Abstract
We investigated the effects of short-term exposure to physiological levels of 17β-estradiol and testosterone on vasocontractile responses in porcine coronary artery rings.
Concentration-response curves to endothelin-1, 5-hydroxytryptamine, the thromboxane analogue U46619 and KCl were constructed in endothelium-intact and endothelium-disrupted artery rings.
Thirty minutes exposure to 17β-estradiol (1 and 30 nM) significantly attenuated vasoconstriction to endothelin-1, 5-hydroxytryptamine and U46619. Conversely, the same concentrations of testosterone significantly potentiated responses elicited by these contractile agents. These inhibitory effects of 17β-estradiol and enhancing actions of testosterone on contractions were endothelium-independent. KCl-mediated contractions were unaffected by the presence of either sex hormones.
The oestrogen receptor antagonists, tamoxifen (10 μM) and ICI 182,780 (10 μM), were unable to reverse the inhibitory influence 1 nM 17β-estradiol had on the agonist-mediated contractile responses. Similarly, the androgen receptor antagonists, flutamide (10 μM) and cyproterone acetate (10 μM), failed to affect the potentiating activities of 1 nM testosterone.
The alteration in vasoconstrictive responses observed following acute exposure to either 1 nM 17β-estradiol and 1 nM testosterone were apparent even in the presence of the protein synthesis inhibitor cycloheximide (10 μM) and the transcription inhibitor actinomycin D (10 μM).
In conclusion, we report a unique type of sex hormone action on the coronary vasculature. These events occur at low nanomolar concentrations of 17β-estradiol and testosterone, are insensitive to conventional sex hormone receptor antagonists, are not blocked by de novo protein synthesis inhibitors and have rapid time-courses that are uncharacteristic of classical genomic activities.
Keywords: 17β-estradiol, testosterone, vasoconstriction, endothelium, smooth muscle, swine
Introduction
The increase in prevalence of coronary artery disease (CAD) in women after menopause (Barrett-Connor, 1997), reversible by oestrogen replacement therapy (Ettinger et al., 1996; Stampfer et al., 1991), suggests an important connection between endogenous oestrogen and the risk of CAD. Indeed, oestrogen treatment not only favourably alters lipoprotein profiles (Ettinger et al., 1996) but also enhances endothelium-dependent (Lieberman et al., 1994) and endothelium-independent relaxation (Gilligan et al., 1994). In spite of the growing evidence for a causal link between the decrease in oestrogen levels and the incidence of CAD, the underlying mechanisms for this phenomenon remain to be fully elucidated.
Interestingly, while it is widely recognized that men are generally more susceptible to CAD, the possibility that androgens could have detrimental effects on the vasculature has received relatively little attention. Indeed, what little literature exists on the effects of testosterone in the development of CAD is contradictory with some indicating positive (Khaw & Barrett-Connor, 1991; Duell & Bierman, 1990) and others negative (Phillips et al., 1994; Larsen et al., 1993) actions. Recently, Herman et al. (1997) reported that androgen deprivation in adult men enhanced endothelium-dependent relaxation. Conversely, administration of testosterone to hypercholesterolaemic rabbits and monkeys impaired endothelium-dependent relaxation and promoted the progression of atherosclerosis (Hutchison et al., 1997; Adams et al., 1995).
Previous studies have demonstrated that only very high concentrations (micromolar range) of 17β-estradiol can produce direct vasorelaxation actions (Han et al., 1995; Jiang et al., 1992). In contrast, we recently reported that a physiological concentration (1 nM) of 17β-estradiol could potentiate relaxation of porcine coronary artery rings in vitro (Teoh et al., 1999). Inasmuch as vasomotor activity is a critical balance between vasorelaxing and vasoconstricting effects, we were interested to determine if agonist-stimulated contractile responses would also be influenced by shortterm incubation with 17β-estradiol and testosterone. In this study, we have utilized the same in vitro porcine coronary artery model.
Methods
Tissue preparation
Hearts from pigs of either sex (50–80 kg) were collected from a local abattoir in cold, modified Krebs-Henseleit solution (composition in mM: 120 NaCl, 4.76 KCl, 1.18 MgSO4, 1.25 CaCl2, 25 NaHCO3, 1.18 NaH2PO4 and 5.5 glucose). Left anterior descending and right coronary arteries were dissected free of fat and connective tissue and cut into 3 mm ring segments. Ring samples were then mounted on two stainless steel hooks in 5 ml organ baths. One of these hooks was attached to a force transducer (Model FT03, Grass Instrument Co., Quincy, MA, U.S.A.) to measure changes in isometric tension. In experiments requiring endothelium-disrupted rings, porcine coronary arteries were perfused at a rate of 1 ml min−1 for 30 s with either 0.5% Triton X-100 or Krebs-Henseleit solution before being cut into 3 mm ring segments. Tissues were maintained at 37°C in oxygenated (95% O2/5% CO2) Krebs-Henseleit solution at a resting tension of 2.0 g. Samples were allowed an equilibration period of at least 100 min during which tension was adjusted to 2.0 g and bathing solution was periodically changed.
