Abstract
Male, Sprague-Dawley rats were actively immunized with novel angiotensin vaccines, and their pressor responses to exogenous angiotensin I (AI) and angiotensin II (AII) were assessed in vivo. Serum antibody titres were also measured.
The most effective vaccine consisted of an AI analogue conjugated with a tetanus toxoid carrier protein and adjuvanted with aluminium hydroxide. When this vaccine was injected on days 0, 21 and 42, pressor responses to AI on day 63 were significantly inhibited (maximum, 8.9 fold shift), but responses to AII were unaffected. The anti-angiotensin antibody titre was increased 32,100 fold, and, uniquely, these antibodies also cross-reacted with angiotensinogen.
These findings indicate that active immunization against AI may be a useful approach for treating cardiovascular disorders involving the renin-angiotensin system.
Keywords: Angiotensin vaccines, pressor responses, antibody titres, angiotensinogen
Introduction
The ability of angiotensin converting-enzyme (ACE) inhibitors (see Brown & Vaughan, 1998 for review) and AT1-receptor antagonists (see Messerli et al., 1996, for review) to influence cardiovascular status in hypertensive conditions is consistent with an important role for the renin-angiotensin system (RAS) in physiological and pathophysiological states.
The clinical effectiveness of ACE inhibitors has been demonstrated in a range of cardiovascular conditions such as hypertension and heart failure, but there are undesirable class associated side effects e.g. dry cough and first dose hypotension (see Sunman & Sever, 1993, for review). The AT1-receptor antagonists are better tolerated, although the long-term effects of augmented AT2-receptor activation, which may occur with prolonged use of AT1-receptor antagonists, are not known (see Stroth & Unger, 1999; Horiuchi et al., 1999, for review). Moreover, even with well tolerated oral drugs, poor compliance is a significant constraint in delivering effective treatment for chronic conditions. Thus, other means of suppressing RAS function in diseases such as hypertension, for example, may have advantages over current therapy. An approach of interest is modulation of RAS function by active immunization, using vaccines based on novel analogues of angiotensin I (AI) or angiotensin II (AII).
As early as 1968, studies on the effects of passive and active immunization against angiotensins were instituted (see Michel et al., 1989, for review). Although passive immunization can cause suppression of responses to exogenous AII and AI, the effects of active immunization were variable, particularly with regard to influence on blood pressure in hypertensive models (Michel et al., 1989). It is feasible the heterogeneity of responses reported relates to variable and limited immunogenicity of the vaccines used.
Therefore, in the present study, analogues of AI and AII were conjugated to carrier proteins which are good immunogens. These immunoconjugates were adjuvanted and used to immunize rats in the expectation, based on prior studies, that they would generate a strong anti-angiotensin immune response. Once the anti-angiotensin antibody titres were determined the possible degree of inhibition of the pressor response to AI was assessed. Subsequently, the most active formulations were investigated with respect to the cross-reactivity with angiotensinogen of the antibodies generated, and the relative selectivity of their effects to suppress in vivo pressor responses to AI and AII. Some of the results herein have been presented to the British Pharmacological Society (Gardiner et al., 1998).
Methods
Angiotensin vaccine preparation
The two angiotensin hormone peptide identified as AI and AII, were prepared using a Symphony peptide synthesiser (Protein Technologies Inc., U.S.A.). The appropriate amount of each peptide was weighed out, dissolved in phosphate buffered saline (PBS buffer) and mixed with either of the three activated carrier proteins.
The carrier proteins used in this study, purchased in solution, were tetanus toxoid (TT) (Chiron Behring, Germany), keyhole limpet haemocyanin (KLH) (Biosyn, Germany) and non toxic recombinant diphtheria toxin (DT) (Chiron Behring, Germany). To activate for conjugation, an appropriate amount of each carrier protein was mixed with an excess of S-MBS bivalent linker (Pierce, U.S.A.). Following activation, the carrier proteins were separated from the remaining reaction components by size exclusion chromatography on Sephadex G-25 matrix columns (Pharmacia, Sweden).
To conjugate, the activated carrier proteins were mixed with an excess of the AI and/or AII peptide analogues. Following the reaction each conjugate was separated from the remaining free peptide by size exclusion chromatography on Sephadex G-25 matrix columns (Pharmacia, Sweden).
