Abstract
Platelet-activating factor (PAF), an inflammatory mediator, plays an important role in mediating intestinal injury. However, it remains unclear whether PAF has a function in the intestine. The production of PAF by normal intestine and by unstimulated intestinal epithelial cell lines suggests that PAF may have a regulatory function in the normal bowel.
In this study we investigated the role of PAF in modulating intestinal mucosal permeability in rats. Lumen-to-blood transit of FD-4 (dextran 4400), (an index of intestinal permeability), was assessed in sham-operated rats and rats injected with PAF (1.25 μg kg−1, i.v., a dose insufficient to induce intestinal injury).
PAF-induced villus cytoskeletal changes were examined by staining the intestine for F-actin. The effect of PAF on tyrosine phosphorylation of the junctional protein E-cadherin was examined by immunoprecipitation. Some rats were pretreated with AG1288 (a tyrosine kinase inhibitor) before PAF injection, and mucosal permeability change was assessed.
To investigate the role of endogenous PAF upon mucosal permeability, we studied the effect of PAF antagonists on (intraluminal) glucose-induced increase in mucosal permeability.
We found that low dose PAF: (a) alters the cytoskeletal structure of intestinal epithelium, (b) causes the influx of FD4 from intestinal lumen to systemic circulation, (c) induces tyrosine phosphorylation of E-cadherin and cadherin-associated proteins. Glucose-induced mucosal permeability increase is abolished by using two structurally different PAF antagonists.
These results suggest that endogenous PAF modulates macromolecular movement across the intestinal mucosal barrier, probably via tyrosine phosphorylation of E-cadherin and cytoskeletal alteration of enterocytes.
Keywords: Platelet-activating factor, intestinal permeability, protein tyrosine phosphorylation, E-cadherin, small intestine
Introduction
The intestinal epithelium functions as a selective barrier that permits the absorption of nutrients, electrolytes, and water but prevents the entry of toxins, antigens, proteinase, and micro-organisms from the lumen into the systemic circulation (Bjarnason, 1994; Bjarnason et al., 1995). Impairment of the mucosal barrier function is often seen during pathological conditions such as circulatory shock and anaphylatic shock (Bjarnason, 1994; Bjarnason et al., 1995), and can be induced experimentally in animals by injecting endotoxin or pro-inflammatory cytokines such as tumour necrosis factor-α (Fink, 1991). In certain physiological conditions, this selective permeability may be altered reversibly, and various small peptides and antigens permeate across intestinal epithelia mainly through paracellular pathways by passive processes. For example, at high concentration of luminal glucose, cell junctions in the intestinal epithelium dilate (Madara & Pappenheimer, 1987; Pappenheimer & Reiss, 1987). In addition, coadminstration of glucose with antigens enhances antigen uptake and augments mucosal immune responsiveness (Zhang & Castro, 1992). However, the molecular mechanism of this glucose-induced transient increase in permeability in the normal intestine remains unclear.
The control of paracellular pathways in the epithelium is determined by proteins in the zona occludens and zona adherens (ZA), which interact with perijunctional actin (Anderson & Van Itallie, 1995). Some components of the junctional complex, including E-cadherin (a transmembrane protein at ZA) and its associated proteins, have been identified (Edelman & Crossin, 1991). A recent study demonstrated a correlation between junctional E-cadherin protein content and paracellular permeability in epithelia (Takeichi, 1991). The crucial role of E-cadherin in the dynamic regulation of paracellular permeability is indicated by the observation that antibodies to E-cadherin block paracellular junction formation and subsequently increase permeability in MDCK cells (Behrens et al., 1985). The junctional complex protein interacts with cytoskeletal actin (Anderson & Van Itallie, 1995; Edelman & Crossin, 1991). In vitro experiments showed that cytochalasin, an inhibitor of actin polymerization, disrupts actin cytoskeleton organization and increases epithelial permeability (Madara et al., 1986).
PAF (platelet-activating factor, paf-acether) is an endogenous phospholipid mediator synthesized by various inflammatory cells, platelets and endothelial cells (Snyder, 1990). Recent reports showed that PAF is also produced by intestinal epithelial cells (Guerrant et al., 1994; Gustafson et al., 1991). A large body of evidence suggests that PAF is involved in the pathogenesis of intestinal injury in various diseases including inflammatory bowel disease and necrotizing enterocolitis (Eliakim et al., 1988; Hsueh et al., 1994). The pathophysiological role of PAF in intestinal injury is also demonstrated by our previous observation that systemically administered PAF causes intestinal necrosis (Gonzalez-Crussi & Hsueh, 1983; Hsueh et al., 1987). Interestingly, PAF is constitutively present in normal small intestinal tissue (Qu et al., 1996), suggesting a physiological role of PAF in the normal bowel. But whether PAF produced in the normal G-I tract has any function at all, is still not known. Moreover, PAF at doses insufficient to cause bowel necrosis triggers the expression of cytokines, and activates transcription factors in the intestine (De Plaen et al., 1998; Huang et al,. 1994; Tan et al., 1994, 1996; Wang et al., 1997).
