Abstract
The pharmacological properties of KATP channels generated by stable co-expression of the sulphonylurea receptor SUR1 and the inwardly rectifying K+ channel Kir6.2 were characterized in HEK-293 cells.
[3H]-Glyburide (glibenclamide) bound to transfected cells with a Bmax value of 18.5 pmol mg−1 protein and with a KD value of 0.7 nM. Specific binding was displaced by a series of sulphonylurea analogues with rank order potencies consistent with those observed in pancreatic RINm5F insulinoma and in the brain.
Functional activity of KATP channels was assessed by whole cell patch clamp, cation efflux and membrane potential measurements. Whole cell currents were detected in transfected cells upon depletion of internal ATP or by exposure to 500 μM diazoxide. The currents showed weak inward rectification and were sensitive to inhibition by glyburide (IC50=0.92 nM).
Metabolic inhibition by 2-deoxyglucose and oligomycin treatment triggered 86Rb+ efflux from transfected cells that was sensitive to inhibition by glyburide (IC50=3.6 nM).
Diazoxide, but not levcromakalim, evoked concentration-dependent decreases in DiBAC4(3) fluorescence responses with an EC50 value of 14.1 μM which were attenuated by the addition of glyburide. Diazoxide-evoked responses were inhibited by various sulphonylurea analogues with rank order potencies that correlated well with their binding affinities.
In summary, results from ligand binding and functional assays demonstrate that the pharmacological properties of SUR1 and Kir6.2 channels co-expressed in HEK-293 cells resemble those typical of native KATP channels described in pancreatic and neuronal tissues.
Keywords: Sulphonylurea receptor, inwardly rectifying potassium channel, potassium channel opener, ATP-sensitive potassium channel, HEK293 cell line, glyburide (glibenclamide)
Introduction
ATP-sensitive potassium (KATP) channels are a family of weak inward rectifiers expressed in diverse cell types including pancreatic β-cells, heart, brain, skeletal and smooth muscle cells where they couple cellular energy metabolism to membrane electrical activity (Noma, 1983). A distinguishing feature of KATP channels is that their activity is inhibited by intracellular ATP concentrations, but activated by an increase in MgADP levels (Nichols et al., 1996; Shyng et al., 1997). Recent evidence indicates that phosphatidylinositol phosphates can profoundly antagonize ATP inhibition of KATP channels (Shyng & Nichols, 1998).
The physiological function of these channels is best understood in the pancreas where they control insulin secretion in response to altered glucose levels (Boyd et al., 1990). Elevation in glucose levels leads to increased glucose transport and metabolism in the β-cell which increases the ATP : ADP ratio resulting in inhibition of the KATP channel, membrane depolarization, activation of voltage-gated calcium channels, calcium influx and insulin secretion. In the heart, potassium channel openers (KCOs) protect ischaemic/reperfused myocardium and evidence suggests that mitochondrial KATP channels may mediate these effects (Garlid et al., 1997; Liu et al., 1998). KATP channels also regulate hypoxia-evoked atrial natriuretic factor secretion in the heart (Xu et al., 1996), cholecystokinin secretion in the smooth muscle (Mangel et al., 1994) and neurotransmitter release in the central nervous system (Amoroso et al., 1990). Recent studies have shown that KATP channels in hypothalamic neurons are targets for activation by leptin, the obese gene product (Spanswick et al., 1997).
During the past 5 years, several KATP channel candidates have been cloned (reviewed in: Bryan & Aguilar-Bryan, 1997; Babenko et al., 1998; Isomoto & Kurachi, 1997; Ashcroft & Gribble, 1998). Reconstitution studies have revealed that the native KATP channel is a complex composed of two components: an inward rectifier K+ channel (Kir) belonging to either Kir6.1 or Kir6.2 and a sulphonylurea receptor (SUR). The Kir subunit is responsible for ion permeation whereas the SUR subunit confers sensitivity to KCOs such as diazoxide and sulphonylureas and may facilitate trafficking of the channel complex (John et al., 1998). Three SURs, SUR1, SUR2A and SUR2B, have been cloned and studied by co-expression with either Kir6.1 or Kir6.2 subunit. The β-cell KATP channel is composed of Kir6.2 and SUR1, assembled possibly in a 1 : 1 (SUR-Kir6x)4 octameric stoichiometry (Clement et al., 1997). Both subunits are required for functional expression of the channel, although removal of a 26-amino acid stretch from the C-terminus of Kir6.2 enables functional expression of the channel in the absence of SUR1 (Tucker et al., 1997). Recent studies have shown that SUR1-Kir6.2 is a major subunit combination of KATP channels not only in the pancreas, but also in neurons including dorsal vagal neurons and leptin-sensitive myenteric neurons (Karschin et al., 1998; Liu & Kirchgessner, 1998). In contrast, the widely accepted molecular composition of the cardiac channel is thought to be SUR2A-Kir6.2, whereas SUR2B in conjunction with Kir6.2 or Kir6.1 is thought to constitute diverse smooth muscle type KATP channels (Isomoto & Kurachi, 1997; Aguilar-Bryan & Bryan, 1999). It is now becoming evident that the C-terminal region of the SUR does not affect sulphonylurea interactions and that the binding sites for both KCOs and sulphonylureas may be associated within the second set (12–17 segments) of transmembrane domains (Dörschner et al., 1999; Uhde et al., 1999). Analysis of radiolabelled glyburide (glibenclamide) binding to the SUR subtypes reveal that occupation of one of the four SUR sites per channel complex is sufficient for the closure of KATP channels (Dörschner et al., 1999).
With the emerging diversity of KATP channel subunits, it is quite possible that each tissue has certain isoforms to play various functions from transmitter release to ischaemic protection. Initial studies of the SUR1-Kir6.2 containing KATP channels have primarily been performed in Xenopus oocytes or in mammalian cells transiently expressing the channel subunits (Inagaki et al., 1995; Shyng et al., 1997). Recently, these KATP channels have been expressed in Spodoptera frugiperda (Sf9) cells using a bacculovirus vector (Mikhailov et al., 1998). However, a detailed evaluation of the biophysical, radioligand binding and pharmacologic properties of these channels and critical comparison with those of native tissues or cell types have not been undertaken. Accordingly, in this study we have examined radioligand binding and functional pharmacology of KATP channels derived by stable co-expression of SUR1 and Kir6.2 subunits in human embryonic kidney 293 cells. Portions of this work were previously presented as an abstract (Gopalakrishnan et al., 1999a).