Experimental protocols
Rings were contracted with 30 mM KCl and relaxed with 1 μM bradykinin. This viability test was repeated, and coronary artery segments that averaged less than 4.0 g contraction and 40% relaxation were discarded from the study. In endothelium-disrupted preparations, rings which relaxed more than 5% were not used. After the effects of KCl and bradykinin were washed out with Krebs-Henseleit solution, samples were incubated with various drugs or vehicle alone. Where necessary, the oestrogen and testosterone receptor antagonists or the de novo protein synthesis inhibitors were introduced into the baths 20 min before addition of vehicle solvent or the appropriate sex steroid. 17β-estradiol and testosterone were added 30 min prior to testing at a final concentration of 1, 30 nM or 1 μM. The supraphysiological concentration of 1 μM was used solely as a means of comparison with earlier work. The role of oestrogen receptors was investigated using the inactive isomer, 17α-estradiol (1 nM and 1 μM) as well as the oestrogen receptor antagonists tamoxifen (10 μM) and ICI 182,780 (7α-[9-[(4,4,5,5,5,-pentafluoropentyl)sulphinyl]nonyl]-estra1,3,5(10)-triene-3,17β-diol; 10 μM). Studies were carried out with the testosterone receptor antagonists, flutamide (10 μM) and cyproterone acetate (10 μM) to investigate the role of the androgen receptor. Where required, rings were incubated with either cycloheximide (10 μM) or actinomycin D (10 μM) to inhibit protein synthesis and transcriptional activity, respectively. Except where noted, all drugs remained present throughout the experiment. In some experiments, ring samples were periodically washed out with Krebs-Henseleit solution over a period of 45 min following incubation with 17β-estradiol or testosterone. Contractions were produced by a stepwise addition of endothelin-1 (ET-1; 0.01–30 nM), 5-hydroxytryptamine (5-HT; 0.01 to 10 μM), the thromboxane analogue U46619 (9, 11-dideoxy-9α-methanoepoxy prostaglandin F2; 0.1 nM to 1 μM) or KCl (10–70 mM). In all cases, each tissue sample was only exposed to one contracting agent.
Drugs and chemicals
With the exception of ICI 182,780 (a gift from Zeneca, Macclesfield, U.K.) and U46619 (from Biomol, Plymouth Meeting, PA, U.S.A.), all drugs and chemicals were purchased from Sigma Chemical Co., St. Louis, MO, U.S.A. Stock solutions of 17α-estradiol, 17β-estradiol, testosterone propionate, ICI 182,780, flutamide and U46619 were made in ethanol. Cyproterone acetate and tamoxifen were dissolved in methanol and 10% ethanol, respectively. The final concentration of ethanol in the bath in each case was always⩽0.2%. The remaining drug stocks were dissolved in water. Where required, stock solutions were further diluted with Krebs-Henseleit solution.
Calculations and statistical analyses
Results are expressed as the mean±s.e.mean where n refers to the number of hearts used in the study. Contraction-response curves were calculated as a percentage of the average of the two initial 30 mM KCl-evoked contractions. Bradykinin-induced relaxation was individually calculated against each respective KCl-elicited contraction, and the average of these two relaxations were used to determine endothelial integrity. Log EC50 values were determined with the aid of a curve-fitting program (SigmaPlot, Jandel Scientific Software, CA, U.S.A.). Analysis of variance (ANOVA) and Dunnett's test were applied where appropriate to determine individual differences between multiple groups of data using a computer statistical package (SPSS, SPSS Inc., Chicago, IL, U.S.A.). A P value of <0.05 was considered significant.
Results
Effects of exogenous 17β-stradiol and testosterone on endothelin-1-, 5-hydroxytryptamine-, U46619- and KCl-mediated contractions
ET-1 (0.01 to 30 nM), 5-HT (0.01–10 μM), U46619 (0.1 nM to 1 μM) and KCl (10–70 mM) elicited concentration-dependent contractions. The maximum contractions obtained by ET-1, 5-HT, U46619 and KCl under control conditions were 7.8±0.5 g (n=14), 4.7±0.3 g (n=18), 8.4±0.6 g (n=15) and 9.8±0.6 g (n=6) respectively. 17β-estradiol (1 and 30 nM) significantly reduced the responses evoked by the three contractile agonists (Figure 1). A supraphysiological concentration of 17β-estradiol, 1 μM, produced a decrease in agonist-induced contraction that was greater than that elicited by 30 nM 17β-estradiol (Figure 1). Although Triton X-100-perfusion increased the overall contractile responses, the inhibitory effect of 17β-estradiol was retained suggesting that the modulatory action of 17β-estradiol was independent of the endothelium (data not shown). The maximum contractile responses and the log EC50 values of 5-HT and U46619 under these conditions are shown in Table 1. Log EC50 values for ET-1 were not determined, as we were unable to obtain a stable maximum response with the concentration range used. In contrast to 17β-estradiol, 17α-estradiol (1 nM to 1 μM) did not have any effect on the concentration-response curves of the three agonists studied (data not shown) and their effects on the maximum contractions as well as log EC50 are summarized in Table 2. Acute exposure to testosterone (1 and 30 nM) for 30 min resulted in significant increase in contractions to ET-1, 5-HT and U46619 (Figure 2). A higher concentration of testosterone (1 μM) also appreciably potentiated agonist-induced contractions. As reflected by data from Triton X-100-perfused tissues, this enhanced response to constricting agents was independent of the endothelium (Table 3). Interestingly, neither 17β-estradiol nor testosterone, at the three concentrations investigated, appreciably affected contractions elicited by KCl (Figure 3).
