Abstract
The influence of several antioxidants (bilirubin, urate, ascorbate, α-tocopherol, glutathione (GSH), Cu/Zn superoxide dismutase (SOD) and the manganese SOD mimic EUK-8) on nitrergic relaxations induced by either exogenous nitric oxide (NO; 10−5 M) or electrical field stimulation (4 Hz; 10 s and 3 min) was studied in the pig gastric fundus.
Ascorbate (5×10−4 M), α-tocopherol (4×10−4 M), SOD (300–1000 u ml−1) and EUK-8 (3×10−4 M) did not influence the relaxations to exogenous NO. In the presence of GSH (5×10−4 M), the short-lasting relaxation to NO became biphasic, potentiated and prolonged. Urate (4×10−4 M) and bilirubin (2×10−4 M) also potentiated the relaxant effect of NO. None of the antioxidants influenced the electrically evoked relaxations.
6-Anilino-5,8-quinolinedione (LY83583; 10−5 M) had no influence on nitrergic nerve stimulation but nearly abolished the relaxant response to exogenous NO. Urate and GSH completely prevented this inhibitory effect, while it was partially reversed by SOD and bilirubin. Ascorbate, α-tocopherol and EUK-8 were without effect.
Hydroquinone (10−4 M) did not affect the electrically induced nitrergic relaxations, but markedly reduced NO-induced relaxations. The inhibition of exogenous NO by hydroquinone was completely prevented by urate and GSH. SOD and ascorbate afforded partial protection, while bilirubin, EUK-8 and α-tocopherol were ineffective.
Hydroxocobalamin (10−4 M) inhibited relaxations to NO by 50%, but not the electrically induced responses. Full protection versus this inhibitory effect was obtained with urate, GSH and α-tocopherol.
These results strengthen the hypothesis that several endogenous antioxidant defense mechanisms, enzymatic as well as non-enzymatic, might play a role in the nitrergic neurotransmission process.
Keywords: pig gastric fundus, LY83583, hydroquinone, hydroxocobalamin, bilirubin, urate, glutathione, superoxide dismutase, EUK-8, nitric oxide
Introduction
Peripheral nitrergic neurotransmission, mediating smooth muscle relaxation, is well established in the gastrointestinal, urogenital and respiratory tracts. The term ‘nitrergic' refers to nerves whose transmitter function depends on the release of NO (Moncada et al., 1997). Although it is accepted that the neurotransmitter, released from nitrergic nerves, is synthetized by neuronal NO-synthase in the nerves (Förstermann & Kleinert, 1995), there still remains doubt on the exact biochemical identity of the nitrergic neurotransmitter (Rand & Li, 1995). This is based on observations that superoxide anion generators and NO-scavengers discriminate between exogenous NO and the nitrergic neurotransmitter: they inhibit the relaxation by exogenous NO but not that by activation of nitrergic nerves (Hobbs et al., 1991; Barbier & Lefebvre, 1992).
It has been suggested that the endogenous neurotransmitter is not free NO but NO linked to a carrier before release into the neuroeffector region. In this region, the carrier would protect NO versus superoxide anion generators and scavengers and release NO upon interaction with the effector smooth muscle cells. S-nitrosothiols seemed the most probable candidates to act as NO carriers (Gibson et al., 1992; Barbier & Lefebvre, 1994) but the similarity between the pharmacological properties of the nitrergic neurotransmitter and S-nitrosothiols was not complete in all tissues (Iversen et al., 1994; De Man et al., 1995). Another possibility is that the nitrergic neurotransmitter is nitrogen monoxide but that it is synthetized as or converted to another redox form i.e. nitrosonium (NO+) or nitroxyl (NO−) before leaving the neuron (Gibson et al., 1995). This hypothesis is difficult to investigate as one has to rely on the assumption that NO− or NO+ donors indeed only act by dissociation of NO− or NO+ and that none of the NO− or NO+ is oxidized or reduced to free radical NO•. The available evidence does not favour NO+ as nitrergic neurotransmitter (Goyal & He, 1998; Li et al., 1999). In the rat anococcygeus, NO− more closely resembles the nitrergic neurotransmitter than NO• but still, it does not behave exactly like the neurotransmitter (Li et al., 1999). Furthermore, NO− as neurotransmitter requires a mechanism for transport through the membrane, as this ionized redox form will not diffuse freely.
Upon irreversible inhibition of the Cu/Zn containing enzyme superoxide dismutase (Cu/Zn SOD) by the Cu-chelator diethyldithiocarbamate (DETCA) in the bovine retractor penis muscle, nitrergic neurotransmission became sensitive to the superoxide generators pyrogallol and hypoxanthine/xanthine oxidase (Martin et al., 1994). Similar results were obtained in the mouse anococcygeus with duroquinone (Lilley & Gibson, 1995) and in the rat gastric fundus with LY83583 (Lefebvre, 1996), suggesting that high levels of tissue Cu/Zn SOD afford protection of the nitrergic transmitter from destruction by superoxide. This mechanism can however not explain the following observations: (1) DETCA-pretreatment did not alter the differential action of superoxide generated by hypoxanthine/xanthine oxidase and pyrogallol in respectively the rat gastric fundus (Lefebvre, 1996) and the rat anococcygeus muscle (La & Rand, 1999); (2) the discriminating effect between exogenous NO and the endogenous nitrergic neurotransmitter of the NO-scavenging agents carboxy-PTIO in the mouse anococcygeus (Lilley & Gibson, 1996) and hydroxocobalamin in the rat gastric fundus (Lefebvre, 1996), the latter still differentiating even in the presence of DETCA. Besides Cu/Zn SOD, other antioxidant defences might act in concert within nitrergically-innervated tissues to protect free radical NO (Gibson & Lilley, 1997). Indeed, reduced glutathione, α-tocopherol and ascorbate were shown to protect NO from some or all of a set of direct NO-scavengers and superoxide anion generators in the mouse anococcygeus (Lilley & Gibson, 1996) and the release of ascorbate and urate upon depolarization was demonstrated in the rat anococcygeus (Lilley & Gibson, 1997).
The aim of this study was to investigate the influence of several antioxidants on nitrergic relaxations in the pig gastric fundus. The antioxidants tested included bilirubin; this bile pigment has a pronounced antioxidant effect (Stocker et al., 1987). It is formed by rapid reduction of biliverdin; the enzyme catalyzing the formation of biliverdin from haeme, haeme oxygenase-2, is colocalized with neuronal NO-synthase in 60–70% of myenteric neurons (Zakhary et al., 1997).