Methods
Stable transfection and cell culture
The amino acid sequence of human SUR1 (obtained from Incyte Pharmaceuticals Inc., Palo Alto, CA, U.S.A.) within the coding region was identical to that deposited in GenBank (Accession No: L78207) and the DNA sequence encoding human Kir6.2 subunit was identical to those previously published by Inagaki et al. (1995). For expression studies, SUR1 and Kir6.2 subunit DNA was subcloned into the mammalian expression vector pcDNA3.1 (Invitrogen) containing the zeocin and G418 resistance markers respectively. Restriction mapping and dideoxysequencing of double stranded DNA verified final constructs. Human embryonic kidney 293 cells (American Type Tissue Collection, Rockville, MD, U.S.A.) were transfected by lipofectamine (GIBCO/BRL, Grand Island, NY, U.S.A.) as previously described (Gopalakrishnan & Molinari, 1998). Briefly, SUR1 and Kir6.2 cDNA samples (1.5 μg) was mixed with 20 μg lipofectamine and used to transfect the 2×106 cells. Forty-eight hours post transfection, cells were split 1 : 10 and geneticin (0.5 mg ml−1) and zeocin (0.1 mg ml−1) were added to the media to select for antibiotic-resistant clones. Clones were picked at random with the aid of cloning rings and further propagated for analysis. Cells were maintained in media containing 250 μg ml−1 neomycin and 100 μg ml−1 zeocin and supplemented with 10% (w v−1) foetal bovine serum, 100 units ml−1 penicillin, 100 μg ml−1 streptomycin and 0.25 mg ml−1 amphotericin B in a humidified cell incubator containing 5% CO2 at 37°C. The RINm5F pancreatic insulinoma (American Type Culture Collection, Rockville, MD, U.S.A.) was cultured in RPMI 1640 medium containing 10% heat-inactivated foetal bovine serum and cells were maintained at 5% CO2.
RT–PCR analysis
RT–PCR was employed to assess the presence of SUR1 and Kir6.2 mRNA in transfected cells. Total RNA from HEK 293 cells transfected with SUR1 and Kir6.2 was isolated using Trizol reagent according to the manufacturer instructions as previously described (Miller et al., 1999; Gopalakrishnan et al., 1999b). Subunit specific primers were designed to SUR1 (accession number L78207) and Kir6.2 (accession number D50582). The oligonucleotide primers used are: SUR1 forward 5′-GCGTGCAAAAGCTAAGCGAG-3′ (1817–1836 bp) 5′-GACGCTTGCGGTT CACAAC-3′ (1951–1933 bp; expected product size, 134 bp) and Kir6.2 forward 5′-ATGCTGTCCCGCAAGGGCATC-3′ (1–21 bp); reverse 5′-GCTGATGATCATG CTCTTGC-3′ (636–617 bp; expected product size, 636 bp). Reverse-transcription PCR (RT–PCR) was performed using 2–4 μl of cDNA in 50 μl reaction containing 0.4 mM each primer, 200 mM each dNTP, and 2.5 units of Taq polymerase (Perkin Elmer, Norwalk, CT, U.S.A.). The cycling conditions were 95°C for 24 s, 55°C for 22 s, 72°C for 78 s for 40 cycles. Appropriate templates were included as positive controls while the reactions lacking reverse transcriptase during first strand synthesis served as a negative control. An aliquot (30 μl) of the RT–PCR product was analysed on a 10% Tris-Borate-EDTA (TBE) polyacrylamide gel and its identity confirmed by DNA sequence analysis.
Membrane preparation and ligand binding
Confluent transfected HEK-293 cells or RINm5F cells were rinsed with ice-cold assay buffer (50 mM Tris HCl, pH 7.2 at 22°C), mechanically disaggregated and homogenized using a Polytron homogenizer for 10 s. The homogenate was centrifuged at 45,000×g for 20 min at 4°C and the pellet resuspended in ice-cold assay buffer. [3H]-Glyburide binding to membranes was carried out in 50 mM Tris HCl buffer in the absence of nucleotides as described previously (Gopalakrishnan et al., 1991). Incubations were carried out in a final 500 μl volume with [3H]-glyburide (0.3 nM) and cell membrane protein (25 μg) for 60 min at room temperature in the presence or absence of unlabelled drugs. For saturation binding, 0.01–8.0 nM radioligand was employed. Specific binding was defined by the addition of 1 μM unlabelled glyburide to a duplicate set of tubes. Incubations were terminated by rapid vacuum filtration over GF/B glass fibre filters, and filters washed three times with 1.5 ml of ice-cold buffer. Bound radioactivity was quantitated by liquid scintillation spectroscopy at an efficiency of 45% (LS 5000 TD, Beckman Instruments, Somerset, NJ, U.S.A.).
Electrophysiology
Whole cell patch clamp was employed to measure the currents in transfected cells. The microelectrodes, pulled from borosilicate glass (0.8–1.1 mm internal diameter), had tip diameter of 2–3 μm and resistance ranging 2–5 MΩ. The intracellular pipette solution contained (mM): KCl 107, MgCl2 1.2, CaCl2 1, EGTA 10, HEPES 5 (pH 7.2 adjusted with KOH; total K+ ∼140 mM). The bath solution contained (mM): KCl 40, NaCl 100, CaCl2 2.6, MgCl2 1.2 and HEPES 5 (pH 7.4 adjusted with NaOH). Different K+ concentrations were obtained by equimolar substitution with NaCl. After a tight-seal was formed, the cell membrane was ruptured and the capacitance transient was integrated on-line to estimate cell capacitance as a measure of cell size. Uncompensated series resistance was typically 3–10 MΩ. Whole cell currents were amplified using Axopatch-200B amplifier (Axon Instruments, Foster City, CA, U.S.A.) and low pass filtered at 2 kHz (−3dB, 4 pole Bessel filter) before digitization by Digidata 1200B at a sampling rate of 10 kHz. Inhibition of glyburide was tested after whole-cell currents had reached steady-state levels with pipette solution containing no ATP.
86Rubidium efflux
Cation flux assays were carried out using cells grown attached to poly-D-lysine coated 24-well culture dishes (Nunc, Naperville, IL, U.S.A.). Cells were plated at a density of 1×105 cells well−1. When confluent, cells were loaded with media containing 86Rb+ (0.2 mCi well−1; NEN-Dupont, Wilmington, DE, U.S.A.) and incubated at 37°C for 4–5 h. Prior to the assay, the loading medium was removed, cells were rinsed three times with 200 μl of assay medium (composition, mM): HEPES 20, NaCl 120, KCl 7, CaCl2 2 and MgCl2 1, pH adjusted to 7.4 with NaOH, and then incubated with 200 μl of medium containing 2-deoxyglucose (10 mM) and oligomycin (0.2 μg ml−1) in the presence or absence of glyburide for 30 min. Radioactivity in the assay medium was detected by gamma counting (Gamma 5500, Beckman Instruments, Fullerton, CA, U.S.A.). Data is expressed as per cent efflux relative to the total amount of radioactivity incorporated.
