Abstract
Experiments were performed to identify the potassium channels involved in the acetylcholine-induced endothelium-dependent hyperpolarization of the guinea-pig internal carotid artery. Smooth muscle and endothelial cell membrane potentials were recorded in isolated arteries with intracellular microelectrodes. Potassium currents were recorded in freshly-dissociated smooth muscle cells using patch clamp techniques.
In single myocytes, iberiotoxin (0.1 μM)-, charybdotoxin (0.1 μM)-, apamin (0.5 μM)- and 4-aminopyridine (5 mM)-sensitive potassium currents were identified indicating the presence of large- and small-conductance calcium-sensitive potassium channels (BKCa and SKCa) as well as voltage-dependent potassium channels (KV). Charybdotoxin and iberiotoxin inhibited the same population of BKCa but a conductance specifically sensitive to the combination of charybdotoxin plus apamin could not be detected. 4-aminopyridine (0.1–25 mM) induced a concentration-dependent inhibition of KV without affecting the iberiotoxin- or the apamin-sensitive currents.
In isolated arteries, both the endothelium-dependent hyperpolarization of smooth muscle and the hyperpolarization of endothelial cells induced by acetylcholine or by substance P were inhibited by 5 mM 4-aminopyridine.
These results indicate that in the vascular smooth muscle cells of the guinea-pig carotid artery, a conductance specifically sensitive to the combination of charybdotoxin plus apamin could not be detected, comforting the hypothesis that the combination of these two toxins should act on the endothelial cells. Furthermore, the inhibition by 4-aminopyridine of both smooth muscle and endothelial hyperpolarizations, suggests that in order to observe an endothelium-dependent hyperpolarization of the vascular smooth muscle cells, the activation of endothelial potassium channels is likely to be required.
Keywords: 4-Aminopyridine, apamin, charybdotoxin, endothelium, EDHF, iberiotoxin, potassium channels, smooth muscle
Introduction
The vascular endothelium controls tone in the underlying smooth muscle cells by releasing various factors including nitric oxide (NO) (Furchgott & Zawadzki, 1980), prostacyclin (Moncada & Vane, 1979) and an unidentified endothelium-derived hyperpolarizing factor (EDHF) (Félétou & Vanhoutte, 1988; Taylor & Weston, 1988). Many observations combine to suggest that the EDHF-induced hyperpolarization of the vascular smooth muscle involves the opening of potassium channels. Thus, it is associated with a decrease in membrane resistance (Bolton et al., 1984; Chen & Suzuki 1989a,1989b), inversely related to the extracellular K+ concentration and cannot be observed at K+ concentrations higher than 25 mM (Chen & Suzuki, 1989a; Nagao & Vanhoutte, 1992; Corriu et al., 1996a). Furthermore, endothelium-dependent hyperpolarizations are associated with an increase in rubidium efflux (Taylor et al., 1988) and prevented by non-selective inhibitors of potassium channels such as tetraethylammonium and tetrabutylammonium (Chen et al., 1991; Nagao & Vanhoutte, 1992; Van de Voorde et al., 1992).
In various tissues, apamin (a specific inhibitor of small conductance calcium-activated potassium channels) alone or in combination with charybdotoxin (a non-specific inhibitor of calcium-activated potassium channels), inhibits the responses attributed to EDHF (Murphy & Brayden, 1995; Garland & Plane, 1996; Corriu et al., 1996a; Zygmunt & Höggestätt, 1996; Chataigneau et al., 1998; Yamanaka et al., 1998; Quignard et al., 1999a). These toxins seem selective as they inhibit EDHF-mediated responses without affecting relaxations or hyperpolarizations produced by endothelial nitric oxide or prostacyclin. Furthermore, the increase in endothelial intracellular calcium produced by acetylcholine was not affected by the two toxins (Yamanaka et al., 1998). Therefore, it has been assumed that the target(s) for the two toxins is on the vascular smooth muscle.
However, calcium-activated potassium channels are also expressed in endothelial cells (Marchenko & Sage, 1996) and in the rat hepatic artery and the rabbit aortic valve, the combination of charybdotoxin plus apamin inhibits endothelial cell hyperpolarization produced by acetylcholine (Edwards et al., 1998; Ohashi et al., 1999). Furthermore, in rat mesenteric artery, charybdotoxin and apamin block EDHF responses if selectively applied to the endothelium (Doughty et al., 1999). Thus, the endothelial actions of the toxins might be responsible for the inhibition of EDHF. Finally, another potassium channel blocker, 4-aminopyridine, inhibits acetylcholine-induced endothelium-dependent hyperpolarization in the isolated coronary artery of the guinea-pig (Eckman et al., 1998). However, in the same blood vessel, 4-aminopyridine inhibits also acetylcholine-induced endothelial cell hyperpolarization (Chen & Cheung 1992a), suggesting again that the endothelial action of the potassium channel blocker could be responsible for the inhibition of EDHF.
The present experiments were therefore designed to study the different types of potassium channels expressed in freshly isolated smooth muscle cells from the guinea-pig carotid artery and to determine the role of endothelial cells in the inhibitory effect of potassium channel blockers.
Methods
Male Hartley guinea-pigs (250–300 g) were anaesthetized by intraperitoneal administration of pentobarbitone (200 mg kg−1) and euthanized by exsanguination.
Patch-clamp studies
The media of guinea-pig carotid artery was dissected from cleaned arteries. The smooth muscle cells were dissociated enzymatically (Quignard et al., 1999a). Whole-cell potassium currents were recorded at room temperature using the patch-clamp technique. The cells were superfused with a solution containing (in mM): NaCl 125, KCl 5, CaCl2 2, MgCl2 1.2, HEPES 10 and glucose 11. In order to record Kv current, a calcium-free solution intracellular solution was used with the following composition (in mM): KCl 130, MgCl2 2, ATP 3, GTP 0.5, HEPES 25, EGTA 10, Glucose 11. In order to record KCa currents, the concentration of EGTA was reduced to 1 mM and CaCl2 (0.5 mM) was added. For the inside-out configuration the intra-pipette solution was (in mM): KCl 130, MgCl2 2, HEPES 10, CaCl2 2, Glucose 11 and the extracellular medium: KCl 130, MgCl2 2, ATP 3, GTP 0.5, HEPES 25, CaCl2 0.01, Glucose 11. For the outside-out configuration these last two solutions were exchanged.