The conjugates were sterilized using 0.2 μm filters (Millipore, U.K.) and the peptide concentration of each determined following protein concentration analysis using a bicinchoninic acid, BCA kit (Pierce, U.S.A.). The vaccines were then formulated yielding the appropriate conjugate peptide concentration using either a diethylaminoethyl cellulose (DEAE) solution or Alhydrogel® (Superfos, S.A., Denmark) as adjuvant and 0.9% (w v−1) saline (Flowfusor®, Fresenius, U.K.) as the vaccine vehicle. Table 1 shows the vaccine formulation and protocol for the first study.
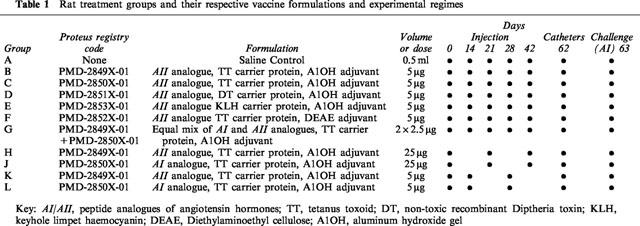
Table 1.
Rat treatment groups and their respective vaccine formulations and experimental regimes
Immunization protocol
Two studies were carried out, but following the same immunization protocol. In both studies male, Sprague-Dawley rats (Harlan Olac, U.K.) were used. In the first study, rats (initial body weight 300–405 g, 11 groups, n=6 in all groups) were injected (0.5 ml s.c.) with saline or angiotensin vaccines on the days indicated in Table 1.
In the second study, the most effective treatment regime from the first study (i.e. PMD-2850, 5 μg was injected on days 0, 21 and 42) was repeated in another group of rats (n=8) together with saline-treated controls (n=8).
In vivo pressor testing
In both studies, on day 62 of the protocol, rats were anaesthetized (sodium methohexitone 40–60 mg kg−1 i.p., supplemented as required) and catheters were implanted in the abdominal aorta (via the ventral caudal artery) and the right jugular vein. Catheters ran subcutaneously to exit at the back of the neck, and then through a flexible spring (for protection) attached to a harness fitted to the rat. The spring was supported by a freely-moving counterbalanced lever. The arterial catheter was connected to a swivel system to allow continuous infusion of saline to maintain patency (Waller et al., 1995). The following day, when animals were conscious, unrestrained and with free access to food and water, cardiovascular responses were assessed.
In the first study, increasing i.v. bolus (0.1 ml) doses of AI (3, 6, 10, 18, 30 and 60 pmol rat−1) (Johnston et al., 1970; Tarpey et al., 1998) were given with sufficient interval (at least 15 min) between doses to allow the acute pressor effects to wane. Measurements of mean arterial blood pressure and heart rate were made immediately before and 0.25, 0.5, 0.75, 1, 2, 3, 4 and 5 min after injection of AI. In each animal, the peak pressor response to AI occurred within the first minute after injection, and this value was used in the assessment of response to AI (see below).
In the second study the procedures followed were as above, but here animals were given increasing bolus doses of AI (10, 20 and 30 pmol rat−1) and increasing doses of AII (5, 10 and 15 pmol rat−1). Animals were randomized to receive AI or AII as the first challenge.
At the end of the experiment rats were terminally anaesthetized (sodium pentobarbitone 100 mg i.v.) and a blood sample was taken by cardiac puncture for the measurement of anti-angiotensin and anti-angiotensinogen antibodies by ELISA.
ELISA analysis of the rat sera
ELISA plate wells (Anachem, U.K.) were coated with either angiotensinogen (Sigma, U.K.) or peptide (AI or AII) equivalents which had been conjugated to bovine serum albumin (BSA) (Sigma, U.K.), as a carrier.
The coated wells were washed throughout with PBS buffer containing 0.2% (v v−1) Tween20 (Sigma, U.K.). Any remaining well space was blocked using PBS buffer containing 3% (w v−1) dried milk powder.
