Abstract
In this study, the effect of 17β-oestradiol on adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP)-dependent protein kinase (PKA) activity was investigated.
Rapid (within 15 min) activation of basal PKA activity was observed in cytosolic fractions by 17β-oestradiol but not by 17α-oestradiol, progesterone or testosterone. This stimulation was abolished by the specific PKA inhibitor PKI but not by the classical oestrogen receptor antagonist tamoxifen.
17β-Oestradiol did not stimulate basal PKA activity in membrane fractions or in cytosolic fractions from male rats.
The increase in cytosolic PKA activity was indirect as (i) it was inhibited by the adenylyl cyclase inhibitor SQ22536, (ii) it was mimicked by forskolin and (iii) 17β-oestradiol did not cause a stimulation of basal PKA activity in either type I or type II commercially available PKA holoenzymes.
Protein kinase Cδ (PKCδ) was directly activated by 17β-oestradiol. The specific PKC inhibitor, bisindolylmaleimide I (GF 109203X), abolished the 17β-oestradiol-induced PKA activation.
17β-Oestradiol stimulated an increase in free intracellular calcium ion concentration ([Ca2+]i) in isolated female but not male rat colonic crypts. This was inhibited by verapamil, nifedipine and zero extracellular [Ca2+] but unaffected by tamoxifen. 17α-Oestradiol, testosterone and progesterone failed to increase [Ca2+]i.
PKC and PKA inhibitors abolished the 17β-oestradiol-induced increase in [Ca2+]i.
These results demonstrate the existence of a novel 17β-oestradiol-specific PKA and Ca2+ signalling pathway, which is both sex steroid- and gender-specific, in rat distal colonic epithelium.
Keywords: Protein kinases; cyclic AMP-dependent; protein kinase C; calcium, intracellular; oestradiol; colon; epithelium
Introduction
The oestrogen receptor (ER) is a member of the steroid hormone receptor superfamily and is thought to regulate gene expression through an interaction with oestrogen responsive elements located within target genes (Ham & Parker, 1989). Following ligand binding, the affinity of the nuclear oestrogen receptor for a particular ERE is increased, thus allowing the receptor protein to serve as an activator of gene transcription. The ER-mediated effects are inhibited by the anti-oestrogen tamoxifen (Rochefort, 1987). This classical pathway for steroid hormone action has a latency of onset of approximately 30 min.
Several reports suggest that oestrogens may be active in previously unsuspected target tissues. The presence of functional ER has been demonstrated in colon, ileum, jejunum and duodenum by reverse transcriptase-polymerase chain reaction (RT–PCR) (Thomas et al., 1993). Also, ligand binding studies have demonstrated the presence of ER in colon (Meggouh et al., 1991), rat renal proximal tubule cells (Stumpf et al., 1980) and whole rat kidney homogenate (Murono et al., 1979). The physiological significance of ER in non-classical target tissues remains to be determined.
The mammalian gastrointestinal tract is capable of absorbing and secreting large volumes of salt and water (Binder et al., 1991). Alterations in colonic salt and water transport can have important implications for total body water and electrolyte balance. There is a dramatic increase in plasma oestrogen concentrations during gestation (Preedy & Aitken, 1961; Roy & Mackay, 1962) – and in other high oestrogen states such as certain phases of the menstrual cycle (Thorn & Engel, 1938), and during use of the combined oral contraceptive pill – and this has been associated with salt and water retention resulting in oedema and hypertension (Katz & Kappas, 1967; Dunn et al., 1975; Laragh, 1976; Fisch & Frank, 1977). Previous studies from our laboratory have demonstrated a down-regulation of net chloride secretion and an up-regulation of sodium reabsorption following addition of 17β-oestradiol in human distal colon (McNamara et al., 1995; 1997).
In contrast to the classical pathway for steroid hormone action, several members of the steroid superfamily, including oestradiol, act through non-genomic as well as genomic mechanisms. Many cellular responses are observed within seconds of hormone administration. One of the earliest reports of rapid non-genomic effects of 17β-oestradiol was made by Szego & Davis (1969). In this study, oestrogen treatment of rats resulted in an acute, very rapid elevation of uterine adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP). 17β-Oestradiol has also been shown to exhibit rapid non-genomic effects in various cell types on: (i) protein kinase C (PKC) activity (Doolan & Harvey, 1996), (ii) activation of the Na+/H+ exchanger and KATP channel (McNamara et al., 1997) and inhibition of Ca2+-sensitive potassium channel activity in mammalian distal colonic epithelium (McNamara et al., 1995; Condliffe et al., 1998), (iii) increased adenylyl cyclase activity and cyclic AMP production in rat pulmonary vascular smooth muscle cells (Farhat et al., 1996), human coronary artery endothelium (Mugge et al., 1993) and rat uterine cells and MCF-7 cells (Aronica et al., 1994), (iv) cyclic AMP and cyclic AMP-dependent protein kinase (PKA) activity (Fernandez et al., 1990), (v) stimulation of inositol phosphate turnover, diacylglycerol production and increases in intracellular free calcium ion concentration [Ca2+]i in female rat osteoblasts (Lieberherr et al., 1993), (vi) increased [Ca2+]i and cyclic AMP production in rat duodenal enterocytes (Picotto et al., 1996), (vii) increased [Ca2+]i in chicken and pig granulosa cells (Morley et al., 1992) and single rat hepatocytes (Sanchez-Bueno et al., 1991), and in human monocytes via the activation of an oestrogen surface receptor (Stefano et al., 1999).