Data were recorded with pClamp6 software (Axon Instruments, U.S.A.) through a RK-400 amplifier (Biologic, France). Passive capacitive currents and leakage currents were subtracted using the P/4 protocol. The cells that showed a leakage current larger than 20 pA, when clamped at a holding potential of −100 mV, were not studied.
Microelectrode studies
The internal carotid artery of the guinea-pig was dissected and cleaned of adherent connective tissues and then was pinned to the bottom of an organ chamber the adventitia upward to record smooth muscle membrane potential. In order to record the membrane potential of endothelial cells, the main carotid artery was dissected with the internal carotid artery and slit open at its junction with the internal branch. Microelectrodes were inserted through this gap and directed toward the endothelial cells of the internal carotid artery as described by Edwards et al. (1998). The tissues were superfused (at 37°C) with a modified Krebs-Ringer bicarbonate solution of the following composition (in mM): NaCl 118.3, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11.1 and EDTA 0.026. Transmembrane potential was recorded by using glass capillary microelectrodes (tip resistance of 30–90 MΩ) filled with KCl (3 M) and connected to the headstage of a recording amplifier (intra 767, WPI). Successful impalements were signalled by a sudden negative drop in potential from the baseline (zero potential reference) followed by a stable negative potential for at least 3 min. All the experiments were performed in the presence of Nω-nitro-L-arginine and indomethacin to inhibit nitric oxide synthase and cyclo-oxygenase, respectively (Corriu et al., 1996b).
Binding
In order to determine the pharmacological profile of carbenoxolone, its inhibitory properties toward the activity of various enzymes and its binding toward various receptors and ionic channels were investigated. These studies were performed by CEREP (Celle L'Evescault, France: http//www.cerep.fr).
Drugs
The following drugs were used: acetylcholine, carbenoxolone, substance-P, indomethacin, Nω-L-nitro-arginine, thiorphan, (Sigma); charybdotoxin, apamin (Latoxan,); 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO, Alexis Biochem); 1-ethyl-2-benzimidazolinone (1-EBIO, Aldrich). Perindoprilat and cromakalim were synthesized in Institut de Recherches Servier (Suresnes, France).
Statistics
Data are shown as mean±s.e.mean; n indicates the number of cells in which membrane potential was recorded. Statistical analysis was performed using Student's t-test for paired or unpaired observations. Differences were considered to be statistically significant when P was less than 0.05.
Results
Patch-clamp studies
Currents in the presence of intracellular calcium (0.5 mM)
The capacity of the carotid arterial myocytes was 27.4±2.9 pF (n=60). With a holding potential of −100 mV, depolarization of the myocytes induced a large whole-cell outward current which was partially inhibited by iberiotoxin (or charybdotoxin), by apamin or by 4-aminopyridine, indicating that it was the resultant of currents carried by different potassium channel types (Figure 1). After inhibition of the current by iberiotoxin (0.1 μM, Figure 1), addition of apamin (0.5 μM) further reduced the current induced by a step-depolarization to +60 mV. Subsequent, additional exposure to charybdotoxin (0.1 μM) did not induce a further inhibition of the whole-cell current but rather an increase which averaged +2±0.3% (n=4, Figure 1). 4-Aminopyridine inhibited the residual current (Figure 1, n=4).
Figure 1.
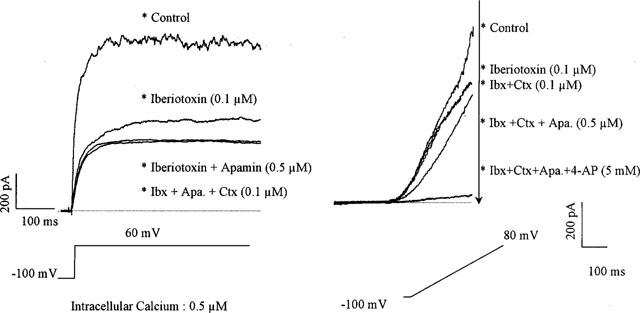
Effect of the combination of different inhibitors of calcium-activated potassium channel in freshly isolated smooth muscle cells of the guinea-pig carotid artery. Left panel: original traces of the large outward current observed in the presence of intracellular calcium (0.5 μM) for a step depolarization from −100 to + 60 mV (whole cell configuration of the patch-clamp technique). This current is partially inhibited by the presence of iberiotoxin (Ibx: 0.1 μM). The addition of apamin (Apa.: 0.5 μM) produces a further inhibition. The subsequent addition of charybdotoxin (Ctx: 0.1 μM) does not produce any further inhibition. However, the addition of 4-aminopyridine produces additional inhibition of the current (data not shown for the sake of clarity). Right panel: original traces of the large global currents observed in presence of intracellular calcium (0.5 μM) for a ramp depolarization from −100 to +80 mV (whole cell configuration of the patch-clamp technique). This current is partially inhibited by the presence of iberiotoxin (0.1 μM). The addition of charybdotoxin (0.1 μM) does not produce any further inhibition. However, the subsequent addition of apamin (0.5 μM) produces a further inhibition. The addition of 4-aminopyridine produces additional inhibition of the current.
With a holding potential of 0 mV (to inactivate voltage-dependent potassium channels, KV), and with a high concentration of intracellular calcium (0.5 μM), depolarization of the myocytes induced a noisy outward current (Figure 2). The current became activated at potentials more positive than +10 mV and its density at +60 mV was 14.6±2.2 pA/pF (n=31). This current was partially inhibited by charybdotoxin (0.1 μM, current density inhibited: 10.6±1.9 pA/pF, per cent inhibition: 75.1±6.2, n=7) or by iberiotoxin (0.1 μM; current density inhibited: 10.4±2.1 pA/pF, per cent inhibition: 80.1±3.3, n=21; Figure 2). The addition of iberiotoxin after charybdotoxin or the addition of charybdotoxin after iberiotoxin did not induce any further inhibition that either toxin alone (current density inhibited 9.8±2.1 and 10.6±2.9, pA/pF, per cent inhibition: 78.2±4.5 and 75.6±6.1, n=3 and 5 for the combination of charybdotoxin plus iberiotoxin and the combination iberiotoxin plus charybdotoxin, respectively).
Figure 2.