Figure 1.
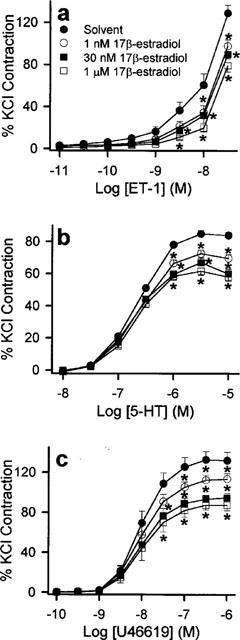
Inhibitory effect of 17β-estradiol on the contractile responses of porcine artery ring segments to (a) ET-1, (b) 5-HT and (c) U46619. Rings were pre-incubated with solvent or 17β-estradiol for 30 min before cumulative additions of the appropriate contractile agent. n=7 to 9 for each treatment group. *P<0.05 vs control data (ANOVA-Dunnett's).
Table 1.
Effects of acute treatment with 17 β-estradiol on the maximum contractions elicited by endothelin-1 (ET-1), 5-hydroxytryptamine (5-HT) or U46619 in endothelium-intact and endothelium-damaged porcine coronary artery
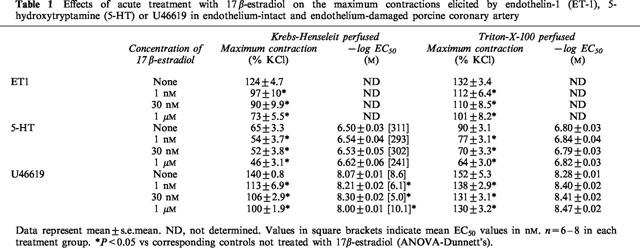
Table 2.
Effects of acute treatment with 17α-estradiol or 17β-estradiol on the maximum contractions elicited by endothelin-1 (ET-1), 5-hydroxytryptamine (5-HT) or U46619
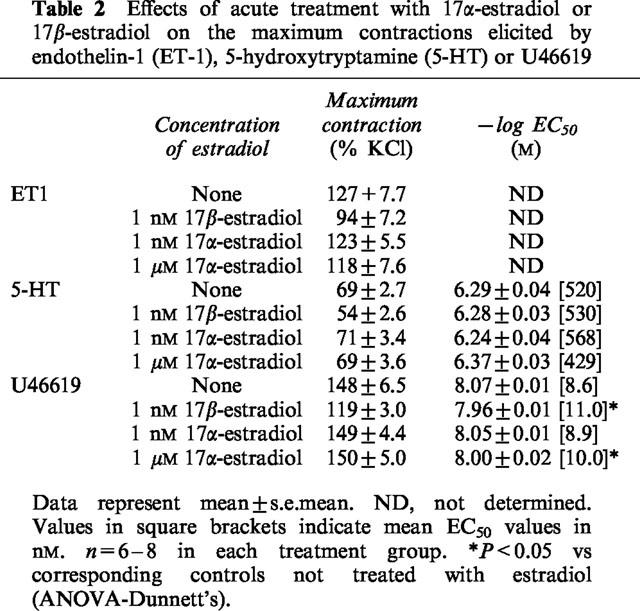
Figure 2.
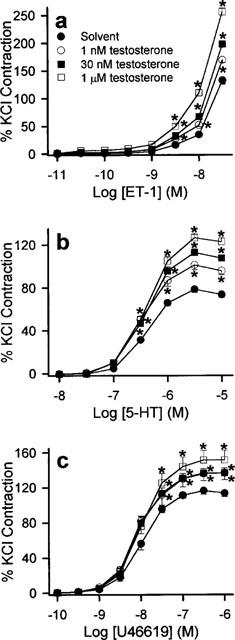
Enhancing effect of testosterone on the contractile responses of porcine coronary artery ring segments to (a) ET-1, (b) 5-HT and (c) U46619. Rings were pre-incubated with solvent or testosterone for 30 min before cumulative additions of the appropriate contractile agent. n=7–9 for each treatment group. *P<0.05 vs control data (ANOVA-Dunnett's).
Table 3.
Effects of acute treatment with testoterone on the maximum contractions elicited by endothelin-1 (ET-1), 5-hydroxytryptamine (5-HT) or U46619 in endothelium-intact and endothelium-damaged porcine coronary artery
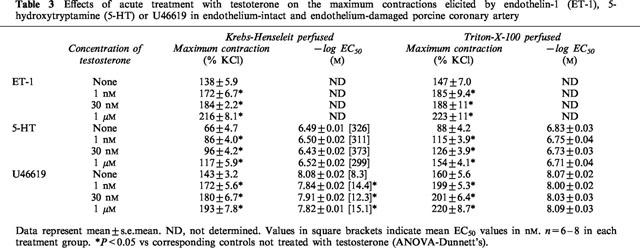
Figure 3.
Effects of (a) 17β-estradiol and (b) testosterone on the contractile responses of porcine coronary artery ring segments to KCl. Rings were pre-incubated with solvent, 17β-estradiol or testosterone for 30 min before cumulative additions of KCl. n=6 for each treatment group.
