (n=3–6). (c) SR59230A treatment increased β3-AR mediated responses in mouse ileum, significantly increasing the Rmax response of the tissue while pEC50 values were significantly different following treatment (2-way ANOVA ***P<0.0001). Points show mean and vertical lines indicate s.e.mean (n=9–11).
The increase in β3-AR mRNA levels in ileum following 4 h SR59230A treatment was accompanied by a significant increase in (−)-[125I]-CYP binding (control: Bmax 63.0±10.2 fmol mg−1 protein, KD 781.7±275.2 pM, n=6; treated: Bmax 115.1±15.5 fmol mg−1 protein, KD 1311±324.4 pM, n=5) (Figure 4a), without significant changes in KD values (2-way ANOVA ***P<0.0001). There was also a significant increase in the Rmax response in ileum in treated (70.6±2.1%; n=11) compared to control mice (61.5±2.3%; n=9) and a small yet significant change in pEC50 values between control (7.27±0.08; n=9) and treated mice (7.49±0.07; n=11) (2-way ANOVA ***P<0.0001) (Figure 5c).
Although 4 h SR59230A treatment produced a significant increase in β3-AR mRNA levels in ileum, it was thought that the change in functional response might be enhanced further by longer treatment with SR59230A. Mice were therefore treated with SR59230A for 24 h. Inconsistent changes in β3-AR mRNA levels were observed following 24 h SR59230A treatment (data not shown). No functional data in ileum could be obtained as the carbachol contraction in ileum was poorly maintained and a large amount of spontaneous activity was observed in tissues originating from treated animals as compared to controls. Examination of binding levels in ileum (Figure 4b) showed that Bmax values were still elevated but the levels were not significantly different from controls (control: Bmax 77.2±8.5 fmol mg−1 protein, KD 593.7±159.8 pM, n=6; treated: Bmax 99.6±13.0 fmol mg−1 protein, KD 902.0±248.6 pM, n=5) (2-way ANOVA ns).
Effect of forskolin on relaxation responses in mouse ileum and β3-AR mRNA in WAT, BAT and ileum
The mechanism whereby β3-AR agonists cause relaxation in ileum is in question and may not involve elevation of cyclic AMP levels. This could explain why β3-AR agonists fail to affect β3-AR mRNA levels in ileum if increased cyclic AMP levels are a prerequisite for β3-AR mRNA reduction. Experiments were therefore performed using forskolin, which increases cyclic AMP by direct activation of adenylate cyclase.
Following 4 h forskolin treatment, β3a-AR mRNA levels were significantly reduced by 40% in both WAT and BAT (*P<0.05), although while β3b-AR mRNA levels were reduced in WAT and BAT, these reductions were not significant. β3a- and β3b-AR mRNA levels were unaltered in ileum following 4 h forskolin treatment (Figure 6). Functionally in ileum (Figure 6c), there was no significant difference in either Rmax or pEC50 values to CL316243 between control (Rmax 70.1±2.6; pEC50 7.44±0.08; n=13) and 4 h forskolin treated mice (Rmax 63.4±2.8; pEC50 7.49±0.10; n=13) (2-way ANOVA ns).
Figure 6.
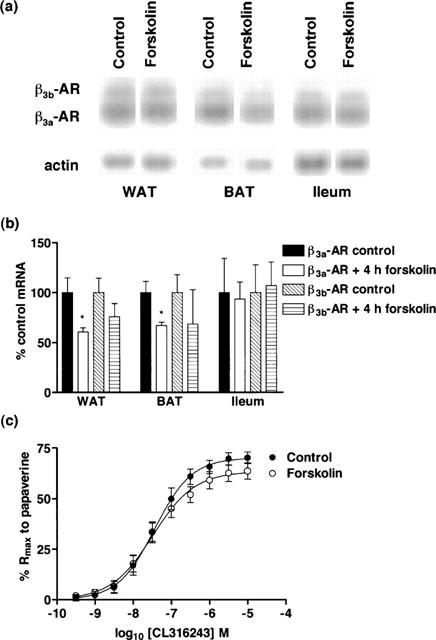
Effect of in vivo 4 h forskolin treatment (3 mg kg−1) on β3a-AR and β3b-AR mRNA levels in WAT, BAT and ileum and on responses to CL316243 in ileum. (a,b) β3a-AR, but not β3b-AR, mRNA levels in WAT and BAT are significantly reduced (*P<0.05) following treatment. β3a- and β3b-AR mRNA levels were unaffected following treatment. Bars show mean±s.e.mean (n=4–6). (c) 4 h forskolin treatment did not alter β3-AR mediated responses in ileum (2-way ANOVA ns). Points show mean and vertical lines indicate s.e.mean (n=13).
