Abstract
Endogenous L-DOPA may act as a neuromodulator contributing to the production of motor activity. We now investigate the effects of the centrally acting aromatic amino acid dopa decarboxylase (AADC) inhibitor NSD-1015 (3-hydroxybenzyl hydrazine) on the motor actions of L-DOPA and dopamine agonist drugs in MPTP treated common marmosets.
Pretreatment with NSD-1015 (10–50 mg kg−1; i.p.) worsened baseline motor deficits in MPTP-treated common marmosets. Similarly, it abolished L-DOPA (5–18 mg kg−1 s.c.) induced locomotor activity and reversal of disability. NSD-1015 pretreatment inhibited dopamine formation and elevated L-DOPA levels in plasma.
The increase in locomotor activity and improvement in disability produced by the administration of the D-1 agonist A-86929 (0.03–0.04 mg kg−1 s.c.) or the D-2 agonist quinpirole (0.05–0.3 mg kg−1 i.p.) was abolished by NSD-1015 (25 mg kg−1 i.p.) pretreatment. While the effects of a low dose combination of A-86929 (0.04 mg kg−1 s.c.) and quinpirole (0.05 mg kg−1 i.p.) were inhibited by NSD-1015 (25 mg kg−1 i.p.), there was little effect on the action of a high dose combination of these drugs (0.08 mg kg−1 A-86929 and 0.1 mg kg−1 quinpirole).
Following central AADC inhibition with NSD-1015 (25 mg kg−1 i.p.), locomotor behaviour induced by administration of high dose combinations of A-86929 (0.08 mg kg−1 s.c.) and quinpirole (0.1 mg kg−1 i.p.) was unaffected by L-DOPA (5 mg kg−1 s.c.) pretreatment.
These results do not support a role for endogenous L-DOPA in spontaneous or drug induced locomotor activity. Rather, they strengthen the argument for the importance of endogenous dopaminergic tone in the motor actions of dopamine agonists.
Keywords: MPTP, marmosets, central aromatic amino acid DOPA decarboxylase, L-DOPA, dopamine agonists, antiparkinsonian activity
Introduction
The primary deficiency of caudate-putamen dopamine content in Parkinson's disease (Bernheimer et al., 1963; 1965; Ehringer, 1960) forms the basis of the use of L-DOPA or dopamine agonist drugs in current therapy. However, L-DOPA is more effective than dopamine agonists in reversing motor symptoms in the later stages of Parkinson's disease (Poewe et al., 1998; Rascol, 1996; Rascol et al., 1998) and this difference raises important issues concerning the mechanisms underlying drug action.
Dopamine acts via at least five receptors, which have been classified into two major families as D-1-like (D-1 and D-5) and D-2-like (D-2, D-3, and D-4) receptors (Kebabian & Calne, 1979). The role of D-3, D-4, and D-5 receptors in motor function remains unclear, thus, only the effects of D-1 and D-2-like receptors will be considered. If decarboxylation of L-DOPA to dopamine and the subsequent stimulation of postsynaptic D-1 and D-2-like dopamine receptors is responsible for antiparkinsonian activity, then dopamine agonists should produce equivalent effects. However, most dopamine agonists used to treat Parkinson's disease (for example, bromocriptine, ropinirole and pramipexole) predominantly stimulate the D-2-like receptor family while, presumably, dopamine formed from L-DOPA occupies both D-1 and D-2 sites (Eden et al., 1991; Keyser et al., 1995; Mierau, 1995; Mierau et al., 1995; Trugman et al., 1991). Importantly, the ability of D-1 and D-2 selective dopamine agonists to produce increased motor activity is dependent on endogenous dopaminergic tone to induce activation of the other dopamine receptor population (Dziewczapolski et al., 1997; Treseder et al., in preparation). Thus, inhibition of tyrosine hydroxylase in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated primates reduces the ability of D-1 and D-2 agonists to reverse motor deficits (Gomez-Mancilla & Bedard, 1991; Treseder et al., 1998). In contrast, the combined administration of D-1 and D-2 agonists or the use of non-selective D-1/D-2 agonists, such as apomorphine or pergolide, produce motor activation which is not abolished by tyrosine hydroxylase inhibition (Gomez-Mancilla & Bedard, 1991; Treseder et al., in preparation). The immediate interpretation is that endogenous dopamine plays an important role in motor activation produced by dopamine agonists and that drug effect declines with the reduction in dopaminergic tone occurring as a result of disease progression.
However, inhibition of tyrosine hydroxylase also inhibits the formation of endogenous L-DOPA (Nakamura et al., 1992), which may act as a neuromodulator to alter motor behaviours. A transmitter role for L-DOPA is supported by its ability to facilitate the release of dopamine and noradrenaline via presynaptic β-adrenoceptors in vitro (Goshima et al., 1990) and to stimulate glutamate and GABA release from rat striatal slices (Aceves et al., 1991; Goshima et al., 1993). L-DOPA also releases glutamate from the striatum of conscious rats (Misu et al., 1995). Although a recognition site or receptor for L-DOPA has not been identified, L-DOPA methyl ester competitively antagonizes the facilitatory effect of L-DOPA on noradrenaline release in rat hypothalamic slices (Goshima et al., 1990). These actions of L-DOPA may be of functional significance, since increased accumulation of endogenous L-DOPA, produced by blockade of central AADC activity using NSD-1015, was reported to potentiate locomotor activity produced by the D-2 agonist quinpirole in normal rats (Yue et al., 1994). In contrast, inhibition of L-DOPA synthesis with AMPT inhibits dopamine agonist-induced locomotion (Dziewczapolski et al., 1997; Gomez-Mancilla & Bedard, 1991; Reavill et al., 1983; Treseder et al., 1998). Furthermore, a sub-threshold dose of L-DOPA potentiated quinpirole-induced locomotor activity following NSD-1015 treatment of both normal and 6-OHDA lesioned rats (Nakamura et al., 1994).
These data suggest a role for endogenous L-DOPA both in the initiation of motor activity and in the actions of dopamine agonist drugs, so explaining why L-DOPA may be more effective in treating Parkinson's disease than dopamine agonists. For this reason, we now explore the involvement of L-DOPA in the actions of the selective D-1 and D-2 dopamine agonists A-86929 and quinpirole in reversing MPTP-induced motor deficits in primates utilizing blockade of central AADC activity by NSD-1015.
Methods
Animals
Adult common marmosets (Callithrix jachus; n=18) of either sex weighing between 300–450 g were used in this study. The animals were housed either alone or in pairs under standard conditions at a temperature of 27°C (±1°C) and 50% relative humidity using a 12 h light-dark circle (light on from 06:00 to 18:00 h). The animals were fed fresh fruit and Mazuri marmoset jelly once daily and had free access to food pellets and water. All procedures were carried out according to current U.K. legislation (Animals Act, 1986).
MPTP treatment
Nigral cell degeneration was induced by subcutaneous injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP) 2 mg kg−1 dissolved in sterile 0.9% saline w v−1 administered once daily for 5 consecutive days. This treatment induces persistent motor dysfunction, which remains stable for 12–18 months and induces akinesia, bradykinesia, postural instability, a loss of vocalization, and in some animals intermittent postural and action tremor (Jenner et al., 1984). After MPTP treatment, the animals made a gradual recovery from the acute effects of MPTP over the following 6–8 weeks. During the treatment and throughout the following weeks the animals were hand fed with Mazuri marmoset jelly and fresh fruit puree until they were able to maintain themselves. At the time of study, all animals displayed a marked reduction in basal locomotor activity, hunched and rigid posture, and a decrease in checking movements of the head.
Drug treatments
Animals were randomized into four groups of 4–6 animals. In group one the behavioural effects of a range of doses of NSD-1015 were investigated. NSD-1015 (10–50 mg kg−1 i.p.) or vehicle was administered and animals were placed in activity cages. Motor behaviour was monitored for 6 h and locomotor activity recorded for 8 h. In a second group (n=6), NSD-1015 (25 mg kg−1 i.p.) or vehicle was administered to a group of six animals. After 45 min, a standard dose of L-DOPA methyl ester (15.6 mg kg−1 equivalent to 12.5 mg kg−1 L-DOPA s.c.) was administered and then after 90 min, 0.5 ml of blood was removed from the femoral vein. The plasma was analysed using HPLC with electrochemical detection for levels of L-DOPA, dopamine, 3-O-methyl-dopa (3-OMD), and DOPAC (Rose et al., 1991).