Effects of oestrogen and testosterone receptor antagonists on acute actions of exogenous 17β-estradiol and testosterone
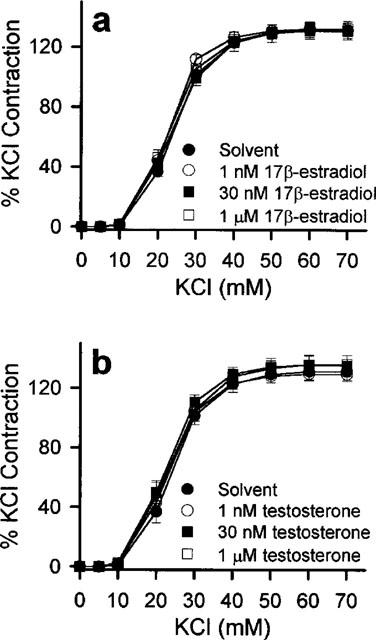
None of the receptor antagonists studied affected the contractions elicited by 5-HT, ET-1 or U46619 (data not shown). Neither tamoxifen nor ICI 182,780 (both added 20 min prior to exposure to 17β-estradiol at 10 μM) significantly reversed the inhibition exerted by 1 nM 17β-estradiol on the agonist-induced contractions (Figure 4). As shown in Figure 5, the potentiating action of 1 nM testosterone on the contractile responses was not antagonized by 20 min preincubation with 10 μM flutamide or 10 μM cyproterone acetate.
Figure 4.
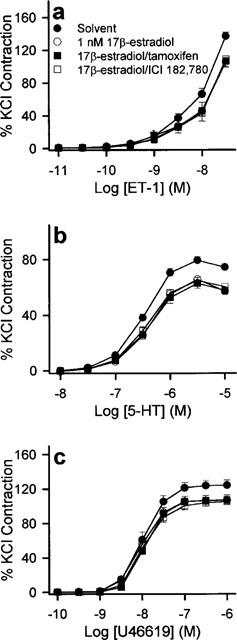
Effects of the oestrogen receptor antagonists, tamoxifen and ICI 182,780, on the acute modulatory effects of 17β-estradiol. Rings were pre-incubated with solvent, 10 μM tamoxifen or 10 μM ICI 182,7801 for 20 min before addition of vehicle solvent or 1 nM 17β-estradiol. Thirty minutes later, they were exposed to increasing concentrations of (a) ET-1, (b) 5-HT and (c) U46619. Contractions were significantly reduced by 1 nM 17β-estradiol. This response was similar to those obtained in the presence of tamoxifen and ICI 182,780. n=6–8 for each treatment group.
Figure 5.
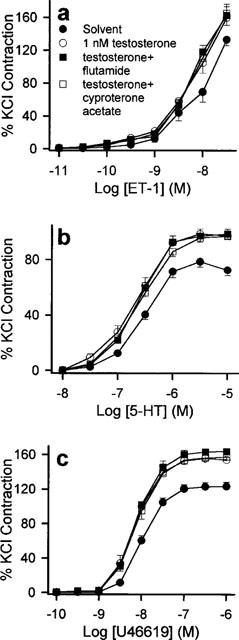
Effects of the testosterone receptor antagonists, flutamide and cyproterone acetate, on the acute modulatory effects of testosterone. Rings were pre-incubated with solvent, 10 μM flutamide or 10 μM cyproterone acetate for 20 min before addition of vehicle solvent or 1 nM testosterone. Thirty minutes later, they were exposed to increasing concentrations of (a) ET-1, (b) 5-HT and (c) U46619. Contractions were significantly potentiated by 1 nM testosterone. This response was similar to those obtained in the presence of flutamide and cyproterone acetate. n=6–8 for each treatment group.
Effects of cycloheximide and actinomycin D on acute actions of exogenous 17β-estradiol and testosterone
Cycloheximide (10 μM) or actinomycin D (10 μM) was added to the baths 20 min prior to addition of 17β-estradiol (1 nM) or testosterone (1 nM). Neither cycloheximide nor actinomycin D, at these concentrations, was able to reverse the actions of either steroid on the contractile responses elicited by ET-1, 5-HT and U46619 (Figures 6 and 7).
Figure 6.
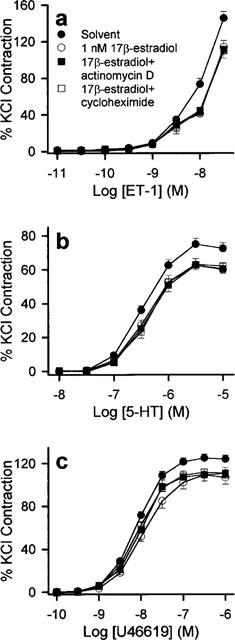
Responses of (a) ET-1, (b) 5-HT and (c) U46619 to 1 nM 17β-estradiol in the absence and presence of 10 μM actinomycin D or 10 μM cycloheximide. Prior to contraction, rings were pre-incubated for 20 min with either actinomycin D or cycloheximide then for 30 min with solvent or 17β-estradiol. Contractions were significantly reduced by 1 nM 17β-estradiol. This response was similar to those obtained in the presence of actinomycin D or cycloheximide. n=6–8 for each treatment group.
Figure 7.