Diluted sera from the vaccinated rats were then incubated in their respective wells. For detection of the AI and AII-BSA conjugates, the rat sera were diluted over a range from 2500–20,000 fold in PBS buffer (Sigma, U.K.). For detection of angiotensinogen, antibodies from rat sera raised to four different AI and AII-TT conjugates were used. These rat antibodies had been purified from sera using a Protein G HiTrap® affinity column (Pharmacia, Sweden), and diluted for use to 0.5 μg ml−1.
Immobilized antibodies were detected in the wells using a rabbit anti-Rat IgG/horseradish peroxidase conjugate diluted in PBS buffer (Sigma, U.K.). The peroxidase chromogenic substrate 3,3′-5,5′-tetra-methyl benzidine (TMB) mixed with 0.5% (v v−1) H2O2 (Sigma, U.K.) in a sodium acetate buffer was incubated in the ELISA plate wells. The reaction was terminated by the addition of 10% (v v−1) H2SO4 (Sigma, U.K.).
Colour generated following reactions between the peroxidase and TMB substrate was determined by absorbency at 450 nm using a Packard plate reader (Packard, U.S.A.). The resulting absorbency readings were statistically analysed to determine the sera antibody titre resulting from each AI and AII conjugate vaccination (see Data analysis).
SDS–PAGE and Western blot analysis
Three SDS-polyacrylamide (10% w v−1) gels were prepared from a bis/acrylamide concentrate cross linked upon reaction with N,N,N′,N′-tetramethylethylenediamine and ammonium persulphate (Sigma, U.K.). The following samples were loaded on to and run through the gels under reducing conditions in the respective lanes. Lane 1=High molecular weight range markers (Sigma, U.K.), Lane 2=2 μg Angiotensinogen (Sigma, U.K.), Lane 3=0.2 μg Angiotensinogen (Sigma, U.K.) and Lane 4=10 μg Rat plasma protein.
One gel was then stained with 0.1% (w v−1) Coomassie R-250 (Sigma, U.K.) and dried between sheets of Cellophan membrane (Pharmacia, Sweden). Samples on the remaining SDS-polyacrylamide gels were blotted by electrophoretic transfer on to Hybond-C extra nitro-cellulose membrane, NCM (Amersham, U.K.). Any remaining NCM space was blocked using PBS buffer (Sigma, U.K.) containing 3% (w v−1) dried milk powder. The two NCMs were then probed with antibodies from either rat sera group C (immunized with AI-TT conjugate PMD 2850) or group A (treated with saline).
Rat antibodies bound with the NCMs were detected using rabbit anti-Rat IgG/horseradish peroxidase conjugate diluted in PBS buffer (Sigma, U.K.). The immobilized peroxidase was reacted with a chemiluminescence reagent (Amersham, U.K.) and the resulting fluorescence identified following exposure to photographic film (Kodak, U.K.).
Data analysis
The maximum change in mean blood pressure relative to the value immediately pre-challenge was calculated for each animal and each AI challenge dose. The AI dose response within each animal was modelled by fitting a 3-parameter logistic:
In this model, the maximum response, ymax, and slope parameter, α, are assumed to be the same for all animals, and d50,ij is the estimated ED50 (i.e. challenge dose giving a half-maximal increase in MAP) for animal j in treatment group i; yijk is the peak change in MAP following challenge with dose dk of AI. Log(d50ij) values were analysed by ANOVA to test for significant differences between treatment groups.
A smaller range of challenge doses was used in the second study, and the maximum response ymax was less well defined by these data (95% confidence interval 41–83 mmHg). However, this did not affect the estimation of the dose-shift of immunized animals relative to controls. The same methods were used to assess the AII dose response in the second study.
Results
In the first study, active immunization caused shifts in AI responses and production of anti-angiotensin antibody titres as shown in Table 2. The most marked effects were seen with PMD-2850, although a higher dose of this conjugate did not produce a greater effect (Figure 1).
Table 2.