Methods
Tissue preparation
Experiments were carried out on isolated circular smooth muscle strips of the porcine gastric fundus. The stomach was removed from healthy 6 month old male castrated pigs, slaughtered at a local abattoir, and transported to the laboratory in ice-chilled physiological salt solution. After the mucosa was removed, strips (15×3 mm) were cut from the fundus in the direction of the circular muscle layer. All tissues were used immediately. Strips were mounted vertically between two platinum plate electrodes under a load of 2 g in 5 or 20 ml organ baths, containing physiological salt solution at 37°C and gassed with 95% O2/5% CO2. The composition of the physiological salt solution was (mM): Na+ 137, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, Cl− 124.1, HCO3− 25, H2PO4− 1.2 and glucose 11.5 (Mandrek & Milenov, 1991). To obtain NANC conditions, atropine (10−6 M) and guanethidine (4×10−6 M) were continuously present in the medium. Changes in length were recorded isotonically via Hugo Sachs B40 Lever transducers type 373 on a Graphtec Linearcorder 8 WR 3500 in the 5 ml baths and via Palmer Bioscience T3 transducers on a Graphtec Linearcorder WR 3701 F in the 20 ml organ baths. Electrical field stimulation (EFS) (40 V, 0.1 ms, 4 Hz) was applied by means of a Hugo Sachs Stimulator I type 215/I in the 5 ml baths and by a Grass S88 stimulator in the 20 ml baths. The tissues were equilibrated for 90 min with rinsing every 15 min before starting the experiment.
Protocols
After the equilibration period, all strips were first contracted with 3×10−7 M 5-hydroxytryptamine (5-HT) and subsequently relaxed by 10−5 M sodium nitroprusside (SNP) before continuing the experimental protocol.
In a first set of investigations, the effect of antioxidants per se on electrical field stimulation (EFS; 40 V, 0.1 ms, 4 Hz, 10 s and 3 min) and on a NO-bolus (10−5 M) was studied as follows. Tissues were rinsed for 1 h; tone was raised again with 3×10−7 M 5-HT and when a stable plateau contraction was obtained, three relaxant stimuli were consecutively studied with a 5 min interval in between: EFS at 4 Hz for 10 s, 10−5 M NO and EFS at 4 Hz for 3 min. After repetitive rinsing for 1 h, the preparations were again contracted with 3×10−7 M 5-HT. When the contraction amplitude was stable, one of the antioxidants was added and after a 2 min incubation period the three relaxant stimuli were studied for the second time. The concentrations of the antioxidants were: SOD, 300 and 1000 u ml−1; ascorbate, 5×10−4 M; α-tocopherol, 4×10−4 M; bilirubin, 2×10−4 M; GSH, 5×10−4 M; urate, 4×10−4 M. The cell permeable manganese superoxide dismutase mimetic EUK-8 was also tested (3×10−4 M). In parallel control tissues, the solvents of the antioxidants were applied.
To study the influence of 6-anilino-5,8-quinolinedione (LY83583; 10−5 M), hydroxocobalamin (10−4 M) and hydroquinone (10−4 M) on the relaxant stimuli, these agents were added 15 min before the third 5-HT-induced contraction, where the relaxant stimuli were tested a second time. To investigate the influence of the antioxidants on the effect of LY83583, hydroxocobalamin and hydroquinone, the latter agents were added 15 min before the third 5-HT-induced contraction, and the antioxidants on top of this contraction 2 min before EFS at 4 Hz for 10 s was applied. The influence of LY83583, hydroxocobalamin and hydroquinone was also tested on sustained relaxation induced by either continuous EFS at 4 Hz or by infusion of NO. We used two different experimental protocols. In the first, continuous NO administration for 10 min was started at the top of the second 5-HT-induced contraction by infusing per 10 s the amount yielding 10−5 M, when given in bolus, into the bath via a Braun infusion pump; LY83583 (10−5 M), hydroxocobalamin (10−4 M) and hydroquinone (10−4 M) or their solvent were injected into the organ bath 15 min before the third 5-HT-induced contraction, at the top of which the NO-infusion for 10 min was repeated. In the second protocol, the above mentioned substances were only added exactly 5 min after start of relaxation induced by NO-infusion or by continuous EFS at 4 Hz applicated at the top of a second 5-HT-induced contraction; both the NO-infusion and the EFS were subsequently sustained for another 10 min.
Drugs
The following drugs were used (supplied by Sigma unless stated otherwise): 6-anilino-5,8-quinolinedione (LY83583; Calbiochem), L-ascorbic acid, atropine sulphate, bilirubin ditaurate (Calbiochem), EUK-8 (Calbiochem), glutathione, guanethidine sulphate, hydroquinone, hydroxocobalamin acetate, 5-hydroxytryptamine creatinine monosulphate (Janssen Chimica), sodium nitroprusside, Cu/Zn superoxide dismutase (from bovine erythrocytes), α-tocopherol, uric acid. Drugs were dissolved in deionized water except LY83583, that was dissolved in 100% ethanol, uric acid that was dissolved in 0.1 M NaOH and α-tocopherol, that was dissolved in dimethylsulphoxide. Solvents themselves were without significant effect at the concentrations used in the experiments unless otherwise indicated. Stock solutions were made of LY83583 (10−2 M) and SOD (100,000 u ml−1); other solutions were prepared on the day of the experiment. A saturated NO solution was prepared as described by Kelm & Schrader (1990), yielding a vial containing NO in a concentration taken to be 2×10−3 M.
Data analysis
Relaxations induced by NO and EFS were expressed as percentage of the relaxation induced by 10−5 M sodium nitroprusside at the beginning of the experiment. Responses in the presence of interfering drugs were related to those obtained before administration of these drugs. Experimental data are expressed as means±s.e.mean and n refers to the number of tissues from different animals. Results within tissues were compared by a paired t-test and results between tissues with an unpaired t-test; a probability value of P<0.05 was considered statistically significant.
Results
Effect of the antioxidants per se
In control tissues, the relaxant responses to EFS at 4 Hz for 10 s, NO (10−5 M) and EFS at 4 Hz for 3 min were well maintained (respectively 98.9±4.3%, 96.2±11.3% and 105.0±3.9%; n=6; Figures 1a and 2). SOD (300 and 1000 u ml−1), α-tocopherol (4×10−4 M), ascorbate (5×10−4 M) and EUK-8 (3×10−4 M) did not influence the relaxation to exogenous NO (Figure 2). In the presence of GSH (5×10−4 M), the short-lasting relaxation to an exogenous bolus of NO (10−5 M) became biphasic (n=6; Figure 1b): the first phase yielded 74.4±10.4% (n=6) of the response before GSH, the second phase (depicted in Figure 2) 154.2±31.2% (n=6). Urate (4×10−4 M) and bilirubin (2×10−4 M) significantly potentiated the relaxant effect of NO to 294.8±60.9% and 151.1±9.3% (n=6; P<0.01; Figures 1c, 2 and 3a). None of the antioxidants tested in this study per se affected the electrically evoked relaxations (Figure 2).
Figure 1.