In another series of experiments using a 2 h forskolin treatment protocol, similar results were obtained with reductions in mean β3-AR mRNA levels in WAT and BAT, with no change in β3-AR mRNA levels or responsiveness to CL316243 in ileum (data not shown).
Effect of dexamethasone treatment of animals on relaxation responses in ileum and on levels of β3-AR mRNA in WAT, BAT and ileum
Mice were treated with the glucocorticoid dexamethasone (1 mg kg−1 i.p. 4 h) to assess whether β3-ARs in gut and adipose tissues are regulated differently by corticosteroids. β3a- and β3b-AR mRNA levels were reduced by more than 40% in BAT, while in WAT β3a-AR mRNA levels were reduced by one-third but β3b-AR mRNA levels remained unchanged. No changes in β3-AR mRNA levels were seen in ileum (Figure 7). Functionally, there was no significant difference in either Rmax or pEC50 values between control (Rmax 61.9±3.3%; pEC50 7.49±0.12; n=5) and dexamethasone treated (Rmax 60.8±2.6%; pEC50 7.58±0.10; n=8) mice (2-way ANOVA ns) (Figure 7c).
Figure 7.
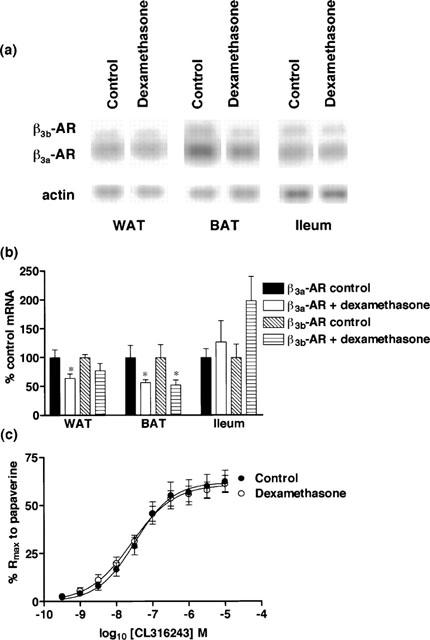
Effect of in vivo dexamethasone treatment (4 h, 1 mg kg−1) on β3a-AR and β3b-AR mRNA levels in WAT, BAT and ileum and on responses to CL316243 in ileum. (a,b) β3a-AR mRNA levels were significantly reduced in WAT (*P<0.05) and BAT (*P<0.05) but not ileum following dexamethasone treatment. β3b-AR mRNA levels were significantly reduced in BAT (*P<0.05) but not in WAT or ileum following treatment. Bars show mean±s.e.mean (n=3–6). (c) Dexamethasone treatment did not affect β3-AR mediated responses (2-way ANOVA ns). Points show mean and vertical lines indicate s.e.mean (n=5–8).
Discussion
Previously we have demonstrated that β3-ARs are markedly down regulated in WAT and BAT of C57BL/6J ob/ob mice compared to +/+ controls, whereas there was no significant difference in mRNA levels in ileum or colon (Evans et al., 1998). A similar pattern of β3-AR regulation was produced in +/+ mice by the administration of dexamethasone indicating that β3-AR expression is controlled differently in adipose tissues and gut. The present study examines the effect of a β3-AR agonist (CL316243), a β3-AR antagonist (SR59230A), forskolin or dexamethasone treatment on mRNA levels and responses in ileum. Since mice also express a β3-AR splice variant, the β3b-AR (Summers et al., 1998; Evans et al., 1999), we have also compared levels of β3a- and β3b-AR mRNA to determine if the regulation of the two β3-AR mRNAs are different.