Although a number of the above studies have documented an increase in adenylyl cyclase activity with subsequent cyclic AMP production and PKA activation in response to 17β-oestradiol, the mechanism by which oestrogen enhances adenylyl cyclase activity has not yet been determined. Previous studies have demonstrated a stimulation of adenylyl cyclase activity by PKC (Jacobowitz et al., 1993; Yoshimura & Cooper, 1993). Since we have demonstrated a stimulation of PKC activity by 17β-oestradiol in rat distal colonic epithelium (Doolan & Harvey, 1996), we investigated whether oestradiol-induced stimulation of adenylyl cyclase, with a subsequent increase in PKA activity, was dependent upon an initial stimulation of PKC by 17β-oestradiol. The effects of 17β-oestradiol on [Ca2+]i in single isolated rat distal colonic crypts was also investigated.
Methods
Materials
[γ-32P]ATP (30 and 3000 Ci μmol−1) and PKC assay kits were purchased from Amersham Pharmacia Biotech (Little Chalfont, Buckinghamshire, U.K.). Kemptide, cyclic AMP, ATP, PKA type II inhibitor from bovine heart (PKI, Walsh inhibitor), PKA holoenzyme type II from bovine heart and type I from rabbit muscle, 8-(6-aminohexyl)amino cyclic AMP, N6-benzoyl cyclic AMP, 8-thiomethyl cyclic AMP, forskolin, 17β-oestradiol, 17α-oestradiol, aldosterone, hydrocortisone, dexamethasone, testosterone, progesterone, 4-hydroxytamoxifen, verapamil, nifedipine and phosphatidylserine were all obtained from Sigma (Poole, Dorset, U.K.). SQ22536, the PKA-inhibitory cyclic AMP analogue adenosine cyclic 3′,5′-phosphorothioate ((Rp)cAMPS), human recombinant PKCα, PKCδ, PKCε and PKCζ, PKCε peptide substrate, bisindolylmaleimide I and chelerythrine chloride were purchased from Calbiochem (Nottingham, U.K.). P81 filter paper was obtained from Whatman (Maidstone, Kent, U.K.). FURA-2 acetoxymethyl ester was obtained from Molecular Probes (Eugene, OR, U.S.A.). All other chemicals were of the highest purity commercially available.
Steroid hormones were dissolved in methanol and stored in aliquots at −20°C until required. The final concentration of methanol in all assays was 0.01%, this concentration of methanol was without effect on PKA or [Ca2+]i.
Tissue preparation
Sprague-Dawley rats were sacrificed by cervical dislocation; the distal colon was removed and the epithelium stripped of smooth muscle and connective tissue by microdissection. Cytosolic and membrane fractions were obtained by homogenization and differential centrifugation using a modification of the method of Aukema et al. (1994). Colonic epithelium was homogenized (on ice) in 6 ml homogenization buffer (5 mM Tris-HCl, pH 7.2, containing (mM): sucrose 250, NaF 50, EDTA 2, EGTA 1, 2-mercaptoethanol 10 and 25 mg l−1 leupeptin) using an Omni homogenizer (Camlab Ltd., Cambridge, U.K.) at 20,000 r.p.m. for 3×40 s. The homogenate was spun at 86,000 g at 4°C for 30 min using a Beckman TL-100 ultracentrifuge. The supernatant was retained and represented the cytosolic fraction. The pellet (membrane fraction) was resuspended in 4 ml homogenization buffer containing 1% Triton X-100 and left on ice for 10 min. The solubilized pellet was centrifuged at 86,000×g at 4°C for 30 min and the supernatant from this spin was designated the solubilized membrane fraction. Protein content of cytosolic and membrane fractions was determined using the Lowry method (Lowry et al., 1951).
Measurement of cyclic AMP-dependent protein kinase (PKA) activity
PKA (EC 2.7.1.37) activity was measured using an assay based on the transfer of the terminal phosphate of [γ-32P]ATP to the synthetic substrate, kemptide (Giembycz & Diamond, 1990). Assays were performed using a modification of the method described (Doolan & Keenan, 1994). Assays were carried out at 30°C in a final volume of 100 μl containing 60 μg protein (cytosol or membrane fraction), assay cocktail (mM): EGTA 0.1, NaF 40, dithiothreitol 1, theophylline 1, sodium arsenate 5, 10 μg kemptide, stimulators and inhibitors as appropriate, in 75 mM phosphate buffer, pH 7.0. (In assays where commercially available PKA isoenzymes were used, the assay cocktail was omitted). Following a 5-min pre-incubation period, the reaction was initiated by addition of 1 mM ATP (containing 1–1.5×107 c.p.m. μmol−1 [γ-32P]ATP) plus 2 mM magnesium acetate. Assays were stopped after 5–60 min and processed using a modification of the method of Witt & Roskoski (1975): assay mixture was spotted onto P81 phosphocellulose ion exchange chromatography discs which were allowed to dry for approximately 30 s and placed in ice-cold 75 mM orthophosphoric acid solution (10 ml filter−1). Filters were washed for 3×10 min in the above solution and 1×5 min in industrial methylated spirit. The filters were allowed to dry and were placed in scintillation vials containing 10 ml Ecolite™. Incorporated radioactivity was determined by liquid scintillation counting. Activities were expressed as pmol phosphate transferred mg protein−1 min−1.
Use of synergistic pairs of site-selective cyclic AMP agonists
The two isoenzymes of PKA (type I and type II) are distinguished in terms of their regulatory subunits which provides the basis for isoenzyme classification (Lohmann & Walter, 1984). Each regulatory subunit contains two different intra-subunit cyclic AMP binding sites, site 1 and site 2, which bind certain cyclic AMP analogues selectively (Beebe et al., 1988). Binding of a cyclic nucleotide at one site stimulates binding at the other site. When two cyclic AMP analogues, each selective for a different binding site, are added in combination to PKA, the enzyme is activated in a synergistic fashion. Since the cyclic AMP analogue specificity of site 1 is different for the two isoenzymes, synergistic activation of type I PKA and type II PKA can be distinguished.