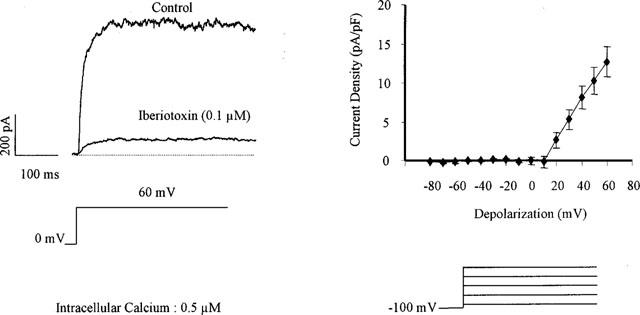
Iberiotoxin-sensitive current in freshly isolated smooth muscle cells of the guinea-pig carotid artery. Left panel: original traces of the large outward current observed in the presence of intracellular calcium (0.5 μM) for a step depolarization from 0 to +60 mV and its inhibition by the presence of iberiotoxin (0.1 μM, whole cell configuration of the patch-clamp technique). Right panel: Current-voltage relationship of the iberiotoxin-sensitive current (n=5).
A potassium current sensitive to apamin (0.5 μm) could also be observed in 50% of the cells (10 out of 20, current density at 60 mV: 2.6±2.1 pA/pF, n=10, Figure 3). In the presence of iberiotoxin to block BKCa, the apamin-sensitive current could still be recorded (1.8±2.1 pA/pF, n=6; Figure 1).
Figure 3.
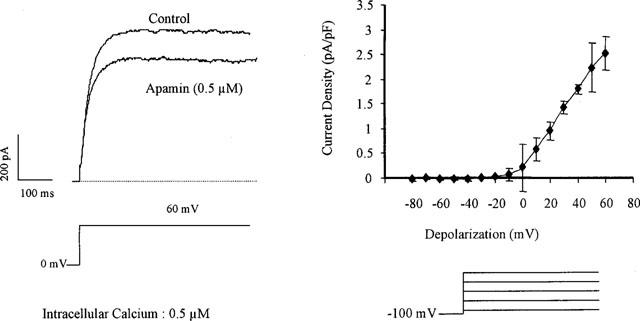
Apamin-sensitive current in freshly isolated smooth muscle cells of the guinea-pig carotid artery. Left panel: original traces of the large outward current observed in the presence of intracellular calcium (0.5 μM) for a step depolarization from 0 to +60 mV and its partial inhibition by the presence of apamin (0.5 μM, whole cell configuration of the patch-clamp technique). Right panel: Current-voltage relationship of the apamin-sensitive current (n=4).
Currents in the presence of a low intracellular calcium concentration
At a holding potential of −100 mV and with a low intracellular calcium concentration, step-depolarizations induced a slowly-inactivating current (Figure 4). Its activation threshold was −30 mV and the current density was 4.5±1.5 pA/pF (n=20) for a step-depolarization to +20 mV. 4-Aminopyridine (0.1–25 mM) inhibited this current in a concentration-dependent manner (Figures 4 and 7). The 4-aminopyridine-sensitive potassium current was voltage-dependent. The voltage for half-inactivation calculated from the steady state curve of the current inhibited by 4-aminopyridine (5 mM) averaged −24±1.5 mV (n=3, Figure 4). An iberiotoxin-sensitive current could be recorded only for depolarizations to potentials more positive than +20 mV (data not shown). 4-Aminopyridine (5 mM) did not affect the potassium currents sensitive to iberiotoxin or apamin (data not shown).
Figure 4.
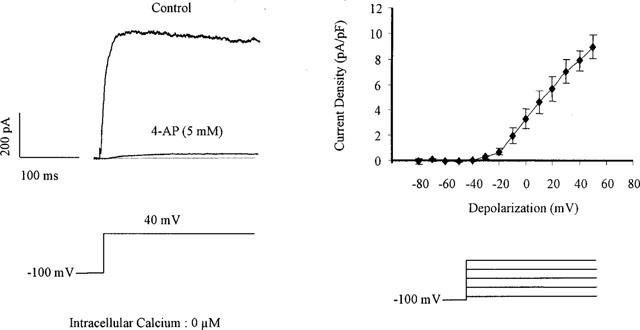
4-Aminopyridine-sensitive current in freshly isolated smooth muscle cells of the guinea-pig carotid artery. Left panel: original traces of the large outward current observed in the presence of very low concentration of intracellular calcium for a step depolarization from −100 to 40 mV and its inhibition by the presence of 4-aminopyridine (4-AP: 5 mM, whole cell configuration of the patch-clamp technique). Right panel: Current-voltage relationship of the 4-aminopyridine-sensitive current (n=7).
Figure 7.
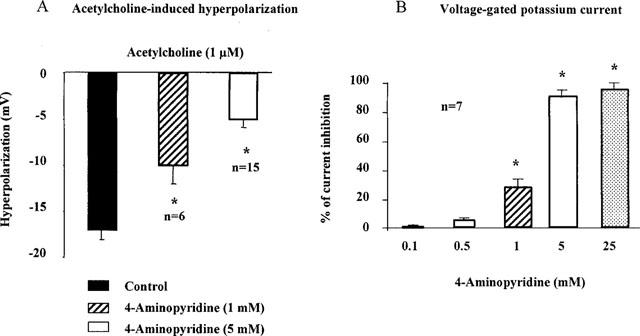
4-Aminopyridine and the smooth muscle cells of the guinea-pig carotid artery. Left panel: endothelium-dependent hyperpolarization of the smooth muscle cells of isolated fragments of internal carotid artery, measured with intracellular microelectrode. Right panel: voltage-gated potassium currents in freshly isolated smooth muscle cells of the guinea-pig carotid artery: presence of very low concentration of intracellular calcium for a step depolarization from −100 to 40 mV (whole cell configuration of the patch-clamp technique). 4-Aminopyridine induces a concentration-dependent inhibition of the acetylcholine-induced hyperpolarization and of the voltage-gated potassium currents.
Currents in inside-out and outside-out patches
Unitary currents through large-conductance, calcium-sensitive potassium channels were recorded in inside-out and outside-out membrane patches. The amplitude of the current and the channel open probability were dependent on the holding potential. With the inside-out configuration, from the current-voltage relationship only one single channel type with a unitary conductance of 280±20 pS (n=5) was observed. With the outside-out configuration, at a stable holding potential of +30 mV the open probability of the unitary curent was reduced by the presence of iberiotoxin (0.1 μM, 0.15±0.05 and 0.02±0.01, n=3 in control and presence of iberiotoxin, respectively; Figure 5). Under the same conditions, application of 4-aminopyridine (5 mM) did not modify the current kinetics (open probability 0.10±0.04 and 0.09±0.04, n=2, in control and presence of 4-aminopyridine, respectively; Figure 5).