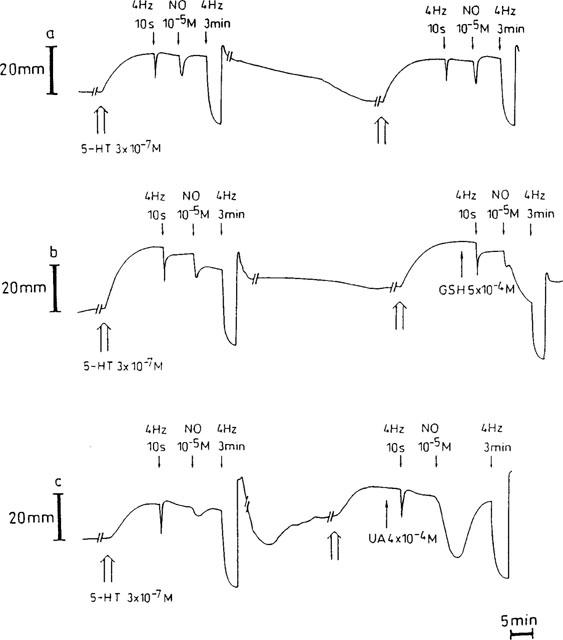
Representative traces showing the responses to electrical field stimulation (40 V, 0.1 ms, 4 Hz, 10 s and 3 min) and to a bolus of exogenous NO (10−5 M) in a time control (a), before and in the presence of 5×10−4 M glutathione (GSH, b) or 4×10−4 M urate (UA, c). During intervals (//—–//), the paper speed was reduced 5 fold.
Figure 2.
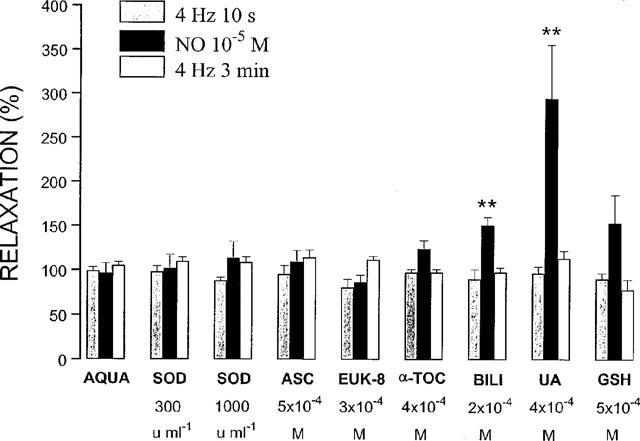
Relaxant responses to electrical field stimulation (40 V, 0.1 ms, 4 Hz, 10 s and 3 min) and exogenous NO (10−5 M) in the presence of antioxidants or aqua, the solvent of most antioxidants. The relaxations are expressed as a percentage of the response to the same stimulus before administration of the antioxidants. All results are the means±s.e.mean of six to eight strips. **P<0.01: significantly different from the response before administration of the antioxidant (paired t-test). SOD=superoxide dismutase; ASC=ascorbate; α-TOC=α-tocopherol; BILI=bilirubin; UA=uric acid; GSH=glutathione.
Figure 3.
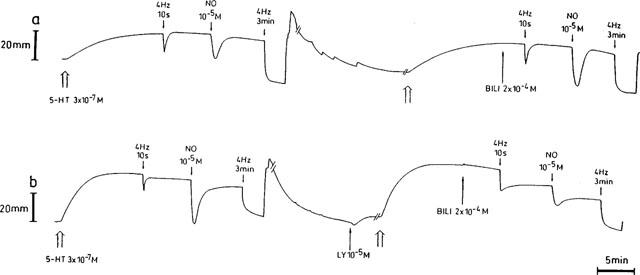
Representative traces showing the effect of 2×10−4 M bilirubin (BILI) on the relaxations induced by electrical field stimulation (40 V, 0.1 ms, 4 Hz, 10 s and 3 min) and exogenous NO (10−5 M) in the absence (a) and in the presence (b) of 10−5 M LY83583. During intervals (//—–//), the paper speed was reduced 5 fold.
Effect of LY83583 and interaction with the antioxidants
Incubation with 10−5 M LY83583 resulted in a slight decrease in tone in most strips but did not alter the amplitude of contraction induced by 3×10−7 M 5-HT. LY83583 (10−5 M) markedly reduced the relaxation elicited by 10−5 M NO to 8.7±4.4% (n=7) of the response before its administration; however, no inhibitory effect on the response to EFS at 4 Hz for 10 s and 3 min was observed (Figure 4a).
Figure 4.
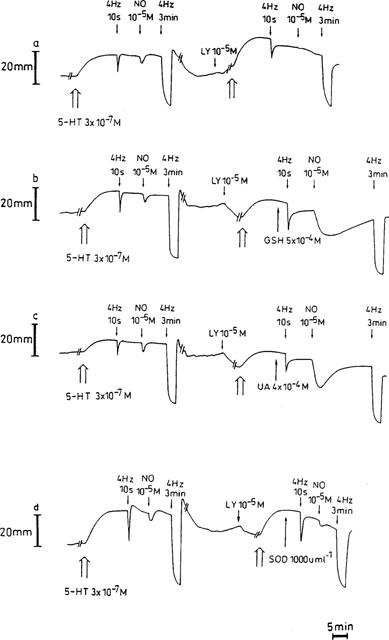
Representative traces showing the influence of 10−5 M LY83583 on the relaxations induced by electrical field stimulation (40 V, 0.1 ms, 4 Hz, 10 s and 3 min) and exogenous NO (10−5 M) (a), and the effect of 5×10−4 M glutathione (GSH, b), 4×10−4 M urate (UA, c) and 1000 u ml−1 SOD (d) on the inhibitory action of LY83583. During intervals (//—–//), the paper speed was reduced 5 fold.
An NO-infusion induced a progressively developing relaxation of the tissues. When repeated in the presence of 10−5 M LY83583, the relaxation was slowed down and the degree of relaxation at 1, 2, 3 and 5 min was significantly lower than before administration of LY83583 (Figure 5b). In the presence of the solvent of LY83583, the response was also significantly decreased at 2 and 3 min of infusion (Figure 5a), but this decrease was significantly less pronounced than in the presence of LY83583 (P<0.05, unpaired t-test). Addition of LY83583 (10−5 M) into the organ bath 5 min after the start of a continuous NO-infusion, instantly reversed the relaxation (Figure 6a), but the reversal was partial (55.6±8.0%; n=6) and disappeared during the further course of the NO-infusion. The solvent of LY83583 had no influence. The relaxation induced by long-lasting EFS at 4 Hz on parallel strips was either not affected by LY83583 administered 5 min after the start of the stimulation (three strips out of six) or only reversed to a very small extent (three strips out of six) yielding a mean reversal of 6.9±4.0%; the solvent of LY83583 was without effect.
Figure 5.