Several studies in WAT and BAT have examined the effects of β-AR agonists on β3-AR regulation. The consensus is that in the short term, β-AR agonists decrease and in the long term do not affect or increase β3-AR mRNA levels. Bengtsson et al. (1996) conducted a detailed study in BAT cell culture and showed that β-AR agonists and forskolin decrease β3-AR mRNA. These effects were rapid, with more than 80% of β3-AR mRNA lost within 2 h of agonist administration. However with continued β-AR agonist treatment, the levels of β3-AR mRNA returned to control levels within 24 h and it was postulated that the effect is associated with the production of a protein transcription factor. These findings are consistent with the present study where short-term administration of a β3-AR agonist, CL316243, decreases β3-AR mRNA in BAT and WAT but the effect is clearly waning after 24 h treatment.
In adipose tissues, changes in β3-AR mRNA levels are correlated with changes in β3-AR responses (Collins et al., 1994; El Hadri et al., 1997; Gettys et al., 1997). In ileum, desensitization of β3-AR mediated responses following β3-AR agonist treatment occurred but was not correlated with changes in β3-AR mRNA or protein as measured by (−)-[125I]-CYP binding. Tachyphylaxis has been observed in several gastrointestinal tissues (Kelly & Houston, 1996; McLaughlin & MacDonald, 1990; 1991; Coleman et al., 1987). In rat distal colon or gastric fundus, a c-r curve to isoprenaline was constructed. Tissues were then washed before exposure to a high concentration of BRL37344. Tissues were further washed before construction of a second c-r curve to isoprenaline. This protocol resulted in the second c-r curve to isoprenaline to be shifted to the right with a decrease in the maximal response to isoprenaline (Kelly & Houston, 1996; McLaughlin & MacDonald, 1991). This desensitization was not observed in rat distal colon when isoprenaline was used to try and mimic the desensitization effects of BRL37344 (Kelly & Houston, 1996). These authors suggested that the desensitization caused by BRL37344 was not due to β3-AR activation but could reflect some type of binding to these receptors since CYP in oesophageal preparations prevented the desensitization effect of BRL37344. Other studies in rat gastric fundus showed that construction of a second c-r curve to BRL37344 resulted in a significant shift to the right (following an interval of 1 h after construction of the first c-r curve), but this shift was hard to quantify since the highest concentration of BRL37344 in the second curve caused only a 30% relaxation response (McLaughlin & MacDonald, 1991). Agonist stimulation of β1- and β2-ARs leads to short-term desensitization (minutes) which is due to phosphorylation of serine and threonine residues in the third cytoplasmic loop and carboxyl-terminal tail of the receptor by cyclic AMP-dependent protein kinase and G-protein receptor kinase 2 (Hausdorff et al., 1989; Freedman et al., 1995). Since the β3-AR lacks most of these serine/threonine residues, (Nahmias et al., 1991; Nantel et al., 1993), the β3-AR would not be expected to undergo desensitization by these mechanisms, so it is unlikely that the decrease in ileal responsiveness following CL316243 administration is due to receptor phosphorylation. Additionally, desensitization due to phosphorylation at β1- and β2-ARs is rapid yet no change in response was observed in ileum following 1 h CL316243 treatment. Since β3-AR mRNA and (−)-[125I]-CYP binding levels were unaffected, down regulation or internalization are also unlikely explanations, especially since a small increase in (−)-[125I]-CYP binding in ileum was observed following 4 h CL316243 treatment. The decrease in response may not be related to signalling at the levels of cyclic AMP or beyond since forskolin treatment (2 and 4 h) was unable to cause the desensitization observed following CL316243. This is clearly different from the situation in cultured BAT adipocytes where forskolin and 8-bromo-cyclic AMP as well as β3-AR stimulation decrease β3-AR mRNA levels (Bengtsson et al., 1996; Granneman & Lahners, 1994). Our results suggest that in ileum desensitization to β3-AR agonist does not involve changes in β3-AR mRNA or receptor levels, but involves changes in signal transduction most probably at the level of the G-protein.