In all subsequent experiments NSD-1015 (25 mg kg−1 i.p.) or vehicle was administered 90 min prior to the injection of: (1) L-DOPA methyl ester (6.25–22.5 mg kg−1 equivalent to 5–18 mg kg−1 of L-DOPA) or vehicle (group 1, n=4). Carbidopa (12.5 mg kg−1 p.o.) was administered 45 min prior to L-DOPA methyl ester or vehicle. In order to determine whether the peripheral decarboxylase inhibitor carbidopa was interfering with the actions of NSD-1015, the experiment was repeated in the absence of carbidopa in those animals receiving NSD-1015. In this case saline vehicle was administered 45 min following NSD-1015 (25 mg kg−1 i.p.) pretreatment; (2) Quinpirole (0.05–0.3 mg kg−1 i.p.) or its vehicle. NSD-1015 (25 mg kg−1 i.p.) or vehicle (group 3, n=4); (3) A-86929 (0.03–0.04 mg kg−1 s.c.) or vehicle (group 4, n=4). Subsequently, these animals received NSD-1015 (25 mg kg−1 i.p.) or vehicle pre-treatment 90 min prior to combinations of quinpirole (0.05 or 0.1 mg kg−1 i.p.) with A-86929 (0.04 or 0.08 mg kg−1 s.c.). Finally, these four animals received NSD-1015 (25 mg kg−1 i.p.) pre-treatment 60 min prior to L-DOPA methyl ester (6.25 mg kg−1 s.c. equivalent to 5 mg kg−1 of L-DOPA). At 90 min A-86929 (0.08 mg kg−1 s.c.) and quinpirole (0.1 mg kg−1 i.p.) were administered in combination.
All experiments were carried out using a Latin square style protocol. A 5 to 7 day recovery period was allowed between experiments.
Measurement of locomotor activity
Locomotor activity was measured simultaneously in four identical aluminium cages (50×60×70 cm) with clear perspex doors. These were equipped with eight horizontally oriented sets of infrared photocells. The number of light beam interruptions due to the animal's movements were accumulated in 10 min intervals and recorded using a Viglen 4DX33 computer. The animals were allowed to acclimatize to the test cage for a minimum of 1 h prior to drug treatment.
Behavioural observations
In parallel to locomotor activity recording, motor behaviour was rated qualitatively through a one-way mirror by trained observers who were blinded with respect to treatment protocol. The degree of motor dysfunction was assessed using a visual disability grading system ranging from 0 to 18, where 0 is normal and 18 is severely disabled. The grading system is scored as follows: alertness (0=normal, 1=reduced, 2=sleepy); head checking movements (0=normal, 1-reduced, 2-absent); posture (0=normal, 1-abnormal trunk, 2=abnormal limbs, 3=abnormal tail, 4=grossly abnormal); balance/co-ordination (0=normal; 1=impaired; 2=unstable; 3=falls); reaction (0=normal, 1=reduced, 2=slow, 3=absent), vocalization (0=normal, 1=reduced, 2=absent). Four animals were observed simultaneously and at the end of each 10 min interval a disability score was recorded for that interval. When assessing the effects of NSD-1015 alone on disability, animals were scored only for the last 10 min of each 30 min interval due to the long duration of this experiment. In all other experiments the animals were monitored continuously.
When assessing the affect of NSD-1015 pretreatment on the motor behaviour induced by L-DOPA, A-86929, and quinpirole locomotor counts were recorded for 12 h and behavioural observations were rated for 4.5 h. Due to the varying time courses of the treatments total locomotor activity and total disability scores were accumulated over a 2 h period for all treatments to enable comparisons and statistical analysis.
Analysis of L-DOPA, dopamine, 3-OMD, and DOPAC content in plasma
Samples of plasma were analysed using high pressure liquid chromatography with electrochemical detection (h.p.l.c./ECD) using the method as previously described (Rose et al., 1988). The chromatography system consisted of a Waters model 510 pump operating at a flow rate of 1 ml min−1, a Spherisorb reverse phase C18 ODS-2 column (25 cm×4.6 mm, particle size 5 μm; Waters U.K.), and an Intro electrochemical detector (Antec, The Netherlands) set to a potential of +0.8 V vs Ag AgCl−1. The mobile phase consisted of 0.1 M sodium phosphate buffer (pH 2.9), 0.1 mM EDTA, 0.74 nM octyl sulphonic acid, and 13% methanol w v−1. All chromatography was carried out at 32.5°C. The detector output was connected to a Unicam 4880 chromatography data handling system (ATI Unicam, U.K.) and samples were quantified based on peak heights compared with standards.
Drugs
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride; Research Biochemicals Inc., U.S.A.) was dissolved in 0.9% w v−1 saline. NSD-1015 (3-hydroxybenzylhydrazine; Aldrich, U.K.) was dissolved in 0.1 M phosphate buffered saline (pH 7.4). Quinpirole hydrochloride (Eli Lilly, U.S.A.) was dissolved in 0.9% w v−1 saline. (−)-trans-9,10-hydroxy-2-propyl-4,5,5a,6,7,11b-hexahydro-3-thia-5-azacyclopent -1- ena [c]phenanthrene hydrochloride (A-86929; Abbott Laboratories, U.S.A.) was dissolved in a few drops of sterile water and then made up to volume in sterile 0.9% w v−1 saline. α-methyl-dopa hydrazine (carbidopa) (Merk, Sharp and Dohme, U.S.A.) was suspended in 0.1% phosphate buffered saline (pH 7.4) for i.p. administration, and suspended in 10% sucrose solution for oral administration. Due to the poor water solubility of L-DOPA, L-DOPA methyl ester was employed. In vivo this compound is rapidly and completely hydrolyzed to L-DOPA in plasma (Brunnerguenat et al., 1995; Stocchi et al., 1992) although in vitro L-DOPA methyl ester acts as a competitive antagonist to the actions of L-DOPA. L-DOPA methyl ester (Chiesi Pharmaceutici, Italy) was dissolved in 0.9% w v−1 saline.
Statistical analysis
The locomotor counts and disability scores were accumulated over the test period, after a given drug administration and were averaged for all animals. The effects of NSD-1015 on motor behaviour were analysed using the Kruskal Wallis one-way ANOVA followed by the Mann–Whitney U-test. All other dose-response relationships were initially analysed by repeated measures two-way ANOVA. If the resulting F value was associated with a probability of less than 5%, a further analysis of data was performed using the Kruskal Wallis one-way ANOVA or Mann–Whitney U-test as appropriate. A students t-test was used to compare plasma levels of L-DOPA, dopamine, 3-OMD and DOPAC.
Results
Effect of NSD-1015 on motor activity
Administration of NSD-1015 (10–50 mg kg−1) to MPTP treated common marmosets produced a dose dependent decrease in locomotor activity (P<0.05, KW=8.8, d.f.=3; P<0.05 at 50 mg kg−1 Mann–Whitney U, Figure 1) lasting for at least 8 h. Motor disability was also dose dependently increased (P<0.05, KW=9.4, d.f.=3; P<0.05 at 25 and 50 mg kg−1 Mann–Whitney U) and marked akinesia, rigidity and loss of balance were observed. At 50 mg kg−1 NSD-1015 induced vomiting in all animals lasting for several hours and therefore a dose of 25 mg kg−1 was chosen for use in further experiments.
Figure 1.