Assays were carried out using a modification of the method described by Beebe et al. (1988). The type I synergistic pair comprised 1.2 μM 8-(6-aminohexyl)amino cyclic AMP (8AHA) and 10 nM N6-benzoyl cyclic AMP (N6BZL). The type II pair comprised 7 nM 8-thiomethyl cyclic AMP (8-S-CH3) and 10 nM N6BZL. The assay conditions were identical to those described above.
The basal activity ratio (activity in the absence divided by activity in the presence of 1 μM cyclic AMP was determined and this value was subtracted from the analogue-stimulated activity ratio. Results were expressed as the increment in activity ratio.
PKC activity assay
PKC assays were performed according to the Amersham protocol. PKC activity was measured using an assay based on the transfer of the terminal phosphate of [γ-32P]ATP to a synthetic substrate present in the reaction mixture. For PKCα (Ca2+-dependent PKC isoform), the peptide substrate from the PKC assay kit was used. For PKCε, PKCδ and PKCζ, the assay mixture contained 0.1 mM EGTA instead of Ca2+ and a PKCε peptide substrate. Basal unstimulated Ca2+/phospholipid-dependent PKC activity was determined for PKCα. For PKCε, PKCδ and PKCζ, stimulated activities were calculated following subtraction of phospholipid-dependent activity.
Preparation of vesicles
Lipid vesicles were prepared as previously described by Slater et al. (1995). Phosphatidylserine solutions in chloroform (100 μg ml−1) were mixed with various steroid hormones (0.1–10 nM in methanol). The solvent mixture was removed under a stream of nitrogen and the steroid-lipid mixture was dispersed by vortex-mixing in 50 mM Tris-HCl, pH 7.4. Vesicle preparations were prepared immediately before use and stored in the dark under nitrogen.
Isolation of rat distal colonic crypts
Rat distal colonic crypts were isolated as previously described in detail (Doolan & Harvey, 1996).
Ca2+ spectrofluorescence
Measurement of [Ca2+]i was performed using the method of FURA-2 spectrofluorescence imaging as previously described in detail (Doolan & Harvey, 1996). A micropipette system was used to apply test solutions to isolated colonic crypts plated on a glass cover slip. Where the effect of (i) the PKA inhibitor, (Rp)cAMPS, (ii) the adenylyl cyclase inhibitor, SQ22536, or (iii) the PKC inhibitor, chelerythrine chloride, were examined, the rat colonic crypts were pre-incubated for 5 or 10 min at room temperature prior to the addition of 17β-oestradiol. In all experiments the test solution was identical to the bathing solution except for the concentration of steroid used. All experiments were performed at room temperature (20–22°C) to minimize dye leakage and colonic crypt disintegration.
Statistical analysis
Measurements of PKA and [Ca2+]i are presented as mean±s.e.mean from the indicated numbers of experiments. Statistically significant differences were determined by ANOVA followed by either Tukey or Dunnett's test, as appropriate, and differences were deemed significant at P0.05.
Results
The presence of PKA activity in membrane and cytosolic fractions isolated from rat distal colonic epithelium was established. Basal PKA activity, in both membrane and cytosolic fractions, was significantly stimulated by cyclic AMP (10 nM–1 μM, n=6–10; Figure 1). This stimulation was abolished in the presence of the specific PKA inhibitor, PKI (5 μg ml−1; Figure 1). Lower concentrations of cyclic AMP (0.1–1 nM) had no effect on basal PKA activity.
Figure 1.
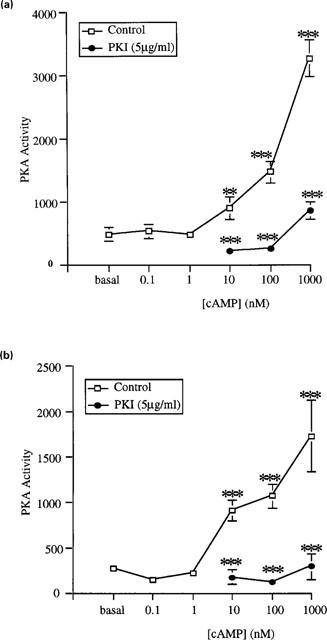
Modulation of cyclic AMP-dependent protein kinase (PKA) activity by cyclic AMP in the absence and presence of PKA type II inhibitor (PKI) in (a) cytosolic and (b) membrane fractions isolated from female rat distal colonic epithelium. Incubations were conducted for 15 min. Results are expressed as PKA activity (pmol phosphate transferred min−1 mg−1 protein). Data are mean±s.e.mean of four independent experiments each performed in duplicate. Asterisks indicate significant differences between cyclic AMP-stimulated and basal activity (□) or between PKI-treated and control preparations (•): **P<0.01, ***P<0.005.
We have identified the PKA isoform present in cytosolic fractions, using synergistic pairs of site-selective cyclic AMP analogues for type I and type II PKA isoforms. For the type I-directed analogue pair, N6BZL and 8-AHA, the increments in activity ratio were 0.13 and 0.12, respectively; an additive effect of these analogues would have yielded a ratio of 0.25. However, the change in activity ratio observed was 0.37 (Figure 2), yielding a synergism quotient (ratio between the observed and the expected response) of 1.48. With the type II-directed analogue pair, N6BZL and 8-S-CH3, the increments in activity ratio were 0.13 and 0.15, respectively; an additive effect of these analogues would have yielded a ratio of 0.28. The observed change in activity ratio was 0.34, however (Figure 2), yielding a synergism quotient of 1.2. We can thus see that both type I and type II PKA isoforms are present in cytosolic fractions.