Treatment with the β3-AR antagonist SR59230A (4 h) markedly increased β3-AR mRNA levels in ileum and BAT but not in WAT. In ileum, there was a dramatic increase in β3-AR mRNA that was associated with an increased relaxation response to CL316243 as well as an increase in (−)-[125I]-CYP binding, suggesting that the sensitization of β3-AR responses in ileum following 4 h SR59230A treatment results from the increase in β3-AR mRNA and receptor levels. However, after 24 h SR59230A treatment β3a-AR mRNA levels in all three tissues returned to or in the case of WAT and ileum fall below control levels (data not shown). This indicates that, as in BAT (Bengtsson et al., 1996), the regulation of the β3-AR is tightly controlled and that with continued β3-AR agonist or antagonist treatment, levels of β3-AR mRNA return towards control levels within 24 h. BAT, in contrast to WAT, is well innervated by the sympathetic nervous system (Lowell & Flier, 1997) and SR59230A antagonizes the thermogenic response in BAT to β3-AR activation (Manara et al., 1996). This suggests that removal of a tonic input from the sympathetic nervous system increases β3-AR mRNA levels. Likewise, the presence of a resting sympathetic tone in ileum that is interrupted by β3-AR blockade may be the mechanism involved in the large increase in β3-AR mRNA levels with SR59230A.
In BAT, the effects of β3-AR agonists, forskolin and dibutyryl cyclic AMP on β3-AR mRNA are consistent with the effect being mediated by increases in intracellular cyclic AMP (Bengtsson et al., 1996; Klaus et al., 1995). Since CL316243 had no effect on β3-AR mRNA levels in ileum, this raised the question of whether cyclic AMP is produced by β3-AR stimulation in ileum and whether β3-AR expression in ileum is sensitive to cyclic AMP. We have previously found in rat ileum that the β3-AR agonists BRL37344 and CGP12177A fail to increase cyclic AMP levels yet they are dramatically increased by forskolin which also produces marked relaxation (unpublished observations). In order to assess whether the lack of effect is due to absence of a cyclic AMP response or to insensitivity to cyclic AMP, mice were treated with forskolin and β3-AR mRNA measured in ileum, WAT and BAT with the latter two tissues acting as controls. Forskolin treatment caused no changes in β3-AR mRNA in ileum and no alteration in the functional response. However, β3-AR mRNA levels were significantly reduced in BAT and WAT following the 4 h treatment, indicating that the treatment was effective in increasing cyclic AMP levels in these tissues. These results favour the interpretation that levels of β3-AR mRNA in ileum, in contrast to those in WAT and BAT, are insensitive to increases in intracellular cyclic AMP.
C57BL/6J ob/ob mice have lower levels of β3-AR mRNA and reduced β3-AR mediated cyclic AMP responses in WAT and BAT compared to their lean counterparts (Collins et al., 1994). This may be explained by high circulating corticosterone levels that are associated with obesity (Bray & York, 1979). In accordance with this, treatment of lean mice with dexamethasone decreases β3-AR mRNA in WAT and BAT (Evans et al., 1998) and treatment of 3T3-F442A adipocytes in culture with dexamethasone (Feve et al., 1992) also decreases β3-AR mRNA levels and β3-AR function. In contrast, in ileum, β3-AR mRNA levels and responses are indistinguishable in lean and obese mice and are not altered by dexamethasone (Evans et al., 1998). The present experiments confirmed, in Swiss mice, that dexamethasone treatment decreased β3-AR mRNA levels in WAT and BAT but had no effect on β3-AR mRNA levels or β3-AR mediated responses in ileum.
We have previously shown that alternate splicing of the mouse β3-AR gene results in the production of a mRNA encoding the β3b-AR (Summers et al., 1998; Evans et al., 1999). In the present study we examined whether agents that are known to decrease β3-AR mRNA levels differentially regulate the two mRNAs. β3b-AR mRNA appears to be regulated similarly to β3a-AR mRNA with some exceptions. Following CL316243 treatment, there was a significant difference in the regulation of β3b-AR mRNA in BAT compared to β3a-AR mRNA following 1 (***P<0.001) and 4 h (*P<0.05) CL316243 treatment. β3a-AR mRNA levels were reduced after 1 and 4 h agonist treatment in BAT whereas β3b-AR mRNA levels were not significantly altered. In WAT and ileum, β3b-AR and β3a-AR mRNA appeared to be regulated similarly by agonist treatment.
