Abstract
The respiratory response to microinjection of tachykinins and analogues into the commissural nucleus of the solitary tract (cNTS) of urethane-anaesthetized rats was investigated in the presence and absence of selective tachykinin NK1, NK2 and NK3 antagonists (RP 67580, SR 48968 and SR 142801, respectively).
All tachykinins, except for the selective NK2 agonist, [Nle10]-NKA(4-10), increased tidal volume (VT). The rank potency order of naturally-occurring tachykinins was neurokinin A (NKA)⩾substance P (SP)>>NKB, whereas the rank order for selective analogues was senktide⩾septide>> [Sar9,Met(O2)11]-SP>>[Nle10]-NKA(4-10). Septide (NK1-selective) and senktide (NK3-selective) were 22 fold more potent (pD2∼12) at stimulating VT than SP (pD2∼10.5).
Tachykinin agonists produced varying degrees of respiratory slowing, independent of changes in VT. At doses producing maximum stimulation of VT, agonists induced either a mild (<10 breaths min−1 decrease; SP and septide), moderate (10–25 breaths min−1 decrease; NKA, NKB and [Sar9,Met(O2]-SP) or severe (∼40 breaths min−1 decrease; senktide) bradypnoea. [Nle10]-NKA(4-10) produced a dose-dependent bradypnoea without affecting VT.
RP 67580 significantly attenuated the VT response to SP (33 pmol) and NKA (10 pmol) but not NKB (100 pmol). In the presence of RP 67580, the mild bradypnoeic response to NKB was significantly enhanced whereas SP and NKA induced a bradypnoea which was not observed in the absence of RP 67580. SR 48968 had no effect on the VT response to SP or NKB, markedly enhanced the VT response to NKA and completely blocked the bradypnoeic response to [Nle10]-NKA(4-10). Only SR142801 attenuated the VT response to NKB.
The present data suggest that all three tachykinin receptors (NK1, NK2 and NK3) are present in the cNTS and are involved in the central control of respiration.
Keywords: Tachykinins, nonpeptide antagonists, nucleus of the solitary tract, respiration
Introduction
The nucleus of the solitary tract (NTS) is a primary site for the integration of many visceral reflexes, including those initiated by the stimulation of arterial chemoreceptors, baroreceptors and pulmonary vagal afferents (Jordan & Spyer, 1986). Tachykinins, including substance P (SP) and neurokinin A (NKA) appear to be key neurotransmitters within the NTS. High levels of SP are found in afferent fibres which originate from baroreceptors and carotid body chemoreceptors and terminate in the NTS (Douglas et al., 1982; Nagashima et al., 1989). Local injection of SP into the NTS stimulates ventilation (Mazzone et al., 1998; Mazzone & Geraghty, 1999a). Moreover, the brain stem concentrations of SP increase during and after hypoxia (Lindefors et al., 1986; Srinivasan et al., 1991). Similarly, NKA and NKB are present in the NTS, although only NKA appears to be derived from afferent neurons (Kalia et al., 1984; Helke & Hill, 1988; Nagashima et al., 1989). Interestingly, although Maubach and Jones (1997) recently reported that NKA is more potent than SP in depolarizing NTS neurons in vitro, the role, if any, played by NKA and NKB in respiratory control has yet to be investigated.
Three tachykinin receptor types have been described based on the order of potency of endogenous tachykinins, i.e. SP>NKA>NKB at NK1 receptors; NKA>NKB>SP at NK2 receptors, and NKB>NKA>SP at NK3 receptors (for review, see Maggi, 1995). In addition, short (324 amino acids) and long (407 amino acids) isoforms of the NK1 receptor have been sequenced and, based on their affinity for the tachykinin analogue septide ([pGlu6,Pro9]-SP(6-11)), ‘septide-sensitive' and ‘septide-insensitive' NK1 receptors (or receptor conformers) have also been proposed (Petitet et al., 1992; Maggi & Schwartz, 1997; Jenkinson et al., 1999). NK1 and NK3 receptors have ben localized to the NTS using autoradiography, in situ hybridization and immunocytochemistry (Stoessl & Hill, 1990; Tsuchida et al., 1990; Nakaya et al., 1994; Carpentier & Baude, 1996; Mazzone et al., 1997). We have recently shown that microinjection of capsaicin (which releases tachykinins from afferent neurons) into the commissural NTS (cNTS) produces a profound bradypnoea which is blocked by the NK3 receptor antagonist, SR 142801, and (to a lesser extent) by the NK2 antagonist, SR 48968 (Mazzone & Geraghty, 1999b). However, although NK2 receptors have been identified in the septum, striatum and spinal cord, localization studies have failed to detect NK2 receptors in the brain stem, including the NTS (Steinberg et al., 1995; 1998; Zerari et al., 1998). Curiously, the depolarizing action of NKA on NTS neurons is blocked by NK1 but not NK2 antagonists, suggesting that NKA may act on NK1 receptors (Maubach & Jones, 1997). Indeed, endogenous tachykinins are notoriously promiscuous, and can stimulate all three receptor types at physiological concentrations (Maggi, 1995).
The aims of the present study were to investigate the effects of tachykinin agonists and antagonists on respiration and characterize the tachykinin receptors involved.
Methods
Surgery
All experimental procedures were approved by the University of Tasmania Ethics Committee (Animal Experimentation; project 97044). A total of 106 male Hooded Wistar rats (240–290 g) were anaesthetized using urethane (0.5 g kg−1 i.p. and 0.5 g kg−1 s.c.) and allowed to breathe room air throughout all procedures. The level of anaesthesia was assessed by monitoring limb withdrawal and head-shake reactions. Body core temperature was kept constant at 37°C by placing the rat in the prone position on a thermostatically controlled water bed. The animal's head was stabilized in a Kopf stereotaxic apparatus and the dorsal aspect of the brain stem and cerebellum was exposed by a midline incision and partial occipital craniotomy. The dura mater was temporarily left intact. Animals were allowed to stabilize for 20 min before continuing.
Respiration, which was spontaneous and rhythmic, was recorded using subcutaneous electrodes (inserted along the sixth intercostal space) and a calibrated impedance converter (UFI, Morro Bay, California, U.S.A.), as previously described by our laboratory (Mazzone et al., 1997; 1998; Mazzone & Geraghty, 1999a,1999b).