One of the functions of PAF in the intestine may be the regulation of mucosal permeability. Previous investigation has shown that infusion of a low dose of PAF (without causing injury) into the splanchnic circulation increased the intestinal mucosal permeability to [51Cr]EDTA (MW <1000 Da) (Kubes et al., 1991). Furthermore, PAF receptor antagonists reduced the early rise in mucosal permeability induced by nitric oxide synthase inhibitors (Kanwar et al., 1994). Interestingly, PAF has been found to stimulate shape change of the endothelium by rearranging the cytoskeleton (Bussolino et al., 1987). Taken together, these observations lead us to hypothesize that: (a) PAF plays an important role in the regulation of mucosal permeability in normal GI tract; and (b) the effect of PAF on the alteration of mucosal permeability is via regulation of the junctional complex and cytoskeletal structure of the intestinal epithelium. In this study we found that a low dose of PAF: (a) alters the cytoskeletal structure of intestinal epithelium, (b) causes the influx of dextran 4400, an inert polymer, from the intestinal lumen to the systemic circulation, and (c) induces tyrosine phosphorylation of E-Cadherin in the small intestine. Further, we found that endogenous PAF may mediate glucose-induced permeability increase of intestinal mucosa.
Methods
Materials
PAF (1-O-hexadecyl-2-acetyl-sn-3-phosphocholine), FITC-dextran 4400 (FD-4), FITC conjugated LPS (FITC-LPS), TRITC conjugated phalloidin, Tyrode's mixture, D-glucose, and molecular biological reagents were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Enhanced Chemical Luminescence (ECL) kit was purchased from Amersham (Arlington Heights, IL, U.S.A.). Murine anti-E-cadherin mAb was purchased from Transduction Labs (Lexington, Kentucky, U.S.A.). Rabbit anti-Tyr(P) antibody (polyclone) was supplied by Pharmingen (San Diego, CA, U.S.A.). Protein A-agarose was obtained from Oncogene Science (Manhasset, NY, U.S.A.). Tyrphostin AG1288, a protein tyrosine kinase inhibitor (Novogrodsky et al., 1994), was obtained from Calbiochem (San Diego, CA, U.S.A.). WEB2170 was a gift from Dr H. Heuer (Boerhinger-Ingelheim, Mainz, Germany). SRI 63-441 was a gift from Sandoz Research Institute (East Hanover, New Jersey, U.S.A.).
Animal experiment and mucosal permeability study (Salzman et al., 1994)
Young male Sprague-Dawley rats (80–120 g) were fasted overnight and anaesthetized with Nembutal (65 mg kg−1, i.p.). The rat was tracheotomized and catheterized via the carotid artery and jugular vein for continuous blood pressure recording, blood sampling, and drug injection. Only animals with stable blood pressure over 100 mmHg were used for the study. After a midline abdominal incision, the terminal ileum was ligated and cannulated for administration of permeability markers (FD-4 and FITC-LPS). Both renal pedicles were ligated to prevent urinary excretion of the permeability markers. The abdominal wall was then closed by suture. The mucosal permeability was determined by measuring the lumen-to-plasma entrance of permeability markers. Tyrode's solution containing permeability markers was slowly instilled into the intestinal lumen (50 ml kg−1) at −60 min. In some experiments, rats were pretreated with Tyrphostin AG1288 (25 mg kg−1, i.p.) 2 h before PAF. PAF (1.25 μg kg−1, i.v.) or saline (1 ml kg−1, i.v.) was injected at time 0. Blood samples (80 μl) were collected at −60, 0, 15, 30, 60, 90, and 120 min, the plasma was diluted 1 : 1000 in PBS (pH 7.0), and the fluorescence was measured with a Perkin-Elmer LS-5 fluorescence spectrophotometer (excitation wavelength 495 nm and emission 520 nm). The animals were sacrificed at 120 min. Since PAF at low doses (1.25 μg kg−1, i.v.) did not cause gross intestinal necrosis, the entire small intestine was examined to rule out microscopic injury. After gentle washing with saline, the small intestine was divided into proximal (duodenum and jejunum), middle (jejunum and ileum), and distal (ileum) portions, each rolled into a spiral, fixed in formalin and processed for histological examination.
F-actin Stain (Ezzell et al., 1993)
After injection with PAF (1.25 μg kg−1, i.v.) or saline (1 ml kg−1), the animals were sacrificed at 0, 5, 15, and 30 min, the intestines were rinsed, fixed at 4°C for 6 h in PBS (pH 7.4) containing 4% paraformaldehyde and 2 mM EGTA, and the tissues were rinsed in PBS-EGTA, tumbled in PBS containing 2 mM EGTA and 0.6 mM sucrose overnight at 4°C. The tissues were then embedded in OCT compound, and cryostat sections (5 μm) were mounted on poly-l-lysine coated slides, extracted with acetone for 2 min at −20°C, and rinsed in PBS containing 2 mM EGTA (PBS-EGTA). F-actin was stained by incubation with PBS-EGTA containing 0.5 μg ml−1 of TRITC-conjugated phalloidin for 30 min at room temperature. After rinsing with PBS-EGTA, the sections were examined with a fluorescence microscope. (The sections were not allowed to dry during staining procedure).