Another difference in the regulation between β3a-AR and β3b-AR mRNA was in ileum following 4 h SR59230A treatment, with a significantly larger increase (*P<0.05) in β3b-AR mRNA levels following treatment compared to β3a-AR mRNA levels. The differential regulation of β3a-AR and β3b-AR mRNA following agonist and antagonist treatment cannot involve actions at the level of transcription and therefore must be related to differences in mRNA processing or stability although this requires further study. Another factor is the relative abundance of β3a-AR transcripts as compared to β3b-AR transcripts. β3b-AR transcripts represent 7.6% of the total β3-AR transcripts in BAT, 21% in WAT and 16% in ileum smooth muscle (Evans et al., 1999; Summers et al., 1998). The small proportion of β3b-AR mRNA in BAT in particular would be expected to cause larger variations in the amounts measured and make it less likely that significant changes would be detected.
In summary, responses of ileum to CL316243 were reduced following CL316243 treatment, indicating the presence of another desensitization mechanism that does not involve changes in β3-AR mRNA or receptor levels. In contrast the increase in β3-AR responsiveness following SR59230A treatment was associated with a marked increase in β3-AR mRNA and receptor levels. The failure of CL316243, forskolin or dexamethasone to alter β3-AR mRNA levels in ileum compared to adipose tissue suggest that distinct regulatory mechanisms are present in ileum compared to WAT and BAT. Furthermore, regulation of the β3-AR in ileum is tightly controlled and appears to involve multiple mechanisms.
Acknowledgments
B.A. Evans is a Faculty of Medicine Research Fellow at Monash University. This work was supported by the National Health and Medical Research Council (NH&MRC).
Abbreviations
- β-AR
β-adrenoceptor
- BAT
brown adipose tissue
- Bmax
maximum number of binding sites
- BRL37344
4-[2-[(2-hydroxy-2-(3-chlorophenyl)ethyl)-amino]propyl]-phenoxyacetic acid
- CGP12177A
(±)-4-(3-t-butylamino-2-hydroxypropoxy)benzimidazol-2-one
- CL31643
(R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-dicarboxylate
- c-r
concentration-response
- CYP
cyanopindolol
- DMSO
dimethylsulfoxide
- Rmax
maximal relaxation
- RT–PCR
reverse transcription-polymerase chain reaction
- SR59230A
3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate
- WAT
white adipose tissue.
References
- ARCH J.R., AINSWORTH A.T., CAWTHORNE M.A., PIERCY V., SENNITT M.V., THODY V.E. Atypical β-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984;309:163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- BENGTSSON T., REDEGREEN K., STROSBERG A.D., NEDERGAARD J., CANNON B. Down-regulation of β3 adrenoceptor gene expression in brown fat cells is transient and recovery is dependent upon a short-lived protein factor. J. Biol. Chem. 1996;271:3366–3375. doi: 10.1074/jbc.271.52.33366. [DOI] [PubMed] [Google Scholar]
- BENSAID M., KAGHAD M., RODRIGUEZ M., LE FUR G., CAPUT D. The rat β3-adrenergic receptor gene contains an intron. FEBS. 1993;318:223–226. doi: 10.1016/0014-5793(93)80516-w. [DOI] [PubMed] [Google Scholar]
- BRAY G.A., YORK D.A. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol. Rev. 1979;59:719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., DENYER L.H., SHELDRICK K.E. β-Adrenoceptors in guinea-pig gastric fundus–are they the same as the ‘atypical' β-adrenoceptors in rat adipocytes. Br. J. Pharmacol. 1987;90:40P. [Google Scholar]
- COLLINS S., DANIEL K.W., ROHLFS E.M., RAMKUMART V., TAYLOR I.L., GETTYS T.W. Impaired expression and functional activity of the β3- and the β1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol. Endocrinology. 1994;8:518–527. doi: 10.1210/mend.8.4.7914350. [DOI] [PubMed] [Google Scholar]
