Abstract
Tyrosine kinases have been proposed as regulators of voltage-operated calcium channels. The effects of a range of structurally different inhibitors of protein tyrosine kinases (PTK) were examined on voltage-operated calcium channel currents (IBa) and pp60c-src kinase (c-src) activity in vitro.
IBa was measured in single myocytes isolated from rabbit ear artery by conventional whole cell voltage-clamp techniques. The activity of purified human c-src was measured in vitro using a non-radioactive assay.
Bath application of tyrphostin-23 and genistein (non-selective PTK inhibitors), bistyrphostin (a receptor-PTK-selective inhibitor) and PP1 (a src family-selective inhibitor) inhibited IBa in a concentration-dependent manner over a range of test membrane potentials. Intracellular application of peptide-A, a peptide inhibitor of c-src also inhibited currents. Inhibitor potency series against IBa was PP1 > genistein > tyrphostin 23 > bistyrphostin.
Tyrphostin-23, genistein, PP1, and peptide-A shifted the steady-state inactivation curves in a hyperpolarized direction without altering their slope. The inhibitors had no significant effects on IBa activation calculated from current-voltage relationships.
The agents inhibited c-src activity in a concentration-dependent manner. The order of potency was PP1 > genistein > peptide-A > tyrphostin-23 > bistyrphostin. The IC50 for inhibition of c-src activity was similar to the IC50 for inhibition of IBa in all cases.
Western blot analysis with a specific antibody to c-src showed the presence of this cytoplasmic tyrosine kinase in rabbit ear artery cells.
A range of structurally dissimilar inhibitors of PTKs inhibit IBa and c-src activity with similar potency. These data provide further evidence implicating endogenous c-src in the modulation of L-type calcium channels in vascular smooth muscle cells.
Keywords: Calcium, calcium channel, L-type, smooth muscle, vascular, pp60src, tyrosine kinase
Introduction
Tyrosine phosphorylation is an important regulator of cell function (Schlessinger & Ullrich, 1992). Protein tyrosine kinases (PTK) are classified into two major groups: receptor-linked PTK, such as receptors for growth factors (e.g. platelet-derived growth factor (PDGF), or epidermal growth factor (EGF)); and non-receptor PTK, such as the src-related PTK and products of Abl and Fes. In vascular smooth muscle cells tyrosine phosphorylation is known to be a critical event in mitogenesis (Tsuda et al., 1991). However, over the past few years it has become evident that tyrosine phosphorylation is not only crucial for regulation of growth-related responses such as gene transcription and cell division, but that it is also important for rapid cellular responses such as cell adhesion (Schlaepfer et al., 1994), migration (Abedi & Zachary, 1995), and smooth muscle contraction (Hughes & Wijetunge, 1998). Furthermore, increased tyrosine phosphorylation is associated with increased intracellular calcium concentration ([Ca2+]i) during cell proliferation and migration. Although the mechanisms linking tyrosine phosphorylation to the changes in [Ca2+]i are not fully understood, in some cases increased opening of L-type calcium channels has been proposed to underlie this effect (Nomoto et al., 1988; Diliberto et al., 1991; Hughes, 1995a).
L-type calcium channels are the major route of calcium entry into differentiated vascular smooth muscle cells and are regulated by a variety of cellular processes including protein phosphorylation (Hughes, 1995b). Recent studies by us, and others have shown that PTK modulate voltage-operated calcium channel currents (IBa) in vascular smooth muscle (reviewed in Hughes & Wijetunge, 1998) and in a variety of other cell types (Estacion & Mordan, 1993; Kusaka & Sperelakis, 1995; Yokoshiki et al., 1995; Cataldi et al., 1996), suggesting that tyrosine phosphorylation may be a ubiquitous regulatory mechanism for calcium channels.
The identity of the endogenous tyrosine kinase responsible for modulation of L-type calcium channels in vascular smooth muscle is uncertain. Intracellular application of pp60c-Src a non-receptor tyrosine kinase has been shown to increase calcium channel currents in rabbit ear artery cells (Wijetunge & Hughes, 1995). Similarly a peptide containing the amino acid motif (pY)EEI, which binds to and activates src family kinases also increased IBa (Wijetunge & Hughes, 1996). Interestingly, smooth muscle cytoplasm has high tyrosine kinase activity (Elberg et al., 1995) and activity of c-src is particularly high in vascular smooth muscle (Di Salvo et al., 1997). The objective in the present study was to examine the effect of PTK inhibitors on IBa and to pharmacologically characterize the endogenous PTK at which they are presumed to act. Furthermore the ability of the same inhibitors to inhibit c-src activity in vitro was investigated to allow comparison with their potency as inhibitors of IBa.