Membrane potential studies
Functional activity of KATP channels expressed in transfected cells were measured by evaluating changes in membrane potential using the bis-oxonol dye DiBAC(4)3 (Molecular Probes) in a 96-well cell-based kinetic assay system, Fluorescent Imaging Plate Reader (FLIPR) as previously described (Gopalakrishnan et al., 1999b). Confluent cells cultured in black clear-bottomed 96-well plates were rinsed twice with 200 μl assay buffer (composition, mM): HEPES 20, NaCl 120, KCl 2, CaCl2 2, MgCl2 1, glucose 5, pH 7.4 at 25°C, containing 5 μM DiBAC4(3) and incubated with 180 μl of buffer in a cell incubator for 30 min to ensure dye distribution across the membrane. Assays were carried out at 37°C and were initiated by the addition of 20 μl of 10× concentration of the test compound. Changes in DiBAC4(3) fluorescence was measured at excitation and emission wavelengths of 488 and 520 nm, respectively (Schroeder & Neagle, 1996) for 25 min. Compounds were added in the presence or absence of metabolic inhibitors (10 mM 2-deoxy-D-glucose and 0.1 μg ml−1 oligomycin) and responses corrected for any background changes in fluorescence by subtracting the corresponding controls. In some experiments, at the end of the initial 25-min period, glyburide was added to examine reversal of KCO effects.
Data analysis and statistics
The IC50 values for the binding displacement was calculated from concentration-response curves using the 4-parameter logistic equation. The Ki values for ligands were estimated from the IC50 values using the Cheng-Prusoff equation Ki=IC50/(1+([glyburide]/KD)) where the KD value is equilibrium binding dissociation constant determined from saturation analysis. In patch clamp studies, the effect of glyburide was evaluated by comparing current before and after treatment in the same cell. The fraction of current remaining (I) was plotted as a fraction of the control current (Io) that was obtained in the absence of glyburide. Concentration-response data were fitted the logistic equation Y=1/[1+(X/a)b where X and Y represent concentration and response respectively and a and b represents the concentration for half-maximal effect and the slope respectively. When comparing group means, a P value <0.05 was considered statistically significant.
Compounds
[3H]-Glyburide (specific activity, 50 Ci mmol−1) and 86Rb+ (specific activity, 1.7 μCi μg−1) was purchased from NEN Life Sciences (Boston, MA, U.S.A.). Glyburide, glipizide, tolbutamide, tolazamide, chlorpropamide, phloxine B, acetohexamide, rose bengal, diazoxide, 2-deoxy glucose and oligomycin were obtained from Research Biochemicals International (Natick, MA, U.S.A.) or from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Stock solutions (10 or 100 mM) of drugs were made in DMSO and diluted to desired concentrations immediately prior to use.
Results
mRNA analysis
RT–PCR analysis of total RNA isolated transfected HEK-293 cells (clone #5) revealed the expected fragment sizes for Kir6.2 (636 bp) and SUR1 (134 bp) (Figure 1). Under these conditions, similar size products were undetectable in cells transfected with plasmids lacking the inserts. Further, negative controls lacking reverse transcriptase in the cDNA synthesis step did not reveal detectable bands (data not shown).
Figure 1.
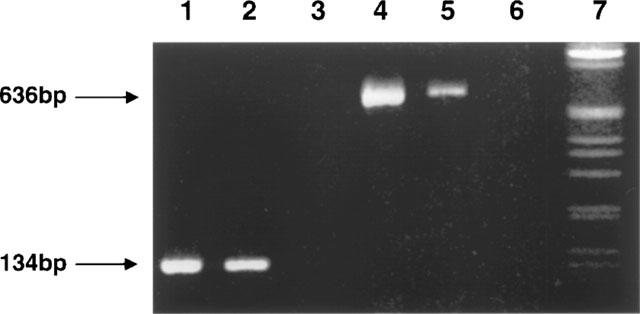
Representative RT–PCR analysis of total cellular RNA isolated from HEK-293 cells transfected with SUR1 and Kir6.2 subunits. RT–PCR products were analysed on a 1% tris-borate-EDTA agarose gel and visualized by ethidium bromide. Lanes 1 and 4, SUR1 and Kir6.2 PCR products amplified from hSUR1 and Kir6.2 templates, respectively; lanes 2 and 5, SUR1 and Kir6.2 PCR products amplified from SUR1/Kir6.2 transfected HEK 293 cells, respectively; lanes 3 and 6 no reverse transcriptase controls for SUR1 and Kir6.2 from transfected HEK 293 cells, respectively; and lane 7, 1 kb DNA ladder. The expected fragment sizes corresponding to Kir6.2 (636 bp) and SUR1 (134 bp) are indicated by arrows.
Pharmacology of [3H]-glyburide binding
[3H]-Glyburide bound to transfected HEK-293 cells in a saturable manner with a Bmax value of 18.46±1.25 pmol mg−1 protein and with a KD value of 0.71±0.08 nM (n=3; Figure 2). Under similar binding conditions, binding to RINm5F cells and rat brain membranes showed Bmax values approximately 30 fold and 180 fold respectively lower than those observed in transfected cells [RINm5F, 596±68 fmol mg−1; n=7; rat brain, 101±7.3 fmol mg−1; n=3]. Importantly, binding properties in this cell line have been maintained in culture for repeated passages and cells could be retrieved from frozen storage without loss in activity.
Figure 2.
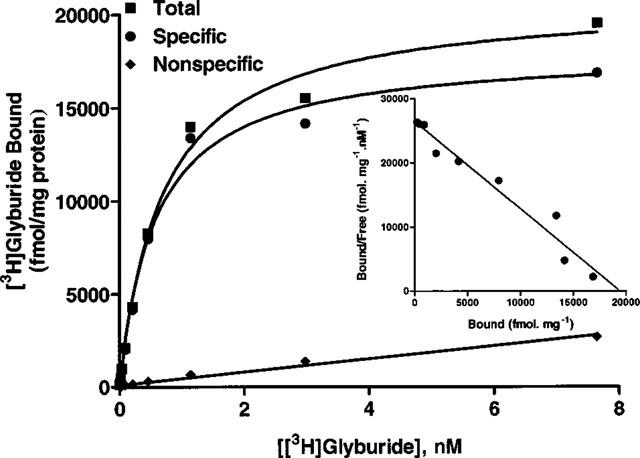
Radioligand binding properties of HEK-293 cells expressing SUR1 and Kir6.2 subunits. Saturation analysis of specific [3H]-glyburide binding data to transfected cell membranes. Inset: Scatchard representation of specific binding data. Shown is a representative plot. The mean Bmax and KD values are summarized in Results.