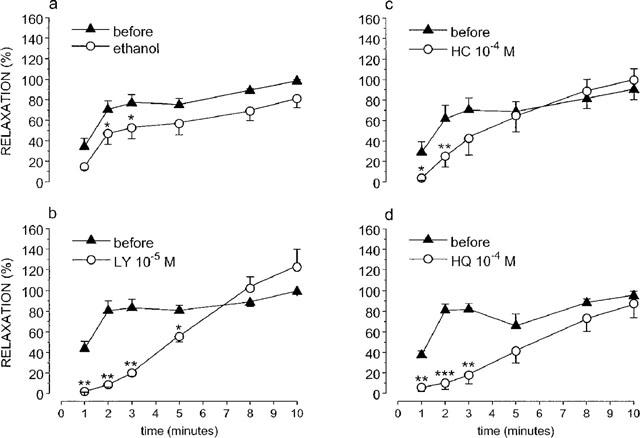
Relaxations induced by exogenous NO infused for 10 min before and in the presence of 10−5 M LY83583 (b) and its solvent ethanol (a), 10−4 M hydroxocobalamin (HC, c) and 10−4 M hydroquinone (HQ, d). The response measured at 1, 2, 3, 5, 8 and 10 min of infusion was expressed as a percentage of the maximum relaxation obtained during the NO-infusion before administration of the drugs. Means±s.e.mean of n=6 are shown. *P<0.05; **P<0.01; ***P<0.001: significantly different versus before.
Figure 6.
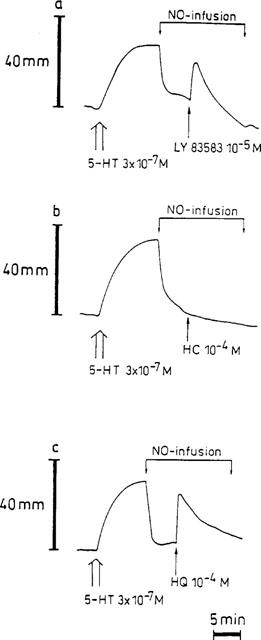
Representative traces showing the influence of 10−5 M LY83583 (a), 10−4 M hydroxocobalamin (HC, b) and 10−4 M hydroquinone (HQ, c) when added 5 min after starting a continuous NO-infusion. The NO-infusion was maintained for 15 min.
The inhibitory effect of LY83583 (10−5 M) on the response to exogenous NO (10−5 M) was partially and concentration-dependently antagonized by 300 and 1000 u ml−1 SOD. The response to NO increased from 8.7±4.4% (n=7) in the presence of LY83583 alone to 24.2±7.0% (n=6) and 34.4±9.5% (n=6; P<0.01) in the combined presence of LY83583 and 300 and 1000 u ml−1 SOD respectively. GSH (5×10−4 M) and urate (4×10−4 M) completely prevented the blocking action of LY83583 versus NO; the response to NO was even significantly increased in comparison to that before administration of the drugs (285.8±47.0% and 264.7±30.7%; n=7; P<0.001; Figures 4b,c and 7); the biphasic relaxation in response to exogenous NO in the presence of GSH alone (see Figure 1b) changed in a monophasic answer in the presence of LY83583 and GSH (Figure 4b). In the presence of LY83583 plus GSH, the relaxation to EFS at 4 Hz for 3 min was significantly reduced (P<0.01). This was the result of an incomplete recovery of tone observed after the foregoing very pronounced NO-induced relaxation in this series, as in an additional series (results not shown), the response to EFS at 4 Hz for 3 min was studied without foregoing NO exposure and was not influenced by LY83583 plus GSH. The addition of bilirubin (2×10−4 M) provided partial protection of NO versus LY83583 (Figures 3b and 7), while ascorbate, α-tocopherol and EUK-8 were without effect.
Figure 7.
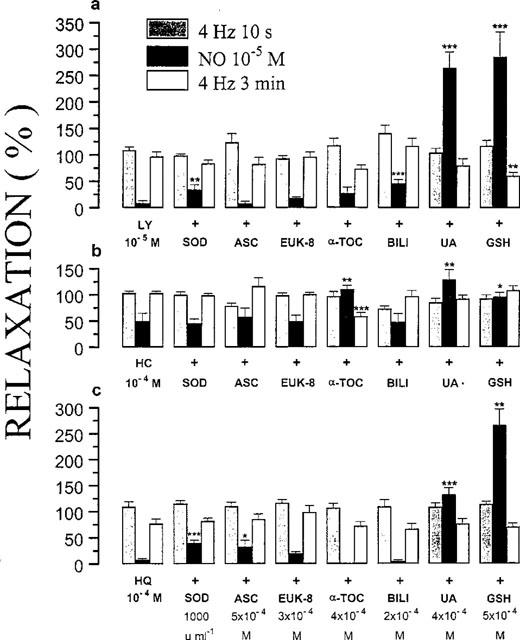
Relaxant responses to electrical field stimulation (40 V, 0.1 ms, 4 Hz, 10 s and 3 min) and exogenous NO (10−5 M), in the presence of 10−5 M LY83583 (a), 10−4 M hydroxocobalamin (b) or 10−4 M hydroquinone (c) alone or plus one of the antioxidants indicated. The relaxations are expressed as a percentage of the response to the same stimulus before administration of the drugs under study. All results are the means±s.e.mean of six to eight strips. *P<0.05; **P<0.01; ***P<0.001: significantly different from the response in the presence of 10−5 M LY83583, 10−4 M HC or 10−4 M HQ alone (unpaired t-test). HC=hydroxocobalamin; HQ=hydroquinone; SOD=superoxide dismutase; ASC=ascorbate; α-TOC=α-tocopherol; BILI=bilirubin; UA=uric acid; GSH=glutathione.
Effect of hydroxocobalamin and interaction with the antioxidants
Hydroxocobalamin (10−4 M) did not affect the basal tone or the 5-HT-induced contraction. Relaxant responses to EFS at 4 Hz for 10 s and 3 min were not inhibited by hydroxocobalamin, while the NO-induced relaxation was reduced to 49.9±15.1% (n=7; Figure 7). The shape of the NO-induced response was changed: the transient, fast relaxation became a slower developing and more or less sustained relaxation. Incubation with hydroxocobalamin (10−4 M) significantly reduced the relaxation elicited by NO-infusion at min 1 and 2 (Figure 5c). When added during both continuous NO-infusion and long-lasting EFS at 4 Hz, no reversal of the relaxation was produced by hydroxocobalamin (Figure 6b).