Methods
Single cells were freshly dispersed from rabbit ear arteries (from New Zealand White rabbits) by an enzymatic method previously described (Hughes & Wijetunge, 1993). 2–3 mm segments of artery were incubated for 50 min in a modified physiological salt solution (PSS) containing (mM): NaCl 130, KCl 6, CaCl2 0.01, MgCl2 1.2, glucose 14, and HEPES 10.7 buffered to pH 7.2 with NaOH, 2 mg ml−1 bovine serum albumin (BSA), 1 mg ml−1 collagenase (130 u mg−1), 0.5 mg ml−1 papain (15 u mg−1) and 5 mM dithiothreitol. Cells were dispersed by trituration and resuspended after centrifugation in PSS containing 1.7 mM CaCl2. Cells were stored on cover slips at 4°C and used within 4–6 h. Patch pipettes were fabricated from borosilicate glass and had resistances of 3–5 MΩ. Calcium channel currents were measured by the whole cell configuration of the voltage clamp technique (Hamill et al., 1981) using a List EPC-7 amplifier, Labmaster A/D interface board with commercially available software (PClamp 5.5, Axon Instruments CA, U.S.A.) on an IBM compatible PC. Internal pipette solution contained (mM): NaCl 126, MgCl2 1.2, EGTA 2, MgATP 2, TEA 10, and HEPES 11 buffered to pH 7.2 with NaOH. A ‘high barium' solution (BaCl2 110 mM, HEPES 10 mM; buffered to pH 7.2 with TEA-OH) was used as the charge carrier to isolate currents carried by calcium channels and to minimise calcium dependent inactivation of these channels (Aaronson et al., 1988; Hughes & Wijetunge 1993). Data were recorded on-line, or on digital audio tape using a DAT recorder (Biologic, France), and analysed off-like after analogue-to-digital conversion using PClamp 5.5 software (Axon Instruments, CA, U.S.A.). The currents were digitally filtered at 2 kHz and leak currents were subtracted digitally, using average values of steady leakage currents elicited by a 10 mV hyperpolarizing pulse (Aaronson et al., 1988). All recordings were made at room temperature (22–25°C). Only cells with no discernible run-down were used for experimentation. Tyrphostin-23, bistyrphostin, genistein and PP1 were applied by bath perfusion and this was done after the control currents had stabilized. Peptide-A was applied via the patch pipette.
Preparation of rabbit ear artery cell lysate
Rabbit cells were isolated as described above for voltage-clamp studies. After centrifugation the cells were resuspended in 50 μl lysis buffer, containing (mM) Tris 50, NaCl 150, EGTA 1, NP-40 1% (v v−1), sodium deoxycholate (19% w v−1), aprotinin, leupeptin, pepstatin (all at 1 μg ml−1) and PMSF (200 μM) and allowed to stand on ice for 5 min prior to homogenization with a glass-on-glass homogenizer. The homogenized cell samples were centrifuged at 4°C at 15,000 × g for 15 min and a protein assay was carried out (BCA assay kit, Pierce). The samples were heated at 95°C for 5 min with 5 × sodium dodecyl sulphate (SDS) sample buffer (Tris-HCl) (pH=6.8) 0.3 mM, β Mercaptoethanol 25% v v−1, SDS 10% v v−1, glycerol 50% v v−1 bromophenol blue 0.01% w w−1. The samples were stored at −20°C until used for gel electrophoresis.
SDS Polyacrylamide Gel Electrophoresis (SDS–PAGE)
SDS–PAGE was carried out using a Bio Rad Protean II system. Known amount of protein samples and molecular weight markers were loaded into wells and the gel was run in SDS running buffer (Tris 25 mM, glycine 192 mM and SDS 1% w v−1, pH=8.3) at 35 V overnight with a cooling system. The separated proteins were transferred onto supported nitrocellulose membrane using a Bio Rad transfer cell using transfer buffer (Tris 25 mM, glycine 192 mM and methanol 20% v v−1 at 35 V overnight. After transfer the membrane was blocked for 1–3 h with 5% BSA in Tris buffered saline (TBS) containing 0.001% v v−1 Tween 20 (TTBS) c-Src was detected by probing the blot with a specific monoclonal antibody to c-src, Mab 327 (2.5 μg ml−1). The primary antibody was bound to a HRP-conjugated-secondary antibody (1/1000 dilution). The antigen-antibody complex was visualized using enhanced chemiluminescence.
Assay of pp60c-src kinase (c-src) activity
Although it was possible to demonstrate the presence of c-src in lysates derived from rabbit ear artery smooth muscle cells, there was insufficient protein to immunoprecipitate useable amounts of c-src for kinase assay. Therefore purified human c-src was used to examine the effects of tyrosine kinase inhibitors, c-src activity was measured using a non-radioactive tyrosine kinase assay kit (Boehringer Mannhein, Germany) according to manufacturer's instructions. The assay utilizes a specific tyrosine kinase substrate (EGPWLEEEEE AYGWMDF) corresponding to the amino acid sequence 1–17 of gastrin. c-src (2U) was allowed to pre-equilibrate for 10 min with the required concentration of inhibitor prior to initiation of the assay by mixing with 5 μM substrate, in a solution containing 5 mM ATP and 50 mM MgCl2.
The assay buffer contained (mM) HEPES/NaOH 20, MgCl2 10, MnCl2 3, DTT 1 and orthovanadate 0.1. Following incubation at 37°C for 30 min the enzyme reaction was terminated by adding 3 mM piceatannol, a PTK inhibitor. Following binding of phosphopeptide onto streptavidin-coated microtitre plate wells, anti-pTyr-peroxidase was added for 1 h. Following addition of peroxidase substrate, absorbance was measured using an ELISA reader at 405 nm. A phosphopeptide standard curve was constructed for every assay by using a range of known concentrations (5 nM–5 μM) of a tyrosine-phosphopeptide provided with the assay kit.