- EL HADRI K., CHARON C., PAIRAULT J., HAUGUEL-DE MOUZON S., QUIGNARD-BOULANGE A., FEVE B. Down-regulation of β3-adrenergic receptor expression in rat adipose tissue during the fasted/fed transition: evidence for a role of insulin. Biochem. J. 1997;323:359–364. doi: 10.1042/bj3230359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMORINE L.J., MARULLO S., BRIEND-SUTREN M.-M., PATEY G., TATE K., DALAVIER-KLUTCHKO C., STROSBERG A.D. Molecular characterization of the human β3-adrenergic receptor. Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- EVANS B.A., PAPAIOANNOU M., ANASTASOPOULOS F., SUMMERS R.J. Differential regulation of β3-adrenoceptors in gut and adipose tissue of genetically obese (ob/ob) C57BL/6J-mice. Br. J. Pharmacol. 1998;124:763–771. doi: 10.1038/sj.bjp.0701867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS B.A., PAPAIOANNOU M., BONAZZI V.R., SUMMERS R.J. Expression of β3-adrenoceptor mRNA in rat tissues. Br. J. Pharmacol. 1996;117:210–216. doi: 10.1111/j.1476-5381.1996.tb15176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS B.A., PAPAIOANNOU M., HAMILTON S., SUMMERS R.J. Alternative splicing generates two isoforms of the β3-adrenoceptor which are differentially expressed in mouse tissues. Br. J. Pharmacol. 1999;127:1525–1532. doi: 10.1038/sj.bjp.0702688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEVE B., BAUDE B., KRIEF S., STROSBERG A.D., PAIRAULT J., EMORINE L.J. Inhibition by dexamethasone of β3-adrenergic receptor responsiveness in 3T3-F442A adipocytes. J. Biol. Chem. 1992;267:15909–15915. [PubMed] [Google Scholar]
- FEVE B., ELHARD K., QIUGNARD-BOULANGE A., PAIRAULT J. Transcriptional down-regulation by insulin of the β3-adrenergic receptor expression in 3T3-F442A adipocytes: a mechanism for repressing the cAMP pathway. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5677–5681. doi: 10.1073/pnas.91.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEDMAN N.J., LIGGETT S.B., DRACHMAN D.E., PEI G., CARON M.G., LEFKOWITZ R.J. Phosphorylation and desensitization of the human β1-adrenergic receptor. J. Biol. Chem. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- GETTYS T.W., WATSON P.M., SEGER L., PADGETT M., TAYLOR I.L. Adrenalectomy after weaning restores β3-adrenergic receptor expression in white adipocytes from C57BL/6J-ob/ob mice. Endocrinology. 1997;138:2697–2704. doi: 10.1210/endo.138.7.5283. [DOI] [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N. Differential adrenergic regulation of β1- and β3-adrenoceptor messenger ribonucleic acids in adipose tissues. J. Endocrinology. 1992;130:109–114. doi: 10.1210/endo.130.1.1309320. [DOI] [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N. Analysis of human and rodent β3-adrenergic receptor messenger ribonucleic acids. Endocrinology. 1994;135:1025–1031. doi: 10.1210/endo.135.3.8070345. [DOI] [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N., CHAUDHRY A. Molecular cloning and expression of the rat β3-adrenergic receptor. Mol. Pharmacol. 1991;40:895–899. [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N., CHAUDHRY A. Characterization of the human β3 receptor gene. Mol. Pharmacol. 1993;44:264–270. [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N., RAO D.D. Rodent and human β3-adrenergic receptor genes contain an intron within the protein-coding block. Mol. Pharmacol. 1992;42:964–970. [PubMed] [Google Scholar]
- HARMS H.H., ZAAGSMA J., DE VENTE V.J. Differentiation of β-adrenoceptors in right atrium, diaphragm and adipose tissue of the rat using stereoisomers of propranolol, alprenolol, nifenolol and practolol. Life Sci. 1977;21:123–128. doi: 10.1016/0024-3205(77)90432-5. [DOI] [PubMed] [Google Scholar]
- HAUSDORFF W.P., BOUVIER M., O'DOWD B.F., IRONS G.P., CARON M.G., LEFKOWITZ R.J. Phosphorylation sites on two domains of the β2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J. Biol. Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- KELLY J., HOUSTON G. β3-Adrenoceptors mediating relaxation of the oesophageal tunica muscularis mucosae and distal colon in the rat: comparative pharmacology and their desensitization by BRL37344. J. Autonomic Pharmacol. 1996;16:205–211. doi: 10.1111/j.1474-8673.1996.tb00424.x. [DOI] [PubMed] [Google Scholar]