Immunoprecipitation and Immunoblotting (Staddon et al., 1995)
Intestinal tissue was lysed with ice-cold lysing buffer (in mM): Tris Cl 2, pH 7.6, containing NaCl 30, EDTA 1, benzamidine 0.2, DTT 1, PMSF 1, leupeptin 10 μg ml−1, aprotinin 10 μg ml−1, soybean trypsin inhibitor 10 μg ml−1, Na3VO4 2, and Nonidet P-40 1%, sonicated for 2 min, centrifuged at 10,000×g for 10 min at 4°C and the pellet of cellular debris was removed. Protein concentration was determined using Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, U.S.A.). Intestinal lysate containing 1 mg of protein was precleaned by incubating with 20 μl of protein A-agarose for 4 h at 4°C, followed by removal of protein A-agarose beads by centrifugation (1500×g for 2 min at 4°C). The protein content was again quantified, and 0.5 mg protein was reacted with 5 μg murine anti-E-cadherin mAb overnight at 4°C, and the immune complexes were precipitated by protein A-agarose beads. The beads bearing the immunoprecipitates were collected by centrifugation, washed four times with lysate buffer, and resuspended in 2× Laemmli's solution containing 2-ME. After spinning at 5000×g for 10 min, the supernatant was resolved by electrophoresis on 10% SDS–PAGE gels, transferred onto a nitrocellulose membrane (Bio-Rad) by electrophoresis overnight at 0.2 A in transfer buffer containing 39 mM glycine, 48 mM Tris base, 0.037% SDS, and 20% methanol. The membrane containing sample proteins was used for immunodetection of tyrosine phosphorylated protein. Briefly, after blocking the residual protein sites on the membrane with 3% skim milk (in PBS) for 60 min at room temperature, the membrane was reacted with polyclonal rabbit anti-phosphotyrosine antibody for 2 h at room temperature. After incubation, the blot was washed four times with PBS containing 0.05% Tween 20 (PBS-T), and incubated with the secondary antibody, a peroxidase-conjugated goat anti-rabbit IgG antibody (Amersham), diluted at 1 : 2000 in PBS-T, for 1 h at room temperature. After additional washing with PBS-T, the tyrosine phosphorylated protein on the blot was detected with a ECL system.
The role of endogenous PAF in glucose-induced increase in mucosal permeability
In this group of experiments, rats were divided into five groups: (a) sham-operated, Tyrode's solution containing 0.4 mg ml−1 of FD-4 was instilled into small intestinal lumen at time 0 (50 ml kg−1), (b) Glucose-treated, Tyrode's solution containing 5% glucose and 0.4 mg ml−1 of FD-4 was instilled into intestinal lumen at time 0 (50 ml kg−1), (c) WEB+glucose-treated. Both WEB2170 (2 mg kg−1, i.v.), a PAF antagonist, and glucose/FD-4 solution (instilled into intestinal lumen) were given at time 0, (d) WEB (i.v.)+Tyrode's solution, at time 0, (e) SRI 63-441+glucose-treated. Both SRI 63-441 (5 mg kg−1, i.v.), a PAF antagonist, and glucose/FD-4 solution (instilled into intestinal lumen) were given at time 0. Blood samples (0.1 ml) were collected at 0 and 60 min, plasma samples were diluted 1 : 1000 in PBS (pH 7.0), and the fluorescence was measured as described above. The animals were sacrificed at 60 min.
Tissue PAF extraction, purification and bioassay (Qu et al., 1998)
Rats were divided into two groups: (a) sham-operated, and (b) glucose-treated. At the end of experiment, the small intestine was removed, flushed with cold saline, homogenized in chloroform-methanol (2 : 1, v v−1), and total tissue lipid was extracted. The lipid residue was reconstituted in a solution containing 10% acetic acid/0.15% Tween-20/1.5% ethanol. PAF was partially purified by C18 column chromatography, dissolved in 50 μl ethanol, reconstituted in 450 μl of saline containing 5 mg ml−1 albumin and quantified by measuring [3H] serotonin release from rabbit platelets as previously described.
Statistic analysis
Data were reported as mean±s.e. mean. Comparisons among multiple groups were made by a one-way analysis of variance (ANOVA) followed by Fisher's protected least significant difference posthoc test. 95% confidence limits (P<0.05) were considered significant.
Results
PAF (1.25 μg kg−1, i.v.) increased FD-4 entry from intestinal lumen into systemic circulation, and its attenuation by tyrosine kinase inhibitor
Sixty minutes following the intraluminal administration of FD-4 in sham-operated rats, a low level of FD-4 entry from the small intestinal lumen into the blood stream became detectable (Figure 1). PAF (1.25 μg kg−1, i.v.) almost tripled the transfer of FD-4 from the lumen to blood within 30 min (P<0.01). The permeability continuously increased after PAF and peaked at 90 min, reaching more than 10 fold the control value (Figure 1). Tyrphostin AG1288 markedly attenuated the PAF effect on intestinal permeability (Figure 1). At the dose (1.25 μg kg−1) used, PAF did not cause LPS entry from intestinal lumen into blood, since in a separate group of experiments with FITC-LPS instilled into the intestinal lumen, no FITC-LPS was detected in the plasma in either sham-operated or PAF-treated rats. Since this dose (1.25 μg kg−1) of PAF does not induce gross intestinal necrosis, the entire small intestine was examined microscopically. We did not find any intestinal injury ranging from epithelial cell detachment to definitive tissue necrosis in the PAF-treated rats.
Figure 1.