Figure 5.
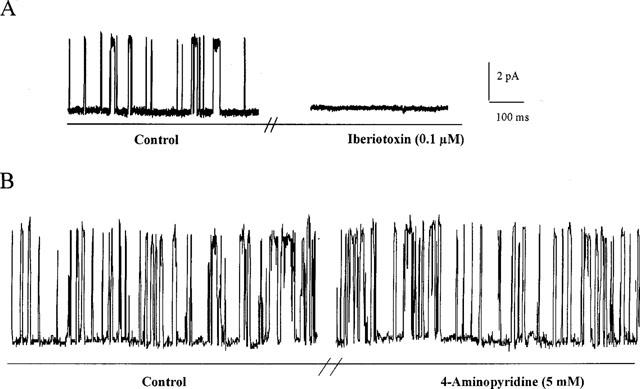
Currents recorded with the outside-out configuration of the patch-clamp technique. At a stable holding potential of +30 mV, unitary currents through large-conductance, calcium-sensitive potassium channels are recorded. (A) The unitary current is abolished by the presence of iberiotoxin (0.1 μM); (B) Under the same conditions, application of 4-aminopyridine (5 mM) did not modify the current kinetics.
Intracellular microelectrode studies
Smooth muscle cells
In the presence of Nω-nitro-L-arginine (100 μM) and indomethacin (5 μM), the resting membrane potential of smooth muscle cells in isolated guinea-pig carotid arteries was −52.7±0.9, n=74 and acetylcholine (1 μM) induced an endothelium-dependent hyperpolarization (17.1±1.0 mV, n=25; Figure 6). In the presence of a higher bath concentration of potassium (35 mM), the cell membrane was depolarized to −32.8±1.9 mV (n=9) and acetylcholine did not evoke significant changes in membrane potential (n=3). In potassium-free solution the membrane potential was −49.8±4.3 mV (n= 6) and acetylcholine induced a significantly larger hyperpolarization (38.0±3.7 mV; n=4) than in the control solution.
Figure 6.
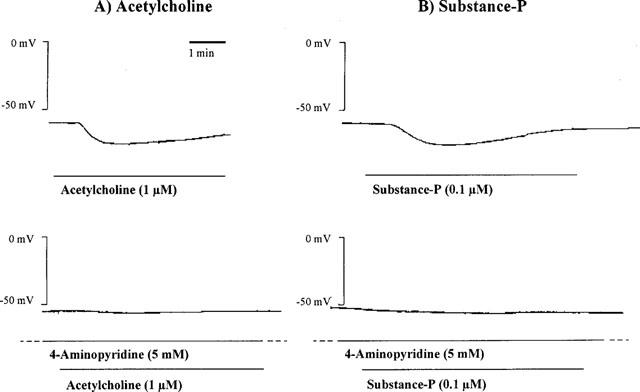
Endothelium-dependent hyperpolarization produced by acetylcholine (1 μM, left panel) and substance P (0.1 μM, right panel) in an isolated guinea-pig internal carotid artery, in presence of L-nitroarginine (100 μM) and indomethacin (5 μM). 4-Aminopyridine (5 mM) produces a significant inhibition of the hyperpolarizations produced by both mediators [experiments involving substance P were performed in the presence of thiorphan (1 μM) and perindoprilat (1 μM)].
4-Aminopyridine alone (up to 5 mM) did not significantly affect the resting membrane potential of vascular smooth muscle cells (−50.9±1.1 mV, n=21). At 1 and 5 mM, it produced a concentration-dependent inhibition of the acetylcholine-induced hyperpolarization (Figures 6 and 7). However, even in the presence of 5 mM 4-aminopyridine there was a residual hyperpolarization (5.1+0.9 mV, n=15) which was not affected by the presence of the NO scavenger, carboxy-PTIO (10 μM, hyperpolarization 5.1±0.6 mV, n=4), charybdotoxin (0.1 μM, hyperpolarization 4.2±1.1 mV, n=3) or apamin (0.1 μM, hyperpolarization 4.7±0.6 mV, n=8).
Under control conditions, substance P did not evoke reproducible endothelium-dependent hyperpolarization when smooth muscle cells were impaled from the adventitial side of the guinea-pig carotid artery (data not shown; n=4). However, in the presence of thiorphan (1 μM) and perindoprilat (1 μM), which did not modify the resting membrane potential (−51.4±0.6 mV, n=11), substance P (0.1 μM) produced consistent and reproducible hyperpolarizations (15.6±0.4 mV, n=11, Figure 6). These were transient (144±8 s, n=5) in contrast to the sustained hyperpolarizations produced by acetylcholine. There was no cross-desensitisation between acetylcholine and substance P (data not shown). 4-Aminopyridine (5 mM) produced a significant inhibition of the hyperpolarization evoked by substance P (3.9± 0.8 mV, n=6, resting membrane potential −51.2±1.7 mV, n=6, Figure 6).
4-Aminopyridine (5 mM) did not significantly affect the hyperpolarization to cromakalim (10 μM; 19.5±2.4 mV, n=3 and 19.9±1.3 mV, n=7 in control and presence of 4-aminopyridine, respectively).
Endothelial cells
In presence of of Nω-nitro-L-arginine (300 μM) and indomethacin (10 μM), the resting membrane potential of carotid artery endothelial cells was −58.7±0.4 mV (n=4). Acetylcholine (10 μM), substance P (100 nM) and levcromakalim (10 μM) produced a hyperpolarization of the endothelial cells (19.4±0.3, 18.4±0.3 and 24.5±0.9 mV, n=4, respectively). Under these conditions, brief exposure to 100 nM substance P did not show marked tachyphylaxis over the time-course of the experiment (Figure 8). Similarly, the responses to 10 μM acetylcholine and levcromakalim were reproducible. However, after 5 min exposure to 5 mM 4-AP, the hyperpolarizations to acetylcholine and to substance P were significantly inhibited while the response to levcromakalim remained unaffected (Figure 8).
Figure 8.