- KLAUS S., MUZZIN P., REVELLI J.-P., CAWTHORNE M.A., GIACOBINO J.-P., RICQUIER D. Control of β3-adrenergic receptor gene expression in brown adipocytes in culture. Mol. Cell. Endorin. 1995;109:189–195. doi: 10.1016/0303-7207(95)03502-x. [DOI] [PubMed] [Google Scholar]
- KRIEF S., LONNQVIST F., RAIMBAULT S., BAUDE B., VAN S.A., ARNER P., STROSBERG A.D., RICQUIER D., EMORINE L.J. Tissue distribution of β3-adrenergic receptor mRNA in man. J. Clin. Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWELL B.B., FLIER J.S. Brown adipose tissue, β3-adrenergic receptors and obesity. Annu. Rev. Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., LEFUR G. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANARA L., CROCI T., LANDI M. β3-Adrenoceptors and intestinal motility. Fundam. Clin. Pharmacol. 1995;9:332–342. doi: 10.1111/j.1472-8206.1995.tb00507.x. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN D.P., MACDONALD A. Evidence for the existence of ‘atypical' β-adrenoceptors (β3-adrenoceptors) mediating relaxation in the rat distal colon in vitro. Br. J. Pharmacol. 1990;101:569–574. doi: 10.1111/j.1476-5381.1990.tb14122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAUGHLIN D.P., MACDONALD A. Characterization of catecholamine-mediated relaxations in rat isolated gastric fundus: evidence for an atypical β-adrenoceptor. Br. J. Pharmacol. 1991;103:1351–1356. doi: 10.1111/j.1476-5381.1991.tb09792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZZIN P., REVELLI J.-P., KUHNE F., GOCAYNE J.D., MCCOMBIE W.R., VENTER J.C., GIACOBINO J.-P., FRASER C.M. An adipose tissue-specific β-adrenergic receptor. J. Biol. Chem. 1991;266:24053–24058. [PubMed] [Google Scholar]
- NAHMIAS C., BLIN N., ELALOUF J.-M., MATTEI M.G., STROSBERG A.D., EMORINE L.J. Molecular characterization of the mouse β3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991;10:3721–3727. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANTEL F., BONIN H., EMORINE L.J., ZILBERFARB V., STROSBERG A.D., BOUVIER M., MARULLO S. The human β3-adrenergic receptor is resistant to short term agonist-promoted desensitization. Mol. Pharmacol. 1993;43:548–555. [PubMed] [Google Scholar]
- ROBERTS S.J., PAPAIOANNOU M., EVANS B.A., SUMMERS R.J. Functional and molecular evidence for β1, β2- and β3-adrenoceptors in human colon. Br. J. Pharmacol. 1997;120:1527–1535. doi: 10.1038/sj.bjp.0701056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS S.J., PAPAIOANNOU M., EVANS B.A., SUMMERS R.J. Characterization of β-adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for β1-, β2- and β3-adrenoceptors in rat ileum. Br. J. Pharmacol. 1999;127:949–961. doi: 10.1038/sj.bjp.0702605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS S.J., RUSSELL F.D., MOLENAAR P., SUMMERS R.J. Characterization and localization of atypical β-adrenoceptors in rat ileum. Br. J. Pharmacol. 1995;116:2549–2556. doi: 10.1111/j.1476-5381.1995.tb17206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILLENCE M.N., MOORE N.G., PEGG G.G., LINDSAY D.B. Ligand binding properties of putative β3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br. J. Pharmacol. 1993;109:1157–1163. doi: 10.1111/j.1476-5381.1993.tb13743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERS R.J., PAPAIOANNOU M., HAMILTON S., EVANS B.A. Alternative splicing generates two isoforms of the β3-adrenoceptor which are differentially expressed in mouse tissues. Br. J. Pharmacol. 1998;123:52P. doi: 10.1038/sj.bjp.0702688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERS R.J., ROBERTS S.J., PAPAIOANNOU M., EVANS B.A. Functional and molecular evidence for β3-adrenoceptors in human and rat gastrointestinal tissues. Br. J. Pharmacol. 1996;117:60P. doi: 10.1038/sj.bjp.0701056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN SPRONSEN A., NAHMIAS C., KRIEF S., BRIEND-SUTREN M.-M., STROSBERG A.D., EMORINE L.J. The promoter and intron/exon structure of the human and mouse β3-adrenergic-receptor genes. Eur. J. Biochem. 1993;213:1117–1124. doi: 10.1111/j.1432-1033.1993.tb17861.x. [DOI] [PubMed] [Google Scholar]