Statistics and data analysis
Current-voltage (I-V) relationships were obtained by repeated, progressive stepwise depolarization to various test potentials for 200 ms from a holding potential of −60 mV. The effect of a drug on I-V relationship was examined after any response to the drug had stabilized. The peak inward current at each test potential was measured. Voltage-dependence of activation was derived from the I-V relationships using a Boltzmann equation:
where I=peak current (pA), g=maximum conductance (nS), Vm=membrane potential (mV), Vr=reversal potential (mV), Vact=potential for half maximal activation (mV) and k=slope factor, by non-linear regression techniques.
The steady-state inactivation curves were obtained from a double-pulse protocol (Aaronson et al., 1988). Following a 6 s conditioning pulse to various potentials to induce steady-state inactivation, currents were evoked by depolarizing to +20 mV from a holding potential of −60 mV. The data were fitted to a Boltzmann equation:
where I is the peak current at any potential, Imax is the maximum peak current, Vinact is the potential at which current is half inactivated, k is a slope factor and Inon is the non-inactivating fraction of current. Data were fitted by non-linear regression analysis.
To investigate the effects of drugs on calcium channel currents, peak currents evoked by a 200 ms depolarization from −60 mV holding potential to 0 mV were measured before and after application of drugs and % change was calculated with respect to pre-drug current. The effect of the inhibitors on currents evoked by depolarization from −60 to −20 mV was also examined, but since the estimates of potency obtained using this protocol did not differ significantly from those obtained with using depolarization to 0 mV only data derived from depolarization to 0 mV have been presented in Results. Concentration-response relations were fitted to a logistic function by non-linear regression analysis and −log (p)EC50 calculated.
All data are presented as means±s.e.means of (n) observations. Statistical comparisons of data were made using Student's t-test for single paired comparisons or multiple repeated measures analysis of variance followed by a Tukey post hoc test as appropriate. P<0.05 was considered statistically significant. Non-linear regression analysis was performed using Prism 2.01 (GraphPad Software, U.S.A.).
Materials
Peptide-A (Calbiochem, U.K.), PP1 (gift from Pfizer, U.S.A.), Anti c-src antibody (Mab 327) (Calbiochem, U.K.). Gel running and transfer systems (BioRad, U.K.). ECL reagents (Amersham, U.K.). All other drugs and chemicals from Sigma, Dorset, U.K. Tyrphostins, genistein and PP1 (4-amino-5- (4-methlyphenyl) -7- (t-butyl) pyrazolo [3,4-d] pyrimidine) were made up in dimethyl sulphoxide (DMSO) and final concentrations from 1–300 μM were made up in the ‘high Ba2+' solution. Stock solutions of Peptide-A (VAPSDSIQAEEWYFGKITRRE) (Sato et al., 1990) were made up in 5% ethanol. Maximum concentrations of DMSO and ethanol used had no effects on currents.
Results
Effects of tyrosine kinase inhibitors on IBa in rabbit ear artery smooth muscle cells
Bath application of tyrphostin-23 inhibited IBa in a concentration-dependent manner (Table 1, Figure 1A). On the basis of the I-V data, tyrphostin-23 did not statistically significantly shift the Vact for activation, although a trend for a shift to more hyperpolarized potentials was seen with the highest concentration of tyrphostin-23 (control Vact=5±2 mV (n=7), 50 μM tyrphostin-23 Vact=−1±4 mV (n=4), 100 μM tyrphostin-23 Vact=−1±3 mV (n=7), 300 μM tyrphostin-23 Vact=−9±2 mV (n=3)). Examination of the steady-state inactivation curves showed that 100 μM tyrphostin-23 shifted the potential for half maximal inactivation (Vinact) in a more hyperpolarized direction, Vinact shifted from −17±1 to −28±2 mV (P<0.001, n=5, Figure 1B), but neither the slope factor nor Inon was significantly altered (slope factor=13±3 mV (control), and 10±1 mV (tyrphostin-23); Inon=6±3 pA (control) and −3±5 pA (tyrphostin-23, n=5)).
Table 1.
The potency of tyrosine kinase inhibitors as inhibitors of IBa or c-Src tyrosine kinase activity
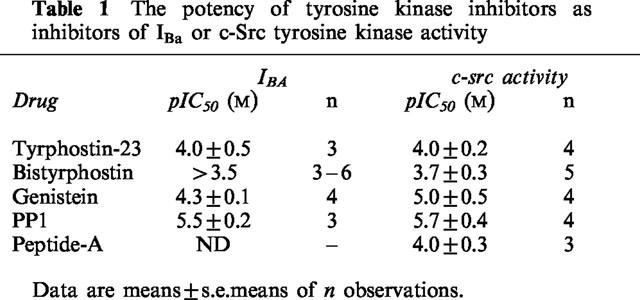
Figure 1.