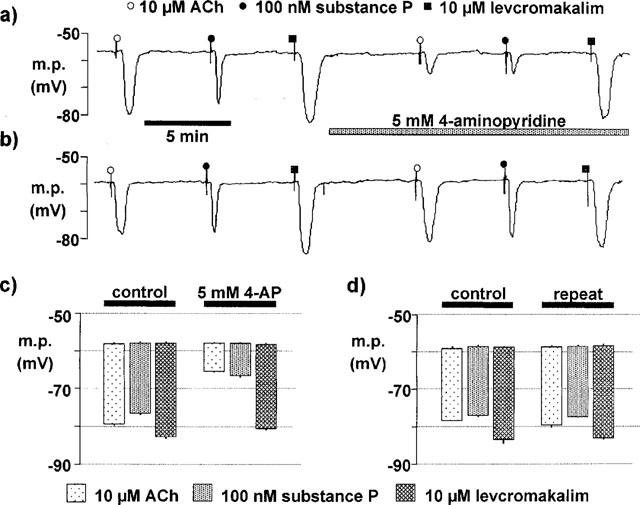
4-Aminopyridine (4-AP) and the endothelial cells of the guinea-pig carotid artery. (a) Original trace showing the hyperpolarizations of an endothelial cell from an isolated fragment of internal carotid artery in response to acetylcholine (ACh: 10 μM), substance P (100 nM) and levcromakalim (10 μM) before and after the administration of 4-aminopyridine (5 mM). (b) Time control showing the reproducibility of the hyperpolarizations of an endothelial cell in response to acetylcholine, substance P and levcromakalim. (c) Summary showing the average membrane potential (m.p.) of the endothelial cells and the average hyperpolarization produced by acetylcholine, substance P and levcromakalim, in control conditions and in the presence of 4-aminopyridine. (d) Summary showing the average membrane potential (m.p.) of the endothelial cells and the average hyperpolarization for time controls. Data are shown as means±s.e.mean (n=4). 4-Aminopyridine (5 mM) induces a significant inhibition of the acetylcholine- and substance P-induced endothelial hyperpolarizations.
The gap junction inhibitor, carbenoxolone (100 μM), did not modify the resting membrane potential of the internal carotid artery endothelial cells (−57.3±0.5 mV, n=5). In the presence of carbenoxolone, acetylcholine (10 μM) hyperpolarized the endothelial cells by 20.5±0.6 mV (n=5). 4-Aminopyridine (5 mM) did not produce any significant changes in the resting membrane potential (−56.6±0.5 mV; n=5) but significantly inhibited the hyperpolarization produced by acetylcholine (6.0±0.9 mV; n=5, P<0.05). In contrast, the hyperpolarization produced by 1-EBIO (600 μM) observed in presence of carbenoxolone was not affected by 4-aminopyridine (Figure 9).
Figure 9.
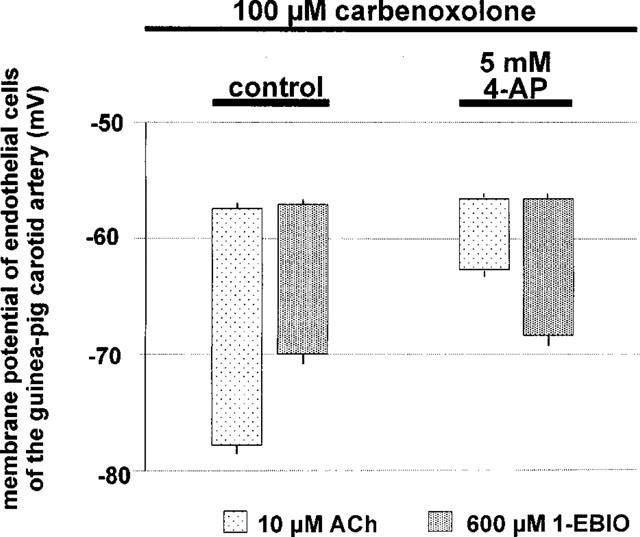
Membrane potential of endothelial cells of the guinea-pig carotid artery in presence of carbenoxolone. Summary showing the average membrane potential (m.p.) of the endothelial cells and the average hyperpolarization produced by acetylcholine (10 μM) and 1-EBIO (600 μM) in control conditions and in the presence of 4-aminopyridine (5 mM). Data are shown as means±s.e. mean (n⩾4). 4-Aminopyridine (5 mM) induces a significant inhibition of the acetylcholine-induced endothelial hyperpolarizations but does not significantly affect 1-EBIO.
Binding
In binding experiments, carbenoxolone (0.1–100 μM), at the highest concentration, tested induced a displacement superior to 50% of mibolerone (59%; ligand of the testosterone receptor) and of picrotoxinine (82%; chloride channel) but exhibited little or no affinity for the other 35 other receptors (including muscarinic) and ion channels (including potassium) tested (data not shown).
Discussion
Calcium-sensitive K+ channels in the smooth muscle
In the present study, two different types of calcium-dependent potassium channel were observed in the smooth muscle cells. Using the whole-cell configuration, a voltage-dependent noisy outward current sensitive to iberiotoxin was recorded. Under the patch configurations inside-out and outside-out, a large unitary conductance potassium channel was observed, the open probability of which was significantly diminished by the same toxin. These observations thus demonstrate the presence of the channel known as ‘BKCa' (Kuriyama et al. 1995). Under the experimental conditions of the present study, charybdotoxin and iberiotoxin each inhibited the same population of potassium channels.
In the presence of intracellular calcium, an apamin-sensitive current was also detected in some muscle cells, even in the presence of iberiotoxin. Such a finding could indicate the presence of the small conductance, calcium-sensitive potassium channel known as SKCa. However, the current carried by classical SKCa channels should be voltage-independent (Latorre et al. 1989), whereas the observed current was voltage-sensitive with an activation threshold of approximately −30 mV. It thus resembles that reported in the smooth muscle cells of the renal arterioles of the rat (Gebremedhin et al. 1996).
A conductance specifically sensitive to the combination of charybdotoxin plus apamin, and which was distinct from BKCa, could not be recorded in the isolated smooth muscle cells of the guinea-pig carotid artery. This confirms previous observations in the rat hepatic artery (Zygmunt et al. 1997) and comforts the hypothesis that the inhibitory effect of the combination of these two toxins on EDHF-mediated responses could be attributed to an effect on the endothelial cells (Edwards et al., 1998; Ohashi et al., 1999; Doughty et al., 1999). However, it cannot be excluded that the presence of a stimulant (EDHF) might be required to observe this conductance in the smooth muscle cells.