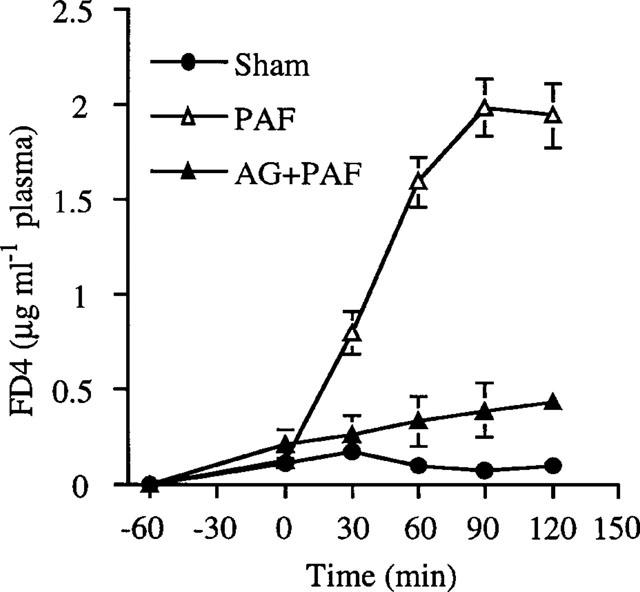
Attenuation of PAF-increased FD-4 entry from intestinal lumen into blood by AG1288. Tyrode's solution containing FD-4 (0.4 mg ml−1) was instilled into intestinal lumen at −60 min. PAF (1.25 μg kg−1, i.v.) was injected at time 0. Tyrphostin AG1288 (25 mg kg−1, i.p.) was given 2 h before PAF. Sham-operated rats were treated with saline (1 ml kg−1). Blood sample (80 μl) was collected at different time points for FD-4 determination. Results are means±s.e.mean (n=4–6).
PAF (1.25 μg kg−1, i.v.) altered cytoskeletal structure of intestinal epithelium
In sham-operated animals, there is an intense, homogeneous and continuous staining of F-actin of the epithelial brush border of the small intestine (Figure 2a). A less intense, linear F-actin staining was also observed along the paracellular junction of intestinal epithelia (Figure 2a). Within 5 min after PAF (1.25 μg kg−1, i.v.) injection, a punctate pattern of F-actin staining was noted focally at the tip of villi (Figure 2b). Thirty minutes after PAF, the linear outline of the brush border as well as paracellular junction at the villus tip became irregular (Figure 2c). Histological examination of the small intestine showed that PAF at the dose used did not cause tissue injury during the 120-min experimental period.
Figure 2.
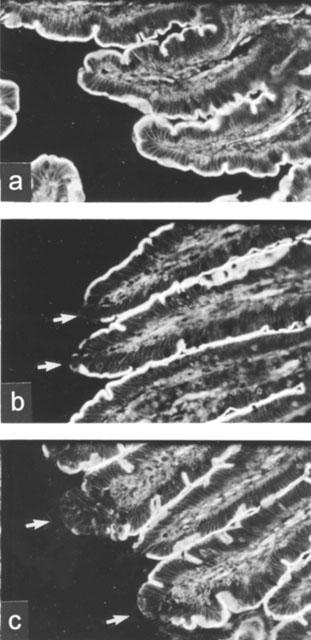
Alteration of intestinal epithelial cytoskeleton after PAF treatment. PAF (1.25 μg kg−1, i.v.) was given at time 0. In sham-operated group, saline (1 ml kg−1) was injected instead of PAF. Three animals were used for each time point. Cryosections of intestinal tissue were stained for F-actin with rhodamine-phalloidin and examined under a fluorescent microscope. Typical sections are shown in (a) (sham-operated, 30 min), (b) (PAF, 5 min) and (c) (PAF, 30 min). Arrows indicate cytoskeletal rearrangement in villus tips. × 200.
PAF enhanced tyrosine phosphorylation of E-cadherin protein
Previous investigations have shown that the function of E-cadherin is regulated by phosphorylation of the tyrosine residues (Staddon et al., 1995). To investigate whether PAF causes tyrosine phosphorylation of E-cadherin, the intestinal lysates from PAF-treated animals as well as from the control were immunoprecipitated with murine mAb anti-E-cadherin protein and immunoblotted with rabbit anti-phosphotyrosine polyclonal antibody. As shown in Figure 3, intestinal E-cadherin protein (120 kDa) was constitutively phosphorylated on tyrosine residues (lane 1). Within 5 min after PAF injection (lane 2), tyrosine phosphorylation of E-cadherin was significantly increased, compared with sham-operated animals. This enhanced phosphorylation remained evident until 30 min after PAF (lanes 3 and 4). Two other proteins with molecular weights of approximately 102 and 98 kDa, which were co-immunoprecipitated with E-cadherin, also showed increased tyrosine phosphorylation.
Figure 3.

PAF enhances tyrosine phosphorylation of E-cadherin and cadherin-associated proteins in rat small intestine. Small intestines were collected from sham-operated (lane 1) and PAF (1.25 μg kg−1, i.v.) -treated rats. Samples in lanes 2, 3, and 4 were isolated from small intestines at 5, 15, or 30 min after PAF injection, respectively. Tyrosine phosphorylation of E-cadherin and cadherin-associated proteins were detected as described in Methods. Arrow heads indicate the presence of tyrosine phosphorylated, cadherin-associated proteins which were co-immunoprecipitated with E-cadherin. The experiment was repeated three times and similar results were obtained.
Systemic physiological changes after PAF treatment
The systemic physiological changes following intraluminal administration of PAF (1.25 μg kg−1, i.v.) are presented in Figure 4. As shown in the figure, PAF caused a transient hypotension which recovers to normal within 60 min (top panel, Figure 4a). A mild hemoconcentration and leukocytosis were observed within 120 min after PAF injection (Figure 4b,c).
Figure 4.