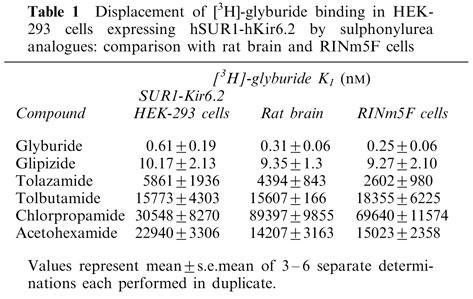
The pharmacology of [3H]-glyburide binding was assessed in detail by displacement analysis of a series of sulphonylurea and fluorescein analogues (Figure 3). The Ki values for binding inhibition are summarized in Table 1. The relative affinities of the various sulphonylurea ligands at the recombinant SUR1-Kir6.2 were, glyburide (0.61 nM)>glipizide>tolazamide>tolbutamide∼acetohexamide∼chlorpropamide (30 μM). Specific binding was also displaced by fluorescein analogues previously described to interact with KATP channels in insulinoma cells (de Weille et al., 1992; Holemans et al., 1995). Phloxine B and rose bengal inhibited [3H]-glyburide binding with Ki values of 449±86 nM and 127±14 nM (n=3), respectively. The affinity values for binding displacement for the various compounds examined including glyburide, glipizide, chlorpropamide, tolazamide showed a good correlation with those observed with pancreatic RINm5F cells (r=0.97) and rat brain (r=0.93) membranes assayed under similar conditions [Figure 3B,C]. In contrast, KCOs including diazoxide and levcromakalim were quite ineffective in displacing [3H]-glyburide binding (up to 100 μM; data not shown) in the absence of MgATP consistent with their lack of interaction with high affinity sulphonylurea binding sites, although these KCOs have been shown to effectively displace [3H]-P1075 binding to SUR2 containing channels when binding assays were carried out in the presence of ATP (Schwanstecher et al., 1998; Hambrock et al., 1999). Although nucleotides have been previously shown to modulate high affinity [3H]-glyburide binding (Gopalakrishnan et al., 1991; Schwanstecher et al., 1998), it should be noted that the present studies were carried out in the absence of nucleotides to permit direct comparisons with similar studies in rat brain and RINm5F cells that were carried out in the absence of nucleotides.
Figure 3.
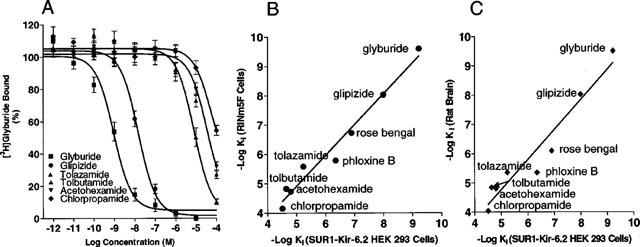
Pharmacological profile of [3H]-glyburide binding in transfected HEK-293 cells (A) Displacement of specific [3H]-glyburide binding by sulphonylurea analogues. Assays were performed using 0.3 nM of [3H]-glyburide with varying concentrations of various sulphonylurea analogues as described in Methods. (B) Correlation of the Ki values in transfected HEK 293 cells with those observed in RINm5F (r2=0.97; slope=1.05±0.07) and (C) with rat brain (r2=0.93; slope=1.03±0.11) membranes. The Ki values were calculated from the IC50 values using the Cheng-Prusoff equation as described in Methods.
Table 1.
Displacement of [3H]glyburide binding in HEK-293 cells expressing hSUR1-hKir6.2 by sulphonylurea analogues: comparison with rat brain and RINm5F cells
Electrophysiology
Upon depletion of internal ATP with patch pipette containing no ATP, increases in inward currents were observed at a holding potential of −80 mV in transfected cells when the cells were bathed with extracellular solution containing 40 mM K+. Similar increases in inward currents were obtained following application of 500 μM diazoxide (data not shown). Under similar conditions, responses were not observed in untransfected cells or in cells co-transfected with corresponding plasmids lacking SUR1 and Kir6.2 inserts (control). Step-pulses from −100 to +100 mV for 400 ms revealed time-independent increases in current at every test potential and a weak inward rectification in the voltage-current relationship. In contrast, in control cells, only endogenous basal currents were observed (Figure 4A). Under the present recording conditions where currents were measured by whole-cell patch clamp method, channel run down was negligible. The current density in transfected HEK-293 cells obtained by normalizing maximal current to cell capacitance is 258.2±54 pA/pF (n=20) is about 1.6 fold greater than that measured in RINm5F cells (161.1±66, n=3) under similar conditions.
Figure 4.
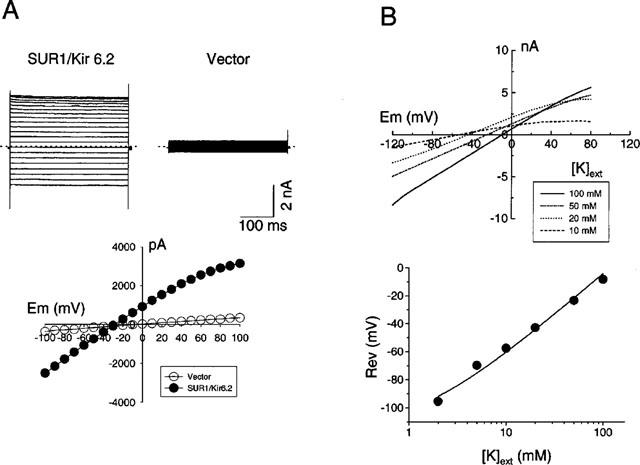
Electrophysiological properties of KATP channels expressed in HEK-293 cells. (A) Cells were held at −20 mV and whole cell currents recorded from cells at test potentials ranging from −100 to +100 mV with extracellular solution containing 40 mM K+ from transfected and control cells. The weak inward rectification of the I-V curve is also depicted. (B) Change in reversal potential as a function of extracellular K+. Whole cell currents were measured at varying extracellular K+ (2–100 mM) and the reversal potential is plotted as a function of K+ concentration. The data was fitted to a modified Goldman equation Erev=59 log (Ko+αNao)/Ki where α is the apparent selectivity ratio, PNa/PK (0.01±0.005; n=7) and Ko and Nao are the extracellular concentrations of K+ and Na+ ions respectively and Ki represents the intracellular concentration of K+ ions.
To verify whether the current responses in transfected cells were mediated by K+ ions, the reversal potentials were measured at different external K+ concentrations. Elevations in external [K+] shifted the reversal potential value to potentials that are more positive. As shown in Figure 4B, the relationship between Erev and log10[K+]o was nearly linear. A PNa/PK ratio of 0.01±0.005 (n=7) was obtained by fitting the data to the modified Goldman equation indicating that the channel is highly K+-selective. (Hodgkin & Horowicz, 1959).
The amplitude of whole cell currents was reduced with increasing concentrations of glyburide (Figure 5). However, at concentrations of glyburide greater than 10 nM, the current responses were not further attenuated. The reductions in current amplitude were similar at all ranges of test potentials (−100 to +100 mV) indicating that the inhibitory effect of glyburide is voltage-independent. The IC50 value for inhibition of whole cell current responses by glyburide was 0.92±0.01 nM (Hill coefficient, 1.0±0.2; n=6).
Figure 5.
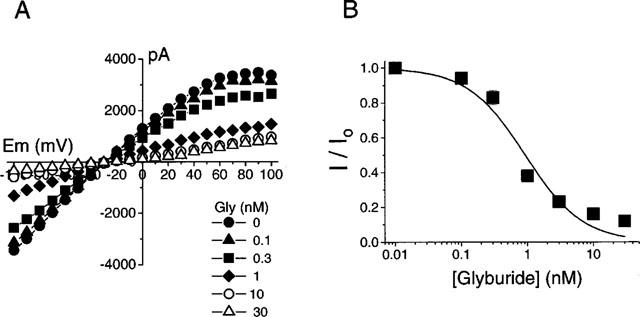
Inhibition of SUR1-Kir6.2 channels in transfected cells by glyburide. (A) The reduction in amplitude of whole cell currents with increasing concentrations of glyburide at each test potential. (B) Concentration-response curve for the inhibition of current amplitude by glyburide (at −100 mV). The fraction of current remaining (I) was plotted as a fraction of the control current (Io) that was obtained in the absence of glyburide. The IC50 value of glyburide is 0.92±0.01 nM (Hill coefficient=1.0±0.2; n=6).