K+-channel inhibition by 4-aminopyridine
In the present study, a current typical of that carried by delayed rectifier channels (KV) was present in the smooth muscle cells of the guinea-pig carotid artery. It exhibited a threshold voltage for activation of approximately −30 mV and inactivated with slow kinetics. However, the rapidly inactivating (type A) channel was not observed under our experimental conditions (for review see Kuriyama et al., 1995). The recorded current (IK(V)) could be inactivated using a holding potential of 0 mV and was inhibited by 4-aminopyridine (for review, see Faraci et al., 1998).
Inhibition of EDHF by 4-AP
In the guinea-pig carotid artery, acetylcholine and substance P induced an endothelium-dependent hyperpolarization which has been attributed to EDHF (Corriu et al., 1996a; Chataigneau et al., 1998) and which is inversely related to the extracellular potassium concentration. In the guinea-pig carotid artery, previous reports suggested that endothelium-dependent hyperpolarization in response to substance P were observed exclusively when impalements were performed from the intimal side of the vessel (Zhang et al., 1994; Corriu et al., 1996b). The present study shows that in the presence of inhibitors of neutral endopeptidase and angiotensin converting enzyme, the hyperpolarization to substance P can be recorded even when the impalements are performed from the adventitial side. The presence of thiorphan and perindoprilat prevents enzymatic degradation of substance P by the vascular wall, thus permitting it to reach the endothelial cells even when given from the adventitial side. The transient nature of the hyperpolarization to substance P can be attributed to a rapid desensibilization of the endothelial tachykinin receptors since acetylcholine still can induce hyperpolarization in the presence of substance P.
In the present study, the endothelium-dependent hyperpolarization of smooth muscle cells by acetylcholine or substance P was inhibited by 4-aminopyridine. Although EDHF-mediated changes resistant to this agent have been described in some vessels (Petersson et al., 1997; Murphy & Brayden 1995; Ohlmann 1997), 4-aminopyridine inhibits responses attributed to EDHF in the guinea-pig coronary, porcine coronary and rat mesenteric arteries (Eckman et al. 1998; Shimizu & Paul, 1998; Cheung et al., 1999). A possible explanation for these apparent discrepancies could be the use of different concentrations of 4-aminopyridine. Thus, the present study clearly shows that substantial inhibition of EDHF requires a concentration of 5 m 4-aminopyridine whereas 1 mM produces only minor inhibition (Nishiyama et al., 1998).
In millimolar concentrations, 4-aminopyridine can, depending on the tissue studied, block or activate muscarinic receptors (Urquhart & Broadley, 1991; Navarro-Polanco & Sanchez-Chapula, 1997) but an action on these is unlikely to explain its inhibition of EDHF. Thus, at 5 mM, 4-aminopyridine did not significantly reduce the affinity of acetylcholine in the guinea-pig trachea (pD2: −5.6±0.1 and −6.1±0.1, n=4 in absence and presence of 5 mM of 4-aminopyridine, respectively; unpublished observations). Furthermore, the inhibition of endothelium-dependent hyperpolarizations to substance P (which interacts with NK1 receptors) cannot be attributed to an inhibitory effect at muscarinic receptors. Under the present experimental conditions, 4-aminopyridine is unlikely to interact with the other populations of potassium channel studied. Indeed, this compound did not affect the smooth muscle hyperpolarizations produced by cromakalim, indicating that it does not inhibit KATP. Furthermore, in the patch-clamp experiments performed on isolated smooth muscle cells of the guinea-pig carotid artery, 4-aminopyridine did not inhibit IBK(Ca), ISK(Ca) or the inwardly rectifying potassium channel (Quignard et al., 1999b).
Therefore, as the concentration of this agent required to inhibit IK(V) was the same as that necessary to antagonize EDHF, it could indicate that the target for EDHF is the smooth muscle KV. However, its activation threshold of −30 mV means that this channel is unlikely to be able to sustain a hyperpolarization which shifts the membrane potential into the region of −80 mV.
Endothelial cell actions of 4-aminopyridine
Impalement of endothelial cells with microelectrodes showed that acetylcholine or substance P produced endothelial cell hyperpolarizations which were also markedly inhibited by 4-aminopyridine. These findings confirm those of an earlier study in which 4-aminopyridine was found to inhibit the endothelial hyperpolarization produced by acetylcholine in the guinea-pig coronary arteries (Chen & Cheung, 1992a). Carbenoxolone, a succinate salt of glycyrrhetinic acid, is an inhibitor of gap junction (Yamamoto et al., 1998; 1999; Taylor et al., 1998). This compound has also been described as an inhibitor of both cyclic GMP- and cyclic AMP-dependent phosphodiesterases (Vapaatalo et al., 1978), of Na+/K+-ATPase (Terasawa et al., 1992) and 11β-hydroxysteroid-dehydrogenase (Walker et al., 1991). However, in binding experiments, carbenoxolone exhibited little or no affinity for 35 different receptors (including muscarinic) and ion channels (including potassium). Furthermore, in the guinea-pig carotid artery and in the rat hepatic and mesenteric arteries, the effect of carbenoxolone and GAP 27 (a peptide inhibitor of gap junction; Chaytor et al., 1998) on EDHF-mediated responses were similar (Edwards et al., 1999a). Collectively these data suggest that under our experimental conditions the effect of carbenoxolone should most likely being attributed to gap junction inhibition than to other undesirable side effects.
Since this inhibition of substance P-induced endothelial cell hyperpolarization by 4-aminopyridine, is still observed in the presence of carbenoxolone, the recorded changes in membrane potential must have originated in the endothelial cells and not have been conducted to the endothelium from the smooth muscle cells (Dora et al., 1997). Furthermore, 1-EBIO a specific opener of calcium activated potassium channel of intermediate conductance (Cai et al., 1998; Jensen et al., 1998) produced an endothelial hyperpolarization in presence of carbenoxolone. This benzimidazolone does not activate BKca and does not hyperpolarize smooth muscle cells (Edwards et al., 1999b) further suggesting that the membrane potential is recorded from endothelial cells.