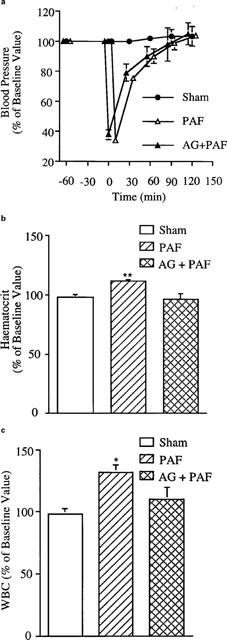
Changes in mean arterial pressure (a), hematocrit (b) and WBC count (c) after administration of PAF. See Figure 1 legend for treatment administered. *P<0.05 vs sham-operated animals and **P<0.01 vs sham-operated animals. Results are means + s.e.mean (n=4–6).
Endogenous PAF mediates glucose-induced increase of mucosal permeability
As shown in Figure 5, instillation of Tyrode's solution containing 5% glucose significantly increased FD-4 entry from the gut lumen into the blood stream within 60 min (P<0.01). The FD-4 entry induced by glucose instillation was prevented by WEB2170 or SRI 63-441, two structurally different PAF antagonists. There were no significant changes of physiological parameters in all groups (sham-operated, glucose, WEB2170, WEB plus glucose, and SRI 63-441 plus glucose) (data not shown). PAF is constitutively present in the intestinal tissue of normal rats (440.5±281 pg g tissue−1, n=4). There was no significant change of tissue PAF content 1 h after intraluminal instillation of 5% glucose (469.0±256 pg g tissue−1, n=5).
Figure 5.
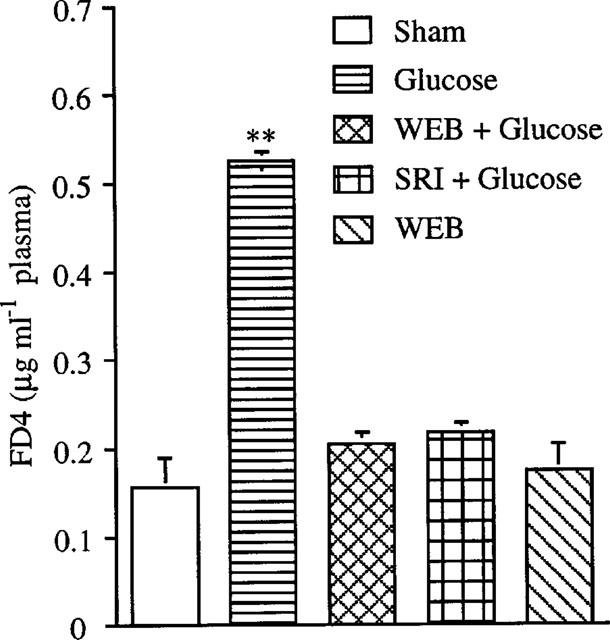
PAF antagonists inhibit glucose-induced FD-4 entry from intestinal lumen into systemic circulation. Tyrode's solution containing 5% glucose and 0.4 mg ml−1 FD-4 was instilled into intestinal lumen at time 0. No glucose was added to Tyrode's solution in the sham-operated group. PAF antagonists, either WEB2170 (2 mg kg−1, i.v.) or SRI 63-441 (5 mg kg−1, i.v.), were given at time 0. Blood samples (0.1 ml) were collected at time 0 and end of the experiment for FD-4 determination. **P<0.01 vs sham-operated animals. Results are means±s.e.mean (n=4–5).
Discussion
PAF is a well recognized inflammatory mediator, whose pathophysiological role in intestinal injury and inflammation has been well established (Eliakim et al., 1988; Hsueh et al., 1994; Snyder, 1990). However, whether PAF has any role in the normal gastro-intestinal tract has not been elucidated. Our previous studies have shown that a low level of PAF is constitutively present in normal rat intestinal tissue (Qu et al., 1996). Furthermore, several intestinal epithelial cell lines synthesize PAF in vitro (Guerrant et al., 1994; Gustafson et al., 1991). Both human and rat intestinal epithelial cells express PAF receptor (Kotelevets et al., 1998; Wang et al., 1999). Moreover, we recently showed that PAF at doses beneath that causing intestinal injury activates transcription factors and induces the gene transcription of cytokines in the intestine (De Plaen et al., 1998; Huang et al,. 1994; Tan et al., 1994, 1996; Wang et al., 1997). These observation suggest an additional role for this potent inflammatory mediator. A novel function of PAF in normal rat intestine demonstrated in the present study is regulation of intestinal mucosal permeability.