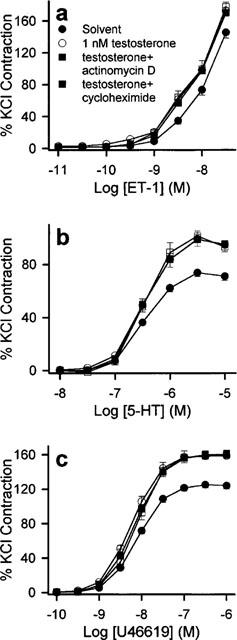
Responses of (a) ET-1, (b) 5-HT and (c) U46619 to 1 nM testosterone in the absence and presence of 10 μM actinomycin D or 10 μM cycloheximide. Prior to contraction, rings were pre-incubated for 20 min with actinomycin D or cycloheximide then for 30 min with solvent or testosterone. Contractions were significantly enhanced by 1 nM testosterone. This response was similar to those obtained in the presence of actinomycin D or cycloheximide. n=6–8 for each treatment group.
Effects of washout on acute actions of exogenous testosterone and 17β-estradiol
Following 30 min incubation with 17β-estradiol (1 nM) or testosterone (1 nM), rings were washed with Krebs-Henseleit solution over a period of 45 min to remove hormones from the bathing medium. After the washout period, contractions induced by ET-1, 5-HT, or U46619 returned towards values obtained prior to the exposure to 17β-estradiol or testosterone (data not shown).
Discussion
Our main finding is that contractions elicited by ET-1, 5-HT and U46619, but not KCl, in porcine coronary artery rings are attenuated by 17β-estradiol and potentiated by testosterone after 30 min exposure. More importantly, these modulatory effects were evident with physiologically relevant concentrations of these hormones and did not appear to involve the classical cytosolic steroid receptors or result from nuclear translation and transcription.
A striking feature of the current work is the correlation between the lower concentrations of 17β-estradiol applied and the physiological levels in castrated and ovariectomized pigs (∼0.2 nM; Amos & Beattie, 1985) as well as pregnant pigs (2–10 nM; Robertson & King, 1974). Interestingly, according to the case records of the Massachusetts General Hospital (1986), the total plasma concentration of oestrogen in women (84–325 pM) and that of testosterone in men (10–38 nM) are also similar to those used in our study.
While there are many studies concerned with the vascular effects of oestrogen, most have focused on its influence on vasorelaxation rather than vasoconstriction. Indeed, earlier work carried out on in vitro preparations of rabbit and porcine coronary artery preparations have indicated that high supraphysiological (micromolar) concentrations of 17β-estradiol are required to elicit direct vasorelaxation (Han et al., 1995; Jiang et al., 1992). Similarly, in vitro contractile responses of rings from rabbit coronary arteries (Jiang et al., 1992) and human internal mammary arteries (Mugge et al., 1997) were only reduced by concentrations of 17β-estradiol beyond the physiological range. In contrast, we have found in the present study that a much lower concentration (1 nM) of 17β-estradiol can acutely reduce agonist-induced vasocontraction in porcine coronary arteries. Furthermore, experiments carried out on Triton X-100-perfused ring segments indicate that this effect is independent of the endothelium. To the best of our knowledge, only one group has had complementary in vivo results to ours (Sudhir et al., 1997). In that study, Sudhir and colleagues demonstrated that in pigs, short-term (10 min) intracoronary infusion with 1 nM 17β-estradiol inhibited vasoconstriction to ET-1. Of relevance is the study that in vitro incubation with low concentrations of oestrogen and progesterone for 12–96 h in isolated primate coronary artery vascular muscle cells reduced reactivity to 5-HT and U46619 (Minshall et al., 1998). The major difference between this study and our data is a longer incubation time was used in the former study suggesting the involvement of genomic events while our study precluded a significant role of the classical steroid receptors (see Discussion).
The evidence concerning the effects of testosterone on blood vessels is still contradictory. In vivo experiments have suggested that androgens can enhance vasoconstriction (Baker et al., 1978; Greenberg et al., 1974) or reduce relaxation (McCredie et al., 1998; Herman et al., 1997). With respect to in vitro findings, Lamping & Nuno (1996) found that 1 μM testosterone was ineffective against ET-1-induced constrictions in isolated canine coronary microvessels. Others, on the other hand, have reported that micromolar levels of testosterone can elicit endothelium-independent relaxation in agonist-precontracted rabbit (Yue et al., 1995) and rat (Costarella et al., 1996) aortic rings. However, a fact worth noting is that the concentration range of testosterone within which these in vitro observations were made was once again in the supraphysiological region. Although our results from acute treatment with testosterone differ from those reported in terms of the concentrations used (Costarella et al., 1996; Yue et al., 1995), the endothelium-independent nature of the modulatory actions of testosterone is synonymous throughout these studies.