Median AI bolus (pmol rat−1)) to achieve half-maximal increase in mean blood pressure (ED50) and anti-AI antibody titres for each group of treated rats
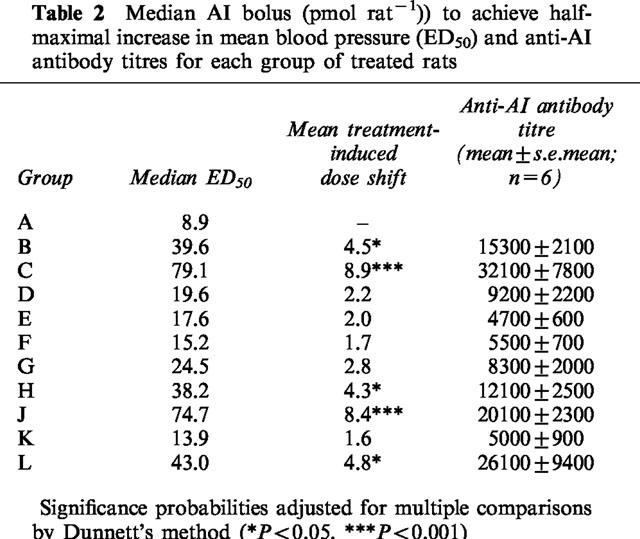
Figure 1.
Pressor responses to AI in conscious rats treated with saline (control; n=6) or with PMD-2850 (see Table 1) at a dose of 5 μg (Group C, Table 1; low dose; n=6) or 25 μg (Group J, Table 1; high dose; n=6). Error bars show the standard error of the mean. Statistics for the data are shown in Table 2.
It is notable that the anti-angiotensin antibodies generated with PMD-2850 as an immunogen also bound to angiotensinogen (renin substrate), but others did not (Figure 2).
Figure 2.
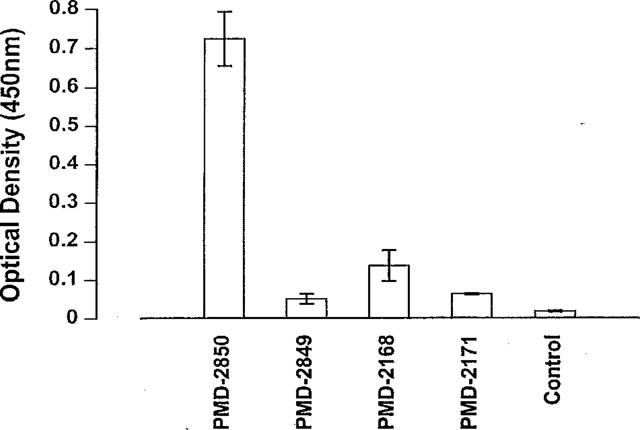
Results from ELISA showing binding to angiotensinogen of rat antisera raised against vaccines containing analogues of angiotensin peptides, encoded PMD-2850, PMD-2849, PMD-2168 and PMD-2171 (see Table 1 for PMD-2850 and PMD-2849 vaccine codes). Error bars show the standard error of the mean.
Antibodies generated with PMD-2850 as an immunogen recognise ⩾0.2 μg of angiotensinogen by Western blot analysis (Figure 3). However, such an indication of antibody selectivity depends on complete protein transfer to, and epitope availability on, the NCM. Antibodies in sera prepared from rats treated with saline did not recognize angiotensinogen.
Figure 3.
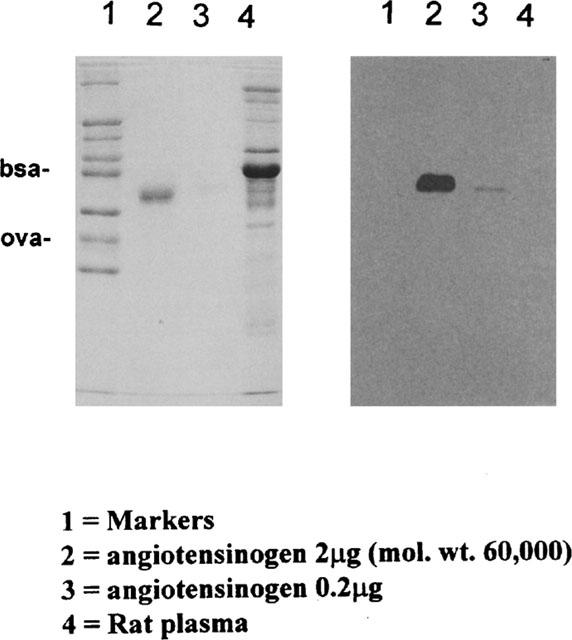
An SDS–PAGE protein stain (left-hand panel) and an immunoblot (right-hand panel) demonstrating that the anti-angiotensin antibodies generated with PMD-2850 (see Table 1) as immunogen also binds to angiotensinogen. The markers in Lane 1 were bovine serum albumin (bsa; mol. wt. 66,000) and ovalbumin (ova; mol. wt. 46,000).