The reduction by hydroxocobalamin (10−4 M) of the response to NO (10−5 M) was fully prevented by 4×10−4 M urate, 5×10−4 M GSH (with a monophasic answer to NO) and 4×10−4 M α-tocopherol (Figure 7). In contrast, SOD (1000 u ml−1), ascorbate (5×10−4 M), EUK-8 (3×10−4 M) and bilirubin (2×10−4 M) showed no protective action against the NO-scavenging capacity of hydroxocobalamin (Figure 7). The relaxation to EFS at 4 Hz for 3 min was significantly attenuated (P<0.001) by the combination hydroxocobalamin (10−4 M) plus α-tocopherol (4×10−4 M). This effect was due to a non-recuperation of tone after application of the second relaxant stimulus (NO) in this series; in an additional series (results not shown), the response to EFS at 4 Hz for 3 min was studied without foregoing NO exposure and was not influenced by hydroxocobalamin plus α-tocopherol.
Effect of hydroquinone and interaction with the antioxidants
Hydroquinone (10−4 M) had a moderate relaxant effect per se when administered on non-contracted tissues; the 5-HT-induced contraction however did not change in amplitude. Hydroquinone clearly differentiated between exogenous NO and nitrergic nerve stimulation: the relaxant response to NO (10−5 M) was nearly abolished (7.8±2.6% of the control value; n=8), while responses to EFS at 4 Hz were unaffected (Figure 7).
Hydroquinone (10−4 M) markedly slowed down the relaxation induced by continuous NO-infusion at min 1, 2 and 3 of the infusion (Figure 5d). When hydroquinone was injected into the organ bath during NO-infusion, an instant partial reversal (61.4±16.7%; n=6) of the relaxation occurred that weaned off during the course of the infusion (Figure 6c); this effect was not seen when hydroquinone was given during long-lasting EFS at 4 Hz.
Ascorbate (5×10−4 M) and SOD (1000 u ml−1) partially reversed the inhibitory effect of 10−4 M hydroquinone on relaxations induced by 10−5 M exogenous NO (Figure 7). Urate (4×10−4 M) and GSH (5×10−4 M) granted full protection of NO versus the action of hydroquinone (Figure 7). In five strips out of 7, the response to exogenous NO in the presence of hydroquinone and GSH had a biphasic course; only the value of the second phase was taken in account to show the mean response to NO for this series in Figure 7. α-Tocopherol (4×10−4 M), bilirubin (2×10−4 M) and EUK-8 (3×10−4 M) did not change the inhibitory effect of hydroquinone.
Discussion
At the level of the proximal stomach, nitric oxide (NO) is thought to be involved in receptive relaxation as a non-adrenergic non-cholinergic inhibitory neurotransmitter (i.e. nitrergic neurotransmission) (Lefebvre, 1993). In order to fulfil this neurotransmitter function, the reactive NO molecule has to diffuse from its neuronal site of synthesis to its target enzyme soluble guanylate cyclase in the neighbouring effector smooth muscle cells, thereby encountering numerous molecules which can potentially decrease its bioavailability. Among these molecules, a prominent role is played by the superoxide anion; this reduced a chemically active form of oxygen is produced during mitochondrial respiration and other normal metabolic cell processes. When produced in proximity, the superoxide anion radical and NO can chemically interact at an almost diffusion-limited rate (6.7×109 M−1 s−1) resulting in the abolition of the biological activity of NO and the generation of peroxynitrite (Huie & Padmaja, 1993).
It has been proposed that endogenous antioxidants protect the nitrergic neurotransmitter from superoxide anions and even NO-scavenging molecules (Martin et al., 1994; Lilley & Gibson, 1996; 1997). It was the aim of this study in the pig gastric fundus to investigate the potential influence of several antioxidants on nitrergic relaxations induced by either electrical field stimulation or exogenous NO. To the antioxidants already studied before in this context (SOD, ascorbate, α-tocopherol, urate and GSH) were added bilirubin and the synthetic agent EUK-8. The substances were tested on relaxations induced by exogenous NO and by EFS in NANC conditions. Previous experiments had indeed shown that the relaxant responses to EFS at 4 Hz for 10 s, 3 min or 15 min are largely nitrergic (Lefebvre et al., 1995; Lefebvre & Vandekerckhove, 1998).
Influence of the antioxidants per se
Exogenously added Cu/Zn SOD did not enhance the NO-induced relaxations or the electrically induced NANC-relaxations in the pig gastric fundus, suggesting that the endogenous amount of SOD is sufficiently high to protect the nitrergic neurotransmitter from superoxide mediated destruction; this is in accordance with previous results obtained in the rat anococcygeus (La & Rand, 1999), the rat gastric fundus (Lefebvre, 1996) and the bovine retractor penis (Liu et al., 1994). In the presence of GSH, the short-lasting relaxation to an exogenous bolus of NO (10−5 M) became biphasic. Furthermore, the second part of this relaxation was potentiated and prolonged in comparison to the NO-induced relaxation in the absence of GSH. This effect is most likely due to the fact that our experimental conditions in the pig gastric fundus favour the formation of S-nitrosoglutathione from exogenous NO and GSH. This process of nitrosothiol formation (Kharitonov et al., 1995) might serve to stabilize free radical NO, thereby prolonging its lifetime and potentiating its biological activity. A somewhat identical phenomenon was observed in the presence of urate: an exogenous bolus of NO produced a significantly potentiated and prolonged relaxation of the gastric fundus strips. Two hypotheses can be considered for this observation. First, although urate does not bind free radical NO (Becker, 1993), a nitrated uric acid derivative might be generated in conditions of urate abundance, slowly releasing NO and inducing relaxation of smooth muscle preparations. A similar nitrosation of uric acid, requiring peroxynitrite as nitrosating agent, has already been reported; the uric acid derivative resulted in endothelium-independent vasorelaxation of rat thoracic aorta and its activity was completely blocked by the NO-scavenger oxyhaemoglobin (Skinner et al., 1998). A second hypothesis is provided by findings that antioxidants such as thiols or ascorbate enhance activation of soluble guanylate cyclase, presumably by maintaining the iron of the metalloporphyrin in a reduced state, thereby facilitating the reaction between NO and (ferrous) haem (Hobbs, 1997). Indeed, also urate with its inherent antioxidant potential might prevent oxidation of the haem group to a ferric state, the latter process resulting in loss of enzyme activity. However, as urate and GSH had no influence on relaxations elicited by EFS in our study, and as ascorbate was without effect on relaxations induced by both EFS and NO, this hypothesis can be rejected in the pig gastric fundus. The effect of GSH and urate on exogenous NO was not seen with the endogenous nitrergic neurotransmitter, released by EFS. This might be related to the short distance the endogenous nitrergic neurotransmitter has to travel to reach the smooth muscle cells in comparison to exogenous NO. The potentiating effect of bilirubin on exogenous NO might be due to its antioxidant effect, the non-effect on the endogenous nitrergic neurotransmitter then being related to its optimal antioxidant protection.