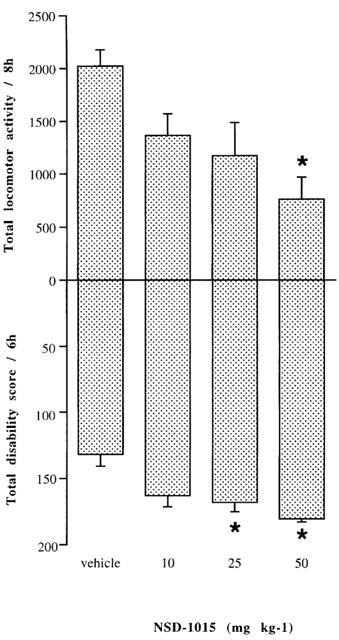
Effects of NSD-1015 (10, 25 or 50 mg kg−1 s.c.) or vehicle (v) on mean total locomotor counts over an 8 h period, and on mean total disability scores recorded over 6 h in MPTP-lesioned marmosets. The results are shown as the mean±s.e.mean for four animals. NSD-1015 50 mg kg−1 significantly decreased total locomotor counts (*P<0.05) vs saline vehicle and 25 and 50 mg kg−1 significantly increased mean total disability scores (P<0.05) vs saline vehicle.
Effect of NSD-1015 pretreatment on plasma levels of L-DOPA, 3-OMD, dopamine and DOPAC induced by L-DOPA administration
Pretreatment with NSD-1015 (25 mg kg−1) increased plasma L-DOPA (P<0.01, t=4.4, d.f.=5) and 3-OMD (P<0.01, t=5.6, d.f.=5) concentrations. At 90 min following L-DOPA administration levels of L-DOPA and 3-OMD had more than doubled compared to those in vehicle pretreated animals (Figure 2A). Dopamine was detectable in plasma 90 min after L-DOPA administration but in animals pretreated with NSD-1015 plasma levels of dopamine were markedly decreased and dopamine was detected in the plasma of only one animal (P<0.05, t=3.0, d.f.=5, Figure 2B). NSD-1015 (25 mg kg−1) pretreatment reduced the plasma DOPAC content, with an approximately 90% reduction occurring at 90 min following L-DOPA administration compared to vehicle pretreatment (P<0.01, t=3.9, d.f.=5).
Figure 2.
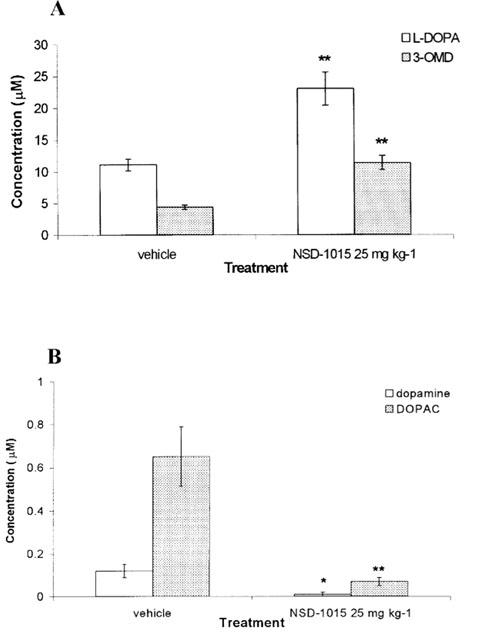
Effect of NSD-1015 (25 mg kg−1 i.p.) pretreatment on plasma levels of (A) L-DOPA and 3-OMD, and (B) dopamine, and DOPAC, following the administration of L-DOPA (12.5 mg kg−1 s.c.). NSD-1015 was administered 45 min prior to L-DOPA. A blood sample was taken after a further 45 min following L-DOPA treatment. The results are shown as mean±s.e.mean for six animals. NSD-1015 25 mg kg−1 significantly increased plasma L-DOPA and 3-OMD concentrations (**P<0.01) vs saline vehicle and significantly decreased plasma dopamine and DOPAC (*P<0.05 and **P<0.01 respectively) vs saline vehicle.
Effect of NSD-1015 on L-DOPA induced motor behaviour
Administration of L-DOPA (5–18 mg kg−1) plus carbidopa (12.5 mg kg−1) dose dependently increased locomotor activity in MPTP-lesioned marmosets (P<0.05, KW=8.5, d.f.=3, Figure 3A,B). The reversal of motor deficits was maximal 0.5–1 h after L-DOPA administration. In addition, motor disability was markedly reversed (P<0.05, KW=10.4, d.f.=3, Figure 3A,B). Natural purposeful behaviour was observed at the lowest L-DOPA dose (5 mg kg−1), although at higher doses hyperactivity/stereotyped behaviour and fleeting dyskinesias were observed.
Figure 3.
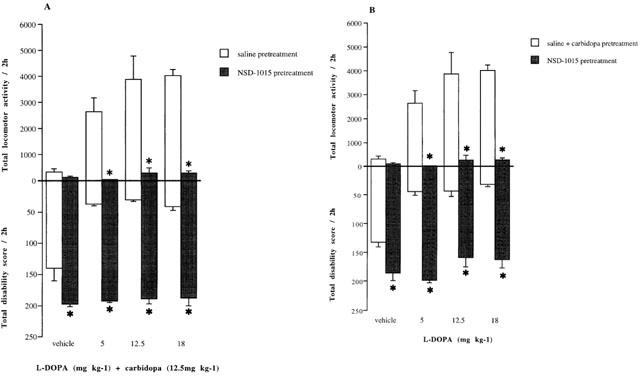
(A) The effects of NSD 1015 (25 mg kg−1 i.p.) or vehicle pretreatment on locomotor activity and disability scores induced by L-DOPA (5–18 mg kg−1 s.c.) and carbidopa (12.5 mg kg−1 i.p.) accumulated over 2 h (n=4) in MPTP-lesioned marmosets. (B) The effects of NSD-1015 (25 mg kg−1 i.p.), in the absence of carbidopa on locomotor activity and disability scores induced by L-DOPA (5–18 mg kg−1 s.c.) accumulated over 2 h (n=4) in MPTP-lesioned marmosets. The data are expressed as mean±s.e.mean *P<0.05 vs saline pretreatment group.
Pretreatment with NSD-1015 (25 mg kg−1) markedly attenuated locomotor activity induced by L-DOPA (5–18 mg kg−1) plus carbidopa (12 mg kg−1). NSD-1015 pretreatment also markedly inhibited L-DOPA plus carbidopa-induced reversal of motor disability compared to saline treated animals. (P<0.05, Mann–Whitney U, Figure 3A). Furthermore, administration of L-DOPA (5–18 mg kg−1) without carbidopa but with NSD-1015 (25 mg kg−1) pretreatment produced no increase in locomotor activity or reversal of motor deficits (P<0.05, Mann–Whitney U, Figure 3B).
Effect of NSD-1015 on A-86929 induced motor behaviour
Administration of A-86929 (0.03 or 0.04 mg kg−1) increased locomotor activity compared to vehicle treated animals (P<0.05, KW=8.1, d.f.=3, Figure 4A). The onset of activity occurred within 10–20 min of drug administration and lasted approximately 2 h (data not shown). Similarly, A-86929 (0.03 or 0.04 mg kg−1) produced a comparable decrease in motor disability (P<0.05, KW=7.5, d.f.=3, Figure 4A). Motor behaviour produced by A-86929 was naturalistic and lacked stereotypies and dyskinesias and resembled that of normal marmosets.
Figure 4.
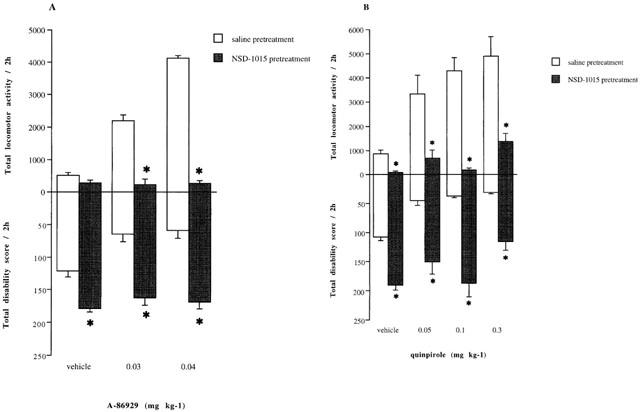
(A) The effects of NSD-1015 (25 mg kg−1 i.p.) or vehicle on locomotor activity and disability scores induced by A-86929 (0.03–0.04 mg kg−1 s.c.) accumulated over 2 h (n=4) in MPTP-lesioned marmosets. (B) The effects of NSD-1015 (25 mg kg−1 i.p.) or saline pretreatment on locomotor activity and disability scores induced by quinpirole (0.05–0.3 mg kg−1 i.p.) accumulated over 2 h (n=4) in MPTP-lesioned marmosets. The data are expressed as mean±s.e.mean *P<0.05 vs saline pretreatment group.