Injection of agents
The dura mater was cut and retracted. A glass micropipette was mounted in a micromanipulator and using obex as a reference point, the needle was inserted into the cNTS: AP −15 to −15.3 mm; L 0 to 0.3 mm relative to bregma and 0.4 mm into the dorsal surface of the brain stem (Paxinos & Watson, 1986). To limit the action of agents to the caudal NTS, all agonists and antagonists were injected in a total volume of 100 nl (1 μl SP diffuses approximately 1.3 mm lateral and 0.5 mm ventrodorsal from the injection site; Mazzone et al., 1998) using a microprocessor-controlled pump (UltraMicroPump II, World Precision Instruments). Agonist dose-response curves were constructed for tachykinins; SP (1–330 pmol), NKA (330 fmol–330 pmol), NKB (10 pmol–1 nmol) and for selective tachykinin receptor agonists; [Sar9,Met(O2)11]-SP (3.3–330 pmol), septide (100 fmol–10 pmol), [Nle10]-NKA(4-10) (100 pmol–1 nmol) and senktide (100 fmol–10 pmol). To minimize the number of animals used in the study, each animal received a maximum of three doses of a single agonist with at least 70 min between injections. A minimum of eight rats was used to construct each agonist log dose-effect curve.
To further characterize tachykinin receptors in the NTS, the effects of selective nonpeptide NK1, NK2 and NK3 receptor antagonists (RP 67580, SR 48968 and SR 142801, respectively) on the central respiratory actions of tachykinins were also investigated. SP, NKA and NKB were chosen for these studies since we wished to determine which receptors these agonists interact with in vivo to modify respiration. The respiratory responses to (approximate) ED50 doses of SP, NKA and NKB were recorded for 60 min. After 10 min recovery, either the vehicle (25% ethanol in normal saline) or a 5 fold excess of antagonist was injected and allowed to act for 10 min prior to a second injection of agonist (the construction of dose-response curves in the presence of multiple doses of antagonist was not feasible). An excess dose of antagonist was employed since SP, NKA and NKB generally have greater affinity for NK1, NK2 and NK3 receptors, respectively, than the corresponding nonpeptide antagonist (vis, RP 67580, SR 48968 and SR 142801; Emonds-Alt et al., 1992; 1995; Garret et al., 1991; Maggi, 1995).
At the end of each experiment, rats were killed with an overdose of pentobarbitone and the brain stems were removed and rapidly frozen. Cryostat-cut serial sections were thaw-mounted onto gelatine-coated slides and stained with thionine to ascertain the exact injection site.
Data analysis
Prior to removal of the brain stem, a polyethylene tube attached to a 5 ml syringe was inserted into the trachea and the impedance converter was calibrated by graded inflation of the lung. Respiratory movements were measured over a 20 s time interval and then converted to frequency, tidal volume (VT) and minute ventilation (VE). Volumes were subsequently normalized to body weight. Dose-response curves were constructed by plotting the maximum change against the log dose of agonist. Subsequently, the dose of agonist producing 50% of the maximum response (ED50) and pD2 (−log ED50) for each agonist was determined using PRISM™ Software (version 2.0, GraphPad Software Inc, San Diego, U.S.A.). For antagonist studies, maximum changes in frequency, VT and VE induced by SP, NKA and NKB in the absence and presence of antagonists were compared using the paired Student t-test. P<0.05 was considered statistically significant.
Drugs and materials
All tachykinin agonists were purchased from Auspep (Melbourne, Australia) and were >95% pure. Nonpeptide antagonists were generous gifts: (3aR,7aR)-2-(1-imino-2-(2-methoxy-phenyl)-ethyl)-7,7-diphenyl-4 perhydroisoindolone (RP 67580) from Dr C. Garret, Rhône-Poulenc Rorer, Vitry sur Seine, France; (S)-N-methyl-N[4-(4-acetylamino-4-phenyl piperidino)-2-(3,4-dichlorophenyl) butyl]benzamide (SR 48968) and (R)-(N)-[1-[3-[1-benzoyl-3-(3,4-dichlorophenyl)piperidin - 3 - yl]propyl] - 4 - phenylpiperidin-4-yl]-N-methylacetamide (SR 142801) from Dr X. Emonds-Alt, Sanofi Recherche, Montpellier, France. All agents were dissolved in 25% ethanol in normal saline and stored in frozen aliquots. All other reagents were of analytical grade.
Results
Respiratory response to microinjection of SP, NKA and NKB into the cNTS
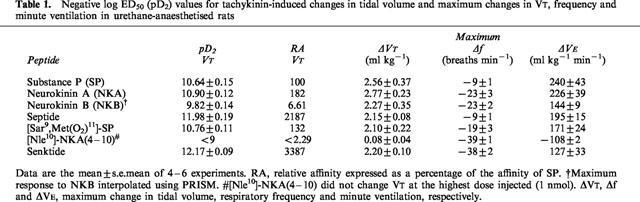
Microinjection of SP, NKA and NKB, into the cNTS produced a dose-dependent increase in VT which peaked between 8 and 10 min after injection and returned to pre-injection values within 30 min (for example, refer to Figure 1a). The maximum VT responses to SP and NKA were similar (2.56 and 2.77 ml kg−1 above basal, respectively), although a maximum VT response to NKB was not observed since the dose-response curve did not plateau at the highest dose (1 nmol) injected (Figure 2a; Table 1). The rank order of potency for increasing VT (based on pD2 values) was NKA⩾SP>NKB (Table 1). In addition to increasing VT, both NKA and NKB produced a dose-related reduction (P<0.05) in respiratory frequency whereas the effect of SP on frequency, even at the higher doses, was minimal (Figure 2b; Table 1). Since stimulation of VT predominated over the decrease in frequency, the three agonists produced a dose-dependent increase in VE with a rank order of potency, NKA⩾SP>NKB (Figure 2c).
Figure 1.
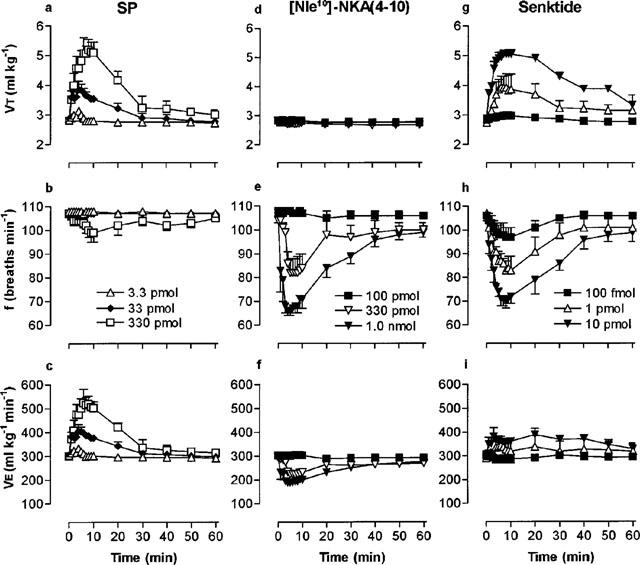
Respiratory response to microinjection of low, medium and high doses of SP (a,b,c), [Nle10-NKA(4-10) (d,e,f) and senktide (g,h,i) into the cNTS of spontaneously breathing, urethane-anaesthetized rats. Each point represents the mean tidal volume (VT), frequency (f) or minute ventilation (VE) of 3–5 rats. Vertical lines show the s.e.mean. Where error bars are not obvious, they are within the symbol.