The effects observed with low concentrations of the steroids are consistent with receptor-mediated events. Indeed, our observations that receptor-independent contractions elicited by KCl were unaffected by 17β-estradiol and testosterone suggests that these interactions could occur at the receptor level or may involve alterations in subsequent signal transduction mechanisms. In any case, the insensitivity of KCl-induced contractions to these sex hormones indicates that a non-specific influence on smooth muscle function cannot account for our observations. This is further supported by our findings that the inhibitory properties of 17β-estradiol were stereospecific. However, the rapid time frame (30 min incubation time) is uncharacteristic of slow-acting nuclear steroid hormone receptors. Furthermore, the effects were insensitive to the oestrogen receptor antagonists, tamoxifen and ICI 182,780. In parallel studies, the enhancing effects of testosterone on contractions were also retained in the presence of the testosterone receptor antagonists, flutamide and cyproterone acetate. Classical steroid receptors would necessitate the utilization of transcriptional and translational pathways. Their involvement is unlikely in view of the inability of actinomycin D and cycloheximide to influence the actions of 17β-estradiol and testosterone. Furthermore, interactions with steroid receptors would not be susceptible to washing out with Krebs-Henseleit solution as was observed in this study. Both oestrogen (Kim-Schulze et al., 1996; Karas et al., 1994) and testosterone (Fujimoto et al., 1994; Stumpf, 1990; Traish et al., 1986) receptors have been identified in the endothelial and smooth muscle cells of the vasculature. However, recent immunohistochemical data suggest the classical oestrogen receptor is absent in human coronary arteries (Collins et al., 1995). Nonetheless, our data indicate that neither the classical oestrogen receptor nor the established testosterone steroid receptor can be involved in the acute modulatory responses recorded in our model.
It would be intriguing to speculate that the effects we observed may involve some as yet unidentified membrane steroid receptors. Indeed, the existence of a membrane oestrogen receptor has already been postulated in hypothalamic (Gu & Moss, 1996), neostriatal (Mermelstein et al., 1996) and medial amygdalal (Nabekura et al., 1986) neurons. Interestingly, the rapid actions of oestrogen observed in our model coincide with the pharmacodynamic properties of the putative oestrogen membrane receptor (Gu & Moss, 1996; Mermelstein et al., 1996; Nabekura et al., 1986). Furthermore, the pharmacological responses described in these neuronal preparations–fast stereospecific actions with nanomolar concentrations of 17β-estradiol, insensitivity to the oestrogen receptor antagonists tamoxifen and ICI 164,384 as well as the inability of actinomycin D and cycloheximide to reverse these influences–mirror those we report in the current work.
Oestrogen deficiency is associated with increased risk of CAD development in postmenopausal women (Barrett-Connor, 1997) while animal models indicate that androgen administration exacerbates atherogenesis (Adams et al., 1995) and vasoconstrictor responses (Matsuda et al., 1994; Schror et al., 1994). Recent evidence further demonstrate that prolonged oestrogen administration enhances arterial relaxation in male-to-female transsexuals (McCrohon et al., 1997; New et al., 1997) while antiandrogen therapy improved vasodilatation in men suffering from prostate cancer (Herman et al., 1997). It would therefore appear that circulating levels of both 17β-estradiol and testosterone have substantial influence over vasomotor function. These data collectively suggest that physiological concentrations of 17β-estradiol exert positive effects on the vasculature. In contrast, the same levels of testosterone could produce deleterious effects.
The porcine hearts used in the current work were from a local abattoir and we were unable to determine or control the sex distribution. While we recognize this as a limitation of our study, the data presented were reproducible in every batch of hearts studied. As such, it is unlikely that the responses to 17β-estradiol and testosterone we observed in our model are dependent on the gender of the pigs. Another consideration is the current study was conducted in the absence of blood components as in most in vitro studies. Hence it is unlikely that oestrogen and testosterone may exert effects on blood coagulation pathways under in vivo situation which can alter the responsiveness of blood vessels to constricting agents.
The current work was primarily concerned with the short-term influence of physiologically relevant concentrations of 17β-estradiol and testosterone on vasocontraction in vitro. We have demonstrated that circulating levels (1 and 30 nM) of 17β-estradiol and testosterone can acutely inhibit and enhance agonist-mediated vasocontraction respectively. Inasmuch as the modulatory effects observed occurred rapidly and were insensitive to recognized oestrogen and androgen receptor antagonists, it would suggest that they were not mediated by the classical oestrogen and testosterone receptors. Moreover, the reproducibility of the responses in the presence of actinomycin D and cycloheximide support the concept that these actions were independent of gene-mediated events. In conclusion, our data provide further evidence for a beneficial vascular role of physiological concentrations 17β-estradiol. They also support the concept that circulating levels of testosterone may have adverse effects on the vascular wall. Hence, the modulating actions exerted by both steroid hormones in our system may partly account for the sexual dimorphism observed in the incidence of cardiovascular disease.
Acknowledgments
A Committee on Research and Conference Grant and University of Hong Kong Block Grant supported part of this study. H. Teoh and S.W.S. Leung are the recipients of a post-doctoral fellowship and a studentship respectively from the University of Hong Kong. We gratefully acknowledge the technical assistance rendered by Mr Godfrey S.K. Man. All authors are members of the Institute of Cardiovascular Science and Medicine, University of Hong Kong.