Pretreatment with NSD-1015 (25 mg kg−1) reduced locomotor activity induced by A-86929 (P<0.05, Mann–Whitney U). The A-86929-induced reversal of motor disability was also inhibited by NSD-1015 pretreatment (P<0.05, Mann–Whitney U, Figure 4A.
Effect of NSD-1015 on quinpirole induced motor behaviour
Administration of quinpirole (0.05–0.3 mg kg−1) to MPTP-lesioned marmosets dose dependently increased locomotor activity (P<0.05, KW=10.2, d.f.=3, P<0.05 at all doses, Figure 4B). Activity was apparent within 2–10 min after drug administration and lasted approximately 2–3 h. In addition, a dose dependent improvement in motor disability was observed compared to vehicle treated animals (P<0.05, KW=10.4, d.f.=3, Figure 4B). At the highest dose of quinpirole, the animals exhibited some stereotyped movements in the form of continuous climbing and attention focusing and transient dyskinesias were observed.
Pretreatment with NSD-1015 markedly inhibited quinpirole- (P<0.05, Mann–Whitney U, 0.05–0.3 mg kg−1) induced locomotor activity compared to vehicle treated animals. Similarly, NSD-1015 pretreatment prevented the reversal of motor disability produced by quinpirole administration (P<0.05, Mann–Whitney, U, Figure 4B).
Effect of NSD-1015 on activity induced by A-86929 and quinpirole administered in combination
Administration of A-86929 (0.03 or 0.08 mg kg−1) in combination with quinpirole (0.05 or 0.1 mg kg−1) produced a dose dependent increase in locomotor activity compared to vehicle treated animals (P<0.05, KW=9.8, d.f.=3, Figure 5A). An equivalent reversal of disability was observed following both low dose and high dose combinations of A-86929 and quinpirole to MPTP-lesioned marmosets (P<0.05, KW=7.4, d.f.=3, Figure 5A). Generally, behaviour was purposeful and stereotypes were not observed. However, at the higher doses of A-86929 and quinpirole in combination mild dyskinesia occurred.
Figure 5.
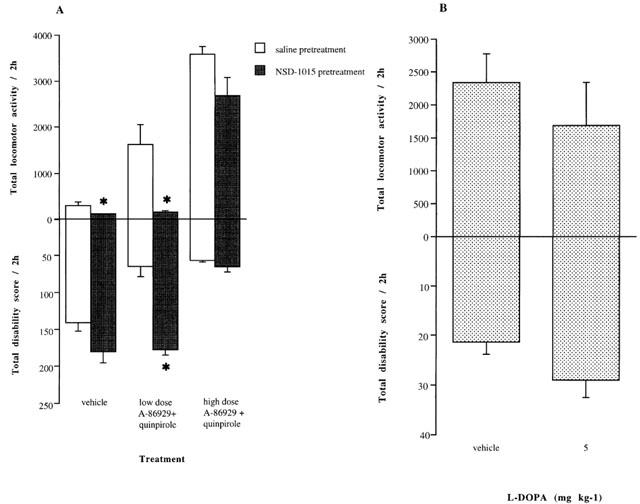
(A) The effects of NSD-1015 (25 mg kg−1 i.p.) or saline pretreatment on locomotor activity and disability scores induced by A-86929 (0.04–0.08 mg kg−1 s.c.) co-administered with quinpirole (0.05–0.1 mg kg−1 i.p.) accumulated over 2 h (n=4) in MPTP-lesioned marmosets. (B) The effects of a subthreshold dose of L-DOPA (5 mg kg−1 s.c.) on locomotor activity and disability scores induced by A-86929 (0.04 mg kg−1 s.c.) and quinpirole (0.1 mg kg−1 i.p.) following inhibition of AADC with NSD-1015 (25 mg kg−1 i.p.) MPTP-lesioned marmosets. NSD-1015 was administered 60 min prior to L-DOPA, and 30 min later A86929 and quinpirole were administered combination. Results are accumulated over 2 h (n=4). The data are expressed as mean±s.e.mean *P<0.05 vs saline pretreatment group.
NSD-1015 pretreatment abolished the reversal of motor disability produced by the low dose agonist combination (P<0.05, Mann–Whitney U, Figure 5A). Locomotor counts were markedly attenuated and disability scores were increased in animals pretreated with NSD-1015 compared to vehicle treated controls. However, NSD-1015 had very little effect on the action of A-86929 and quinpirole administration in combination at the higher dose (0.1 and 0.08 mg kg−1 respectively). Locomotor counts were non-significantly reduced compared to vehicle treated animals. Similarly, motor disability was largely unaffected with only a small increase over the observation period (Figure 5A).
Effect of L-DOPA on activity induced by A-86929 and quinpirole administered in combination
Following NSD-1015 pretreatment, the administration of A-86929 (0.08 mg kg−1) in combination with quinpirole (0.1 mg kg−1) produced an increase in locomotor activity and improvement in behavioural disability, which lasted approximately 2 h. Pretreatment with L-DOPA (5 mg kg−1) had very little effect on locomotor activity (Figure 5B).
Comparison of the effects of NSD-1015 on locomotor activity induced by dopaminergic therapies
The effects of NSD-1015 pretreatment on doses of D-1 and/or D-2 agonists (A-86929 0.04 mg kg−1, quinpirole 0.1 mg kg−1, A-86929 0.08 mg kg−1 plus quinpirole 0.1 mg kg−1 and L-DOPA 5 mg kg−1), which produced an equal, increase locomotor activity and reversal of disability were compared. NSD-1015 had little effect on motor behaviour induced by combinations of D-1 and D-2 agonists whilst severely inhibiting activity produced by the agonists given alone or motor activity produced by L-DOPA (P<0.05, Mann–Whitney U, Figure 6).
Figure 6.
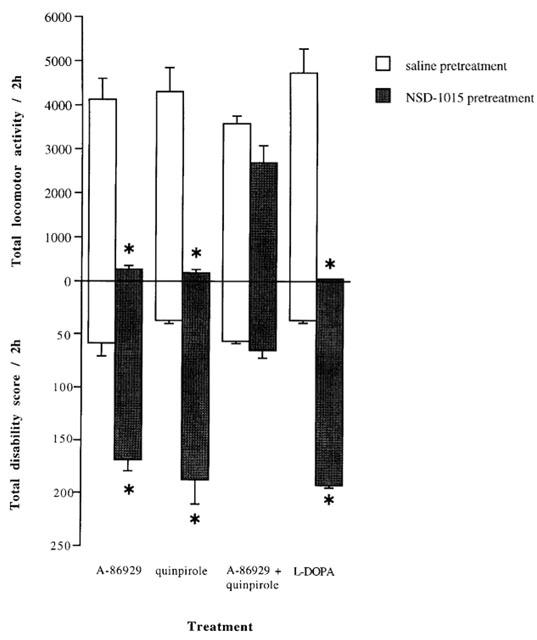
The effects of NSD-1015 (25 mg kg−1 i.p.) or saline pretreatment on locomotor activity and disability scores induced by A-86929 (0.04 mg kg−1 s.c.), quinpirole (0.1 mg kg−1 i.p.), A-86929 (0.08 mg kg−1 s.c.) and quinpirole (0.1 mg kg−1 i.p.), and L-DOPA (5 mg kg−1 s.c.) accumulated over 2 h (n=4) in MPTP-lesioned marmosets. Doses were chosen to give comparable levels of locomotor activity and disability scores. The data are expressed as mean±s.e.mean *P<0.05 vs saline pretreatment group.