Figure 2.
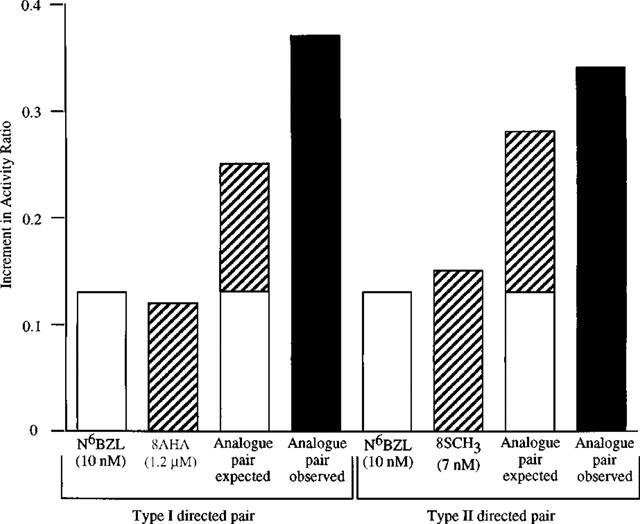
Synergism of PKA activation in cytosolic fractions isolated from female rat distal colonic epithelium using both a type I- and a type II-directed analogue pair. Cytosolic fractions were incubated with type I PKA activators (N6BZL, 8AHA or a combination of both) or with type II PKA activators N6BZL, 8-S-CH3 or a combination of both). The ratio between the observed and the expected response to the paired analogues represents the extent of synergism. Basal activity ratio (determined in the absence and presence of 1 μM cyclic AMP) was 0.27 and was subtracted from all values. Results are expressed as increment in activity ratio. This is a representative experiment that was repeated six times with similar results.
We then examined the effect of 17β-oestradiol on basal PKA activity in cytosolic and membrane fractions. Rapid (within 15 min) activation of basal PKA activity was observed in cytosolic fractions by 17β-oestradiol (0.1–100 nM; Figure 3a). No increase in basal PKA activity was observed with lower concentrations of 17β-oestradiol (0.1–10 pM). This PKA stimulation was significantly inhibited by PKI (Figure 3a). In contrast to the results obtained in cytosolic fractions, 17β-oestradiol (0.1–100 nM) failed to stimulate basal PKA activity in membrane fractions (data not shown).
Figure 3.
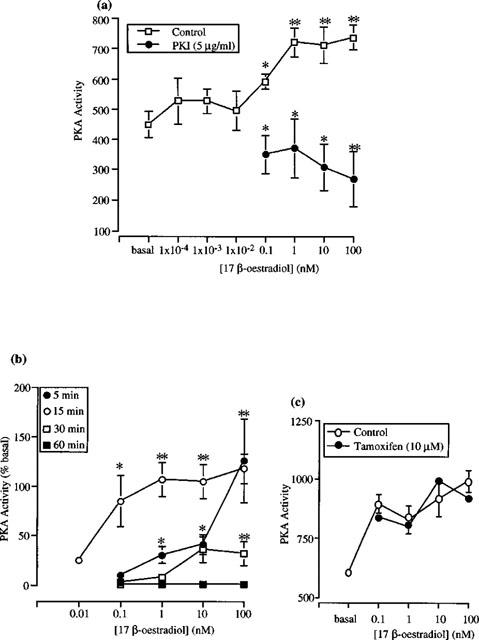
Activation of PKA by 17β-oestradiol in cytosolic fractions of female rat distal colonic epithelium. (a) Activation within 15 min in the absence and presence of 5 μg ml−1 PKI. (b) Effects on 17β-oestradiol measured after 5, 15, 30 or 60 min. Data are presented as per cent increase above basal activity. (c) Activation within 15 min in the absence and presence of 10 μM 4-hydroxytamoxifen. Data are expressed as PKA activity (pmol phosphate transferred min−1 mg−1 protein) (a,c) or per cent increase above basal activity (b). Data are mean±s.e.mean of 5–9 independent experiments each performed in duplicate. Asterisks indicate significant differences between 17β-oestradiol-stimulated and basal activity or between PKI-treated and control preparations. *P<0.05, **P<0.01.
We have also examined the effect of oestradiol on basal PKA activity over a longer time course of 30–60 min (Figure 3b). Following a 30 min incubation, a significant stimulation of basal PKA activity was observed with supraphysiological concentrations of 17β-oestradiol (10–100 nM). No stimulation of PKA activity was observed, however, with lower concentrations of the hormone (0.1–1 nM). At the 60-min time point, 17β-oestradiol (0.1–100 nM) failed to stimulate basal PKA activity.
To examine whether stimulation of PKA activity could be detected following a shorter incubation time, the effect of 17β-oestradiol on basal PKA activity was examined following a 5-min exposure to the hormone. Significant stimulation of basal PKA activity was observed with 17β-oestradiol (1–100 nM), although no stimulation was observed at 0.1 nM 17β-oestradiol (Figure 3b). The classical oestrogen receptor antagonist 4-hydroxytamoxifen (10 μM) did not inhibit the 17β-oestradiol induced stimulation of PKA activity (Figure 3c).