Cation-efflux
Metabolic poisoning by treatment with 2-deoxyglucose (10 mM) and oligomycin (0.25 μg ml−1) triggered a significant efflux of 86Rb+ efflux in transfected cells (Figure 6A). This was not observed in cells cotransfected with the corresponding plasmids lacking SUR1 and Kir6.2 inserts (Figure 6A, insert), confirming that the observed responses are specific to expression of the transgenes. No significant differences in basal efflux (∼30%) were noted between SUR1-Kir6.2 transfected and control HEK-293 cells. Specific efflux stimulated by metabolic blockade was inhibited by glyburide with an IC50 value of 3.58±0.92 nM (n=5; Figure 6B) suggesting that the efflux is mediated through KATP channels. The basal efflux was unaffected by glyburide. This value is similar to that obtained for inhibition of 2-deoxyglucose-oligomycin evoked efflux in the RINm5F cells (1.77±0.58 nM; n=10) which is comparable to the values reported previously by Schmid-Antomarchi et al. (1987).
Figure 6.
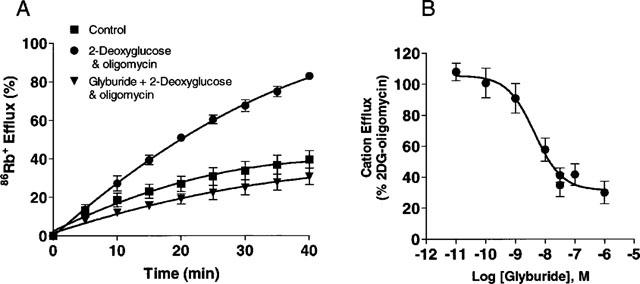
Cation efflux in HEK-293 cells transfected with SUR1 and Kir6.2 subunits. (A) Shown is the net 86Rb+ efflux under control conditions, following addition of 10 mM 2-deoxyglucose and 0.25 μg ml−1 oligomycin in the absence or presence of glyburide in transfected cells. Inset: Responses of cells co-transfected with corresponding plasmids lacking inserts. (B) Inhibition of cation efflux responses in transfected cells by glyburide. Data shown are means±s.e.mean of 3–5 determinations each carried out in duplicate.
FLIPR-based membrane potential studies
The functional expression of KATP channels in transfected HEK-293 cells was also evaluated by assessing membrane potential changes in response to KCOs. Assessment of membrane potential changes using potentiometric dyes such as DiBAC4(3) in the FLIPR enables studies on KATP channels in a rapid and high-throughput manner (Schroeder & Neagle, 1996). Addition of diazoxide evoked concentration-dependent decreases in membrane potential as assessed by DiBAC4(3) fluorescence in transfected cells. In initial experiments, it was found that fluorescence responses of diazoxide was more pronounced when applied in presence of 2-deoxyglucose and oligomycin. Accordingly, experiments were carried out under conditions of metabolic inhibition and KCO-evoked effects were derived by subtracting responses from corresponding controls that were treated with metabolic inhibitors alone. Similar effects have been noted previously by two electrode voltage clamp studies where diazoxide in presence of azide potentiates SUR1-Kir6.2 currents by some 130% (Narwal et al., 1997). The EC50 value of diazoxide in transfected cells was determined to be 14.06±1.66 μM (n=5). Similar experiments carried out using the RINm5F cells showed a similar enhancement of diazoxide evoked responses in the presence of metabolic inhibitors. The EC50 value for diazoxide in RINm5F cells was determined to be 10.94±3.78 μM (n=3) which is close to those observed in transfected cells. In contrast, levcromakalim that have been shown to effectively activate smooth muscle type KATP channels (Quayle et al., 1997; Gopalakrishnan et al., 1999b) evoked only a modest maximal 20% change in DiBAC4(3) fluorescence (Figure 7B).
Figure 7.
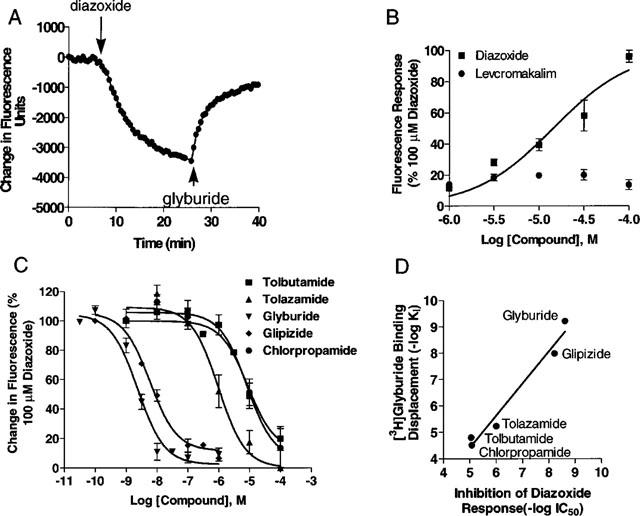
Pharmacology of membrane potential responses in SUR1-Kir6.2 transfected HEK-293 cells assessed by the bisoxanol dye, DiBAC4(3). (A) Representative experiment showing typical changes in DiBAC4(3) fluorescence upon addition (indicated by down arrows) of diazoxide (100 μM) and its attenuation (indicated by up arrows) by the addition of glyburide (5 μM). Experiments were carried out in the presence of 10 mM 2-deoxyglucose and 0.1 μg ml−1 oligomycin as described in Methods. Diazoxide-evoked fluorescence changes were derived by subtracting responses from control cells treated with metabolic inhibitors alone. (B) Concentration response curves for fluorescence changes evoked by diazoxide and levcromakalim in transfected cells. Data is normalized to the response evoked by 100 μM diazoxide. (C) Concentration response for the inhibition of 100 μM diazoxide-evoked responses by glyburide (IC50=1.6±0.4 nM), glipizide (5.6±1.0 nM), chlorpropamide (20.3±8.0 μM), tolazamide (4.2±1.0 μM) and tolbutamide (12.5±5.3 μM). Depicted are mean±s.e.mean of 3–6 separate determinations. (D) Correlation of IC50 values for inhibition of diazoxide-evoked functional responses and the Ki values for displacement of [3H]-glyburide binding in transfected cells (r2=0.96; slope=1.21±0.12).
Diazoxide-evoked responses were readily attenuated by the addition of glyburide (Figure 7A). Diazoxide-evoked responses were inhibited in a concentration-dependent manner by various sulphonylurea analogues including glyburide, glipizide, tolazamide and tolbutamide (Figure 7C). The rank order potency of sulphonylureas for inhibition of diazoxide-evoked responses (Figure 7D) showed a good correlation (r2=0.96) with their corresponding [3H]-glyburide binding affinities in the transfected cells.