Discussion
These studies suggest that L-DOPA itself does not contribute to the motor actions produced by administration of this amino acid and that, contrary to previous work, endogenous L-DOPA does not function to modulate the effects of postsynaptic dopamine receptor occupation (Nakamura et al., 1994; Nakazoto & Akiyama, 1989). Rather, the suppression of the actions of dopamine agonists following central AADC inhibition supports a role for endogenous dopamine in controlling motor activation.
Blockade of central AADC activity in MPTP-lesioned marmosets increased the severity of established motor deficits. This presumably reflects a further reduction in the dopamine content of remaining dopaminergic neurones in the caudate-putamen in MPTP treated animals (Gnanalingham et al., 1995; Ueki et al., 1989). Treatment of MPTP-treated primates, or patients with Parkinson's disease with peripheral AADC inhibitors, such as carbidopa or benserazide, had no effect on motor behaviour (Butcher et al., 1972), whereas NSD-1015 clearly worsened motor deficits. This shows that NSD-1015 must be having a central effect on motor behaviour. The worsening of motor symptoms, which occurred following central AADC inhibition, might have been due to an accumulation of endogenous L-DOPA. However, this would seem unlikely as a similar increase in motor deficits was observed following administration of the tyrosine hydroxylase inhibitor, AMPT (Blanchet et al., 1993; Treseder et al., 1998). Furthermore, these data clearly show that when exogenous L-DOPA is administered following inhibition of central AADC there is no further increase in motor deficit, which would be expected to occur if L-DOPA itself caused a worsening of motor symptoms.
The reduction in motor activity following NSD-1015 pretreatment of MPTP-treated marmosets may also reflect a reduction in dopamine content of areas outside the striatum. For example, the mesolimbic and mesocortical dopaminergic tracts, which are less affected following MPTP-treatment or in idiopathic Parkinson's disease, may also contribute to the genesis of motor activity (Björklund & Lindvall, 1984). These forebrain structures are highly interconnected and the dopamine pathways are seen to provide modulation of widespread limbic- and cortical-striatal circuits involved in motivated behaviour (Jones & Robbins, 1992; Wu et al., 1993; Wu & Brudzynski 1995). The nucleus accumbens has a primary role in the ventral striatal system and intraacumbens injections of quinpirole alters movement initiation in rats (Crescimanno et al., 1998). Importantly, while mesolimbic and mesocortical dopaminergic pathways are relatively spared by MPTP treatment, there is an initial transient but marked reduction in mesolimbic dopamine content, the recovery from which parallels an improvement in motor function (Rose et al., 1989a,1989b). So the effect of NSD-1015 may be to significantly reduce the involvement of these areas by inhibiting dopamine formation.
The administration of L-DOPA following pretreatment with NSD-1015 resulted in an accumulation of L-DOPA in plasma and a concomitant fall in the levels of dopamine and DOPAC, indicating that AADC was effectively inhibited. Furthermore, studies in rats have shown that AADC activity is comparably inhibited both centrally and peripherally following the administration of NSD-1015 (Treseder et al., 1999). Central AADC inhibition abolished the reversal of motor deficits induced by administration of L-DOPA also indicating effective enzyme inhibition. The elevation of plasma 3-OMD levels produced by NSD-1015 in this study suggests that increased amounts of L-DOPA were being metabolised through the catechol-O-methyl transferase pathway (Kaakkola et al., 1992; Rose et al., 1988; 1991).
The inhibition of L-DOPA-induced motor activity in MPTP treated marmosets following central AADC blockade does not support the concept that L-DOPA itself contributes to motor behaviour. Rather it confirms the classical view that antiparkinsonian activity of L-DOPA is due to dopamine formation and an action on dopamine D-1 and D-2 receptors (Hornykiewicz, 1974; Lorenz et al., 1972).
The concept that D-1 and D-2 receptors interact with each other is supported by morphological data displaying the presence of synaptic contacts between the D-1-modulated direct striatal outflow pathway and the D-2-modulated indirect pathway (Yung et al., 1996). Indeed, very recently it has been demonstrated that D-2 receptors can modulate the phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein in rat striatal slices (Lindskog et al., 1999). In the present study, both the selective D-1 receptor agonist A-86929 (Michaelides et al., 1995; Michaelides et al., 1995; Shiosaki et al., 1995) and the selective D-2 receptor agonist quinpirole (Gomez-Mancilla & Bedard, 1991; Loschmann et al., 1992; Vermeulen et al., 1994) effectively reversed motor deficits in MPTP-treated marmosets as previously reported (Blanchet et al., 1993; Elliott et al., 1992; Loschmann et al., 1991; 1992; Michaelides et al., 1995; Michaelides et al., 1995; Shiosaki et al., 1995). These findings confirm that both D-1 and D-2 agonists administration can produce an antiparkinsonian response in MPTP treated primates as occurs in patients with Parkinson's disease (Bedard et al., 1997; Grondin et al., 1997; Hagan et al., 1997; Jenner, 1995; Kebabian et al., 1992; Loschmann et al., 1991; 1992; Rascol, 1996; Rascol et al., 1997; Shiosaki et al., 1995; Smith et al., 1992). However, the effects of A-86929 or quinpirole were abolished by central AADC inhibition. The inhibition of quinpirole-induced motor activity produced by NSD-1015 in this study contrasts with work carried out in normal rats where the activity of quinpirole was enhanced by AADC inhibition (Yue et al., 1994). The reason for this conflicting result is unclear.
The inhibition of D-1 or D-2-mediated motor behaviour resulting from central AADC blockade in MPTP-lesioned marmosets is, most likely, due to the reduction in endogenous dopaminergic tone which normally provides stimulation at both D-1 and D-2 receptors. This is in agreement with our previous findings (Treseder et al., 1998) and with those of Blanchet et al. (1993) where D-1 or D-2 induced motor behaviour was markedly inhibited by AMPT in MPTP treated primates. Whereas when D-1 and D-2 agonists were coadministered, or the mixed D-1/D-2 agonist apomorphine was administered, the reduction in the reversal of motor deficit produced by AMPT was only mild (Blanchet et al., 1993; Treseder et al., in preparation). In addition, behavioural studies in normal and 6OHDA lesioned rats have shown that AMPT severely inhibited locomotor activity induced by selective D-2 agonists, whilst activity of the D-1/D-2 agonist pergolide was much more resistant to the effects of AMPT (Dziewczapolski et al., 1997; Gershanik et al., 1983; Robertson & Robertson, 1987). Similarly, our results clearly show that when D-1 and D-2 agonists are co-administered, at doses, which produce comparable anti-Parkinsonian activity to the selective agonists alone, their activity is resistant to the inhibitory effects of central AADC inhibition.
The importance of dual D-1 and D-2 receptor stimulation in the genesis of motor activity in MPTP lesioned monkeys is further supported by data from studies using selective dopamine antagonists where simultaneous administration of the selective D-1 antagonist SCH23390 with quinpirole decreased the antiParkinsonian effect of the dopamine agonist (Akai et al., 1995; Elliott et al., 1992) indicating that linkage exists between D-1 and D-2 receptors. In this study, the increase in locomotor activity and reversal of motor disability occurring after the low dose combination of A-86929 and quinpirole were abolished by central AADC inhibition, while the reversal of motor deficit induced by the high dose agonist combination was unaffected. This may indicate that a critical level of stimulation is required at both D-1 and D-2 receptors for the reversal of motor deficits to occur.