Figure 4a shows the significant inhibitory effect of the adenylyl cyclase inhibitor, SQ22536, on 17β-oestradiol-induced PKA stimulation. In contrast to these results, however, no inhibition of the oestradiol-induced stimulation of PKA activity was observed with other adenylyl cyclase inhibitors, 2′ : 3′-dideoxyadenosine (100 nM or 1 μM) or MDL 12-330A (200 μM; data not shown). The stimulation of PKA activity by 17β-oestradiol could be mimicked by forskolin (2 and 10 μM); this stimulation was also significantly inhibited by SQ22536 (Figure 4b). These results indicate an indirect effect of 17β-oestradiol on PKA activity. To test this, we examined the effect of oestradiol on commercially available PKA holoenzymes: type I from rabbit muscle and type II from bovine heart. 17β-oestradiol (1 nM) had no stimulatory effect on basal PKA activity of either type I or type II holoenzymes (Table 1). Similar results were obtained for 17β-oestradiol (0.1–100 nM; data not shown).
Figure 4.
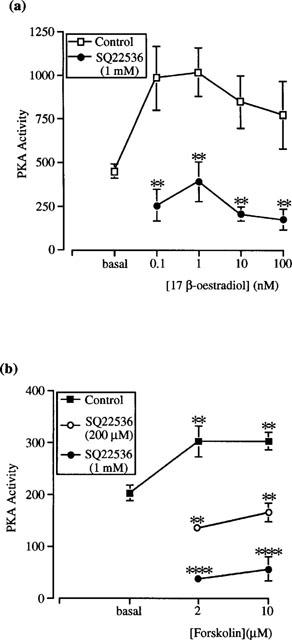
Inhibition of (a) 17β-oestradiol- and (b) forskolin-stimulated PKA activity in cytosolic fractions of female rat distal colonic epithelium by the membrane-permeant adenylyl cyclase inhibitor, SQ22536. Experiments were conducted for 15 min. Results are expressed as PKA activity (pmol phosphate transferred min−1 mg−1 protein). Data are mean±s.e.mean of 3–6 independent experiments each performed in duplicate. Asterisks indicate significant differences between SQ22536-treated and control preparations. **P<0.01, ****P<0.001.
Table 1.
Lack of effect of 17β-oestradiol on PKA holoenzyme activity
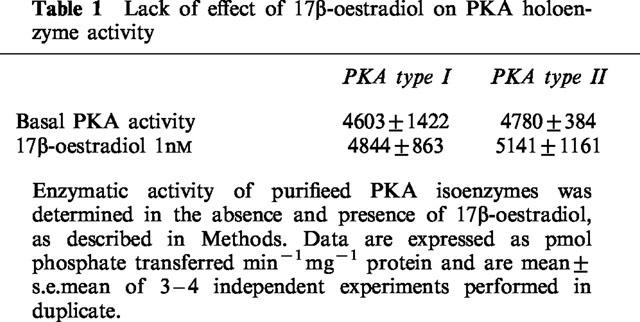
The specific PKC inhibitor, bisindolylmaleimide I (GF 109203X, 25 nM), significantly inhibited the 17β-oestradiol-induced stimulation of PKA activity (Figure 5a). In contrast, however, bisindolylmaleimide I had no inhibitory effect on cyclic AMP-stimulated PKA activity (Figure 5b).
Figure 5.
Effects of the specific PKC inhibitor bisindolylmaleimide I (GF109203X, BIM; 25 nM) on (a) 17β-oestradiol- and (b) cyclic AMP-stimulated PKA activity in cytosolic fractions from rat distal colonic epithelium. Results are expressed as per cent increase above basal activity. Data are mean±s.e.mean of 5–6 independent experiments. **P<0.01.
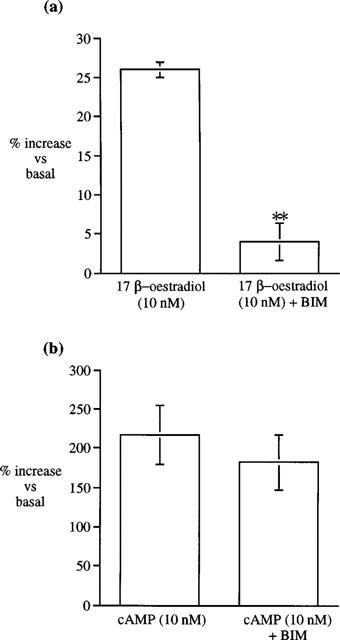
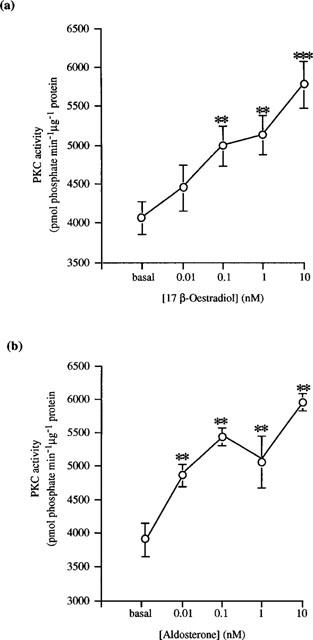
Studies from other laboratories have demonstrated a direct activation of PKC by another steroid hormone, 1α,25-dihydroxyvitamin D3 in a cell-free assay system (Slater et al., 1995). We examined the direct effect of 17β-oestradiol on the PKC isoforms α, δ, ε and ζ – known to be present in distal colonic epithelium (Davidson et al., 1994; Jiang et al., 1995; Verstovek et al., 1998). The following experiments were performed in a cell free assay system containing only purified commercially available enzyme, appropriate substrate peptides, co-factors and lipid vesicles. We observed a stimulatory effect on basal PKCα activity following the addition of 17β-oestradiol (0.1–10 nM; Figure 6a) and aldosterone (0.01–10 nM; Figure 6b). Neither 17β-oestradiol, 17α-oestradiol nor aldosterone stimulated the activity of either PKCε or PKCζ (data not shown). In contrast, however, we could demonstrate a direct significant stimulation of basal PKCδ activity following the addition of 17β-oestradiol (0.1–10 nM; Figure 7a). Both the stereoisomer 17α-oestradiol (10 nM; Figure 7b) and aldosterone (0.1–10 nM; Figure 7a) were without effect on basal PKCδ activity.