In the second study, active immunization with PMD-2850 suppressed responses to AI (P>0.05), but had no significant effect on those to AII (Figure 4).
Figure 4.
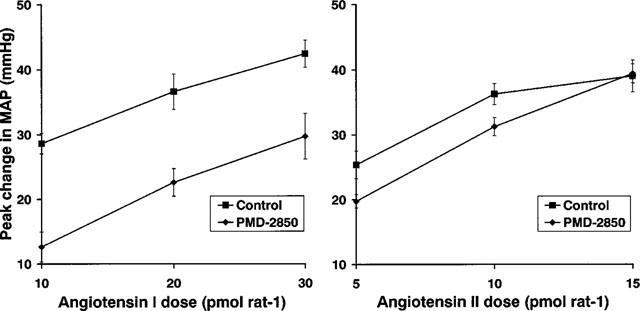
Pressor responses to AI or AII in conscious rats treated with saline (control; n=8) or with PMD-2850 (see Table 1) at a dose of 5 μg (n=8). Error bars show the standard error of the mean.
Discussion
In the present work, active immunization with novel immunogens against AI indicates that the suppression of in vivo responses to exogenous AI and the anti-angiotensin antibody titre show a loose correspondence, in as much as the immunogen, PMD-2850 (AI analogue, tetanus toxoid carrier protein, AlOH adjuvant), caused the biggest shift in the dose-response to AI and generated the highest antibody titres. However, immunization with a higher dose of PMD-2850 did not cause a greater effect, and immunization with PMD-2850 on days 0, 14 and 28 caused less of a shift in the AI dose-response, yet a similar increase in antibody titre to that seen when animals were immunized on days 0, 21 and 42 (Tables 1 and 2). Clearly, further studies are needed to determine the affinities of the antibodies generated in each of the treatment groups, and to clarify the relation between change in response to AI and antibody titre. However, it is unlikely that the properties of the antibodies raised by challenge with PMD-2850 would vary with dose or timing of immunization.
Nonetheless there was clearly something qualitatively different about the antibodies raised by the challenge with PMD-2850, since they showed cross-reactivity with angiotensinogen, whereas others did not. Furthermore, this cross-reactivity was not an expression of a lack of selectivity of effect since active immunization with PMD-2850 had no significant influence on responses to exogenous AII, when responses to AI were clearly suppressed. However, we cannot infer from these observations that the antibodies do not bind AII. It could be, for example, that administration of exogenous AII causes rapid activation of AT1-receptors, such that binding to the antibodies does not suppress the peak response. Thus, the apparently selective inhibition of the response to AI could be a reflection of the requirement for it to be converted to AII to exert an effect, thereby allowing a greater time for the antibodies to sequester the peptide (AI and AII) and suppress the response.
Previous studies have dealt with the topic of the present investigation but have not addressed the same questions. Thus, Johnston et al. (1970) showed that rats could be actively immunized against AII and, while this reduced pressor responses to exogenous AII, it did not affect the development or maintenance of renal hypertension. Johnston et al. (1970) did not investigate active immunization against AI.
Oates et al. (1974) studied rats actively immunized against AII, or AI and AII, but not animals specifically immunized against AI. Rats immunized against AI and AII showed diminished pressor responses to both peptides, but the development of renal hypertension was unaffected.
In the present study, the fact that the antibodies raised by immunization with PMD-2850 cross-reacted with angiotensinogen was unexpected but, presumably, could relate to the greater ability of this immunogen to suppress responses to exogenous AI. If immunization with PMD-2850 produces antibodies which react with endogenous angiotensinogen, and suppress the ability of endogenous AI to influence cardiovascular status (to the same extent as seen here with exogenous peptide), then it would be expected to be more effective than previous active immunization protocols (Johnston et al., 1970; Oates et al., 1974) in lowering blood pressure in hypertensive rat models and, potentially, in man. Indeed, recent studies with PMD-2850 have shown that active immunization of male spontaneously hypertensive rats causes significant although limited lowering of resting diastolic arterial blood pressure, compared to non-immunized animals (Smits et al., 1999).