Other neurotransmitter systems may also be involved in the reduction in D-1 or D-2 agonist- or L-DOPA-induced motor activity, which occurred following NSD-1015 treatment. Although dopamine is involved directly in the genesis of motor activity, the noradrenergic system is believed to act synergistically in the facilitation and modification of this behaviour. For example, studies in dopamine-depleted rodents have shown that by directly inhibiting noradrenaline synthesis, the activity of L-DOPA is attenuated (Dolphin et al., 1975) indicating that the noradrenaline, which is synthesized from L-DOPA, also plays a role in motor behaviours. Indeed, other studies in MPTP treated primates have shown that treatment with either the α-2 autoreceptor agonist clonidine, or with the β-blocker propranolol result in a reduction in the antiParkinsonian activity of L-DOPA (Gomez-Mancilla & Bedard, 1993) indicating that a link exists between the reduction in noradrenergic stimulation and a decrease in motor activity. The fact that the motor activity produced by direct D-1 or D-2 receptor stimulation in MPTP treated marmosets was reduced by central AADC inhibition may therefore be due to a depressed activity in the noradrenergic system. However, a fall in noradrenaline synthesis would not explain the resistance of the actions of D-1 and D-2 agonists, when administered in combination, to the effects of NSD-1015.
Thus, contrary to reports, L-DOPA itself does not appear to have a neuromodulatory role in motor function. Rather these data strengthen the argument for the importance of endogenous dopamine tone in the actions of D-1 and D-2 agonists.
Acknowledgments
This study was supported by grants for the Parkinson's Disease Society and the National Parkinson Foundation, Miami.
Abbreviations
- A-86929
((−) - trans - 9,10 - hydroxy - 2 - propyl - 4,5,5a,6,7,11b-hexahydro-3-thia-5- azacyclopent-1-ena[c]phenanthrene hydrochloride)
- AADC
(L-aromatic amino acid decarboxylase)
- AMPT
(α-methyl-para-tyrosine)
- carbidopa
(α-methyl-dopa hydrazine)
- MPTP
(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride)
- NSD-1015
(3-hydroxybenzyl hydrazine)
- 3-OMD
(3-O-methyl-dopa)
References
- ACEVES J., FLORAN B., FONG D., SIERRA A., HERNANDEZ S., MARISCAL S. L-DOPA stimulates the release of [3H] gamma-aminobutyric acid in the basal ganglia of 6-OHDA lesioned rats. Neurosci. Lett. 1991;121:223–226. doi: 10.1016/0304-3940(91)90690-u. [DOI] [PubMed] [Google Scholar]
- AKAI T., OZAWA M., YAMAGUCHI M., MIZUTA E., KUNO S. Behavioral involvement of central dopamine D-1 and D-2 receptors in 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned Parkinsonian cynomolgus monkeys. Jap. J. Pharmacol. 1995;67:117–124. doi: 10.1254/jjp.67.117. [DOI] [PubMed] [Google Scholar]
- BEDARD P.J., GOMEZ-MANCILLA B., BLANCHET P., CALON F., GRONDIN R., GAGNON C., FALARDEAU P., GOULET M., MORISSETTE M., ROUILLAERD C., PAOLO T.D.Dopamine agonists as first line therapy of Parkinsonism in MPTP monkeys Beyond the Decade of the Brain 19972Royal Tunbridge Wells, Wells Medical Limited; 101–113.Olanow C. W. and Obeso J.A. (eds.) [Google Scholar]
- BERNHEIMER H., BIRKMAYER W., HORNYKIEWICZ O. Zur Biochemie des Parkinson-Syndroms des Menschen. Klin. Wschr. 1963;41:465–469. [Google Scholar]
- BERNHEIMER H., BIRKMAYER W., HORNYKIEWICZ O., JELLINGER K., SEITELBERGER F. Proc. 8th Inter. Congress of Neurology, Vienna. Wiener Medizinische Akademie; 1965. Zur Differenzierung des Parkinson-Syndroms: Biochemisch-Neurohistolisch Vergleichsunterssuchungen. [Google Scholar]
- BJÖRKLUND A., LINDVALL O. Handbook of Chemical Neuroanatomy, 2, Classical Transmitters in the CNS 1984Amsterdam, Elsevier; 55–122.Part 1, Dopamine containing systems in the CNS. Björkland, A. & Hökfelt, T. (eds.) [Google Scholar]
- BLANCHET P., BEDARD P.J., BRITTON D.R., KEBABIAN J.W. Differential effect of selective D-1-dopamine and D-2-dopamine receptor agonists on levodopa-induced dyskinesia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-exposed monkeys. J. Pharmcol. Exp. Therapeut. 1993;267:275–279. [PubMed] [Google Scholar]
- BRUNNERGUENAT M., CARRUPT P. A., LISA G., TESTA B., ROSE S., THOMAS K., JENNER P., VENTURA P. Esters of L-Dopa–structure-hydrolosis relationships and ability to induce circling behaviour in an experimental-model of Hemiparkinsonism. J. Pharm. Pharmacol. 1995;47:861–869. doi: 10.1111/j.2042-7158.1995.tb05755.x. [DOI] [PubMed] [Google Scholar]
- BUTCHER L.L., ENGEL J., FUXE K. Behavioural, biochemical and histological analysis of the effects of monoamine precursors after peripheral decarboxylase inhibition. Brain Research. 1972;41:387–411. doi: 10.1016/0006-8993(72)90510-0. [DOI] [PubMed] [Google Scholar]
- CRESCIMANNO G., EMMI A., AMATO G. Effects of intraacumbens microinjections of quinpirole on head turning and circling movement in the rat. Pharmacology Biochemistry and Behavior. 1998;60:829–834. doi: 10.1016/s0091-3057(98)00033-1. [DOI] [PubMed] [Google Scholar]
- DOLPHIN A.C., JENNER P., MARSDEN C.D. The relative importance of domamine and noradrenaline receptor stimulation for the restoration of motor activity in reserpine or a-methyl-p-tyrosine pre-treated mice. Pharm. Biochem. Behav. 1975;4:661–670. doi: 10.1016/0091-3057(76)90217-3. [DOI] [PubMed] [Google Scholar]
- DZIEWCZAPOLSKI G., MORA M.A., MENALLED L.B., STEFANO F.J.E., RUBINSTEIN M., GERSHANIK O.S. Threshold of dopamine content and D-1 receptor stimulation necessary for the expression of rotational behaviour induced by D-2 receptor stimulation under normo and supersensitive conditions. Naunyn-Schmiedebergs Arch. Pharmacol. 1997;355:30–35. doi: 10.1007/pl00004914. [DOI] [PubMed] [Google Scholar]
- EDEN R., COSTALL B., DOMENEY A., GERRARD P., HARVEY C., KELLY M. Preclinical pharmacology of ropinirole (SK-AND-F-101468-A) a novel dopamine-D2 agonist. Pharm. Biochem. Behav. 1991;38:147–154. doi: 10.1016/0091-3057(91)90603-y. [DOI] [PubMed] [Google Scholar]
- EHRINGER H., HORNYKIEWICZ O. Verteilung von Noradrenaline und Dopamin (3-Hydroxytyramine) in Gehirn des Menschen und ihr Verhalten bei Erkrankungen des Extrapyramidalen Systems. Klin. Wschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- ELLIOTT P.J., WALSH D.M., CLOSE S.P. Dopamine-D1 and D2 receptor interactions in the MPTP-treated marmoset. Neurosci. Lett. 1992;142:1–4. doi: 10.1016/0304-3940(92)90606-8. [DOI] [PubMed] [Google Scholar]
- GERSHANIK O., HEIKKILA R.E., DUVOISIN R.C. Effects of dopamine depletion on rotational behavior to dopamine agonists. Brain Res. 1983;261:358–360. doi: 10.1016/0006-8993(83)90645-5. [DOI] [PubMed] [Google Scholar]