Influence of LY83583, hydroxocobalamin and hydroquinone
LY83583, hydroxocobalamin and hydroquinone showed the discriminating effect between exogenous NO and the nitrergic neurotransmitter mentioned in the introduction. None of the three agents affected EFS, performed both for short periods (10 s) and longer terms (3 and 10 min) of stimulation. While LY83583 (10−5 M) and hydroquinone (10−4 M) nearly abolished the relaxant response to a bolus of exogenous NO (10−5 M), hydroxocobalamin at 10−4 M reduced the amplitude of this NO-induced relaxation to about 50% and at the same time changed the fast and transient relaxation profile into a more or less sustained one. This marked increase in duration of the NO-induced response is quite similar to that described by Jenkinson et al. (1995), corroborating their hypothesis that hydroxocobalamin reversibly sequesters NO to form nitrosocobalamin. Our finding that hydroxocobalamin does not reverse the relaxation upon addition during continuous NO-infusion can be explained in two ways: either the formed nitrosocobalamin liberates its NO relatively fast, supporting the view of a reversible binding (Rochelle et al., 1995), or the rate of NO-infusion is sufficiently high to overcome the scavenging effect of 10−4 M hydroxocobalamin even if the binding is irreversible. In contrast with hydroxocobalamin, LY83583 and hydroquinone instantly reversed the relaxation elicited by continuous NO-infusion. This corroborates that their mode of action implies a mechanism other than direct NO-scavenging, most likely the generation of superoxide. Still, the reversal by LY83583 and hydroquinone was only temporary showing that continuous NO supply can overwhelm their effect.
Interaction of the antioxidants with LY83583, hydroquinone and hydroxocobalamin
Addition of exogenous Cu/Zn SOD resulted in a partial reversal of the inhibitory action of LY83583 and hydroquinone on a bolus of exogenous NO; this observed reversal supports the proposal that, at least under the experimental conditions used in the pig gastric fundus, these compounds act via production of superoxide anions. Indeed, the mechanism of action of hydroquinone may differ between tissues: NO-scavenging activity is suggested in the mouse anococcygeus (Lilley & Gibson, 1995) and in the rat gastric fundus (Lefebvre, 1996), while superoxide anion production is proposed in the bovine retractor penis muscle (Paisley & Martin, 1996). Cu/Zn SOD failed to reverse the inhibitory effect of hydroxocobalamin, indicating that direct free radical scavenging constitutes the NO-blocking action of this latter substance. As LY83583 and hydroquinone can generate superoxide anions extracellularly as well as intracellularly (Boersma et al., 1994) and exogenous Cu/Zn SOD cannot enter cells, we examined the effect of EUK-8, a synthetic cell-permeable mimetic of the mitochondrial Mn SOD. Mn SOD is the mitochondrial isozyme that disposes of superoxide generated by respiratory chain activity. Mn SOD immunoreactivity is demonstrated in nerve cells and smooth muscle cells in the stomach wall of rats (Fang & Christensen, 1995) and in nerve cells in the myenteric plexus of the opossum oesophagus (Thomas et al., 1996). The enzyme effectively catalyzes the dismutation of superoxide with a rate constant of 1.8×109 M−1 s−1 (Forman & Fridovich, 1973). These findings together with the demonstration that it is colocalized with NADPH diaphorase (a marker for NO-synthase) in the gastrointestinal tract (Fang & Christensen, 1995), support the theory that Mn SOD might be involved in protecting the nitrergic neurotransmission process in the stomach. However, in the pig gastric fundus, EUK-8 did not prevent the inhibitory effect of LY83583 and hydroquinone. This contrasts markedly with results obtained in rat aortic segments treated with DETCA to induce an endogenous flux of superoxide: EUK-8 fully restored endothelium-derived NO-mediated arterial relaxation in response to acetylcholine, while Cu/Zn SOD had only a partial effect (Jackson et al., 1998). Since DETCA is known to block both the Cu/Zn containing extracellular and intracellular isozyme of SOD but not the Mn requiring isozyme, the total recovery provided by EUK-8 is probably due to compensation for the loss of intracellular Cu/Zn SOD activity. Incomplete cellular access and/or poor sensitivity of the tissue may account for the complete lack of superoxide dismutation by EUK-8 in the pig gastric fundus. We therefore increased the experimental concentration of EUK-8 up to 5×10−4 M, but tissues responded with marked phasic activity interfering with the relaxant responses.
The bile pigment bilirubin partially reversed the inhibitory effect of LY83583 versus exogenous NO. The protective effect versus LY83583 is associated with the bilirubin moiety of bilirubin ditaurate since taurine alone is ineffective (results not shown). Bilirubin is formed in mammals via a pathway involving two enzymes. The microsomal haeme oxygenase catalyzes the cleavage of ferriprotoporphyrin IX (haeme) to form ferrous iron, carbon monoxide and biliverdin; subsequently, biliverdin is rapidly reduced by the cytosolic enzyme biliverdin reductase to bilirubin (Stocker et al., 1990). This machinery is found integrally in myenteric plexus neurons: biliverdin reductase is present in large functional excess in all tissues and about 70% of neurons of the myenteric plexus costain for constitutive haeme oxygenase-2 and neuronal nitric oxide synthase (Zakhary et al., 1996; 1997). Bilirubin can be added to the list of physiological antioxidants, proposed by Lilley & Gibson (1996), which have been demonstrated to preserve the biological actions of NO. Bilirubin interacts chemically with superoxide with a second-order rate constant estimated to be 2.3×104 M−1 s−1 (Robertson & Fridovich, 1982). This is 105 times less than the rate at which superoxide reacts with NO, suggesting that the concentration of bilirubin would have to exceed that of NO 105 times to become effective for the protection of NO versus superoxide. Still, the protecting capacity of antioxidants versus superoxide is not only determined by their intrinsic chemical reactivity toward radicals, but is equally influenced by the site of the antioxidant, the concentration and mobility at the microenvironment, and the interaction with other antioxidants (Niki et al., 1995). Bilirubin will probably be located into neuronal membranes or alternatively, it can be a cytosolic antioxidant bound to glutathione S-transferases. Cytosolic glutathione S-transferases are present in various mammalian organ systems, including the nervous system (Johnson et al., 1993), and participate in the intracellular binding and/or transport of a broad range of endogenous molecules such as hormones, neurotransmitters and lipophilic compounds including bilirubin (Abramovitz et al., 1988). This potential presence of bilirubin in both lipophilic and hydrophilic cell compartments with the possibility of migrating between those two phases, enhances its antioxidant potency.