Figure 2.
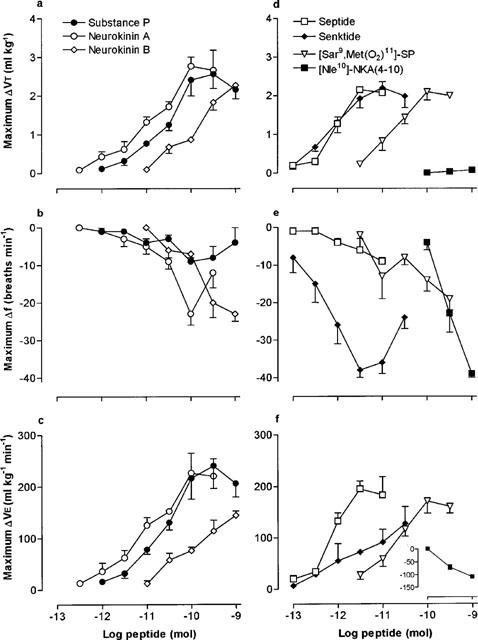
Log dose-response curves for naturally-occurring tachykinins (a–c) and receptor-selective synthetic agonists (d–f), microinjected into the cNTS of spontaneously breathing, urethane-anaesthetized rats. Each point is the mean maximum agonist-induced change in tidal volume (ΔVT), frequency (Δf) or minute ventilation (ΔVE) of 3–8 rats. Vertical lines show the s.e.mean. Where error bars are not obvious, they are within the symbol.
Table 1.
Negative log ED50 (pD2) values for tachykinin-induced changes in tidal volume and maximum changes in VT, frequency and minute ventilation in urethane-anaesthetised rats
Respiratory response to microinjection of selective tachykinin receptor agonists into the cNTS
The selective NK3 receptor agonist, senktide, and selective NK1 receptor agonists, [Sar9,Met(O2)11]-SP and septide increased VT in a dose-dependent manner (Figure 2d). The rank order of potency was senktide⩾septide>>[Sar9,Met (O2)11]-SP and the three agonists produced almost identical maximum VT responses (Table 1). Interestingly, although SP and [Sar9,Met(O2)11]-SP increased VE to similar maxima and with almost identical affinity (Table 1), [Sar9,Met(O2)11]-SP had a longer duration of action (data not shown).
Senktide caused a profound bradypnoea (36% decrease in frequency) which was dose-related up to 33 pmol (Figures 1g, 2e). Higher doses of senktide were less effective at reducing frequency. In contrast, septide and [Sar9,Met(O2)11]-SP only reduced frequency by 8 and 18%, respectively. Maximum VE responses obtained for septide and [Sar9,Met(O2)11]-SP were similar (∼65% increase) whereas senktide increased VE by only 42% due to the marked bradypnoea caused by the NK3 receptor agonist (Figure 2f; Table 1).
The selective NK2 receptor agonist, [Nle10]-NKA(4-10), had no effect on VT, even at the highest dose injected (1 nmol) but caused a dose-dependent bradypnoea (Figures 1d,e and 2d,e) and a concomitant reduction in VE (inset, Figure 2f).
Effect of selective nonpeptide NK1, NK2 and NK3 antagonists on the respiratory response to microinjection of SP into the cNTS
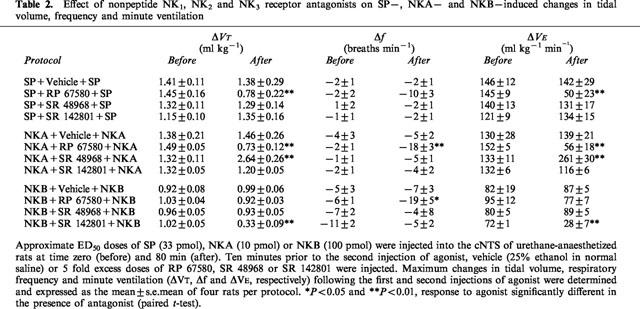
Microinjection of SP (33 pmol) before and 10 min after microinjection of vehicle (25% EtOH in normal saline) produced only minor reductions in respiratory frequency (−2 breaths min−1) but increased VT and hence, VE to a similar extent (Figure 3; Table 2). In the presence of the selective NK1 receptor antagonist, RP 67580 (165 pmol), the VE response to SP was significantly (P<0.01) attenuated (68% decrease) due to a markedly reduced VT response (46% decrease) and a greater reduction (−10 breaths min−1) in frequency (Figure 3a,b; Table 2). In contrast, the selective NK2 and NK3 receptor antagonists, SR 48968 (165 pmol) and SR 142801 (165 pmol), respectively, had no effect on the respiratory response to SP (Figure 3c–f). Furthermore, all antagonists were without effect on baseline respiration (data not shown).
Figure 3.
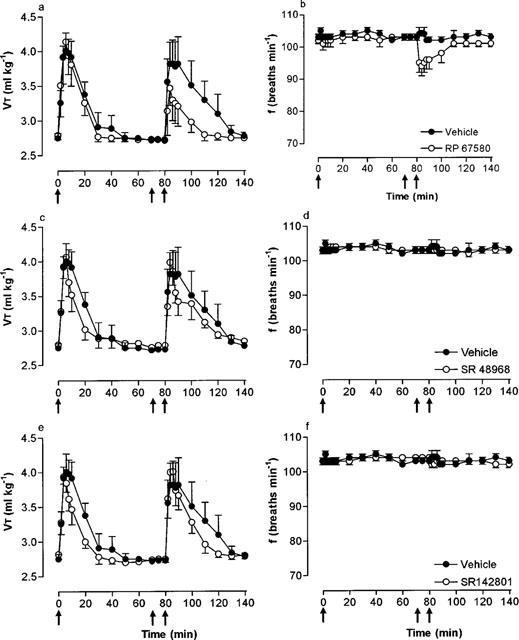
Effect of the NK1, NK2 and NK3 receptor antagonists, RP 67580 (165 pmol), SR 48968 (165 pmol) and SR 142801 (165 pmol), respectively, on tidal volume (VT) and respiratory frequency (f) responses to microinjection of SP (33 pmol) into the cNTS of spontaneously breathing, urethane-anaesthetized rats. SP was injected into the cNTS at time zero (first arrow) and 80 min (third arrow). Ten minutes prior to the second injection of SP, vehicle (25% ethanol in normal saline) or RP 67580 (a,b), SR 48968 (c,d) or SR 142801 (e,f) were injected (second arrow). Values are the mean of four rats. Vertical lines show s.e.mean. Where error bars are not obvious, they are within the symbol.