- GNANALINGHAM K.K., MILKOWSKI N.A., SMITH L.A., HUNTER A.J., JENNER P., MARSDEN C.D. Short-Term and Long-Term Changes in Striatal and Extrastriatal Dopamine uptake sites in the MPTP-treated common marmoset. Eur. J. Pharmacol. 1995;277:235–241. doi: 10.1016/0014-2999(95)00086-z. [DOI] [PubMed] [Google Scholar]
- GOMEZ-MANCILLA B., BEDARD P.J. Effect of D1 and D2 agonists and antagonists on dyskinesia produced by L-DOPA in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys. J. Pharmacol. Exp. Therap. 1991;259:409–413. [PubMed] [Google Scholar]
- GOMEZ-MANCILLA B., BEDARD P.J. Effect of nondopaminergic drugs On L-DOPA-induced dyskinesias in MPTP-treated monkeys. Clin. Neuropharmacol. 1993;16:418–427. doi: 10.1097/00002826-199310000-00004. [DOI] [PubMed] [Google Scholar]
- GOSHIMA Y., NAKAMURA S., MISU Y. L-DOPA facilitates the release of endogenous norepinephrine and dopamine via presynaptic B1 and B2-adrenoceptors under essentially complete inhibition of L-aromatic amino acid decarboxylase in rat hypothalamic slices. Jap. J. Pharmacol. 1990;53:47–56. doi: 10.1254/jjp.53.47. [DOI] [PubMed] [Google Scholar]
- GOSHIMA Y., OHNO K., NAKAMURA S., MIYAMAE T., MISU Y., AKAIKE A. L-DOPA induces Ca2+-dependent and tetrodotoxin-sensitive release of endogenous glutamate from rat striatal slices. Brain Res. 1993;617:167–170. doi: 10.1016/0006-8993(93)90631-v. [DOI] [PubMed] [Google Scholar]
- GRONDIN R., BEDARD P.J., BRITTON D.R., SHIOSAKI K. Potential therapeutic use of the selective dopamin D-1 receptor agonist, A-86929: An acute study in Parkinsonian levodopa-primed monkeys. Neurology. 1997;49:421–426. doi: 10.1212/wnl.49.2.421. [DOI] [PubMed] [Google Scholar]
- HAGAN J.J., MIDDLEMISS D.N., SHARPE P.C., POSTE G.H. Parkinson's disease: Prospects for improved drug therapy. Trends Pharmacol. Sci. 1997;18:156–163. doi: 10.1016/s0165-6147(97)01050-x. [DOI] [PubMed] [Google Scholar]
- HORNYKIEWICZ O. The mechanisms of action of L-DOPA in Parkinson's disease. Life Sci. 1974;15:1249–1259. doi: 10.1016/0024-3205(74)90306-3. [DOI] [PubMed] [Google Scholar]
- JENNER P. The rationale for the use of dopamine agonists in Parkinsons-disease. Neurology. 1995;45:6–12. doi: 10.1212/wnl.45.3_suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- JENNER P., RUPNIAK N.M.J., ROSE S., KELLY E., KILPATRICK G., LEES A., MARDSEN C.D. 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-induced Parkinsonism in the common marmoset. Neurosci Lett. 1984;50:85–90. doi: 10.1016/0304-3940(84)90467-1. [DOI] [PubMed] [Google Scholar]
- JONES G., ROBBINS T. Differential-effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor-activity. Pharmacol. Biochem. Behav. 1992;43:887–895. doi: 10.1016/0091-3057(92)90422-c. [DOI] [PubMed] [Google Scholar]
- KAAKKOLA S., TUOMAINEN P., WURTMAN R.J., MANNISTO P.T. Effects of systemic carbidopa on dopamine synthesis in rat hypothalamus and striatum. J. Neur. Trans-Parkinsons Disease and Dementia Section. 1992;4:143–154. doi: 10.1007/BF02251477. [DOI] [PubMed] [Google Scholar]
- KEBABIAN J.W., BRITTON D.R., DENINNO M.P., PERNER R., SMITH L., JENNER P., SCHOENLEBER R., WILLIAMS M. A-77636 - a potent and selective dopamine-D1 receptor agonist with antiParkinsonian activity in marmosets. Eur. J. Pharmacol. 1992;229:203–209. doi: 10.1016/0014-2999(92)90556-j. [DOI] [PubMed] [Google Scholar]
- KEBABIAN J.W., CALNE D.B. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- KEYSER J.D., BACKER J.D., WILCZAK N., HERROELEN L. Dopamine Agonists used in the treatment of Parkinson's disease and their selectivity for the D1, D2, and D3 dopamine receptors in the human striatum. Neur. Psychopharmacol. Biol. Psychiat. 1995;19:1147–1154. doi: 10.1016/0278-5846(95)00232-4. [DOI] [PubMed] [Google Scholar]
- LINDSKOG M., SVENNINGSSON P., FREDHOLM B., GREENGARD P., FISONE G. Activation of dopamine D-2 receptors decrease DARPP-32 phosphorylation in striatonigral and striatopallidal projection neurons via different mechanisms. Neuroscience. 1999;88:1005–118. doi: 10.1016/s0306-4522(98)00411-4. [DOI] [PubMed] [Google Scholar]
- LORENZ K., CHASE T., COLBURN R., KOPIN I. L-DOPA in parkinsonism. Neurology. 1972;22:688–696. doi: 10.1212/wnl.22.7.688. [DOI] [PubMed] [Google Scholar]
- LOSCHMANN P.A., SMITH L.A., LANGE K.E., JAHNIG P., JENNER P., MARSDEN C.D. Motor-activity following the administration of selective D-1 and D-2 dopaminergic drugs to normal common marmosets. Psychopharmacology. 1991;105:303–309. doi: 10.1007/BF02244422. [DOI] [PubMed] [Google Scholar]
- LOSCHMANN P.A., SMITH L.A., LANGE K.W., JAHNIG P., JENNER P., MARSDEN C.D. Motor-activity following the administration of selective D-1 and D-2 dopaminergic drugs to MPTP-treated common marmosets. Psychopharmacology. 1992;109:49–56. doi: 10.1007/BF02245479. [DOI] [PubMed] [Google Scholar]
- MICHAELIDES M.R., ASIN K.E., BIANCHI B., BRITTON D.R., DIDOMENICO S., HODGES L., HONG Y.F., LIN C.W., MIKUSA J., MILLER T., NIKKEL A., SMITH L., STASHKO M., WILLIAMS M., JENNER P., SHIOSAKI K. A-86929 - a potent and selective dopamine (DA) D1 agonist with efficacy in rodent and primate models of Parkinsons-disease (PD) Abstracts of Papers of the American Chemical Society. 1995;209:83-MEDI. [Google Scholar]
- MICHAELIDES M.R., HONG Y., DIDOMENICO S., ASIN K.E., BRITTON D.R., LIN C.W., WILLIAMS M., SHIOSAKI K. (5aR,11bs)-4,5,5a,6,7,11b-Hexahydro-2-propyl-3-thia-5-azacyclopent-1-ena[c]-phenanthrene-9,10-diol (A-86929)–a potent and selective dopamine D1 agonist that maintains behavioural efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431) J. Med. Chem. 1995;95:3445–3447. doi: 10.1021/jm00018a002. [DOI] [PubMed] [Google Scholar]
- MIERAU J. Pramipexole–a dopamine-receptor agonist for treatment of Parkinson's-disease. Clin. Neuropharmacol. 1995;18:S195–S206. [Google Scholar]
- MIERAU J., SCHNEIDER F., ENSINGER H., CHIO C., LAJINESS M., HUFF R. Pramipexole binding and activation of cloned and expressed dopamine D-2, D-3 and D-4 receptors. Eur. J. Pharmacol. Mol. Pharmacol. Section. 1995;290:29–36. doi: 10.1016/0922-4106(95)90013-6. [DOI] [PubMed] [Google Scholar]
- MISU Y., OKUMURA Y., GOSHIMA Y., YUE J.L., UEDA H. Nanomolar L-DOPA induces Ca2+-dependent and tetrodotoxin-sensitive release of glutamate from striata of conscious rat. J. Neurochem. 1995;65:S154. [Google Scholar]
- NAKAMURA S., GOSHIMA Y., YUE J.L., MISU Y. Transmitter-like basal and K+-evoked released of 3,4-dihydroxyphenylalanine from the striatum in conscious rats studied by microdialysis. J. Neurochem. 1992;58:270–275. doi: 10.1111/j.1471-4159.1992.tb09306.x. [DOI] [PubMed] [Google Scholar]