It is known that the gate function of paracellular junctions in small intestinal epithelium is under dynamic regulation (Madara, 1988). Madara & Pappenheimer (1987) have shown that glucose induces dilation within paracellular junctions of enterocytes (Madara et al., 1987; Pappenheimer & Reiss, 1987). Furthermore, several other investigators demonstrated that glucose triggers contraction of peri-junctional actomyosin, enhancing absorption of oligopeptides and uptake of antigens by enterocytes (Pappenheimer et al., 1994; Pappenheimer & Volpp, 1992; Zhang & Castro, 1992). In the present study, we confirmed that intestinal mucosal permeability is increased at a physiologically relevant luminal concentration of glucose, and further demonstrated that this permeability increase could be abolished by two structurally different PAF antagonists. These observations suggest that endogenous PAF in normal small intestine may play a role in the regulation of mucosal permeability to lumenal antigens, thereby enhancing their access to mucosal immune system. However, despite the fact that glucose-induced permeability increase could be completely blocked by PAF antagonists, we could not demonstrate a significant increase of tissue PAF content from the basal level after glucose instillation. The most likely explanation for this discrepancy is that the increase of PAF production may be local and transient, and thus cannot be detected by measuring the PAF content of the massive bowel tissue. Another possible reason is that glucose instillation may cause only PAF release from the intracellular pool (as reflected by the constitutive presence of PAF in normal intestine) to the cell surface or the extracellular matrix, but not new PAF synthesis. Thus, the total PAF content in the intestinal tissue remains unchanged. The cellular source of PAF in normal small intestine includes intestinal epithelial cells, endothelial cells, mast cells, macrophages, and other inflammatory cells. Mast cells may be of special importance, as suggested by the observations that PAF-induced intestinal necrosis resembles that observed in IgE-sensitized (mast cell-mediated) rats, and PAF antagonists ameliorate the intestinal hemorrhagic necrosis in rat passive anaphylaxis (Pellon et al., 1994). Additionally, animals sensitized to nematode antigens display an increase in epithelial short-circuit current when challenged with the antigen. Although this response is largely dependent on mast cell mediators, it is also tetrodotoxin-sensitive, implying a role of nerves and their interaction with mast cells (McKay & Bienenstock, 1994). Moreover, capsaicin-induced hypotension in N. brasiliensis-sensitized rats also depends on PAF release from mast cells (Mathison & Davison, 1993), indicating a cross-talk between mast cells and nerves in the gastro-intestinal tract through the release of chemical mediators such as PAF.
To directly prove the effect of PAF on mucosal permeability, we injected PAF at a dose (1.25 μg kg−1) below that causing irreversible shock. Although PAF at this low dose still induced a brief, transient hypotension, histological examination confirmed that it did not cause any microscopic injury of the small intestine. Moreover, the mucosal barrier to endotoxin remained intact. In contrast, the mucosal permeability to small inert polymers (4400 Da) was increased 15 min after PAF injection. Similarly, Kubes et al. (1991) demonstrated that low dose of PAF which did not cause histological injury of the small intestine, increased intestinal permeability to low molecular weight substance such as EDTA. Since PAF at low doses does not induce circulatory shock, systemic inflammation, or intestinal injury, this action of PAF may be operative in ‘physiological' or ‘pre-pathological' conditions, e.g., during minor or inconsequential insults in the intestinal tract.
The mechanism by which PAF regulates mucosal permeability was partly elucidated in the present study. Administration of low dose PAF caused progressive structural alterations of the F-actin-based cytoskeleton of small intestinal epithelia. The change begins as early as 5 min after PAF administration and lasts through the entire experimental period. This structural change temporally correlated with increased tyrosine phosphorylation of intestinal E-Cadherin, a crucial component of the adherens junction complex. As a result, the paracellular permeability is increased, providing access of luminal contents to the systemic circulation. Although there have been previous reports showing PAF increasing intestinal mucosal permeability (Kubes et al., 1991), this is the first study investigating the molecular mechanism of this PAF effect.
The integrity of the paracellular seal requires structural and functional linkage between the actin cytoskeleton and the adherens junction (Aberle et al., 1996). In the present study, we demonstrated that F-actin staining was rapidly (within 5 min) lost focally in the brush border at the villus tip after PAF injection. In addition, the F-actin staining within paracellular junction of the villus tip also became irregular. In contrast, the cytoskeletal structure was not disrupted in the crypt region. Thus, the villus tip may be the primary target of the PAF action, where permeability alteration occurs.
A major finding of this study is that PAF increases tyrosine phosphorylation of E-cadherin protein, which correlates temporally with the altered mucosal cytoskeletal structure and permeability. In addition, PAF also caused increased tyrosine phosphorylation of several other intestinal proteins which co-immunoprecipitated with E-cadherin. It is possible that these proteins correspond to catenin α (102 kDa) and β (98 kDa), paracellular junction-associated plaque proteins of the ZA (Anderson & Van Itallie, 1995; Edelman & Crossin, 1991), since catenin α and β could be co-immunoprecipitated with E-cadherin (Behrens et al., 1993; Matsuyoshi et al., 1992; Staddon et al., 1995). E-cadherin and other proteins of the ZA are constitutively phosphorylated on tyrosine residues (Aberle et al., 1996). Previous investigations indicated that the function of the E-cadherin/catenin complex is regulated by tyrosine phosphorylation (Behrens et al., 1993; Matsuyoshi et al., 1992). Several intracellular mediators including protein tyrosine kinase and protein tyrosine phosphatase are involved in the regulation of paracellular permeability (Adamson, 1996; Staddon et al., 1995). In vitro studies demonstrate an association between an enhanced tyrosine phosphorylation of components of E-cadherin/catenin complex and increased epithelial cell permeability (Staddon et al., 1995). Although there has been no previous studies demonstrating the relationship between PAF-induced tyrosine phosphorylation and mucosal permeability, protein tyrosine kinase has been shown to be involved in the PAF-induced increase of microvascular permeability (Kim & Duran, 1995). Further, the involvement of tyrosine phosphorylation reactions in the signal transduction pathway of PAF receptor has been well established (Chao & Olson, 1993). Our present study showed that pretreatment of animals with a protein tyrosine kinase inhibitor attenuated PAF-enhanced intestinal permeability. Thus, it is probable that PAF-induced phosphorylation of tyrosine residues of E-cadherin/catenin complex accounts for the alteration of mucosal permeability.
Acknowledgments
This work was supported by NIH grants DK34574 and HD31840 and a grant from The Children's Memorial Institute for Education and Research.