Effect of tyrphostin-23 on calcium channel currents. (A) The current traces evoked from a holding potential of −60 mV depolarizing to a test potential of 0 mV for 200 ms in a single rabbit ear artery cell. (B) The I-V relationship of calcium channel currents in a single rabbit ear artery cell. I-V relationships were derived by depolarizing to various test potentials for 200 ms from a holding potential of −60 mV as shown in the protocol (inset). I-V data were measured at least 3–4 min after establishment of whole cell mode when the size of the currents had stabilized. Data are representative of 3–7 similar observations. (C) Steady-state inactivation of calcium channels. Steady-state inactivation curves were derived by using a double pulse protocol (inset). Cells were held at various conditioning voltages for 6 s to induce inactivation, then held for 10 ms at the holding potential of −60 mV before currents were evoked by a 200 ms depolarizing step to +20 mV as shown in the voltage protocol. IBa normalized with respect to IBa evoked by a −80 mV conditioning potential is plotted against the conditioning potential. Data are means±s.e.means of five separate experiments.
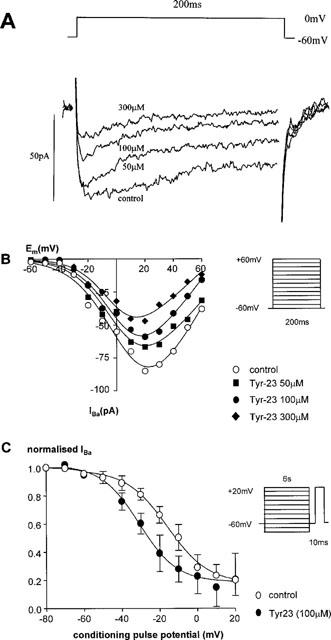
Bistyrphostin, which is reported to be more selective towards some receptor tyrosine kinases, notably the receptor for epidermal growth factor (EGFR) (Levitzki & Gilon, 1991), also inhibited calcium channel currents concentration-dependently (Table 1, Figure 2), although less potently than tyrphostin-23. In view of the low potency of bistyrphostin it was not possible to calculate a precise IC50, but it was estimated to be in excess of 300 μM. In view of the low potency of bistyrphostin the effect of this agent on activation and inactivation of IBa was not examined.
Figure 2.
Effect of bistyrphostin on calcium channel currents. (A) The current traces evoked from a holding potential of −60 mV depolarizing to a test potential of 0 mV for 200 ms in a single rabbit ear artery cell. (B) The I-V relationship of calcium channel currents in a single rabbit ear artery cell. I-V relationships were derived as described in the legend for Figure 1. I-V data were measured at least 3–4 min after establishment of whole cell mode when the size of the currents had stabilized. Data are representative of 3–6 similar observations.
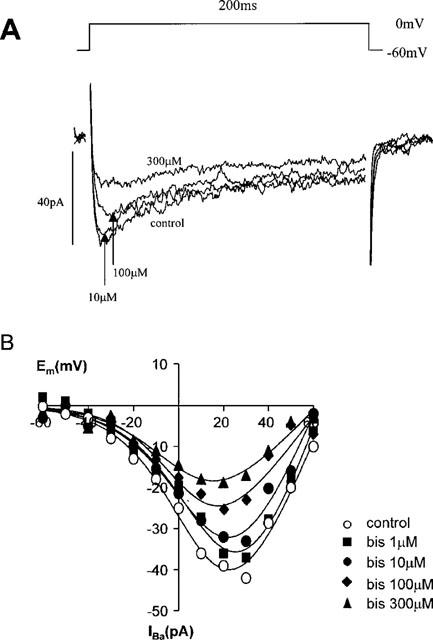
Bath application of the non-selective tyrosine kinase inhibitor, genistein (50–300 μM) caused a concentration-dependent inhibition of IBa (Table 1, Figure 3A). Genistein (50–300 μM) had no significant effect on the voltage-dependence of activation (Vact for control=13±2 mV, and genistein=8±5, −1±5 and −3±2 mV for 50, 100 and 300 μM respectively) (n=4–5). Steady-state inactivation curves showed that 50 μM genistein induced a shift in the Vinact to hyperpolarized potentials, from −19±3 to −31±5 mV (P=0.004, n=5, Figure 3B). Slope factor was not significantly altered (8±4 mV (control), 5±4 mV (genistein n=5); nor was Inon (12±3 pA (control) and 8.2±1 pA (genistein, n=5)).
Figure 3.
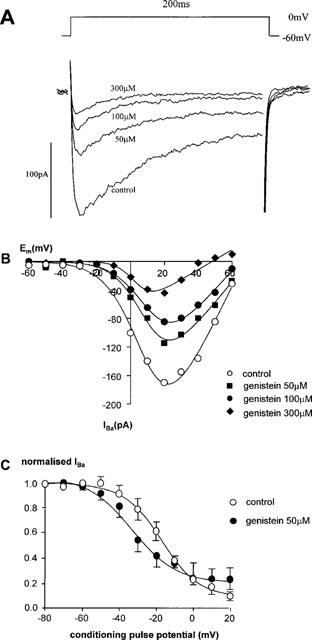
Effect of genistein on calcium channel currents. (A) The current traces evoked from a holding potential of −60 mV depolarizing to a test potential of 0 mV for 200 ms in a single rabbit ear artery cell. (B) The I-V relationship of calcium channel currents in a single rabbit ear artery cell. I-V relationships were derived as described in the legend for Figure 1. I-V data were measured at least 3–4 min after establishment of whole cell mode when the size of the currents had stabilized. Data are representative of 4–5 similar observations. (C) Steady-state inactivation of calcium channels. Steady-state inactivation curves were derived by using a double pulse protocol as described in the legend for Figure 1. Data are means±s.e.means of five separate experiments.