- NAKAMURA S., YUE J., GOSHIMA Y., MIYAMAE T., UEDA H., MISU Y. Non-effective doses of exogenously applied L-DOPA itself stereoselectively potentiates postsynaptic D2 receptor-mediated locomotor activities in conscious rats. Neurosci. Lett. 1994;170:22–26. doi: 10.1016/0304-3940(94)90229-1. [DOI] [PubMed] [Google Scholar]
- NAKAZOTO T., AKIYAMA A. Effect of exogenous L-DOPA on behavior in the rat: an in vivo voltammetric study. Brain Res. 1989;490:332–338. doi: 10.1016/0006-8993(89)90250-3. [DOI] [PubMed] [Google Scholar]
- POEWE W.H., RASCOL O., BROOKS D.J., BRUNT E.R., KORCZYN A.D., STOCCHI F. Ropinirole in the treatment of early Parkinson's disease: A 6-month interim report of a 5-year levodopa-controlled study. Movement Disorders. 1998;13:39–45. doi: 10.1002/mds.870130111. [DOI] [PubMed] [Google Scholar]
- RASCOL O.A double blind L-DOPA controlled study of ropinirole in de novo patients with Parkinson's disease Movement Disorders. 199611139P510 [Google Scholar]
- RASCOL O., BLIN O., DESCOMBES S., SOUBROUILLIARD C., FABRE N., VIALLET F., THALAMAS C., AZULAY J.P., LAFNITZEGGER K., FREDRICK E., WRIGHT S., NUTT J. ABT-431, a selective D1 agonist has efficacy in patients with Parkinson's disease. Neurology. 1997;48:32004. doi: 10.1002/1531-8249(199906)45:6<736::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- RASCOL O., BROOKS D.J., BRUNT E.R., KORCZYN A.D., STOCCHI F. Ropinirole in the treatment of early Parkinson's disease: A 6-month interim report of a 5-year levodopa-controlled study. Movement Disorders. 1998;13:39–45. doi: 10.1002/mds.870130111. [DOI] [PubMed] [Google Scholar]
- REAVILL C., JENNER P., MARSDEN C.D. Differentiation of dopamine agonists using drug-induced rotation in rats with unilateral or bilateral 6-hydroxydopamine destruction of ascending dopamine pathways. Biochem. Pharmacol. 1983;32:865–870. doi: 10.1016/0006-2952(83)90589-0. [DOI] [PubMed] [Google Scholar]
- ROBERTSON G.S., ROBERTSON H.A. D1 and D2 dopamine agonist synergism–Separate sites of actions. Trends Pharmacol. Sci. 1987;8:295–299. [Google Scholar]
- ROSE S., JENNER P., MARSDEN C.D. The effect of carbidopa on plasma and muscle levels of L-DOPA, dopamine, and their metabolites following L-DOPA administration to rats. Movement Disorders. 1988;3:117–125. doi: 10.1002/mds.870030203. [DOI] [PubMed] [Google Scholar]
- ROSE S., JENNER P., MARSDEN C.D. Peripheral pharmacokinetic handling and matabolism of L-DOPA in the rat–the effect of route of administration and carbidopa pretreatment. J. Pharm. Pharmacol. 1991;43:325–330. doi: 10.1111/j.2042-7158.1991.tb06698.x. [DOI] [PubMed] [Google Scholar]
- ROSE S., NOMOTO M., JENNER P., MARSDEN C.D. Transient depletion of nucleus accumbens dopamine content may contribute to initial akinesia induced by MPTP in common marmosets. Bioch. Pharmacol. 1989a;38:3677–3681. doi: 10.1016/0006-2952(89)90572-8. [DOI] [PubMed] [Google Scholar]
- ROSE S., NOMOTO M., KELLY E., KILPATRICK G., JENNER P., MARSDEN C.D. Increased caudate dopamine turnover may contribute to the recovery of motor function in marmosets treated with dopaminergic neurotoxin MPTP. Neurosci. Lett. 1989b;101:305–310. doi: 10.1016/0304-3940(89)90550-8. [DOI] [PubMed] [Google Scholar]
- SHIOSAKI K., JENNER P., ASIN K. ABT-431: The diacyl prodrug of A86929, a potent and selective dopamine D1 receptor agonist: In vitro characterisation and effects in animal models of Parkinson's disease. J. Pharmacol. Exp. Therapeut. 1995;276:150–160. [PubMed] [Google Scholar]
- SMITH L.A., KEBABIAN J., BRITTON D., WILLIAMS M., DENINNO M., JENNER P., MARSDEN C.D.A77636, a full D1 dopamine agonist, reverses MPTP-induced motor deficits in common marmosets Br. J. Pharmacol. 199210766-P661330166 [Google Scholar]
- STOCCHI F., RUGGIERI S., CARTA A., RYATT J., QUINN N., JENNER P., MARSDEN C., D'AGNOLI A.Intravenous boluses and continuous infussions of L-Dopa methyl-ester in fluctuating patients with Parkinsons-disease 19927249–256.Movement Disorders [DOI] [PubMed] [Google Scholar]
- TRESEDER S.A., ROSE S., JENNER P. The central DOPA decarboxylase inhibitor, NSD-1015, does not present L-DOPA-induced circling behaviour in 60HDA-lesioned rats. Br. J. Pharmacol. 1999;127:24. doi: 10.1046/j.0953-816x.2000.01370.x. [DOI] [PubMed] [Google Scholar]
- TRESEDER S.A., SMITH L., JACKSON M., JENNER P., MARSDEN C.D. The actions of L-DOPA and dopamine agonsits following dopamine depletion in MPTP-treated common marmosets. Br. J. Pharmacol. 1998;123:251. [Google Scholar]
- TRUGMAN J.M., JAMES C.L., WOOTEN G.F. D1/D2 dopamine receptor stimulation by L-DOPA–A (C-14)-2-deoxyglucose autoradiographic study. Brain. 1991;114:1429–1440. doi: 10.1093/brain/114.3.1429. [DOI] [PubMed] [Google Scholar]
- UEKI A., CHONG P.N., ALBANESE A., ROSE S., CHIVERS J.K., JENNER P., MARSDEN C.D. Further treatment with MPTP does not produce Parkinsonism in marmosets showing behavioral recovery from motor deficits induced by an earlier exposure to the toxin. Neuropharmacology. 1989;28:1089–1097. doi: 10.1016/0028-3908(89)90122-6. [DOI] [PubMed] [Google Scholar]
- VERMEULEN R.J., DRUKARCH B., SAHADAT M.C.R., GOOSEN C., WOLTERS E.C., STOOF J.C. The Dopamine-D-1 agonist Skf-81297 and the Dopamine-D-2 agonist Ly-171555 act synergistically to stimulate motor behavior of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Lesioned Parkinsonian rhesus-monkeys. Movement Disorders. 1994;6:664–672. doi: 10.1002/mds.870090613. [DOI] [PubMed] [Google Scholar]
- WU M.B., BRUDZYNSKI S. Mesolimbic dopamine terminals and locomotor-activity induced from the subiculum. Neuroreport. 1995;6:1601–1604. doi: 10.1097/00001756-199508000-00004. [DOI] [PubMed] [Google Scholar]
- WU M., BRUDZYNSKI S., MOGENSEN G. Functional interaction of dopamine and glutamate in the nucleus-accumbens in the regulation of locomotion. J. Physiol. Pharmacol. 1993;71:407–413. doi: 10.1139/y93-061. [DOI] [PubMed] [Google Scholar]
- YUE J., NAKAMURA S., UEDA H., MISU Y. Endogenously released L-DOPA itself tonically functions to potentiate postsynaptic D2 receptor mediated locomotor activities of conscious rats. Neurosci. Lett. 1994;170:107–110. doi: 10.1016/0304-3940(94)90250-x. [DOI] [PubMed] [Google Scholar]
- YUNG K., SMITH A., LEVEY A., BOLAM J. Synaptic connections between spiny neurones of the direct and indirect pathways in the neostriatum of the rat: Evidence from dopamine receptor and neuropeptide immunostaining. Eur. J. Neurosci. 1996;8:861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]