Figure 6.
Concentration-response curves for the activation of PKCα activity by (a) 17β-oestradiol and (b) aldosterone. Results are expressed as PKC activity (pmol phosphate transferred min−1 μg−1 protein. Data are mean±s.e.mean of three determinations. **P<0.01, ***P<0.005.
Figure 7.
Effects of (a) 17β-oestradiol or aldosterone and (b) 17α-oestradiol on PKCδ activity. Results are expressed as PKC activity (pmol phosphate transferred min−1 μg−1 protein). Data are mean±s.e.mean of three determinations. *P<0.05, **P<0.01.
The specificity of the 17β-oestradiol response was also examined. No increase in PKA activity was observed in the presence of the stereoisomer 17α-oestradiol or the sex steroid hormones progesterone or testosterone (0.1–100 nM). Similarly, neither the mineralocorticoid hormone, aldosterone, nor the glucocorticoid hormones, hydrocortisone or dexamethasone (0.1–100 nM), had any effect on basal PKA activity (data not shown).
As well as being stereospecific, the PKA response to 17β-oestradiol was also gender-specific. Neither 17β-oestradiol nor testosterone (0.1–100 nM) had any effect on basal PKA activity in cytosolic fractions isolated from male Sprague-Dawley rat distal colonic epithelium (Figure 8).
Figure 8.
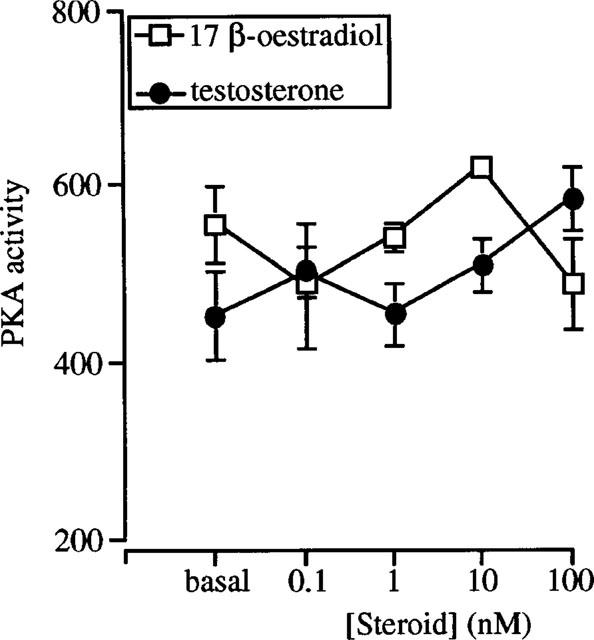
Lack of effect of 17β-oestradiol or testosterone on PKA activity in cytosolic fractions isolated from male rat distal colonic epithelium. Experiments were conducted for 15 min. Results are expressed as PKA activity (pmol phosphate transferred min−1 mg−1 protein). Data are mean±s.e.mean of 4–6 independent experiments each performed in duplicate.
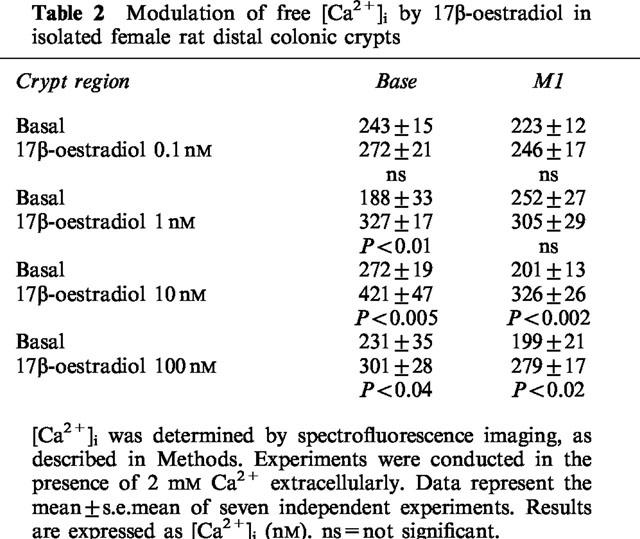
We examined the effects of 17β-oestradiol on [Ca2+]i in single isolated crypts from female rat distal colonic epithelium. Ca2+ (2 mM) was present in all cases in the external bathing solution unless otherwise stated. Following the addition of 17β-oestradiol, an increase in [Ca2+]i was observed solely at the base of the colonic crypt; following addition of higher concentrations of 17β-oestradiol, an increase was also observed in the middle M1 region of the crypt. A concentration-response relationship was observed, with concentrations of 17β-oestradiol in the range 0.1–100 nM. No increase in [Ca2+]i was observed at the lowest concentration of 17β-oestradiol tested (0.1 nM). 17β-Oestradiol (1 nM) induced a significant increase in [Ca2+]i in the base of the crypt, with no significant increase observed in any other crypt region. Higher concentrations of 17β-oestradiol (10–100 nM) produced a significant increase in [Ca2+]i at both the base and M1 region of the crypt (Table 2).