Bath application of a novel, src family-selective inhibitor, PP1 (10 nM–5 μM), caused a concentration-dependent inhibition of IBa (Table 1, Figure 4A). PP1 had no significant effect on Vact (−2±2 mV (control), −4±2 mV (500 nM PP1) (n=4)). However, PP1 shifted the steady-state inactivation curve to more hyperpolarized potentials (Figure 4B), so that in the presence of PP1 (500 nM) Vinact =−29±1 mV compared with −17±2 mV in control (n=3). Slope factor (control=9±1 mV, 500 nM PPI=10± 1 mV), (n=3) and Inon (control=8±1 pA, 500 nM PP1=1 ±1 pA; n=3) were unaffected by PP1. It was not possible to determine −log IC50 for Peptide-A as there were non-specific effects of vehicle (ethanol) when concentrations in excess of 100 μM) were used. However 100 μM Peptide A induced almost 50% inhibition, consequently this concentration was used to examine the effects of Peptide A on activation and inactivation of IBa. Peptide A also had no significant effect on the voltage-dependence of activation (Figure 5A; Vact for control =−2±2 mV, 100 μM peptide-A =−6±2 mV, n=3). Examination of the steady-state inactivation curves again showed a shift of Vinact in the negative direction by peptide-A (−35±5 mV, n=3) compared to a Vinact of −18±5 mV in control cells (Figure 5B) without altering the slope or non-inactivating component.
Figure 4.
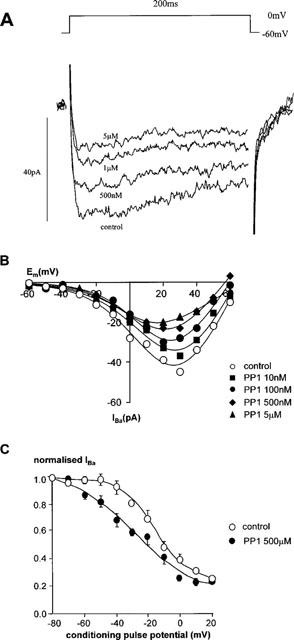
Effect of PP1 on calcium channel currents. (A) The current traces evoked from a holding potential of −60 mV depolarizing to a test potential of 0 mV for 200 ms in a single rabbit ear artery cell. (B) The I-V relationship of calcium channel currents in a single rabbit ear artery cell. I-V relationships were derived as described in the legend for Figure 1. I-V data were measured at least 3–4 min after establishment of whole cell mode when the size of the currents had stabilized. Data are representative of three similar observations. (C) Steady-state inactivation of calcium channels. Steady-state inactivation curves were derived by using a double pulse protocol as described in the legend for Figure 1. IBa normalized with respect to IBa evoked by a −80 mV conditioning potential is plotted against the conditioning potential. Data are means±s.e.means of three separate experiments.
Figure 5.
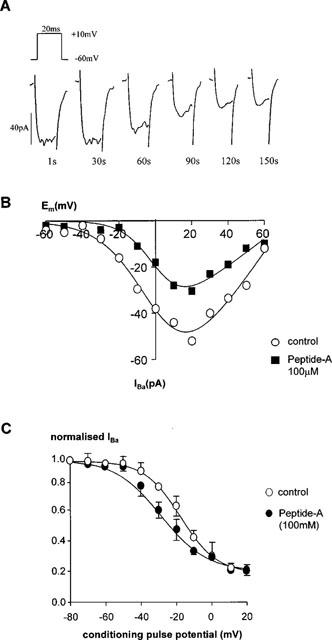
Effect of peptide A on calcium channel currents. (A) Effect of 100 μM peptide-A on a single rabbit ear artery cell. Currents were evoked every 1 s using a 20 ms pulse to +10 mV from a holding potential of −60 mV (upper inset). Peptide-A (100 μM) was applied by inclusion in the intracellular pipette solution. Current traces recorded at 1, 30, 60, 90, 120 and 150 s are shown. (B) The effect of peptide-A on the I-V relationship of calcium channel currents. I-V relationships were derived as described in the legend for Figure 1. The figure shows a representative control cell and a cell dialysed with peptide-A. I-V data were measured when the size of the currents had stabilized. Control and peptide-A-dialysed cells were derived from the same batch of cells. Data are representative of 3–4 similar observations. (C) Steady-state inactivation of calcium channels. Steady-state inactivation curves were derived by using a double pulse protocol as described in the legend for Figure 1. Data are means±s.e.means of three separate experiments.
Effect of tyrosine kinase inhibitors on purified pp60c-src tyrosine kinase activity
The results with the putative src-selective inhibitors, peptide-A and PP1 suggested that endogenous pp60c-Src might be involved in the effects of the tyrosine kinase inhibitors in these cells. The presence of pp60s-Src in cell lysates was demonstrated by Western blot analysis with a specific anti-Src antibody (Mab 327) (Figure 6).
Figure 6.