On the basis of these findings, pre-clinical development studies are proceeding with the intention to initiate Phase I clinical trials during 1999.
Acknowledgments
This work was funded by Protherics Molecular Design Ltd.
Abbreviations
- AI
angiotensin I
- AII
angiotensin II
- ACE
angiotensin converting-enzyme
- BSA
bovine serum albumin
- DEAE
diethylaminoethyl cellulose
- DT
diptheria toxin
- ELISA
Enzyme linked immunosorbent assay
- i.v.
intra-venous
- i.p.
intra-peritoneal
- KLH
keyhole limpet haemocyanin
- NCM
nitrocellulose material
- RAS
renin-angiotensin system
- s.c. sub-cutaneous; SDS-PAGE
sodium dodecyl-sulphate polyacrylamide gel electrophoresis
- S-MBS
sulpho-m-Maleimidobenzoyl-N-hydrosuccinimide ester
- TMB
3,3,′-5,5′-tetra-methyl benzidine
- TT
tetanus toxoid
References
- BROWN N.J., VAUGHAN D.E. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., AUTON T.R., DOWNHAM M.R., SHARP H.L., KEMP P.A., MARCH J.E., RUSHTON A., BENNETT T., GLOVER J.F. Effects of active immunization against angiotensin peptides on the pressor effects of exogenous angiotensin I (A1) in conscious rats. Br. J. Pharmacol. 1998;124:119P. doi: 10.1038/sj.bjp.0703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIUCHI M., AKISHITA M., DZAU V.J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- JOHNSTON C.I., HUTCHINSON J.S., MENDELSOHN F.A. Biological significance of renin angiotensin immunization. Circ. Res. 1970;26–27 Suppl II:II-215–II-222. [PubMed] [Google Scholar]
- MESSERLI F.H., WEBER A., BRUNNER H.R. Angiotensin II receptor inhibition. Arch. Intern. Med. 1996;156:1957–1965. [PubMed] [Google Scholar]
- MICHEL J.-B., GUETTIER C., READE R., SAYAH S., CORVOL P., MÉNARD J. Immunologic approaches to blockade of the renin-angiotensin system: a review. Am. Heart J. 1989;117:756–767. doi: 10.1016/0002-8703(89)90767-9. [DOI] [PubMed] [Google Scholar]
- OATES H.F., STOKES G.S., STOREY B.G., GLOVER R.G., SNOW B.F. Renal hypertension in rats immunized against angiotensin I and angiotensin II. J. Exp. Med. 1974;139:239–248. doi: 10.1084/jem.139.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITS J., AUTON T., SHARP J., DOWNHAM M., GLOVER J., JANSSEN B. Anti-hypertensive properties of angiotensin immunotherapeutic in spontaneous hypertension in rats. FASEB J. 1999;13:A483. [Google Scholar]
- STROTH U., UNGER T. The renin-angiotensin system and its receptors. J. Cardiovasc. Pharmacol. 1999;33 Suppl 1:S21–S28. doi: 10.1097/00005344-199900001-00005. [DOI] [PubMed] [Google Scholar]
- SUNMAN W., SEVER P.S. Non-angiotensin effects of angiotensin-converting enzyme inhibitors. Clin. Sci. 1993;85:661–670. doi: 10.1042/cs0850661. [DOI] [PubMed] [Google Scholar]
- TARPEY S.B., BENNETT T., RANDALL M.D., GARDINER S.M. Differential effects of endotoxaemia on pressor and vasoconstrictor actions of angiotensin II and arginine vasopressin in conscious rats. Br. J. Pharmacol. 1998;123:1367–1374. doi: 10.1038/sj.bjp.0701751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLER J., GARDINER S.M., JOSE J., BENNETT T. Lack of effect of TNF antibodies on the cardiovascular sequelae of lipopolysaccharide infusion in conscious rats. Br. J. Pharmacol. 1995;116:2487–2495. doi: 10.1111/j.1476-5381.1995.tb15100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