Table 2.
Modulation of free [Ca2+]i by 17β-oestradiol in isolated female rat distal colonic crypts
To investigate the specificity of the oestradiol response, the effects of the stereoisomer 17α-oestradiol, testosterone and progesterone (all at 10 nM) were examined. No increase in basal [Ca2+]i was observed in any region of the colonic crypt following the addition of these hormones (Figure 9a). Similarly, no increase in [Ca2+]i was observed following oestradiol addition when the crypts were bathed in a Ca2+-free external bathing solution. In these experiments, crypts were pre-incubated in Ca2+-free buffer for 1 min, followed by addition of 17β-oestradiol. The L-type Ca2+ channel blockers, verapamil (10 μM) and nifedipine (100 nM), inhibited the oestradiol-induced increase in free [Ca2+]i (Figure 9b).
Figure 9.
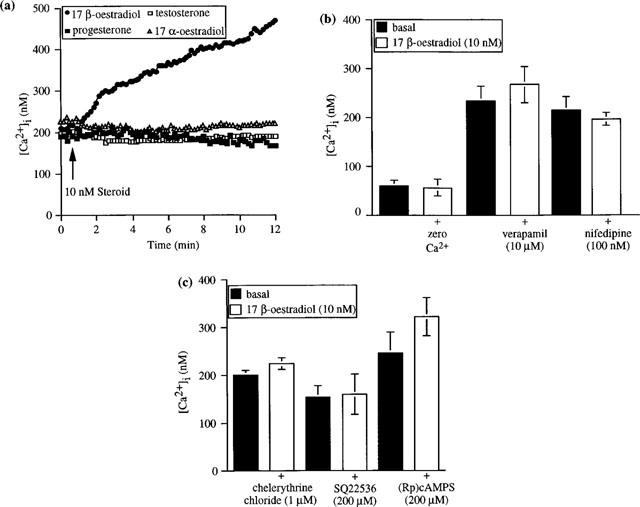
(a) Modulation of [Ca2+]i by 17β-oestradiol (10 nM), 17α-oestradiol (10 nM), testosterone (10 nM) and progesterone (10 nM), in the presence of 2 mM Ca2+ externally, in the base region of a single isolated rat distal colonic crypt. Data are from a representative experiment that was repeated seven times for each hormone. (b) Inhibition of the stimulatory effect of 17β-oestradiol on [Ca2+]i in zero Ca2+ externally or following a 5-min pre-incubation with verapamil or nifedipine. Data are mean±s.e.mean of six independent experiments. (c) Inhibition of the stimulatory effect of 17β-oestradiol on [Ca2+]i following pre-incubation with the protein kinase C inhibitor, chelerythrine chloride, the adenylyl cyclase inhibitor, SQ22536, or the PKA-inhibitory cyclic AMP analogue, (Rp)cAMPS. Data are mean±s.e.mean of six independent experiments.
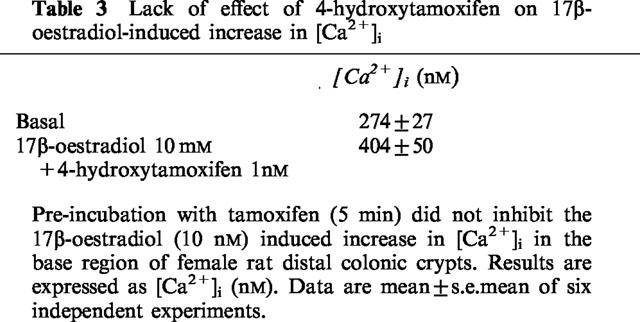
The increase in [Ca2+]i induced by oestradiol is very rapid and would not be consistent with a genomic response via the ER. Pre-incubation of rat distal colonic crypts for 5 min with the classical ER antagonist 4-hydroxytamoxifen (10 μM) did not block the oestradiol-induced increase in [Ca2+]i (Table 3).
Table 3.
Lack of effect of 4-hydroxytamoxifen on 17β-oestradiol-induced increase in [Ca2+]i
A 5-min pre-incubation of the colonic crypts with (i) the membrane-permeant PKC inhibitor, chelerythrine chloride (1 μM), or (ii) the adenylyl cyclase inhibitor, SQ22536 (200 μM), or (iii) a 10-min pre-incubation with the inhibitory cyclic AMP analogue, (Rp)cAMPS (200 μM), completely abolished the oestradiol-induced stimulatory effect on [Ca2+]i in rat distal colonic crypts (Figure 9c).
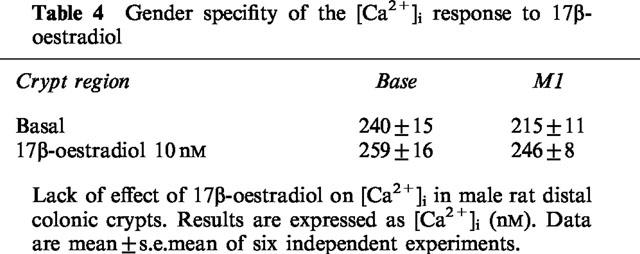
The stimulatory effect of 17β-oestradiol on [Ca2+]i was also gender-specific. No increase in [Ca2+]i was observed following 17β-oestradiol (10 nM) addition in crypts isolated from male rat distal colonic epithelium (Table 4).
Table 4.