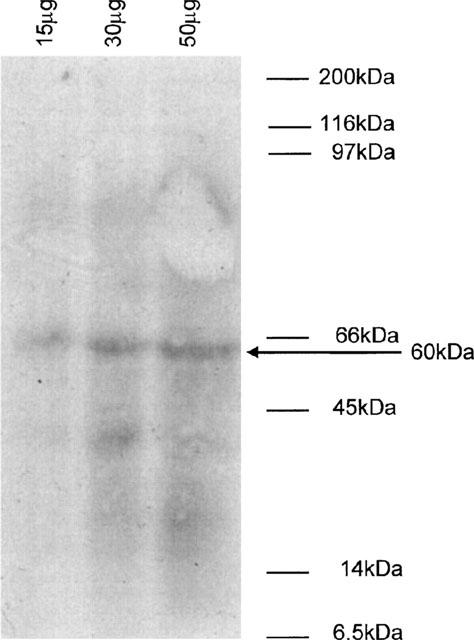
Detection of pp60c-src by Western blot analysis of rabbit ear artery cell lysates. Cell lysates were prepared from freshly isolated rabbit ear artery cells as described in the Methods. 15, 30 and 50 μg of protein were run on a 10% polyacrylamide gel and immunoblotted with an anti-c-Src antibody (Mab327, 2.5 μg ml−1). Proteins were detected using an HRP-linked secondary antibody followed by enhanced chemiluminescence. Figure shown is representative of three separate experiments. The position of the molecular weight markers are shown on the right. The band marked with an arrow corresponds to a protein with an approximate molecular weight of 60 kDa identified by the anti-c-Src antibody.
The activity of the inhibitors on purified pp60c-src tyrosine kinase activity was then examined. All the inhibitors inhibited pp60c-src tyrosine kinase activity in a concentration-dependent manner. The calculated −log potencies derived from these studies were comparable to their potency as inhibitors of IBa and are shown in Table 1.
Discussion
Previous studies using similar experimental conditions to those in the current study, showed that IBa in rabbit ear artery cells, is carried almost exclusively by L-type calcium channels (Wijetunge et al., 1998) and hence the effects of the tyrosine kinase inhibitors on IBa reflect actions on this channel type. In these experiments a range of structurally different protein tyrosine kinase inhibitors were shown to inhibit IBa in rabbit ear artery smooth muscle cells in a concentration-dependent manner. The same agents also inhibited c-src activity with very similar potency and this is taken as evidence implicating inhibition of c-src in the effects of the inhibitors on IBa.
In comparing the potency of the inhibitors used in this study with those reported in the literature, it should be noted that both the electrophysiological studies and kinase assays were conducted using millimolar concentrations of ATP. The concentration of ATP used in assays is known to influence the apparent potency of inhibitors (Niu & Lawrence, 1997) and the low concentrations of ATP used in some studies may account for reported differences in potency. The potency of genistein found in these studies is similar to that reported to inhibit calcium channel currents in rat portal vein (Liu et al., 1997), rat ventricular cells (Yokoshiki et al., 1995) and myometrial cells (Kusaka & Sperelakis, 1995). In contrast, daidzein, a structurally similar compound, which does not inhibit tyrosine kinases, had no effect on IBa in a previous study of rabbit ear artery cells (Wijetunge et al., 1992). The potency of PP1 is of particular interest, since this agent has been reported to be selective for src-family kinases, particularly Lck, Fyn and Hck (Hanke et al., 1996). Lck and Hck are reported to have relatively restricted distribution (Thomas & Brugge, 1997), however Fyn has been reported to be present in rat smooth muscle in one study (Elberg et al., 1995). The relatively low potency of PP1 (∼1 μM) is inconsistent with Fyn contributing to modulation of L-type calcium channels in ear artery cells, and is in keeping with our and previous (Hanke et al., 1996) estimates of the potency of PP1 against c-src activity.
It is also worth noting that tyrphostin-23 and genistein, which are both considered relatively non-selective inhibitors of tyrosine kinases, have relatively low potency as inhibitors of c-src compared with their reported potency against receptor tyrosine kinases such as EGFR (Levitzki & Gilon, 1991). This emphasises that even ‘non-selective' inhibitors of tyrosine kinases may display varying potency depending on the tyrosine kinase studied and/or the assay condition.
Overall, these data provide strong evidence for c-src being responsible for modulation of L-type calcium channels in vascular smooth muscle, particularly when taken in conjunction with previous observations which have shown that IBa is increased by intracellular application of purified c-src (Wijetunge & Hughes, 1995), or a peptide that activates endogenous c-src (Wijetunge & Hughes, 1996). The idea that c-src may regulate L-type calcium channels receives further support from the recent observation that c-src can be found in immunoprecipitates of the α1c subunit of the L-type calcium channel isolated from colonic smooth muscle (Hu et al., 1998). Hence there is both functional and biochemical evidence closely linking c-src to the pore-forming α1c subunit of the L-type calcium channel.
Precisely how c-src modulates the L-type channel is uncertain. Single channel studies in inside-out patches isolated from rat portal vein cells (Liu & Sperelakis, 1997) have demonstrated that genistein reduces the open probability of L-type channels without altering unit amplitude or slope conductance. At present the effects of other inhibitors or activators have not been studied at the single channel level. The data presented here indicate that all tyrosine kinase inhibitors shift the steady-state inactivation relationship to more hyperpolarized potentials without affecting activation parameters. Although there was no statistically significant effect of any inhibitor on the apparent reversal potential considering all concentrations, high concentrations of tyrphostin23 and genistein did appear to affect the apparent reversal potential. If this is an effect it seems likely that this represents a non-specific action related to the high concentration of inhibitor used. A similar effect of genistein on steady-state inactivation of calcium channel current has also been reported in other cells types (Yokoshiki et al., 1995; Liu et al., 1997). These data could be taken to implicate c-src in modulating the inactivation process of L-type channels and imply a possible role for tyrosine phosphorylation/dephosphorylation in L-type calcium channel inactivation. Further studies are required to explore this possibility. Several recent studies have suggested that c-src can influence gating of a variety of ion channels (Fischer & Machen, 1996; Kohr & Seeburg, 1996; Fadool et al., 1997; Fitzgerald & Dolphin 1997), and so this kinase may represent a ubiquitous modulator of ion channels in many cell types.