Abbreviations
- ANOVA
analysis of variance
- CAD
coronary artery disease
- ET-1
endothelin-1
References
- ADAMS M.R., WILLIAMS J.K., KAPLAN J.R. Effects of androgens on coronary artery atherosclerosis and atherosclerosisrelated impairment of vascular responsiveness. Arterioscler. Thromb. Vasc. Biol. 1995;15:562–570. doi: 10.1161/01.atv.15.5.562. [DOI] [PubMed] [Google Scholar]
- AMOS M.S., BEATTIE C.W.Influence of gonadal steroid hormones upon the growth of melanoma in Sinclair swine Swine in Biomedical Research 1985New York: Plenum Press; 689–698.ed. Tumbleson, M.E. pp [Google Scholar]
- BAKER P.J., RAMEY E.R., RAMWELL P.W. Androgen-mediated sex differences of cardiovascular responses in rats. Am. J. Physiol. 1978;235:H242–H246. doi: 10.1152/ajpheart.1978.235.2.H242. [DOI] [PubMed] [Google Scholar]
- BARRETT-CONNOR E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- CASE RECORDS OF THE MASSACHUSETTS GENERAL HOSPITAL Weekly clinicopathological exercises. Normal reference values. N. Engl. J. Med. 1986;314:39–49. doi: 10.1056/NEJM198601023140108. [DOI] [PubMed] [Google Scholar]
- COLLINS P., SHEPARD M., BEALE C.M., DOWSETT M. The classical estrogen receptor is not found in human coronary arteries. Circulation. 1995;92 Suppl I:I–38. [Google Scholar]
- COSTARELLA C.E., STALLONE J.N., RUTECKI G.W., WHITTIER F.C. Testosterone causes direct relaxation of rat thoracic aorta. J. Pharmacol. Exp. Ther. 1996;277:34–39. [PubMed] [Google Scholar]
- DUELL P.B., BIERMAN E.L. The relationship between sex hormone and high-density lipoprotein cholesterol levels in healthy adult men. Arch. Intern. Med. 1990;150:2317–2320. [PubMed] [Google Scholar]
- ETTINGER N., FRIEDMAN G.D., BUSH T., QUESENBERRY C.P., JR Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet. Gynecol. 1996;87:6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO R., MORIMOTO I., MORITA E., SUGIMOTO H., ITO Y., ETO S. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J. Steroid Biochem. Mol. Biol. 1994;50:169–174. doi: 10.1016/0960-0760(94)90025-6. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., BADAR D.M., PANZA J.A., QUYYUMI A.A., CANNON R.O., III Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- GREENBERG S., GEORGE W.R., KADOWITZ P.J., WILSON W.R. Androgen-induced enhancement of vascular reactivity. Can. J. Physiol. Pharmacol. 1974;52:14–22. doi: 10.1139/y74-003. [DOI] [PubMed] [Google Scholar]
- GU Q., MOSS R.L. 17β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J. Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN SZ., KARAKI H., OUCHI Y., AKISHITA M., ORIMO H. 17β-estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation. 1995;91:2619–2626. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- HERMAN S.M., ROBINSON J.T.C., MCCREDIE R.J., ADAMS M.R., BOYER M.J., CELERMAJER D.S. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arterioscler. Thromb. Vasc. Biol. 1997;17:2004–2009. doi: 10.1161/01.atv.17.10.2004. [DOI] [PubMed] [Google Scholar]
- HUTCHISON S.J., SUDHIR K., CHOU T.M., SIEVERS R.E., ZHU B.Q., DEEDWANIA P.C., GLANTZ S.A., PARMLEY W.W., CHATTERJEE K. Testosterone worsens endothelial dysfunction associated with hypercholesterolemia and environmental tobacco smoke exposure in male rabbit aorta. J. Am. Coll. Cardiol. 1997;29:800–807. doi: 10.1016/s0735-1097(96)00570-0. [DOI] [PubMed] [Google Scholar]
- JIANG C., SARREL P.M., POOLE-WILSON P.A., COLLINS P. Acute effect of 17β-estradiol on rabbit coronary artery contractile responses to endothelin-1. Am. J. Physiol. 1992;263:H271–H275. doi: 10.1152/ajpheart.1992.263.1.H271. [DOI] [PubMed] [Google Scholar]
- KARAS R.H., PATTERSON B.L., MENDELSOHN M.E. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994;89:1943–1950. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- KHAW K.T., BARRETT-CONNOR E. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arterioscler. Thromb. 1991;11:489–494. doi: 10.1161/01.atv.11.3.489. [DOI] [PubMed] [Google Scholar]
- KIM-SCHULZE S., MCGOWAN K.A., HUBCHAK S.C., CID M.C., MARTIN M.B., KLEINMAN H.K., GREENE G.L., SCHNAPER H.W. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation. 1996;94:1402–1407. doi: 10.1161/01.cir.94.6.1402. [DOI] [PubMed] [Google Scholar]
- LAMPING K.G., NUNO D.W. Effects of 17β-estradiol on coronary microvascular responses to endothelin-1. Am. J. Physiol. 1996;271:H1117–H1124. doi: 10.1152/ajpheart.1996.271.3.H1117. [DOI] [PubMed] [Google Scholar]