Recent studies (Edwards et al., 1998; Ohashi et al., 1999) have shown that the site of action of the combination of charybdotoxin plus apamin is likely to be small and intermediate conductance Ca2+-sensitive potassium channels (SKCa and IKCa, respectively) present on endothelial cells. 4-AP did not inhibit the effects of 1-EBIO indicating that IKCa are not affected by the potassium channel blocker. Furthermore, in the present study, the inhibitory effect of 4-aminopyridine was not increased by apamin or charybdotoxin, suggesting that 4-aminopyridine acts on endothelial cell but independently to the targets of these two toxins possibly at a step proximal to the activation of SKCa and IKCa. A detailed analysis of the exact site of action of 4-aminopyridine was beyond the scope of this study. However, agonist-induced hyperpolarization of endothelial cells is dependent upon the influx of extracellular calcium and the release of calcium from intracellular pools (Mehrke & Daut, 1990; Chen & Cheung, 1992b). It is therefore tempting to speculate that 4-aminopyridine inhibits this endothelial cell Ca2+ release process, evidence for which was recently reported (Wood & Gillespie, 1998).
Conclusions
The present study suggests that the agonist-induced smooth muscle hyperpolarization attributed to EDHF in the guinea-pig internal carotid artery is dependent on the hyperpolarization of vascular endothelial cells. The site of the inhibitory action of 4-aminopyridine is unlikely to be the smooth muscle delayed rectifier channel. Instead, the effects of this agent are probably exerted on the endothelial cell, also the site at which charybdotoxin and apamin inhibit EDHF.
Abbreviations
- 4-AP
4-aminopyridine
- BKCa
large conductance calcium-activated potassium channels
- carboxy-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- 1-EBIO
1-ethyl-2-benzimidazolinone
- Kv
voltage dependent potassium channels
- SKCa
small conductance calcium-activated potassium channels
References
- BOLTON T.B., LANG R.J., TAKEWAKI T. Mechanism of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J. Physiol. 1984;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAI S., GARNEAU L., SAUVE R. Single-channel characterization of the pharmacological properties of the K(Ca2+) channel of intermediate conductance in bovine aortic endothelial cells. J. Membr. Biol. 1998;163:147–158. doi: 10.1007/s002329900379. [DOI] [PubMed] [Google Scholar]
- CHATAIGNEAU T., FÉLÉTOU M., DUHAULT J., VANHOUTTE P.M. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br. J. Pharmacol. 1998;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. 1998;508:561–73. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., CHEUNG D.W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ. Res. 1992a;70:257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- CHEN G., CHEUNG D.W. Pharmacological distinction of the hyperpolarization response to caffeine and acetylcholine in guinea-pig coronary endothelial cells. Eur. J. Pharmacol. 1992b;223:33–38. doi: 10.1016/0014-2999(92)90815-l. [DOI] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H. Some electrical properties of the endothelium-dependent hyperpolarisation recorded from rat arterial smooth muscle cells. J. Physiol. 1989a;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H. Direct and indirect action of acetylcholine and histamine on intrapulmonary artery and vein smooth muscles of the rat. Jap. J. Physiol. 1989b;39:51–65. doi: 10.2170/jjphysiol.39.51. [DOI] [PubMed] [Google Scholar]
- CHEN G., YAMAMOTO Y., MIWA K., SUZUKI H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am. J. Physiol. 1991;260:H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- CHEUNG D.W., CHEN G., BURNETTE E., LI G.Potassium channels involved in hyperpolarization and relaxation induced by endothelium-derived hyperpolarizing factor in rat mesenteric arteries Endothelium-Derived Hyperpolarizing Factor 19992Amsterdam: The Netherlands, Harwood Academic Publishers; 209–217.P.M. Vanhoutte, ed. ppin press [Google Scholar]
- CORRIU C., FÉLÉTOU M., CANET E., VANHOUTTE P.M. Endothelium-derived factors and hyperpolarization of the carotid artery of the guinea-pig. Br. J. Pharmacol. 1996a;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRIU C., FÉLÉTOU M., CANET E., VANHOUTTE P.M. Inhibitors of the cytochrome P450-monooxygenase and endothelium-dependent hyperpolarisations in the guinea-pig isolated carotid artery. Br. J. Pharmacol. 1996b;117:607–610. doi: 10.1111/j.1476-5381.1996.tb15233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA K.A., DOYLE M.P., DULING B.R. Elevation of intracellular calcium in smooth muscle causes endothelial generation of NO in arterioles. Proc. Ntl. Acad. Sci. U.S.A. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- ECKMAN D.M., HOPKINS N.O., MCBRIDE C., KEEF K.D. Endothelium-dependent relaxation and hyperpolarization in guinea-pig coronary artery: role of epoxyeicosatrienoic acid. Br. J. Pharmacol. 1998;124:181–189. doi: 10.1038/sj.bjp.0701778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., FÉLÉTOU M., GARDENER M.J., THOLLON C., VANHOUTTE P.M., WESTON A.H. Role of gap junction in the responses to EDHF in rat and guinea-pig small arteries. Br. J. Pharmacol. 1999a;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., GARDENER M.J., FÉLÉTOU M., BRADY G., VANHOUTTE P.M., WESTON A.H. Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br. J. Pharmacol. 1999b;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARACI F.M., HEISTAD D.D. Regulation of the cerebral circulation: role of endothelium and potassium channel. Physiol. Rev. 1998;78:54–75. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- FÉLÉTOU M., VANHOUTTE P.M. Endothelium-dependent hyperpolarisation of canine coronary smooth muscle. Br. J. Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F.Relative importance of endothelium-derived hyperpolarizing factor for the relaxation of vascular smooth muscle in different arterial beds Endothelium-Derived Hyperpolarizing Factor 19961Amsterdam, The Netherlands: Harwood Academic Publishers; 173–179.ed. P.M. Vanhoutte. pp [Google Scholar]
- GEBREMEDHIN D., KALDUNSKI M., JACOBS E.R., HARDER D., ROMAN R.J. Coexistance of two types of calcium activated potassium channels in rat renal arterioles. Am. J. Physiol. 1996;270:F69–F81. doi: 10.1152/ajprenal.1996.270.1.F69. [DOI] [PubMed] [Google Scholar]