In conclusion these data show a close correspondence between the potency of a range of tyrosine kinase inhibitors on IBa and c-src activity and provide further evidence linking c-src to L-type calcium channel opening in smooth muscle.
Acknowledgments
This work was supported by the Wellcome Trust.
Abbreviations
- BSA
bovine serum albumin
- [Ca2+]i
intracellular calcium concentration
- c-src
pp60src kinase
- DMSO
dimethyl sulphoxide
- ECL
enhanced chemiluminescence
- EGF
epidermal growth factor
- ELISA
enzyme linked immunosorbent assay
- g
maximum conductance
- HRP
horseradish peroxidase
- IBa
voltage-operated calcium channel currents
- Inon
non-inactivating component of current
- I-V
current-voltage
- k
slope factor
- Mab
monoclonal antibody
- PDGF
platelet-derived growth factor
- PMSF
phenylmethylsulphonylfluoride
- PP1
(4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine)
- PSS
modified physiological salt solution
- PTK
protein tyrosine kinase
- SDS–PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- TBS
Tris buffered saline
- TBS
Tris buffered saline containing 0.001% Tween 20
- TEA
tetraethylammonium
- V
membrane potential
- Vact
membrane potential for half maximal activation
- Vinact
membrane potential for half maximal inactivation
- Vr
reversal potential
References
- AARONSON P.I., BOLTON T.B., LANG R.J., MACKENZIE I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal calcium and high barium solutions. J. Physiol. 1988;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABEDI H., ZACHARY I. Signalling mechanisms in the regulation of vascular cell migration. Cardiovasc. Res. 1995;30:544–556. [PubMed] [Google Scholar]
- CATALDI M., TAGLIALATELA M., GUERRIERO S., AMOROSO S., LOMBARDI G., DI RENZO G., ANNUNZIATO L. Protein-tyrosine kinases activate while protein-tyrosine phosphatases inhibit L-type calcium channel activity in pituitary GH3 cells. J. Biol. Chem. 1996;271:9441–9446. doi: 10.1074/jbc.271.16.9441. [DOI] [PubMed] [Google Scholar]
- DI SALVO J., NELSON S.R., KAPLAN N. Protein tyrosine phosphorylation in smooth muscle: A potential coupling mechanism between receptor activation and intracellular calcium. Soc. Exp. Biol. Med. 1997;214:285–301. doi: 10.3181/00379727-214-44097. [DOI] [PubMed] [Google Scholar]
- DILIBERTO P.A., HUBBERT T., HERMAN B. Early PDGF-induced alterations in cytosolic free calcium are required for mitogenesis. Res. Commun. Chem. Path. Pharmacol. 1991;72:3–12. [PubMed] [Google Scholar]
- ELBERG G., LI J., LEIBOVITCH A., SHECHTER Y. Non-receptor cytosolic tyrosine kinases from various rat tissues. Biochim. Biophys. Acta. 1995;1269:299–306. doi: 10.1016/0167-4889(95)00124-8. [DOI] [PubMed] [Google Scholar]
- ESTACION M., MORDAN L.J. Expression of voltage-gated calcium channels correlates with PDGF stimulated calcium influx and depends upon cell density in C3H 10t1/2 mouse fibroblasts. Cell Calcium. 1993;14:161–171. doi: 10.1016/0143-4160(93)90085-k. [DOI] [PubMed] [Google Scholar]
- FADOOL D.A., HOLMES T.C., BERMAN K., DAGAN D., LEVITAN I.B. Tyrosine phosphorylation modulates current amplitude and kinetics of a neuronal voltage-gated potassium channel. J. Neurophysiol. 1997;78:1563–1573. doi: 10.1152/jn.1997.78.3.1563. [DOI] [PubMed] [Google Scholar]
- FISCHER H., MACHEN T.E. The tyrosine kinase p60c-src regulates the fast gate of the cystic fibrosis transmembrane conductance regulator chloride channel. Biophys. J. 1996;71:3073–3082. doi: 10.1016/S0006-3495(96)79501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD E.M., DOLPHIN A.C. Regulation of rat neuronal voltage-dependent calcium channels by endogenous p21-ras. Eur. J. Neurosci. 1997;9:1252–1261. doi: 10.1111/j.1460-9568.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch clamp techniques for high resolution current recordings from cells and cell free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HANKE J.H., GARDNER J.P., DOW R.L., CHANGELIAN P.S., BRISSETTE W.H., WERINGER E.J., POLLOK B.A., CONNELLY P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck and Fyn-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- HU X.Q., SINGH N., MUKHOPADHYAY D., AKBARALI H.I. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J. Biol. Chem. 1998;27:5337–5342. doi: 10.1074/jbc.273.9.5337. [DOI] [PubMed] [Google Scholar]