Table 2.
Effect of nonpeptide NK1, NK2 and NK3 receptor antagonists on SP−, NKA− and NKB−induced changes in tidal volume, frequency and minute ventilation
Effect of selective nonpeptide NK1, NK2 and NK3 antagonists on the respiratory response to microinjection of NKA into the cNTS
Microinjection of NKA (10 pmol) increased VT but had no significant effect on frequency (−4 breaths min−1). Moreover, a second NKA injection (10 min after injection of vehicle) produced similar changes in both VT and frequency (Table 2). In the presence of RP 67580 (50 pmol), NKA produced a bradypnoea (−18 breaths min−1) and the VT response was significantly attenuated (Figure 4a,b). Thus, in the presence of RP 675680, NKA caused only a minor increase in VE. In contrast, SR 48968 (50 pmol) had no effect on frequency but significantly (P<0.01) enhanced the VT (and hence VE) response to NKA (Figure 4c,d; Table 2). SR 142801 was without effect on the respiratory response to NKA (Figure 4e,f).
Figure 4.
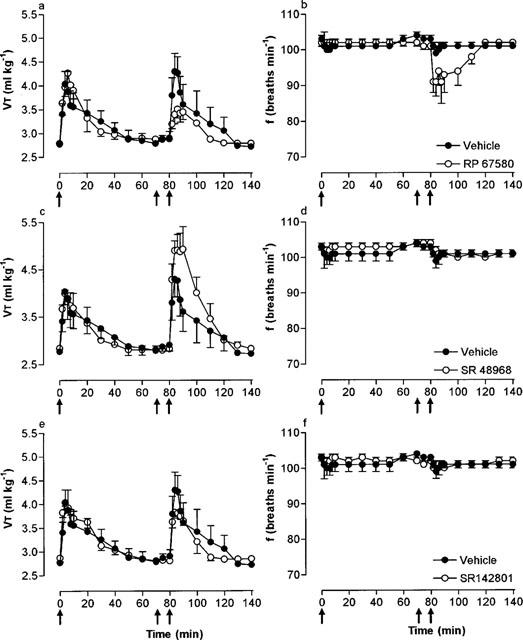
Effect of the NK1, NK2 and NK3 receptor antagonists, RP 67580 (50 pmol), SR 48968 (50 pmol) and SR 142801 (50 pmol), respectively, on tidal volume (VT) and respiratory frequency (f) responses to microinjection of NKA (10 pmol) into the cNTS of spontaneously breathing, urethane-anaesthetized rats. NKA was injected into the cNTS at time zero (first arrow) and 80 min (third arrow). Ten minutes prior to the second injection of NKA, vehicle (25% ethanol in normal saline) or RP 67580 (a,b), SR 48968 (c,d) or SR 142801 (e,f) were injected (second arrow). Values are the mean of four rats. Vertical lines show s.e.mean. Where error bars are not obvious, they are within the symbol.
Additional experiments were performed using the NKA analogue, [Nle10]-NKA(4-10), to assess the selectivity of this agonist for NK2 receptors. Microinjection of [Nle10]-NKA(4-10) (330 pmol) produced a typical bradypnoea (frequency after 4 min, 87±3 breaths min−1; pre-injection control, 106±1 breaths min−1, n=3) without affecting VT (2.8–3.0 ml kg−1). In the presence of SR 48968 (1 nmol), the bradypnoea was completely blocked (frequency after 4 min, 103±1 breaths min−1).
Effect of selective nonpeptide NK1, NK2 and NK3 antagonists on the respiratory response to microinjection of NKB into the cNTS
Microinjection of NKB (100 pmol) before and 10 min after microinjection of vehicle produced only minor reductions in respiratory frequency (5–7 breaths min−1) but increased VT and hence, VE to a similar extent (Figure 5; Table 2). In the presence of RP 67580 (500 pmol), the VT response to NKB was unchanged but the bradypnoea was significantly enhanced (maximum, −19 breaths min−1; Figure 5a,b). The exaggerated bradypnoeic response to NKB resulted in a reduced VE response in the presence of RP 67580 (Table 2).
Figure 5.
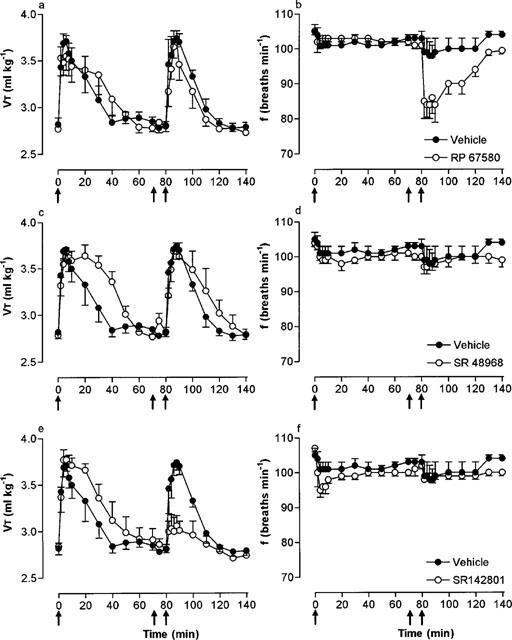
Effect of the NK1, NK2 and NK3 receptor antagonists, RP 67580 (500 pmol), SR 48968 (500 pmol) and SR 142801 (500 pmol), respectively, on tidal volume (VT) and respiratory frequency (f) responses to microinjection of NKB (100 pmol) into the cNTS of spontaneously breathing, urethane-anaesthetized rats. NKB was injected into the cNTS at time zero (first arrow) and 80 min (third arrow). Ten minutes prior to the second injection of NKB, vehicle (25% ethanol in normal saline) or RP 67580 (a,b), SR 48968 (c,d) or SR 142801 (e,f) were injected (second arrow). Values are the mean of four rats. Vertical lines show s.e.mean. Where error bars are not obvious, they are within the symbol.
SR 48968 (500 pmol) had no effect on the respiratory response to NKB (Figure 5c,d). In contrast, SR 142801 (500 pmol) significantly (P<0.01) reduced the stimulation of VT (68% decrease) and hence, VE (61% decrease) following injection of NKB observed in the absence of the antagonist (Figure 5e,f; Table 2).