Discussion
In this study, we have stably expressed and characterized the pharmacology of KATP channels formed by the association of SUR1 and Kir6.2 in HEK 293 cells. The pharmacological properties of recombinant KATP channels expressed in HEK-293 cells studied by a variety of methods including voltage-sensitive fluorescent probes, whole cell patch clamp, cation efflux and ligand binding collectively demonstrate that channels derived from co-expression of the SUR1 with Kir6.2 resemble the pharmacology of KATP channels functionally expressed in pancreatic/neuronal tissues.
Whole cell patch clamp studies show that the KATP channels reconstituted in HEK-293 cells by stable co-expression of SUR1 and Kir6.2 subunits are functional with properties similar to those previously described in pancreatic β-cells in terms of its higher sensitivity to glyburide and activation by depletion of internal ATP. In the absence of ATP in the pipette solution, a progressive increase in whole cell currents were observed in transfected cells which were sensitive to inhibition by nanomolar concentrations of glyburide. The weak inward rectification properties observed are similar to those previously reported for KATP channels in RINm5F cells (Ciani & Ribalet, 1988). In most inside-out patches, the KATP channel activity decreases rapidly (rundown) following patch isolation and that phosphorylation is required to maintain channel availability (Ashcroft & Rorsman, 1991). In this study, currents were measured in the whole-cell patch clamp mode wherein the cytosolic factors are much preserved and channel rundown was negligible.
While patch clamp analysis would certainly be a superior approach for biophysical characterization, both membrane potential and cation efflux measurements permit development of functional assays amenable to substantially higher throughput. The results obtained from analysis of transfected cells using both membrane potential and cation efflux assays are in support of results observed from current measurements. Diazoxide was found to decrease fluorescence responses in the FLIPR in a concentration-dependent manner with potencies similar to those observed in native KATP channels expressed in RINm5F cells (Miller et al., 1999). The potency of diazoxide is also in the range reported in transient expression studies in COS cells (60 μM; Inagaki et al., 1995) and in oocytes expressing SUR1-Kir6.2 combinations, where diazoxide evoked an almost 2 fold increase in KATP currents (Gribble et al., 1997).
In cation efflux studies, addition of oligomycin and 2-deoxyglucose to deplete cells of [ATP]i by blocking oxidative phosphorylation and glycolysis resulted in a significant increase in 86Rb+ efflux due to opening of KATP channels. The sensitivity of metabolic depletion-evoked cation efflux to glyburide agrees well with those observed in RINm5F cells or in transient expression studies of SUR1 and Kir6.2 (1.8 nM; Inagaki et al., 1995). This is some 20–200 fold higher than those observed in cardiovascular tissues or with KATP channels derived by co-expression of Kir6.2 with SUR2A or SUR2B isoforms (Inagaki et al., 1996; Okuyama et al., 1998; Dörschner et al., 1999). It is to be noted that glyburide was without effect on the basal efflux or on endogenous current responses which may be contributed by other Kir channels present in HEK-293 cells (Ammala et al., 1996).
Radioligand binding analysis revealed that [3H]-glyburide binding in transfected cells is of high affinity, typical of those described in membranes isolated from pancreatic β-cells, β-cell lines, several neuro-endocrine cells and in various brain regions (reviewed in Ashcroft & Ashcroft, 1992; Gopalakrishnan et al., 1993). The ligand affinities of sulphonylurea analogues in transfected cells compare well with those observed in RINm5F cells and in rat brain membranes and are approximately 300–500 fold higher than those required for interaction with the SUR2 isoforms (Meyer et al., 1999). Detailed analysis of the structural requirements for sulphonylurea analogues with SUR subtypes have shown that the selectivity of sulphonylurea analogues such as glyburide for SUR1 may be attributed to lipophilic substitution on the urea moiety (Meyer et al., 1999). The observed close correlation of the displacement of [3H]-glyburide binding by several sulphonylurea analogues in the SUR1-Kir6.2 transfected cells with those in RINm5F cells and in rat brain suggest that these binding sites are closely related in these tissues and that SUR1 may be an integral component not only of pancreatic, but also of neuronal KATP channels. The presence of high densities of [3H]-glyburide binding sites in substantia nigra, neocortex, hippocampus and cerebellar molecular layer together with the high degree of overlap in the expression of SUR1 and Kir6.2 in these regions support the notion that these subunits could form a major class of KATP channel in the brain with diverse physiologic roles (Karschin et al., 1997). Direct evidence for the presence of SUR1-containing KATP channels in nervous tissues including rat dorsal vagal neurons (Karschin et al., 1998) and glucoresponsive myenteric neurons of the gut have been presented (Liu & Kischgessner, 1998).
The density of receptors expressed in transfected cells is some 30–150 fold higher than those found in pancreatic insulinoma RINm5F and in the rodent brain. For example, the Bmax value of [3H]-glyburide was about 30 fold higher in transfected cells compared to RINm5F cells. However, an estimation of the current densities by patch clamp show that the transfected cells show only about a 1.6 fold higher current density compared to those expressed in RINm5F cells. This suggests that many SUR subunits that may not be coupled to form functional channels, or that not all SURs may be expressed on the cell surface to form functional KATP channels. Although sulphonylureas exert their primary action on β-cell electrical activity through closure of plasma membrane KATP channels, there is evidence for plasma membrane-independent pathways in glucose/sulphonylurea-induced insulin secretion, as for example, those involving intracellular sulphonylurea receptors (Eliasson et al., 1996).
During recent years, it has increasingly become evident that the diversity of KATP channels results from the assembly of various SUR and Kir6.n subunits (Bryan & Aguilar-Bryan, 1997; Isomoto et al., 1996). SUR1 and SUR2-based KATP channels are distinguished by their differential sensitivities to sulphonylurea analogues, whereas SUR2A-Kir6.n channels are distinguished from the SUR2B-containing channels by their differential sensitivities to KCOs such as nicorandil and diazoxide (Isomoto & Kurachi, 1997). Transient expression studies have shown that SUR1 and Kir6.2 subunits co-associate to form a heterooctamer that shows a higher sensitivity to ATP and glyburide compared to cardiac and smooth muscle type KATP channels (Inagaki et al., 1995; 1996). In the present investigation, depletion of [ATP]i by metabolic inhibition or by dialysis evoked significant KATP channel activity as measured by either whole cell patch clamp, cation flux or membrane potential studies. In the battery of functional assays investigated in the present study, KATP channels were inhibited by glyburide with an IC50 value ranging 1–3 nM. The apparent partial (∼85%) reversal by glyburide noted in fluorescence change measurements in the FLIPR might be attributed to the limited duration of the data collection period subsequent to the addition of glyburide as the partitioning of the dye is a relatively slower process.