- HUGHES A.D. Increase in tone and intracellular Ca2+ in rabbit isolated ear artery by platelet-derived growth factor. Br. J. Pharmacol. 1995a;114:138–142. doi: 10.1111/j.1476-5381.1995.tb14917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES A.D. Calcium channels in vascular smooth muscle cells. J. Vasc. Res. 1995b;32:353–370. doi: 10.1159/000159111. [DOI] [PubMed] [Google Scholar]
- HUGHES A.D., WIJETUNGE S. The action of amlodipine on voltage-operated calcium channels in vascular smooth muscle. Br. J. Pharmacol. 1993;109:120–125. doi: 10.1111/j.1476-5381.1993.tb13540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES A.D., WIJETUNGE S. Role of tyrosine phosphorylation in excitation-contraction coupling in vascular smooth muscle. Acta. Physiol. Scand. 1998;164:457–469. doi: 10.1046/j.1365-201X.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- KOHR G., SEEBURG P.H. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J. Physiol. Lond. 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSAKA M., SPERELAKIS N. Inhibition of L-type calcium current by genistein, a tyrosine kinase inhibitor, in pregnant rat myometrial cells. Biochemica et Biophysica Acta. 1995;1240:196–200. doi: 10.1016/0005-2736(95)00191-3. [DOI] [PubMed] [Google Scholar]
- LEVITZKI A., GILON C. Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol. Sci. 1991;12:171–174. doi: 10.1016/0165-6147(91)90538-4. [DOI] [PubMed] [Google Scholar]
- LIU H., SPERELAKIS N. Tyrosine kinases modulate the activity of single L-type calcium channels in vascular smooth muscle cells from rat portal vein. Can. J. Physiol. Pharmacol. 1997;75:1063–1068. [PubMed] [Google Scholar]
- LIU H., LI K., SPERELAKIS N. Tyrosine kinase inhibitor, genistein, inhibits macroscopic L-type calcium currents in rat portal vein smooth muscle cells. Can. J. Physiol. Pharmacol. 1997;75:1058–1062. doi: 10.1139/cjpp-75-9-1058. [DOI] [PubMed] [Google Scholar]
- NIU J., LAWRENCE D.S. L-Dopa: a powerful nonphosphorylatable tyrosine mimetic for pp60c-src. J. Am. Chem. Soc. 1997;119:3844–3845. [Google Scholar]
- NOMOTO A., MUTOH S., HAGIHARA H., YAMAGUCHI I. Smooth muscle cell migration induced by inflammatory cell products and its inhibition by a potent calcium antagonist, nilvadipine. Atherosclerosis. 1988;72:213–219. doi: 10.1016/0021-9150(88)90083-4. [DOI] [PubMed] [Google Scholar]
- SATO K., MIKI S., TACHIBANA H., HAYASHI F., AKIYAMA T., FUKAMI Y. A synthetic peptide corresponding to residues 137 to 157 of p60v-src inhibits tyrosine-specific protein kinases. Biochem. Biophys. Res. Commun. 1990;171:1152–1159. doi: 10.1016/0006-291x(90)90805-w. [DOI] [PubMed] [Google Scholar]
- SCHLAEPFER D.D., HANKS S.K., HUNTER T., VAN-DER-GEER P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER J., ULLRICH A. Growth factor signalling by receptor tyrosine kinases. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- THOMAS S.M., BRUGGE J.S. Cellular functions regulated by src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- TSUDA T., KAWAHARA Y., SHI K., KOIDE M., ISHIDO Y., YOKOYAMA M. Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS. Lett. 1991;285:44–48. doi: 10.1016/0014-5793(91)80721-e. [DOI] [PubMed] [Google Scholar]
- WIJETUNGE S., AALKJAER C., SCHACHTER M., HUGHES A.D. Tyrosine kinase inhibitors block calcium channel currents in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1992;189:1620–1623. doi: 10.1016/0006-291x(92)90262-j. [DOI] [PubMed] [Google Scholar]
- WIJETUNGE S., LYMN J., HUGHES A.D. Effect of inhibition of tyrosine phosphatases on voltage-operated calcium channel currents in rabbit isolated ear artery cells. Br. J. Pharmacol. 1998;124:307–316. doi: 10.1038/sj.bjp.0701840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIJETUNGE S., HUGHES A.D. pp60src increases voltage-operated calcium channel currents in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1995;217:1039–1044. doi: 10.1006/bbrc.1995.2874. [DOI] [PubMed] [Google Scholar]
- WIJETUNGE S., HUGHES A.D. Activation of endogenous c-src or a related tyrosine kinase by intracellular (pY)EEI peptide increases voltage-operated calcium channel currents in rabbit ear artery cells. FEBS Lett. 1996;399:63–66. doi: 10.1016/s0014-5793(96)01177-5. [DOI] [PubMed] [Google Scholar]
- YOKOSHIKI H., SUMII K., SPERELAKIS N. Inhibition of L-type calcium current in rat ventricular cells by the tyrosine kinase inhibitor, genistein and its inactive analogue diadzein. J. Mol. Cell. Cardiol. 1995;28:807–814. doi: 10.1006/jmcc.1996.0075. [DOI] [PubMed] [Google Scholar]