Abbreviations
- FD-4
FITC conjugated dextran 4400
- PAF
platelet activating factor
- ZA
zona adherens
References
- ABERLE H., SCHWARTZ H., KEMLER R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J. Cell. Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- ADAMSON R.H. Protein tyrosine phosphorylation modulates microvessel permeability in frog mesentery. [Review] Microcirculation. 1996;3:245–247. doi: 10.3109/10739689609148297. [DOI] [PubMed] [Google Scholar]
- ANDERSON J.M., VAN ITALLIE C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- BEHRENS J., BIRCHMEIER W., GOODMAN S.L., IMHOF B.A. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J. Cell Biol. 1985;101:1307–1315. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEHRENS J., VAKAET L., FRIIS R., WINTERHAGER E., VAN ROY F., MAREEL M.M., BIRCHMEIER W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BJARNASON I. Intestinal permeability. Gut. 1994;35:S18–S22. doi: 10.1136/gut.35.1_suppl.s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BJARNASON I., MACPHERSON A., HOLLANDER D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- BUSSOLINO F., CAMUSSI G., AGLIETTA M, , BRAQUET P., BOSIA A., PESCARMONA G., SANAVIO F., D'URSO N., MARCHISIO P.C. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J. Immunol. 1987;139:2439–2446. [PubMed] [Google Scholar]
- CHAO W., OLSON M.S. Platelet-activating factor: receptors and signal transduction. Biochem. J. 1993;292:617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PLAEN I.G., TAN X.D., CHANG H., QU X.W., LIU Q.P., HSUEH W. Intestinal NF-kappa-B is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim. Biophys. Acta. 1998;1392:185–192. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- EDELMAN G.M., CROSSIN K.L. Cell adhesion molecules: implications for a molecular histology. Annu. Rev. Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- ELIAKIM R., KARMELI F., RAZIN E., RACHMILEWITZ D. Role of platelet-activating factor in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine and prednisolone. Gastroenterology. 1988;95:1167–1172. doi: 10.1016/0016-5085(88)90346-0. [DOI] [PubMed] [Google Scholar]
- EZZELL R.M., CARTER E.A., YARMUSH M.L., TOMPKINS R.G. Thermal injury-induced changes in the rat intestine brush border cytoskeleton. Surgery. 1993;114:591–597. [PubMed] [Google Scholar]
- FINK M.P. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit. Care. Med. 1991;19:627–641. doi: 10.1097/00003246-199105000-00009. [DOI] [PubMed] [Google Scholar]
- GONZALEZ-CRUSSI F., HSUEH W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am. J. Pathol. 1983;112:127–135. [PMC free article] [PubMed] [Google Scholar]
- GUERRANT R.L., FANG G.D., THIELMAN N.M., FONTELES M.C. Role of platelet activating factor in the intestinal epithelial secretory and chinese hamster ovary cell cytoskeletal responses to cholera toxin. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9655–9658. doi: 10.1073/pnas.91.20.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSON C., KALD B., SJODAHL R., TAGESSON C. Phospholipase C from Clostridium perfringens stimulates formation and release of platelet-activating factor (PAF-acether) in cultured intestinal epithelial cells (INT 407) Scand. J. Gastroenterology. 1991;26:1000–1006. doi: 10.3109/00365529109003948. [DOI] [PubMed] [Google Scholar]
- HSUEH W., CAPLAN M.S., SUN X., TAN X., MACKENDRICK W., GONZALEZCRUSSI F. Platelet-activating factor, tumor necrosis factor, hypoxia and necrotizing enterocolitis. Acta Paediatr. 1994;396:S11–S17. doi: 10.1111/j.1651-2227.1994.tb13234.x. [DOI] [PubMed] [Google Scholar]
- HSUEH W., GONZALEZ-CRUSSI F., ARROYAVE J.L. Plateleta-ctivating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- HUANG L., TAN X., CRAWFORD S.E., HSUEH W. Platelet-activating factor and endotoxin induce tumour necrosis factor gene expression in rat intestine and liver. Immunology. 1994;83:65–69. [PMC free article] [PubMed] [Google Scholar]
- KANWAR S., WALLACE J.L., BEFUS D., KUBES P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am. J. Physiol. 1994;266:G222–G229. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- KIM D., DURAN W. Platelet-activating factor stimulates protein tyrosine kinase in hamster cheek through pouch microcirculation. Am. J. Physiol. 1995;268:H399–H403. doi: 10.1152/ajpheart.1995.268.1.H399. [DOI] [PubMed] [Google Scholar]
- KOTELEVETS L., NOE V., BRUYNEEL E., MYSSIAKINE E., CHASTRE E., MAREEL M., GESPACH C. Inhibition by platelet-activating factor of src- and hepatocyte growth factor-dependent invasiveness of intestinal and kidney epithelial cells: phosphatidylinositol 3′-kinase is a critical mediator of tumor invasion. J. Biol. Chem. 1998;273:14138–14145. doi: 10.1074/jbc.273.23.14138. [DOI] [PubMed] [Google Scholar]
- KUBES P., ARFORS K.E., GRANGER D.N. Platelet-activating factor-induced mucosal dysfunction: role of oxidants and granulocytes. Am. J. Physiol. 1991;260:G965–G971. doi: 10.1152/ajpgi.1991.260.6.G965. [DOI] [PubMed] [Google Scholar]
- MADARA J.L. Tight junction dynamics: is paracellular transport regulated. Cell. 1988;53:497–498. doi: 10.1016/0092-8674(88)90562-4. [DOI] [PubMed] [Google Scholar]