On the basis of the general hypothesis that products of degradation metabolic pathways may play a useful biological role (as also exemplified by bilirubin in this study), and as urate is released upon depolarization of nerve cells in the rat anococcygeus (Lilley & Gibson, 1997), the effect of uric acid, another degradation product, was tested versus the three differentiating agents LY83583, hydroxocobalamin and hydroquinone. Urate (in the physiological pH range, about 99% of the total uric acid is present as the monovalent anion urate) displays powerful antioxidant activity, evidenced by its ability to scavenge reactive oxygen species (Ames et al., 1981) and to chelate iron, therefore, preventing iron-catalyzed oxidation (Davies et al., 1986). As high levels of urate are excreted via gastric and intestinal juices (Ames et al., 1981), it was of interest to find that in the pig gastric fundus urate provided an overall protection of exogenous NO against both superoxide and scavenger mediated inactivation. The tripeptide thiol GSH, a major determinant of intracellular redox state and antioxidant defences (Meister & Anderson, 1983), exhibited the same protective profile as urate: in its presence, LY83583, hydroquinone and hydroxocobalamin could not reduce the NO-induced relaxation. Whether direct interaction of GSH and urate with noxious oxygen radicals and scavenging molecules or the chemical nitrosation process with formation of respectively S-nitrosoglutathione and a nitrated uric acid derivative, as described above, is responsible for these results, requires further research.
The antioxidant vitamins ascorbate and α-tocopherol showed only a partial reversal of the NO-blocking effect of respectively hydroquinone and hydroxocobalamin. The limited effect of ascorbate contrasts with results in the mouse anococcygeus, where ascorbate was able to reverse the inhibitory effect versus exogenous NO of all superoxide anion generators and NO-scavengers tested (Lilley & Gibson, 1996). This suggests that the importance of the antioxidants involved in the protection of nitrergic neurotransmission might differ from tissue to tissue. Regional differences in antioxidants with a concomitant different use of antioxidant mechanisms to withstand oxidative stress have also been reported in the cardiovascular system (Palace et al., 1999).
Conclusion
Our results in the pig gastric fundus indicate that several antioxidant defence systems can preserve the biological actions of exogenous NO when confronted with superoxide anions or scavenging molecules. Whether SOD as enzymatic and GSH, urate, bilirubin, ascorbate and α-tocopherol as non-enzymatic antioxidants might contribute to the resistance of the endogenous nitrergic neurotransmitter versus NO-inhibitors, requires further studies with tissues in which the antioxidants have been selectively depleted or inhibited, since the other possibilities described in the introduction can not yet be completely ruled out.
Acknowledgments
E.E. Colpaert is a research assistant of the Fund for Scientific Research Flanders. The study was financially supported by grant No. 3G0031.96 from the Fund of Scientific Research Flanders, grant 011A1696 from the Special Investigation Fund of the Gent University and by Interuniversity Pole of Attraction Programme P4/16 (Services to the Prime Minister – Federal Services for Scientific, Technical and Cultural Affairs).
Abbreviations
- DETCA
diethyldithiocarbamate
- EFS
electrical field stimulation
- GSH
glutathione
- 5-HT
5-hydroxytryptamine
- LY83583
6-anilino-5,8-quinolinedione
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- NO+
nitrosonium cation
- NO−
nitroxyl anion
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
References
- ABRAMOVITZ M., HOMMA H., ISHIGAKI S., TANSEY F., CAMMER W., LISTOWSKY I. Characterization and localization of glutathione-S-transferases in rat brain and binding of hormones, neurotransmitters, and drugs. J. Neurochem. 1988;50:50–57. doi: 10.1111/j.1471-4159.1988.tb13228.x. [DOI] [PubMed] [Google Scholar]
- AMES B.N., CATHCART R., SCHWIERS E., HOCHSTEIN P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. U.S.A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBIER A.J.M., LEFEBVRE R.A. Effect of LY83583 on relaxation induced by non-adrenergic non-cholinergic nerve stimulation and exogenous nitric oxide in the rat gastric fundus. Eur. J. Pharmacol. 1992;219:331–334. doi: 10.1016/0014-2999(92)90315-u. [DOI] [PubMed] [Google Scholar]
- BARBIER A.J.M., LEFEBVRE R.A. Influence of S-nitrosothiols and nitrate tolerance in the rat gastric fundus. Br. J. Pharmacol. 1994;111:1280–1286. doi: 10.1111/j.1476-5381.1994.tb14884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER B.F. Towards the physiological function of uric acid. Free Rad. Biol. Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- BOERSMA M.G., BALVERS W.G., VERVOORT J., RIETJENS I.M.C.M. NADPH cytochrome reductase catalysed redox cycling of 1,4-benzoquinone; hampered at physiological conditions, initiated at increased pH values. Biochem. Pharmacol. 1994;47:1949–1955. doi: 10.1016/0006-2952(94)90068-x. [DOI] [PubMed] [Google Scholar]
- DAVIES K.J.A., SEVANIAN A., MUAKKASSAH-KELLY S.F., HOCHSTEIN P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem. J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAN J.G., BOECKXSTAENS G.E., DE WINTER B.Y., MOREELS T.G., MISSET M.E., HERMAN A.G., PELCKMANS P.A. Comparison of the pharmacological profile of S-nitrosothiols, nitric oxide and the nitrergic neurotransmitter in the canine ileocolonic junction. Br. J. Pharmacol. 1995;114:1179–1184. doi: 10.1111/j.1476-5381.1995.tb13331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANG S., CHRISTENSEN J. Manganese superoxide dismutase and reduced nicotinamide adenine dinucleotide diaphorase colocalize in the rat gut. Gastroenterology. 1995;109:1429–1436. doi: 10.1016/0016-5085(95)90627-4. [DOI] [PubMed] [Google Scholar]
- FORMAN H.J., FRIDOVICH I. Superoxide dismutase: a comparison of rate constants. Arch. Biochem. Biophys. 1973;158:396–400. doi: 10.1016/0003-9861(73)90636-x. [DOI] [PubMed] [Google Scholar]
- FÖRSTERMANN U., KLEINERT H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:351–364. doi: 10.1007/BF00172772. [DOI] [PubMed] [Google Scholar]
- GIBSON A., BABBEDGE R., BRAVE S.R., HART S.L., HOBBS A.J., TUCKER J.F., WALLACE P., MOORE P.K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br. J. Pharmacol. 1992;107:715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., BRAVE S.R., MCFADZEAN I., TUCKER J.F., WAYMAN C. The nitrergic transmitter of the anococcygeus - NO or not. Arch. Int. Pharmacodyn. 1995;32:39–51. [PubMed] [Google Scholar]
- GIBSON A., LILLEY E. Superoxide anions, free-radical scavengers, and nitrergic neurotransmission. Gen. Pharmacol. 1997;28:489–493. doi: 10.1016/s0306-3623(96)00281-9. [DOI] [PubMed] [Google Scholar]