SUR1-Kir6.2 containing KATP channels serve as an essential regulator of stimulus-secretion coupling in pancreatic β-cells (Bryan & Aguilar-Bryan, 1997). Studies in mice lacking Kir6.2 (Kir6.2 −/−) have shown that neither glucose or tolbutamide stimulated [Ca2+]i or insulin secretion suggesting that the rapid rise of Ca2+ in response to glucose or sulphonylureas in pancreatic β cells depends on closure of endogenous KATP channels containing the Kir6.2 subunit (Miki et al, 1999). Our present studies utilizing stably transfected HEK-293 cells subunits further demonstrate that ligand binding and functional pharmacology of the KATP channels derived from Kir6.2 and SUR1 resemble those typical of native channels described in pancreatic β-cells and neuronal tissues.
Acknowledgments
The authors thank Incyte Pharmaceuticals Inc. (Palo Alto, CA, U.S.A.) for providing the hSUR1 clone.
Abbreviations
- DiBAC4(3)
Bis-(1,3-dibutylbarbituric acid)trimethine oxonol
- DMEM
Dulbecco's modified Eagle's medium
- EC50
molar concentration of test compound for 50% activation of response
- FLIPR
fluorescent imaging plate reader
- IC50
molar concentration of test compound for 50% inhibition of response
- KATP
ATP-sensitive K+ channel
- KCO
potassium channel opener
- Kir
inwardly rectifying K+ channel
- RT–PCR
reverse transcriptase-polymerase chain reaction
- SUR
sulphonylurea receptor
References
- AGUILAR-BRYAN L., BRYAN J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 1999;20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- AMMALA C., MOORHOUSE A., ASHCROFT F.M. The sulfonylurea receptor confers diazoxide sensitivity on the inwardly rectifying K+ channel Kir6.1 expressed in human embryonic kidney cells. J. Physiol. 1996;494:709–714. doi: 10.1113/jphysiol.1996.sp021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMOROSO S., SCHMID-ANTOMARCHI H., FOSSET M., LAZDUNSKI M. Glucose, sulfonylurea receptors and neurotransmitter release. Role of ATP-sensitive K+ channels. Science. 1990;247:852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., RORSMAN P. Electrophysiology of the pancreatic β-cell. Prog. Biophys. Mol. Biol. 1991;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- ASHCROFT S.J.H., ASHCROFT F.M. The sulfonylurea receptor. Biochim. Biophys. Acta. 1992;1175:45–59. doi: 10.1016/0167-4889(92)90008-y. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., GRIBBLE F.M. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- BABENKO A.P., AGUILAR-BRYAN L., BRYAN J. A view of SUR/KIR6.x KATP channels. Annu. Rev. Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- BOYD A.E., AGUILAR-BRYAN L., NELSON D.A. Molecular mechanisms of action of glyburide on the beta cell. Am. J. Med. 1990;89 Suppl. 2A:3S–9S. doi: 10.1016/0002-9343(90)90330-g. [DOI] [PubMed] [Google Scholar]
- BRYAN J., AGUILAR-BRYAN L. The ABCs of ATP-sensitive potassium channels–more pieces of the puzzle. Curr. Opin. Cell Biol. 1997;9:553–559. doi: 10.1016/s0955-0674(97)80033-6. [DOI] [PubMed] [Google Scholar]
- CIANI S., RIBALET B. Ion permeation and rectification in ATP-sensitive channels from insulin-secreting cells (RINm5F): effects of K+, Na+ and Mg2+ J. Mem. Biol. 1988;103:171–180. doi: 10.1007/BF01870947. [DOI] [PubMed] [Google Scholar]
- CLEMENT J.P., KUNJILWAR K., GONZALEZ G., SCHWANSTECHER M., PANTEN U., AGUILAR-BRYAN L., BRYAN J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- DE WEILLE J., MULLER M., LAZDUNSKI M. Activation and inhibition of ATP-sensitive K+ channels by fluorescein derivatives. J. Biol. Chem. 1992;267:4557–4563. [PubMed] [Google Scholar]
- DÖRSCHNER H., BREKARDIN E., UHDE I., SCHWANSTECHER C., SCHWANSTECHER M. Stoichiometry of sulfonylurea-induced ATP-sensitive potassium channel closure. Mol. Pharmacol. 1999;55:1060–1066. doi: 10.1124/mol.55.6.1060. [DOI] [PubMed] [Google Scholar]
- ELIASSON L., RENSTROM E., AMMALA C., BERGGREN P.O., BERTORELLO A.M., BOKVIST K., CHIBALIN A., DEENEY J.T., FLATT P.R., GABEL J., GROMADA J., LARSSON O., LINDSTROM P., RHODES C.J., &5 RORSMAN P. PKC-dependent stimulation of exocytosis by sulfonylureas in pancreatic beta cells. Science. 1996;271:813–815. doi: 10.1126/science.271.5250.813. [DOI] [PubMed] [Google Scholar]
- GARLID K.D., PAUCEK P., YAROV-YAROVOY B., MURRAY H.N.M., DARBENZIO R.B., D'ALONZO A.J., LODGE N.J., SMITH M.A., GROVER G.J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive potassium channels. Possible mechanisms of cardioprotection. Circ. Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- GOPALAKRISHNAN M, MOLINARI E.J.Expression of cloned receptors in mammalian cell lines Current Protocols in Pharmacology 1998NY: John Wiley; 6.3.1–6.3.13.Unit 6.3 [DOI] [PubMed] [Google Scholar]
- GOPALAKRISHNAN M., JANIS R.A., TRIGGLE D.J. ATP-sensitive potassium channels: pharmacological properties, regulation and therapeutic potential. Drug Dev. Res. 1993;28:95–127. [Google Scholar]
- GOPALAKRISHNAN M., JOHNSON D., JANIS R.A., TRIGGLE D.J. Characterization of binding of the ATP-sensitive potassium channel ligand, [3H]-glyburide, to neuronal and muscle preparations. J. Pharmacol. Exp. Ther. 1991;257:1162–1171. [PubMed] [Google Scholar]
- GOPALAKRISHNAN M., MOLINARI E., SHIEH C.-C., MONTEGGIA L.M., ROCH J.-M., SCOTT V.E.S., ANDERSON K.A., SULLIVAN J.P., BRIONI J.D. Stable expression of the sulfonylurea receptor SUR1 and the inward rectifier Kir6.2: Pharmacology of ligand binding and function. Biophys. J. 1999a. p. A74.