- LARSEN B.A., NORDESTGAARD B.G., STENDER S., KJELDSEN K. Effect of testosterone on atherogenesis in cholesterol-fed rabbits with similar cholesterol levels. Atherosclerosis. 1993;99:49–86. doi: 10.1016/0021-9150(93)90053-w. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN E.H., GERHARD M.D., UEHATA A., WALSH B.W., SELWYN A.P., GANZ P., YEUNG A.C., CREAGER M.A. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann. Intern. Med. 1994;121:936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- MATSUDA M., RUFF A., MORINELLI T.A., MATHUR R.S., HALUSHKA P.V. Testosterone increases thromboxane A2 receptor density and responsiveness in rat aortas and platelets. Am. J. Physiol. 1994;267:H887–H893. doi: 10.1152/ajpheart.1994.267.3.H887. [DOI] [PubMed] [Google Scholar]
- MCCREDIE R.J., MCCROHON J.A., TURNER L., GRIFFITHS K.A., HANDELSMAN D.J., CELERMAJER D.S. Vascular reactivity is impaired in genetic females taking high-dose androgens. J. Am. Coll. Cardiol. 1998;32:1331–1335. doi: 10.1016/s0735-1097(98)00416-1. [DOI] [PubMed] [Google Scholar]
- MCCROHON J.A., WALTERS W.A.W., ROBINSON H.T.C., MCCREDIE R.J., TURNER L., ADAMS M.R., HANDELSMAN D.J., CELERMAJER D.S. Arterial reactivity is enhanced in genetic males taking high dose estrogens. J. Am. Coll. Cardiol. 1997;29:1432–1436. doi: 10.1016/s0735-1097(97)00063-6. [DOI] [PubMed] [Google Scholar]
- MERMELSTEIN P.G., BECKER J.B., SURMEIER D.J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINSHALL R.D., MIYAGAWA K., CHADWICK C.C., NOVY M.J., HERMSMEYER K. In vitro modulation of primate coronary vascular muscle cell reactivity by ovarian steroid hormones. FASEB J. 1998;12:1419–1429. doi: 10.1096/fasebj.12.13.1419. [DOI] [PubMed] [Google Scholar]
- MUGGE A., BARTON M., FIEGUTH H.G., RIEDEL M. Contractile responses to histamine, serotonin, and angiotensin II are impaired by 17β-estradiol in human internal mammary arteries in vitro. Pharmacology. 1997;54:162–168. doi: 10.1159/000139483. [DOI] [PubMed] [Google Scholar]
- NABEKURA J., OOMURA Y., MINAMI T., MIZUNO Y., FUKUDA A. Mechanism of the rapid effect of 17β-estradiol on medial amygdala neurons. Science. 1986;233:226–229. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- NEW G., TIMMINS K.L., DUFFY S.J., TRAN B.T., O'BRIEN R.C., HARPER R.W., MEREDITH I.T. Long-term estrogen therapy improves vascular function in male to female transsexuals. J. Am. Coll. Cardiol. 1997;29:1437–1444. doi: 10.1016/s0735-1097(97)00080-6. [DOI] [PubMed] [Google Scholar]
- PHILLIPS G.B., PINKERNELL B.H., JING T.Y. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler. Thromb. 1994;14:701–706. doi: 10.1161/01.atv.14.5.701. [DOI] [PubMed] [Google Scholar]
- ROBERTSON H.A., KING G.J. Plasma concentration of progesterone, oestrone, oestradiol-17β and oestrone sulphate in the pig at implantation, during pregnancy and at parturition. J. Reprod. Fertil. 1974;40:133–141. doi: 10.1530/jrf.0.0400133. [DOI] [PubMed] [Google Scholar]
- SCHROR K., MORINELLI T.A., MASUDA A., MATSUDA K., MATHUR R.S., HALUSHKA P.V. Testosterone treatment enhances thromboxane A2 mimetic induced coronary artery vasoconstriction in guinea pigs. Eur J. Clin. Invest. 1994;24:50–52. doi: 10.1111/j.1365-2362.1994.tb02428.x. [DOI] [PubMed] [Google Scholar]
- STAMPFER J.M., COLDITZ F.A., WILLETT W.C., MANSON J.E., ROSNER B., SPEIZER F.E., HENNEKENS C.H. Postmenopausal estrogen therapy and cardiovascular disease. N. Engl. J. Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- STUMPF W.E. Steroid hormones and the cardiovascular system: direct actions of estradiol, progesterone, testosterone, gluco- and mineralocorticoids, and soltriol (vitamin D) on central nervous regulatory and peripheral tissues. Experientia. 1990;46:13–25. doi: 10.1007/BF01955408. [DOI] [PubMed] [Google Scholar]
- SUDHIR K., KO E., ZELLNER C., WONG H.E., HUTCHISON S.J. Physiological concentrations of estradiol attenuate endothelin1-induced coronary vasoconstriction in vivo. Circulation. 1997;96:3626–3632. doi: 10.1161/01.cir.96.10.3626. [DOI] [PubMed] [Google Scholar]
- TEOH H., LEUNG S.W.S., MAN R.Y.K. Short-term exposure to physiological levels of 17β-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc. Res. 1999;42:224–231. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- TRAISH A.M., MULLER R.E., WOTIZ H.H. Binding of 7 alpha, 17 alpha-dimethyl-19-nortestosterone (mibolerone) to androgen and progesterone receptors in human and animal tissues. Endocrinol. 1986;118:1327–1333. doi: 10.1210/endo-118-4-1327. [DOI] [PubMed] [Google Scholar]
- YUE P., CHATTERJEE K., BEALE C., POOLE-WILSON P.A., COLLINS P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation. 1995;91:1154–1160. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]