- JENSEN B.S., STROBAEK D., CHRISTOPHERSEN P., JORGENSEN T.D., HANSEN C., SILAHTAROGLU A., OLESEN S.P., AHRING P.K. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am. J. Physiol. 1998;275:C848–C856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., NABATA H. Pharmacological and physiological significance of ion channels and factors that modulate then in vascular tissues. Pharmacol. Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- LATORRE R., OBERHAUSER A., LABARCA P., ALVAREZ O. Varieties of calcium-activated potassium channels. Ann. Rev. Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Calcium-activated potassium channels in the endothelium-of intact rat aorta. J. Physiol. (London) 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHRKE G., DAUT J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J. Physiol. 1990;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., VANE J.R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2 and prostacyclin. Pharmacol. Rev. 1979;30:293–331. [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J. Physiol. 1995;489:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAO T., VANHOUTTE P.M. Hyperpolarisation as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J. Physiol. 1992;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAVARRO-POLANCO R.A., SANCHEZ-CHAPULA J.A. 4-Aminopyridine activates potassium currents by activation of a muscarinic receptor in feline atrial myocytes. J. Physiol. 1997;498:663–678. doi: 10.1113/jphysiol.1997.sp021891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIYAMA M., HASHITANI H., FUKUTA H., YAMAMOTO Y., SUZUKI H. Potassium channels activated in the endothelium-dependent hyperpolarization in guinea-pig coronary artery. J. Physiol. 1998;510:455–65. doi: 10.1111/j.1469-7793.1998.455bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHASHI M., SATOH K., ITOH T. Acetylcholine-induced membrane potential changes in endothelial cells of rabbit aortic valve. Br. J. Pharmacol. 1999;126:19–26. doi: 10.1038/sj.bjp.0702262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHLMANN P., MARTINEZ M.C., SCHNEIDER F., STOCLET J.C., ANDRIANTSITOHAINA R. Characterization of endothelium-derived relaxaing factors released by bradykinin in human resistance arteries. Br. J. Pharmacol. 1997;121:657–664. doi: 10.1038/sj.bjp.0701169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSSON J., ZYGMUNT P.M., HÖGESTÄTT E.D. Characterization of the potassium channels involved in EDHF-mediated relaxation in cerebral arteries. Br. J. Pharmacol. 1997;120:1344–1350. doi: 10.1038/sj.bjp.0701032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUIGNARD J-F., CHATAIGNEAU T., CORRIU C., DUHAULT J., FÉLÉTOU M., VANHOUTTE P.M.Potassium Channels Involved in EDHF-Induced Hyperpolarization of the Smooth Muscle Cells of the Isolated Guinea-Pig Carotid Artery Endothelium-Derived Hyperpolarizing Factor 1999bVol 2Amsterdam: Harwood Academic Publishers; 201–208.P.M. Vanhoutte, ed. pp [Google Scholar]
- QUIGNARD J-F., FÉLÉTOU M., THOLLON C., VILAINE J-P., DUHAULT J., Vanhoutte P.M. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br. J. Pharmacol. 1999a;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMIZU S., PAUL R.J. The endothelium-dependent, substance P relaxation of porcine coronary arteries resistant to nitric oxide synthesis inhibition is partially mediated by 4-aminopyridine-sensitive voltage dependent potassium channels. Endothelium. 1998;5:287–295. doi: 10.3109/10623329709052593. [DOI] [PubMed] [Google Scholar]
- TAYLOR H.J., CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Inhibition of the gap junctional component of endothelium-dependent relaxations in rabbit iliac artery by 18β-glycyrrhetinic acid. Br. J. Pharmacol. 1998;125:1–3. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR S.G., SOUTHERTON J.S., WESTON A.H., BAKER J.R.J. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br. J. Pharmacol. 1988;94:853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR S.G., WESTON A.H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol. Sci. 1988;9:272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- TERASAWA T., OKADA T., HARA T., ITOH K. Glycyrrhetinic acid derivatives as potent inhibitors of Na+, K+-ATPase. Synthesis and structure-activity relationships. Eur. J. Med. Chem. 1992;27:345–351. [Google Scholar]
- URQUHART R.A., BROADLEY K.J. Blockade by 4-aminopyridine of the muscarinic-receptor-mediated responses of guinea-pig atria and trachea. J. Pharm. Pharmacol. 1991;43:417–420. doi: 10.1111/j.2042-7158.1991.tb03500.x. [DOI] [PubMed] [Google Scholar]
- VAN DE VOORDE J., VANHEEL B., LEUSEN I. Endothelium-dependent relaxation and hyperpolarisation in aorta from control and renal hypertensive rats. Circ. Res. 1992;70:1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- VAPAATALO H., LINDÉN I.B., METSÄ-KETELÄ T., KANGASAHO M., LAUSTIOLA K. Effect of carbenoxolone on phosphodiesterase and prostaglandin synthetase activities. Experentia. 1978;34:384–385. doi: 10.1007/BF01923050. [DOI] [PubMed] [Google Scholar]
- WALKER B.R., YAU Y.L., BRETT L.P., SECKL J.R., MONDER C., WILIAMS B.C., EDWARDS C.R. 11-betahydroxysteroid dehydrogenase in vascular smooth muscle and heart: implications for cardiovascular response to glucocorticoides. Endocrinology. 1991;129:3305–3312. doi: 10.1210/endo-129-6-3305. [DOI] [PubMed] [Google Scholar]
- WOOD P.G., GILLESPIE J.I. In permeabilised endothelial cells IP3-induced Ca2+ release is dependent on the cytoplasmic concentration of monovalent cations. Cardiovasc. Res. 1998;37:263–270. doi: 10.1016/s0008-6363(97)00207-1. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y., FUKUTA H., NAKAHIRA Y., SUZUKI H. Blockade by 18β-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. J. Physiol. 1998;511:501–508. doi: 10.1111/j.1469-7793.1998.501bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO Y., IMAEDA K., SUZUKI H. Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. J. Physiol. 1999;514:505–513. doi: 10.1111/j.1469-7793.1999.505ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANAKA A., ISHIKAWA T., GOTO K. Characterization of endothelium-dependent relaxation independent of NO and prostaglandins in guinea pig coronary artery. J. Pharmacol. Exp. Ther. 1998;285:480–489. [PubMed] [Google Scholar]
- ZHANG G, YAMAMOTO Y, MIWA K, SUZUKI H. Vasodilation induced by substance P in the guinea-pig carotid artery. Am. J. Physiol. 1994;266:H1132–H1137. doi: 10.1152/ajpheart.1994.266.3.H1132. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., EDWARDS G., WESTON A.H., LARSSON B., HOGESTATT E.D. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of the rat hepatic artery. Br. J. Pharmacol. 1997;121:141–149. doi: 10.1038/sj.bjp.0701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D.Endothelium-dependent hyperpolarization and relaxation in the hepatic artery of the rat Endothelium-Derived Hyperpolarizing Factor 19961Amsterdam: The Netherlands, Harwood Academic Publishers; 191–201.ed. P.M. Vanhoutte pp [Google Scholar]