- GOPALAKRISHNAN M., WHITEAKER K.L, , MOLINARI E.J., DAVIS-TABER R.A., MONTEGGIA L.M., SCOTT V.E.S., BUCKNER S., MILICIC I., CAIN J., POSTL S., BRIONI J.D., SULLIVAN J.P. Pharmacologic and molecular characterization of ATP-sensitive K+ channels (KATP) in guinea-pig bladder smooth muscle cells. J. Pharmacol. Exp. Ther. 1999b;289:551–558. [PubMed] [Google Scholar]
- GRIBBLE F.M., ASHFIELD R., AMMALA C., ASHCROFT F.M. Properties of cloned ATP-sensitive K+ currents expressed in Xenopus oocytes. J. Physiol. 1997;498:87–98. doi: 10.1113/jphysiol.1997.sp021843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBROCK A., LOFFLER-WALZ C., KLOOR D., DELBAR U., HORIO Y., KURACHI Y., QUAST U. ATP-senstive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: Opposite effects of MgADP. Mol. Pharmacol. 1999;55:832–840. [PubMed] [Google Scholar]
- HODGKIN A.L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J. Physiol. 1959;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLEMANS S., FERON O., OCTAVE J., MALOTEAUX J. Interaction of fluorescein derivatives with glibenclamide binding sites in rat brain. Neurosci. Lett. 1995;183:183–186. doi: 10.1016/0304-3940(94)11146-a. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., IV, NAMBA N., INAZAWA J., GONZALEZ G., AGUILAR-BRYAN L., SEINO S., BRYAN S. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMENT J.P., IV, WANG C.-Z., AGUILAR-BRYAN L., BRYAN J., SEINO S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- ISOMOTO S., KONDO C., YAMADA M., MATSUMOTO S., HIGASHIGUCHI O., HORIO Y., MATSUZAWA Y., KURACHI Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J. Biol. Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- ISOMOTO S., KURACHI Y. Function, regulation, pharmacology and molecular structure of ATP-sensitive K+ channels in the cardiovascular system. J. Cardiovasc. Electrophysiol. 1997;8:1431–1446. doi: 10.1111/j.1540-8167.1997.tb01040.x. [DOI] [PubMed] [Google Scholar]
- JOHN S.A., MONCK J.R., WEISS J.N., RIBALET B. The sulfonylurea receptor SUR1 regulates ATP-sensitive Kir6.2 K+ channels linked to the green fluorescent protein in human embryonic kidney cells (HEK 293) J. Physiol. 1998;510:333–345. doi: 10.1111/j.1469-7793.1998.333bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARSCHIN A., BROCKHAUS J., BALLANYI K. KATP channel formation by the sulfonylurea receptors SUR1 with Kir6.2 subunits in rat dorsal vagal neurons in situ. J. Physiol. (Lond.) 1998;509:339–346. doi: 10.1111/j.1469-7793.1998.339bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARSCHIN C., ECKE C., ASHCROFT F.M., KARSCHIN A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- LIU M.-T., KIRCHGESSNER A.ATP-sensitive K+ channels are expressed by glucoresponsive enteric neurons 1998St. Charles, IL; 2nd International Conference on ATP-sensitive potassium channels and diseaseAbstr 226 [Google Scholar]
- LIU Y., SATO T., O'ROURKE B., MARBAN E. Mitochondrial ATP-dependent potassium channels. Novel effectors of cardioprotection. Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- MANGEL A.W., PRPIC V., SNOW N.D., BASAVAPPA S., HURST L.J., SHARARA A.I., LIDDLE R.A. Regulation of cholecystokinin secretion by ATP-sensitive potassium channels. Am. J. Physiol. 1994;267:G595–G600. doi: 10.1152/ajpgi.1994.267.4.G595. [DOI] [PubMed] [Google Scholar]
- MEYER M., CHUDZIAK F., SCHWANSTECHER C., SCHWANSTECHER M., PANTEN U. Structural requirements of sulfonylureas and analogues for interaction with sulfonylureas receptor subtypes. Br. J. Pharmacol. 1999;128:27–34. doi: 10.1038/sj.bjp.0702763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKHAILOV M.V., PROKS P., ASHCROFT F.M., ASHCROFT S.J.H. Expression of functionally active ATP-sensitive K+ channels in insect cells using baculovirus. FEBS Lett. 1998;429:390–394. doi: 10.1016/s0014-5793(98)00640-1. [DOI] [PubMed] [Google Scholar]
- MIKI T., INAGAKI N., NAGASHIMA K., GONOI T., SEINO S.Structure and function of ATP-sensitive potassium channels Potassium Ion Channels: Molecular Structure, Function and Diseases 1999San Diego: Academic Press; 373–385.Kurachi, Y., Jan, L.Y. Lazdunski, M. (eds) [Google Scholar]
- MILLER T.R., DAVIS-TABER R., MOLINARI E.J., WHITEAKER K.L., MONTEGGIA L.M., SCOTT V.E.S., BRIONI J.D., SULLIVAN J.P., GOPALAKRISHNAN M. Pharmacological and molecular characterization of ATP-sensitive K+ channels in the TE671 human medulloblastoma cell line. Eur. J. Pharmacol. 1999;370:179–185. doi: 10.1016/s0014-2999(99)00128-4. [DOI] [PubMed] [Google Scholar]
- NARWAL S., MCHUGH D., MCDONALS R.L., BEECH D.J., SIVAPRASADRAO A. Cloning of a KATP channel from human aorta. Br. J. Pharmacol. 1997. p. 102P. [DOI] [PMC free article] [PubMed]
- NICHOLS C.G., MAKHINA E.N., PEARSON W.L., SHA Q., LOPATIN A.N. Inward rectification and implications for cardiac excitability. Circ. Res. 1996;78:1–7. doi: 10.1161/01.res.78.1.1. [DOI] [PubMed] [Google Scholar]
- NOMA A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- OKUYAMA Y., YAMADA Y., KONDO C., SATOH E., ISOMOTO S., SHINDO T., HORIO Y., KITAKAZE M., HORI M., KURACHI Y. The effects of nucleotides and potassium channel openers on the SUR2A/Kir6.2 complex K+ channel expressed in a mammalian cell line, HEK293T cells. Pfluger's Arch. 1998;435:595–603. doi: 10.1007/s004240050559. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., NELSON M.T., STANDEN N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 1997;77:1165–1231. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- SCHMID-ANTOMARCHI H., DE WEILLE J., FOSSET M., LAZDUNSKI M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J. Biol. Chem. 1987;262:15840–15844. [PubMed] [Google Scholar]
- SCHROEDER K.S., NEAGLE B.D. FLIPR: A new instrument for accurate, high throughput optical screening. J. Biomol. Screen. 1996;1:75–81. [Google Scholar]
- SCHWANSTECHER M., SIEVERDING C., DORSCHNER H., GROSS I., AGUILAR-BRYAN L., SCHWANSTECHER C., BRYAN J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHYNG S., FERRIGNI T., NICHOLS C.G. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHYNG S.L., NICHOLS C.G. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- SPANSWICK D., SMITH M., GROPPI V., LOGAN S.D., ASHFORD M.L.J. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive K+ channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- TUCKER S.J., GRIBBLE F.M., ZHAO C., TRAPP S., ASHCROFT F.M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- UHDE I, , TOMAN A, , GROSS I, , SCHWANSTECHER C, SCHWANSTECHER M. Identification of the potassium channel opener site on sulfonylurea receptors. J. Biol. Chem. 1999;274:28079–28082. doi: 10.1074/jbc.274.40.28079. [DOI] [PubMed] [Google Scholar]
- XU T., JIAO J.H., PENCE R.A., BAERTSCHI A.J. ATP-sensitive potassium channels regulate stimulated ANF secretion in isolated rat heart. Am. J. Physiol. 1996;271:H2339–H2345. doi: 10.1152/ajpheart.1996.271.6.H2339. [DOI] [PubMed] [Google Scholar]