Discussion
The aim of the present study was to characterize tachykinin receptors involved in central (NTS) respiratory control by recording the respiratory response to microinjection of tachykinin receptor agonists in the absence and presence of receptor-selective nonpeptide antagonists. The results suggest that NK1, NK2 and NK3 receptors are involved in the central respiratory actions of tachykinins.
Actions of tachykinin agonists in the cNTS
The role of SP and NK1 receptors in cardiorespiratory control at the NTS level is well documented (Picard et al., 1994; Mazzone et al., 1997; 1998; Mazzone & Geraghty, 1999a). Moreover, although central administration of NKA and NKB alters cardiovascular function, the effects of these tachykinins on respiration have, until now, not been investigated. In the present study, all tachykinin agonists, with the exception of the selective NK2 agonist, [Nle10]-NKA(4-10), increased VT to a similar extent. The rank order of potency of naturally-occurring tachykinins for stimulating VT was NKA⩾SP>NKB. This rank order does not correspond to any of the classical rank orders in monoreceptor systems (for review, see Maggi, 1995). However, the rank order of potency of selective agonists for stimulating VT was senktide⩾septide>>[Sar9,Met(O2)11]-SP>>>[Nle10]-NKA(4-10), indicating that the VT effect is probably mediated by both NK1 and NK3 receptors.
In contrast to VT, the respiratory frequency response varied, depending on which agonist was injected. Moreover, the change in frequency and increase in VT produced by agonists appeared to be independent of each other. SP and selective NK1 receptor agonist, septide, produced a mild bradypnoea at doses which produced close to maximum increases in VT. NKA, NKB and another selective NK1 receptor agonist, [Sar9,Met(O2)11]-SP, yielded a moderate bradypnoea and maximum stimulation of VT (interpolated for NKB). The selective NK3 receptor agonist, senktide, produced a large decrease in frequency but also stimulated VT to a maximum comparable with all NK1 and NK3 agonists. Finally, microinjection of the selective NK2 receptor agonist [Nle10]-NKA(4-10) into the NTS induced a profound bradypnoea similar to that of senktide, but failed to affect VT (at doses up to 1 nmol). Thus, based purely on agonist studies, we propose that the bradypnoeic response to tachykinins most likely involves NK3, and possibly NK2, receptors. However, a minor role for NK1 receptors in tachykinin-induced bradypnoea cannot be excluded as high doses of all NK1 agonists (SP, [Sar9,Met(O2)11]-SP, septide) produced a mild to moderate bradypnoea. Nevertheless, since tachykinins are notoriously promiscuous and are able to stimulate all three tachykinin receptors at high concentrations, the bradypnoeic effect of NK1 agonists may reflect an interaction with NK2 and NK3 receptors.
The present observation that NK2 and NK3 agonists induce bradypnoea highlights two anomalies with regard to tachykinins and tachykinin receptors in the brain stem. Firstly, immunocytochemical studies have shown that all three tachykinins are present in the rat NTS. Nagashima & coworkers (1989) reported that unilateral nodose ganglionectomy caused a significant decrease in the content of SP and NKA but not NKB in the NTS. Thus, NKB is not present in the central terminals of cardiorespiratory afferents, and hence is unlikely to be involved in the initial integration of cardiorespiratory reflexes. A more likely scenario is that descending neurons from the hypothalamus, projections from other brain stem regions (e.g., raphe nuclei) or NTS interneurons release NKB to modify afferent input. Moreover, the respiratory actions of NKB may be extrasynaptic since immunocytochemical studies have shown that NK3 receptors are not synaptically located in the rat NTS (Carpentier & Baude, 1996). Secondly, the bradypnoeic response to [Nle10]-NKA(4-10) was very surprising since in vitro localization studies have yet to demonstrate NK2 receptors at any supraspinal site, although no study has specifically addressed the localization of NK2 receptors in the brain stem (Tsuchida et al., 1990). The action of [Nle10]-NKA(4-10) may represent a nonselective action on NK3 receptors. However, the bradypnoea induced by [Nle10]-NKA(4-10) was blocked by SR 48968, suggesting a specific action at NK2 receptors. Thus, a role for NK2 receptors in the respiratory actions of tachykinins in the rat NTS cannot be dismissed.
Whether respiratory responses to microinjection of tachykinins into the cNTS are due to an interaction with pre- or postsynaptic tachykinin receptors was not investigated in the present study. Indeed, presynaptic tachykinin receptors appear to regulate the release of tachykinins and other neurotransmitters (dopamine) from rat striatal and sympathetic preganglionic neurons (Tremblay et al., 1992; Cammack & Logan, 1996; Jakob & Goldman-Rakic, 1996). Furthermore, preliminary reports from Jones and coworkers suggest that tachykinins can modify synaptic activity by stimulating either local NTS circuits or presynaptic tachykinin (NK1 and/or NK2) receptors, leading to the release of neurotransmitters including glutamate and GABA (see Bailey & Jones, 1999). However, a detailed study addressing the effect(s) of tachykinins on the release of neurotransmitters (e.g. glutamate, aspartate, GABA) in the NTS has yet to be reported.
The validity of injecting multiple doses of tachykinins into an animal may be questioned since desensitization of tachykinin receptors is well documented. For example, following agonist exposure, NK1 receptors in the spinal cord and other preparations are rapidly phosphorylated and internalized (endocytosis) for agonist removal (Jenkinson et al., 1999; Grady et al., 1995; Mantyh et al., 1995). A marked reduction in cellular responsiveness to agonists (desensitization) accompanies receptor phosphorylation and endocytosis. Agonist sensitivity is restored when receptors are dephosphorylated and returned to the cell surface, typically within 60 min. We have provided both functional and autoradiographic evidence that NK1 receptors in the NTS probably undergo similar processing in vivo (Mazzone et al., 1997; 1998). Thus, in the present study, repetitive injections of agonists were performed with a minimum of 70 min recovery between doses to minimize the risk of receptor desensitization. Indeed, neither the frequency nor the VT response to low dose repetitive injections of tachykinin agonists showed evidence of tachyphylaxis. Nevertheless, the bradypnoeic (but not VT) response to single high doses of SP, NKA and senktide appeared to desensitize. Whether this reduction in response was due to receptor phosphorylation and endocytosis or other mechanisms remains to be determined.