- MADARA J.L., BARENBERG D., CARLSON S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J. Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADARA J.L., MOORE R., CARLSON S. Alteration of intestinal tight junction structure and permeability by cytoskeletal contraction. Am. J. Physiol. 1987;253:C854–C861. doi: 10.1152/ajpcell.1987.253.6.C854. [DOI] [PubMed] [Google Scholar]
- MADARA J.L., PAPPENHEIMER J.R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membrane Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- MATHISON R., DAVISON J.S. Capsaicin sensitive nerves in the jejunum of Nippostrongylus brasiliensis-sensitized rats participate in a cardiovascular depressor reflex. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:638–642. doi: 10.1007/BF00167241. [DOI] [PubMed] [Google Scholar]
- MATSUYOSHI N., HAMAGUCHI M., TANIGUCHI S., NAGAFUCHI A., TSUKITA S., TAKEICHI M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J. Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKAY D.M., BIENENSTOCK J. The interaction between mast cells and nerves in the gastointestinal tract. Immunology Today. 1994;15:533–538. doi: 10.1016/0167-5699(94)90210-0. [DOI] [PubMed] [Google Scholar]
- NOVOGRODSKY A., VANICHKIN A., PATYA M., GAZIT A., OSHEROV N., LEVITZKI A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994;264:1319–1322. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J.R., DAHL C.E., KARNOVSKY M.L., MAGGIO J.E. Intestinal absorption and excretion of octapeptides composed of D amino acids. Proc. Natl. Acad. Sci. U.S.A.. 1994;91:1942–1945. doi: 10.1073/pnas.91.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPENHEIMER J.R., REISS K.Z. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J. Membrane Biol. 1987;100:123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J.R., VOLPP K. Transmucosal impedance of small intestine: correlation with transport of sugars and amino acids. Am. J. Physiol. 1992;263:C480–C493. doi: 10.1152/ajpcell.1992.263.2.C480. [DOI] [PubMed] [Google Scholar]
- PELLON M.I., STEIL A.A, , FURIO V., SANCHEZ CRESPO M. Study of the effector mechanism involved in the production of haemorrhagic necrosis of the small intestine in rat passive anaphylaxis. Br. J. Pharmacol. 1994;112:1101–1108. doi: 10.1111/j.1476-5381.1994.tb13197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QU X.-W., HUANG L., BURTHART T., CRAWFORD S.E., CAPLAN M.S., HSUEH W. Endotoxin induces PAF production in the rat ileum: quantitation of tissue PAF by an improved method. Prostaglandins. 1996;51:249–262. doi: 10.1016/0090-6980(96)00020-2. [DOI] [PubMed] [Google Scholar]
- QU X.-W., ROZENFELD R.A., HUANG W., CRAWFORD S.E., GONZALEZ-CRUSSI F., HSUEH W. Interaction of platelet-activating factor, spleen and atrial natriuretic peptide in plasma volume regulation during endotoxaemia in rats. J. Physiol. 1998;512:227–234. doi: 10.1111/j.1469-7793.1998.227bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN A.L., WANG H., WOLLERT P.S., VANDERMEER T.J., COMPTON C.C., DENENBERG A.G., FINK M.P. Endotoxininduced ileal mucosal hyperpermeability in pigs: role of tissue acidosis. Am. Physiol. 1994;266:G633–G646. doi: 10.1152/ajpgi.1994.266.4.G633. [DOI] [PubMed] [Google Scholar]
- SNYDER F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am. J. Physiol. 1990;259:C697–C708. doi: 10.1152/ajpcell.1990.259.5.C697. [DOI] [PubMed] [Google Scholar]
- STADDON J.M., HERRENKNECHT K., SMALES C., RUBIN L.L. Evidence that tyrosine phosphorylation may increase tight junction permeability. J. Cell Sci. 1995;108:609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- TAKEICHI M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- TAN X.-D., SUN X., GONZALEZ-CRUSSI F.X., GONZALEZ-CRUSSI F., HSUEH W. PAF and TNF increase the precursor of NFkappa B p50 mRNA in mouse intestine: quantitative analysis by competitive PCR. Biochim. Biophys. Acta. 1994;1215:157–162. doi: 10.1016/0005-2760(94)90105-8. [DOI] [PubMed] [Google Scholar]
- TAN X.-D., WANG H., GONZALEZ-CRUSSI F.X., CHANG H., GONZALEZ-CRUSSI F., HSUEH W. Platelet activating factor and endotoxin increase the enzyme activity and gene expression of type II phospholipase A2 in the rat intestine. Role of polymorphonuclear leukocytes. J. Immunol. 1996;156:2985–2990. [PubMed] [Google Scholar]
- WANG H., TAN X., CHANG H., GONZALEZ-CRUSSI F., REMICK D.G., HSUEH W. Regulation of platelet-activating factor receptor gene expression in vivo by endotoxin, platelet-activating factor and endogenous tumour necrosis factor. Biochem. J. 1997;322:603–608. doi: 10.1042/bj3220603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H., TAN X.-D., CHANG H., HUANG W., GONZALEZ-CRUSSI F., HSUEH W.Platelet-activating factor receptor mrna is localized in eosinophils and epithelial cells in rat small intestine: regulation by dexamethasone and gut flora Immunology 1999(in press) [DOI] [PMC free article] [PubMed]
- ZHANG S., CASTRO G.A. Boosted mucosal immune responsiveness in the rat intestine by actively transported hexose. Gastroenterology. 1992;103:1162–1166. doi: 10.1016/0016-5085(92)91499-t. [DOI] [PubMed] [Google Scholar]