- GOYAL R.K., HE X.D. Evidence for NO• redox form of nitric oxide as nitrergic inhibitory neurotransmitter in gut. Am. J. Physiol. 1998;275:G1185–G1192. doi: 10.1152/ajpgi.1998.275.5.G1185. [DOI] [PubMed] [Google Scholar]
- HOBBS A.J. Soluble guanylate cyclase: the forgotten sibling. TiPS. 1997;18:484–491. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- HOBBS A.J., TUCKER J.F., GIBSON A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br. J. Pharmacol. 1991;104:645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Rad. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- IVERSEN H.H., GUSTAFSSON L.E., LEONE A.M., WIKLUND N.P. Smooth muscle relaxing effects of NO, nitrosothiols and a nerve-induced relaxing factor released in guinea-pig colon. Br. J. Pharmacol. 1994;113:1088–1092. doi: 10.1111/j.1476-5381.1994.tb17107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON T.S., XU A., VITA J.A., KEANYE J.F., JR Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- JENKINSON K.M., REID J.J., RAND M.J. Hydroxocobalamin and haemoglobin differentiate between exogenous and neuronal nitric oxide in the rat gastric fundus. Eur. J. Pharmacol. 1995;275:145–152. doi: 10.1016/0014-2999(94)00762-v. [DOI] [PubMed] [Google Scholar]
- JOHNSON J.A., BARBARY A.E., KORNGUTH S.E., BRUGGE J.F., SIEGEL F.L. Glutathione S-transferase isoenzymes in rat brain neurons and glia. J. Neurosci. 1993;13:2013–2023. doi: 10.1523/JNEUROSCI.13-05-02013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELM M., SCHRADER J. Control of coronary vascular tone by nitric oxide. Circ. Res. 1990;66:1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- KHARITONOV V.G., SUNDQUIST A.R., SHARMA V.S. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J. Biol. Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- LA M., RAND M.J. Effects of pyrogallol, hydroquinone and duroquinone on responses to nitrergic nerve stimulation and NO in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;126:342–348. doi: 10.1038/sj.bjp.0702277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Non-adrenergic non-cholinergic neurotransmission in the proximal stomach. Gen. Pharmacol. 1993;24:257–266. doi: 10.1016/0306-3623(93)90301-d. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Influence of superoxide dismutase inhibition on the discrimination between NO and the nitrergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 1996;118:2171–2177. doi: 10.1111/j.1476-5381.1996.tb15659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A., SMITS G.J.M., TIMMERMANS J.P. Study of NO and VIP as non-adrenergic non-cholinergic neurotransmitters in the pig gastric fundus. Br. J. Pharmacol. 1995;116:2017–2026. doi: 10.1111/j.1476-5381.1995.tb16406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A., VANDEKERCKHOVE K. Effect of nitroglycerin and long-term electrical stimulation on nitrergic relaxation in the pig gastric fundus. Br. J. Pharmacol. 1998;123:143–149. doi: 10.1038/sj.bjp.0701582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., KARAGIANNIS J., RAND M.J. Comparison of the redox forms of nitrogen monoxide with the nitrergic transmitter in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;127:826–834. doi: 10.1038/sj.bjp.0702540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Inhibition of relaxations to nitrergic stimulation of the mouse anococcygeus by duroquinone. Br. J. Pharmacol. 1995;116:3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus muscle against inhibition by superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of the antioxidants ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU X., GILLESPIE J.S., MARTIN W. Non-adrenergic non-cholinergic relaxation of the bovine retractor penis muscle: role of S-nitrosothiols. Br. J. Pharmacol. 1994;111:1287–1295. doi: 10.1111/j.1476-5381.1994.tb14885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDREK K., MILENOV K. Responses of porcine gastric and duodenal smooth muscle to VIP. J. Autonom. Pharmacol. 1991;11:353–364. doi: 10.1111/j.1474-8673.1991.tb00259.x. [DOI] [PubMed] [Google Scholar]
- MARTIN W., MCALLISTER K.M.H., PAISLEY K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- MEISTER A., ANDERSON M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HIGGS A., FURCHGOTT R. International Union of Pharmacology Nomenclature in Nitric Oxide Research. Pharmacol. Rev. 1997;49:137–142. [PubMed] [Google Scholar]
- NIKI E., NOGUCHI N., TSUCHIHASHI H., GOTOH N. Interaction among vitamin C, vitamin E, and β-carotene. Am. J. Clin. Nutr. 1995;62:1322S–1326S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- PAISLEY K., MARTIN W. Blockade of nitrergic transmission by hydroquinone, hydroxocobalamin and carboxy-PTIO in bovine retractor penis: role of superoxide anion. Br. J. Pharmacol. 1996;117:1633–1638. doi: 10.1111/j.1476-5381.1996.tb15333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALACE V., KUMAR D., HILL M.F., KHAPER N., SINGAL P.K. Regional differences in non-enzymatic antioxidants in the heart under control and oxidative stress conditions. J. Mol. Cell. Cardiol. 1999;31:193–202. doi: 10.1006/jmcc.1998.0859. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Ann. Rev. Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- ROBERTSON P., JR, FRIDOVICH I. A reaction of the superoxide radical with tetrapyrroles. Arch. Biochem. Biophys. 1982;213:353–357. doi: 10.1016/0003-9861(82)90560-4. [DOI] [PubMed] [Google Scholar]
- ROCHELLE L.G., MORANA S.J., KRUSZYNA H., RUSSELL M.A., WILCOX D.E., SMITH R.P. Interactions between hydroxocobalamin and nitric oxide (NO): evidence for a redox reaction between NO and reduced cobalamin and reversible NO-binding to oxidized cobalamin. J. Pharmacol. Exp. Ther. 1995;275:48–52. [PubMed] [Google Scholar]
- SKINNER K.A., WHITE C.R., PATEL R., TAN S., BARNES S., KIRK M., DARLEY-USMAR V., PARKS D.A. Nitrosation of uric acid by peroxynitrite. J. Biol. Chem. 1998;273:24491–24497. doi: 10.1074/jbc.273.38.24491. [DOI] [PubMed] [Google Scholar]
- STOCKER R., MCDONAGH A.F., GLAZER A.N., AMES B.N. Antioxidant activities of bile pigments: Biliverdin and Bilirubin. Methods Enzymol. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]
- STOCKER R., YAMAMOTO Y., MCDONAGH A.F., GLAZER A.N., AMES B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- THOMAS R.M., FANG S., LEICHUS L.S., OBERLEY L.W., CHRISTENSEN J., MURRAY J.A., LEDLOW A., CONCKLIN J.L. Antioxidant enzymes in intramural nerves of the opossum esophagus. Am. J. Physiol. 1996;270:G136–G142. doi: 10.1152/ajpgi.1996.270.1.G136. [DOI] [PubMed] [Google Scholar]
- ZAKHARY R., GAINE S.P., DINERMAN J.L., RUAT M., FLAVAHAN N.A., SNYDER S.H. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKHARY R., POSS K.D., JAFFREY S.R., FERRIS C.D., TONEGAWA S., SNYDER S.H. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]