The existence of ‘septide-sensitive' tachykinin receptors in the NTS
In the present study, septide was 22 times more potent than SP (and [Sar9,Met(O2)11]-SP) at stimulating VT whereas NKA was approximately twice as potent. Furthermore, in a previous study, we reported that septide competed for [125I]-Bolton-Hunter SP binding sites in rat brain stem homogenates with 2000 fold lower affinity than either SP or [Sar9,Met(O2)11]-SP (Mazzone et al., 1997). Similar conflicting observations with septide in other tissues (vis, potent NK1 receptor agonist in functional studies, but an extremely weak competitor for SP binding sites) formed the basis for the proposal of the ‘septide-sensitive' receptor (Petitet et al., 1992; Zeng & Burcher, 1994; Hastrup & Schwartz, 1996; Nguyen-Le et al., 1996a). However, Maggi & Schwartz (1997) suggested that these observations could also be explained if the NK1 receptor existed in two interchangeable conformers. Indeed, whether the action of septide is at a site on the NK1 receptor which is different to SP, or reflects an interaction with a distinct NK1 receptor subtype is the subject of considerable controversy.
In addition to the conflicting functional and binding data, there appear to be differences in the potency of septide and SP at different NK1 receptor populations. Septide is more potent than SP at depolarizing NTS neurons in vitro and at stimulating acetylcholine release from rat striatum (Steinberg et al., 1995; Maubach & Jones, 1997). In contrast, in other areas of the CNS, such as the dorsal motor nucleus of the vagus and in peripheral tissues, including guinea-pig bronchus and myenteric neurons, septide and SP (or [Sar9,Met(O2)11]-SP) are equipotent (Zeng & Burcher, 1994; Maubach & Jones, 1997; Jenkinson et al., 1999). The higher potency of septide compared with SP in some tissues suggests NK1 receptor subtypes may exist. Indeed, substitution of Cys for Gly166 in NK1 receptors expressed in chinese hamster ovary (CHO) cells increases the affinity of NKA and septide relative to SP (Ciucci et al., 1998). Furthermore, while both septide and [Sar9,Met (O2)11]-SP stimulate ventilation in normal rats, septide, but not [Sar9,Met(O2)11]-SP, fails to stimulate ventilation in adult rats treated systemically at birth with the sensory neurotoxin capsaicin (Mazzone & Geraghty, 2000). This latter observation, together with the high affinity of septide and NKA relative to SP demonstrated by the present in vivo study, suggests that septide and [Sar9,Met(O2)11]-SP interact with different (NK1) receptor types (or conformers) in the NTS.
Effects of tachykinin receptor antagonists
To further characterize tachykinin receptors in the rat NTS, the respiratory actions of SP, NKA and NKB were compared in the absence and presence of selective tachykinin receptor antagonist, RP 67580, SR 48968 and SR 142801 (selective for NK1, NK2 and NK3 receptors, respectively; Garret et al., 1991; Emonds-Alt et al., 1992; 1995). The selective NK1 receptor antagonist, RP 67580, attenuated the VT response to SP and NKA but had no effect on the VT response to NKB, suggesting that SP and NKA, but not NKB, interact with NK1 receptors in the NTS. Indeed, the depolarizing action of NKA on NTS neurons in vitro is blocked by NK1 but not NK2 antagonists (Maubach & Jones, 1997). Thus, tachykinins released from sensory neurons in the NTS (SP and NKA), would most likely increase VT due to their preference for NK1 receptors.
Interestingly, ED50 doses of SP, NKA and NKB did not have a significant effect on frequency when injected alone but all induced a bradypnoea in the presence of RP 67580. The mechanisms by which RP 67580 facilitates a tachykinin-induced bradypnoea is unclear. However, a simple explanation is that since all tachykinins can stimulate each of the three tachykinin receptors at physiological concentrations, then blockade of the receptor which appears to have negligible effects on frequency (vis, NK1) may effectively increase the proportion of peptide available to interact with the receptor(s) which decrease frequency (NK3 and, possibly, NK2).
In a previous study, the selective NK2 receptor antagonist, SR 48968, attenuated the bradypnoea which follows microinjection of capsaicin into the NTS, suggesting that NK2 receptors are present in the rat brain stem (Mazzone & Geraghty, 1999b). Furthermore, in the present study, SR 48968 did not affect the VT response to SP or NKB but significantly enhanced the VT response to NKA. This latter observation may seem unusual since selective stimulation of NK2 receptors with [Nle10]-NKA(4-10) failed to alter VT. However, as NKA appears to stimulate VT by interacting with NK1 receptors, blocking the NK2 receptor type which (when stimulated) has no effect on VT may make more agonist available to increase VT via NK1 receptors. Alternatively, NK2 receptor stimulation may act to modify neurotransmitter uptake. Zerari and coworkers (1998) recently demonstrated NK2 receptors on astrocytes in the rat spinal cord and suggested that NKA released from afferent neurons may regulate neurotransmitter (particularly excitatory amino acid) uptake. Although purely hypothetical, a similar scenario would explain the action of SR 48968 in the present study. Blocking astrocytic NK2 receptors in the NTS might impede the uptake of excitatory amino acids and in turn, exaggerate the NK1 receptor-mediated VT response to NKA. A detailed immunocytochemical study addressing the cellular location of NK2 receptors in the NTS may help to clarify the mechanism of action of NKA on respiration.
SR 142801 selectively attenuated only the NKB-induced increase in VT, further supporting a role for NK3 receptors in central respiratory control. Nevertheless, the use of SR 142801 as a NK3 antagonist may be criticised. Firstly, SR 142801 has low affinity for rat NK3 receptors (pA2 ∼7) compared with guinea-pig and human NK3 receptors (pA2 ∼9; Patacchini et al., 1995; Nguyen-Le et al., 1996b). However, in contrast to RP 67580 and CP-96,345, which are selective for rat and human/guinea-pig NK1 receptors, respectively (Barr & Watson, 1993), high affinity nonpeptide antagonists for the rat NK3 receptor are not available. Secondly, SR 142801 may have some agonist activity at high doses (up to 65 nmol) when injected i.c.v. (Cellier et al., 1997). In the present study, SR 142801 (up to 500 pmol) had no effect on basal respiration. Moreover, in a previous study, we showed that SR 142801 (up to 5 nmol) has negligible direct effects on either frequency or VT, but blocks the bradypnoeic actions of capsaicin (Mazzone & Geraghty, 1999b). Thus, we are confident that the reduction in the VT response to NKB in the presence of SR 142801 was due to selective antagonism of NKB at NK3 receptors.
In conclusion, the present study provides evidence that tachykinins play a role in the control of respiration at the level of the NTS. Stimulation of NK1 and NK3 receptors increases VT whereas NK2 and NK3 receptor stimulation produces a bradypnoea. Moreover, the superior potency of septide compared with SP and [Sar9,Met(O2)11]-SP at stimulating VT, suggests that a proportion of NK1 receptors in the NTS are septide-sensitive. The exact role played by individual tachykinins in respiratory control was not assessed. However, in vivo, NK1 (and possibly NK2) receptors are probably activated by SP and NKA released from the central terminals of peripheral chemoreceptor, baroreceptor and/or pulmonary afferents. In contrast, the role played by NKB (and NK3 receptors) is less clear, since NKB does not function as a sensory neurotransmitter, and activation of NK3 receptors appears to be limited to NKB. One possibility is that NKB-containing neurons modify primary afferent input into the NTS. Nevertheless, these data provide evidence that NK3 receptors play a significant role in respiratory control by the brain stem.
Acknowledgments
This study was supported by grants from the National Health and Medical Research Council of Australia and the Australian Research Council. We thank Dr Xavier Emonds-Alt, Sanofi Recherche (Montpellier, France) for the gift of SR 48968 and SR 142801, and Dr Claude Garret, Rhône-Poulenc Rorer (Vitry sur Seine, France) for the gift of RP 67580.
Abbreviations
- cNTS
commissural nucleus of the solitary tract
- f
frequency
- NK
neurokinin
- RA
relative affinity
- SP
substance P
- VE
minute ventilation
- VT
tidal volume
References
- BAILEY C.P., JONES R.S.G. NK2-receptor activation increases spontaneous GABA-release in the rat nucleus tractus solitarius in vitro. Br. J. Pharmacol. 1999;127:48P. [Google Scholar]
- BARR A.J., WATSON S. Non-peptide antagonists, CP-96,345 and RP 67580, distinguish species variants in tachykinin NK1 receptors. Br. J. Pharmacol. 1993;108:233–227. doi: 10.1111/j.1476-5381.1993.tb13466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMMACK C., LOGAN S.D. Excitation of rat sympathetic preganglionic neurones by selective activation of the NK1 receptor. J. Autonom. Nerv. Sys. 1996;57:87–92. doi: 10.1016/0165-1838(95)00103-4. [DOI] [PubMed] [Google Scholar]
- CARPENTIER C., BAUDE A. Immunocytochemical localisation of NK3 receptors in the dorsal vagal complex of rat. Brain Res. 1996;734:327–331. [PubMed] [Google Scholar]
- CELLIER E., BARBOT L., REGOLI D., COUTURE R. Cardiovascular and behavioural effects of intracerebroventricularly administered tachykinin NK3 receptor antagonists in the conscious rat. Br. J. Pharmacol. 1997;122:643–654. doi: 10.1038/sj.bjp.0701435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIUCCI A., PALMA C., MANZINI S., WERGE T.M. Point mutation increases a form of the NK1 receptor with high affinity for neurokinin A and B and septide. Br. J. Pharmacol. 1998;125:393–401. doi: 10.1038/sj.bjp.0702070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS F.L., PALKOVITS M., BROWNSTEIN M.J. Regional distribution of substance P-like immunoreactivity in the lower brainstem of the rat. Brain Res. 1982;245:376–378. doi: 10.1016/0006-8993(82)90821-6. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., BICHON D., DUCOUX J.P., HEAULME M., MILOUX B., PONCELET M., PROIETTO V., VAN BROECK D., VILAIN P., NELIAT G., SOUBRIE P., LE FUR G., BRELIERE J.C. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56:PL27–PL32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., VILAIN P., GOULAOUUC P., PROIETTO V., VAN BROECK D., ADVENIER C., NALINE E., NELIAT G., LE FUR G., BRELIERE J.C. A potent and selective non-peptide antagonist of the neurokinin A (NK2) receptor. Life Sci. 1992;50:PL101–PL106. doi: 10.1016/0024-3205(92)90352-p. [DOI] [PubMed] [Google Scholar]
- GARRET C., CARRUETTE A., FARDIN V., MOUSSAOUI S., PEYRONEL J., BLANCHARD J., LADURON P. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADY E.F., GARLAND A.M., GAMP P.D., LOVETT M., PAYAN D.G., BUNNETT M.W. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Mol. Biol. Cell. 1995;6:509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTRUP H., SCHWARTZ T.W. Septide and neurokinin A are high-affinity ligands on the NK-1 receptor: evidence from homologous versus heterologous binding analysis. FEBS Lett. 1996;399:264–266. doi: 10.1016/s0014-5793(96)01337-3. [DOI] [PubMed] [Google Scholar]
- HELKE C.J., HILL K.M. Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience. 1988;26:539–551. doi: 10.1016/0306-4522(88)90166-2. [DOI] [PubMed] [Google Scholar]
- JAKOB R.L., GOLDMAN-RAKIC P. Presynaptic and postsynaptic subcellular localization of substance P receptor immunoreactivity in the neostriatum of the rat and rhesus monkey (Macaca Mulatta) J. Comp. Neurol. 1996;369:125–136. doi: 10.1002/(SICI)1096-9861(19960520)369:1<125::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- JENKINSON K.M., SOUTHWELL B.R., FURNESS J.B. Two affinities for a single antagonist at the neuronal NK1 tachykinin receptor: evidence from quantitation of receptor endocytosis. Br. J. Pharmacol. 1999;126:131–136. doi: 10.1038/sj.bjp.0702285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN D., SPYER K.M.Brainstem integration of cardiovascular and pulmonary afferent activity Progress in Brain Research 198667New York: Elsevier Science Publishers; 295–314.Cervero, F. & Morrison, J.F.B. (eds) [DOI] [PubMed] [Google Scholar]
- KALIA M., FUXE K., HÖKFELT T., JOHANSSON O., LANG R., GATEN D., CUELLO C., TERENIUS L. Distribution of neuropeptide immunoreactive nerve terminals within the subnuclei of the nucleus of the tractus solitarius of the rat. J. Comp. Neurol. 1984;222:409–444. doi: 10.1002/cne.902220308. [DOI] [PubMed] [Google Scholar]
- LINDEFORS N., YAMAMOTO Y., PANTALEO T., LARGERCRANTZ H., BRODIN E., UNGERSTEDT U. In vivo release of substance P in the nucleus tractus solitarii increases during hypoxia. Neurosci. Letts. 1986;69:94–97. doi: 10.1016/0304-3940(86)90421-0. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., SCHWARTZ T.W. The dual nature of the tachykinin NK1 receptor. Trends Pharmacol. Sci. 1997;18:351–355. doi: 10.1016/s0165-6147(97)01107-3. [DOI] [PubMed] [Google Scholar]
- MANTYH P.W., DEMASTER E., MALHOTRA A., GHILARDI J.R., ROGERS S.D., MANTYH C.R., LIU H., BASBAUM A.I., VIGNA S.R., MAGGIO J.E., SIMONE D.A. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- MAUBACH K.A., JONES R.S.G. Electrophysiological characterisation of tachykinin receptors in the rat nucleus of the solitary tract and dorsal motor nucleus of the vagus in vitro. Br. J. Pharmacol. 1997;122:1151–1159. doi: 10.1038/sj.bjp.0701482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Altered respiratory response to substance P and reduced NK1 receptor binding in the nucleus of the solitary tract of aged rats. Brain Res. 1999a;826:139–142. doi: 10.1016/s0006-8993(99)01247-0. [DOI] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Respiratory action of capsaicin microinjected into the nucleus of the solitary tract: involvement of vanilloid and tachykinin receptors. Br. J. Pharmacol. 1999b;127:473–478. doi: 10.1038/sj.bjp.0702522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., GERAGHTY D.P. Respiratory actions of tachykinins in the nucleus of the solitary tract: effect of neonatal capsaicin pretreatment. Br. J. Pharmacol. 2000;129:1132–1139. doi: 10.1038/sj.bjp.0703173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZONE S.B., HINRICHSEN C.F., GERAGHTY D.P. Substance P receptors in brain stem respiratory centres of the rat: regulation of NK1 receptors by hypoxia. J. Pharmacol. Exp. Ther. 1997;282:1547–1556. [PubMed] [Google Scholar]
- MAZZONE S.B., HINRICHSEN C.F., GERAGHTY D.P. Hypoxia attenuates the respiratory response to microinjection of substance P into the nucleus of the solitary tract of the rat. Neurosci. Lett. 1998;256:9–12. doi: 10.1016/s0304-3940(98)00743-5. [DOI] [PubMed] [Google Scholar]
- NAGASHIMA A., TAKANO Y., TATEISHI K., MATSUOKA Y., HAMAOKA T., KAMIYA H. Cardiovascular roles of tachykinin peptides in the nucleus tractus solitarii of rats. Brain Res. 1989;487:392–396. doi: 10.1016/0006-8993(89)90848-2. [DOI] [PubMed] [Google Scholar]
- NAKAYA Y., KANEKO T., SHIGEMOTO R., NAKANISHI S., MIZUNO N. Immunohistochemical localization of substance P receptor in the CNS of the adult rat. J. Comp. Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- NGUYEN-LE X.K., NGUYEN Q.T., GOBEIL F., JUKIC D., CHRÉTIEN L., REGOLI D. Neurokinin receptors in the guinea pig ileum. Pharmacology. 1996a;52:35–45. doi: 10.1159/000139359. [DOI] [PubMed] [Google Scholar]
- NGUYEN-LE X.K., NGUYEN Q.T., GOBEIL F., PHENG L.H., EMONDS-ALT X., BRELIERE J.C., REGOLI D. Pharmacological characterization of SR 142801: a new non-peptide antagonist of the neurokinin NK-3 receptor. Pharmacology. 1996b;52:283–291. doi: 10.1159/000139393. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., BARTHÒ L., HOLZER P., MAGGI C.A. Activity of SR 142801 at peripheral tachykinin receptors. Eur. J. Pharmacol. 1995;278:17–25. doi: 10.1016/0014-2999(95)00090-8. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The rat brain in stereotaxic coordinates 1986Sydney: Academic Press Inc; (2nd edition) [Google Scholar]
- PETITET F., SAFFROY M., TORRENS Y., LAVIELLE S., CHASSAING G., LOEUILLET D., GLOWINSKI J., BEAUJOUAN J.-C. Possible existence of a new tachykinin receptor subtype in the guinea-pig ileum. Peptides. 1992;13:383–388. doi: 10.1016/0196-9781(92)90125-m. [DOI] [PubMed] [Google Scholar]
- PICARD P., REGOLI D., COUTURE R. Cardiovascular and behavioural effects of centrally administered tachykinins in the rat: characterization of receptors with selective antagonists. Br. J. Pharmacol. 1994;112:240–249. doi: 10.1111/j.1476-5381.1994.tb13058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN M., GOINY M., PANTALEO T., LAGERCRANTZ H., BRODIN E., RUNOLD M., YAMAMOTO Y. Enhanced in vivo release of substance P in the nucleus tractus solatarii during hypoxia in the rabbit: role of peripheral input. Brain Res. 1991;546:211–216. doi: 10.1016/0006-8993(91)91483-h. [DOI] [PubMed] [Google Scholar]
- STEINBERG R., MARCO N., VOUTSINOS B., BENSAID M., RODIER D., SOUILHAC J., ALONSO R., OURY-DONAT F., LE FUR G., SOUBRIE P. Expresion and presence of septal neurokinin-2 receptors controlling hippocampal acetylcholine release during sensory stimulation in rat. Eur. J. Neurosci. 1998;10:2337–2345. doi: 10.1046/j.1460-9568.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- STEINBERG R., RODIER D., SOUILHAC J., BOUGAULT I., EMONDS-ALT X., SOUBRIE P., LE FUR G. Pharmacological characterization of tachykinin receptors controlling acetylcholine release from rat stratium: an in vivo microdialysis study. J. Neurochem. 1995;65:2543–2548. doi: 10.1046/j.1471-4159.1995.65062543.x. [DOI] [PubMed] [Google Scholar]
- STOESSL A.J., HILL D.R. Autoradiographic visualization of NK-3 tachykinin binding sites in the rat brain, utilizing [3H]senktide. Brain Res. 1990;534:1–7. doi: 10.1016/0006-8993(90)90105-k. [DOI] [PubMed] [Google Scholar]
- TREMBLAY L., KEMEL M.L., DESBAN M., GAUCHY C., GLOWINSKI J. Distinct presynaptic control of dopamine release in striosomal- and matrix-enriched areas of the rat striatum by selective agonists of NK1, NK2 and NK3 tachykinin receptors. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11214–11218. doi: 10.1073/pnas.89.23.11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIDA K., SHIGEMOTO R., YOKOTA Y., NAKANISHI S. Tissue distribution and quantitation of the mRNAs for three rat tachykinin receptors. Eur. J. Biochem. 1990;193:751–757. doi: 10.1111/j.1432-1033.1990.tb19396.x. [DOI] [PubMed] [Google Scholar]
- ZENG X.-P., BURCHER E. Use of antagonists for further characterization of tachykinin NK-2, NK-1 and possible ‘septide-sensitive' receptors in guinea pig bronchus. J. Pharmacol. Exp. Ther. 1994;270:1295–1300. [PubMed] [Google Scholar]
- ZERARI F., KARPITSKIY V., KRAUSE J., DESCARRIES L., COUTURE R. Astroglial distribution of neurokinin-2 receptor immunoreactivity in the rat spinal cord. Neuroscience. 1998;84:1233–1246. doi: 10.1016/s0306-4522(97)00548-4. [DOI] [PubMed] [Google Scholar]