Gender specifity of the [Ca2+]i response to 17β-oestradio
Discussion
This paper provides evidence for a rapid non-genomic stimulatory effect of 17β-oestradiol on cytosolic PKA activity and [Ca2+]i in female rat distal colon. The oestradiol-induced stimulatory effect on PKA activity was shown to be indirect, via an initial activation of PKC followed by subsequent activation of adenylyl cyclase. Oestradiol specifically and directly stimulated activity of the protein kinase C isoform PKCδ. We propose, therefore, that stimulation of PKA activity by oestradiol in rat distal colonic epithelium occurs indirectly via PKCδ.
The rapid effects of 17β-oestradiol on PKA and [Ca2+]i are both gender- and sex steroid hormone-specific. The increase in [Ca2+]i following hormone addition is abolished by both PKC and PKA inhibition, indicating an involvement of both kinases in the Ca2+ signalling pathway in colonic crypts. These results show that intracellular signalling for 17β-oestradiol in the distal colon involves changes in [Ca2+]i via activation of both the PKC and the cyclic AMP second messenger pathways.
17β-Oestradiol stimulated PKA activity in cytosolic fractions in a concentration-dependent manner, with a significantly greater effect observed in the nanomolar vs the picomolar concentration range. (Human mid-cycle oestrogen peak levels in plasma range between 0.9 and 2.1 nM (Brown et al., 1987)). In contrast, no stimulation of PKA activity was observed in membrane fractions isolated from female rat distal colonic epithelium following 17β-oestradiol addition. This absence of 17β-oestradiol-induced stimulatory effects on membrane PKA activity cannot be accounted for by a difference in PKA isoform distribution between cytosolic and membrane fractions, as both type I and type II PKA isoforms were identified in cytosolic fractions. Aukema et al. (1994) have also demonstrated the existence of both type I and type II PKA isozymes in proximal and distal rat colonic mucosa isolated from Sprague-Dawley rats. In these studies, the majority of PKA activity in both fractions was type II PKA (89% in cytosol and 96% in membrane fraction), with the remaining PKA activity representing type I PKA.
The oestradiol-induced stimulation of PKA activity is evident within 5–15 min. However, when PKA activity was measured after longer periods of incubation, the increase in activity was only observed with supraphysiological concentrations after 30 min, while no increase in PKA activity was observed after a 60-min incubation with any concentration of oestradiol tested. These results indicate that the stimulatory effect of oestradiol on PKA is transient and complete within 30 min. As the latency of onset of genomic responses is approximately 30 min, these results appear to represent a non-genomic stimulation of PKA activity by oestradiol, independent of the involvement of the classical oestrogen receptor (ER). This conclusion is supported by the fact that the classical oestrogen receptor antagonist 4-hydroxytamoxifen failed to inhibit 17β-oestradiol-induced stimulation of PKA activity.
The stimulation of colonic PKA activity is steroid specific, as (i) the stereoisomer, 17α-oestradiol, (ii) the sex steroid hormones, progesterone and testosterone, (iii) the mineralocorticoid hormone, aldosterone, and (iv) the glucocorticoids, hydrocortisone and dexamethasone, were devoid of activity.
The oestradiol-induced stimulation of cytosolic PKA activity is indirect, via the activation of adenylyl cyclase, as it is inhibited by the adenylyl cyclase inhibitor SQ22536 and mimicked by forskolin. Forskolin stimulates the activity of all the known adenylyl cyclase isoforms. However, the mechanism of this forskolin-induced activation is still unknown. Membrane location of the enzyme may not be important since a soluble, artificially engineered form of the enzyme lacking the two transmembrane domains is still activated by forskolin (Tang & Gilman, 1995; Dessauer & Gilman, 1996; Whisnant et al., 1996). Although it is widely reported that adenylyl cyclases are integral transmembrane glycoproteins (Iyengar, 1993), Finidori-Lepicard et al. (1981) have reported that adenylyl cylcase in Xenopus laevis oocytes is compartmentalized, with 50–65% in the soluble fraction and 20–30% in the plasma membrane fraction. Both particulate and soluble fractions appear equally active. This distribution is similar to that observed in the early stages of germ cell development (Braun & Dods, 1975) and in mature rat testis (Neer, 1978). From the results obtained in this study, adenylyl cyclase activity also appears to be present in cytosolic fractions of rat distal colon, and oestradiol stimulates PKA activity indirectly via adenylyl cyclase activation. The lack of effect of 17β-oestradiol on PKA activity in membrane fractions may therefore be due to the existence of a difference in adenylyl cyclase isoform distribution between membrane and cytosolic fractions in rat distal colonic epithelium.
Previous studies from other laboratories have demonstrated a stimulation of adenylyl cyclase activity by PKC (Jacobowitz & Iyengar, 1994; Jacobowitz et al., 1993; Yoshimura & Cooper, 1993). Lin & Chen (1998) have shown that distinct PKC isoforms (PKCε and PKCμ) mediate the activation of adenylyl cyclase activity in RAW264.7 macrophages. Recent studies have also demonstrated an up-regulation of PKCδ expression in the rat and rabbit corpus luteum by oestrogen (Cutler et al., 1994; Maizels et al., 1992; 1996), indicating a role for PKC in the biological response to oestrogen. Since we have previously demonstrated a rapid non-genomic stimulation of PKC activity in cytosolic fractions of rat distal colonic epithelium (Doolan & Harvey, 1996), it is possible that PKC may be the cytosolic target protein or ‘receptor' responsible for the rapid response to 17β-oestradiol.
Studies from other laboratories have shown an elevation of cyclic AMP levels (within 45 s–5 min), an activation of PKA activity and a PKA-dependent phosphorylation of microsomal proteins isolated from chick soleus muscle by the metabolite of vitamin D3, 1α,25-dihydroxyvitamin D3 (Fernandez et al., 1990). The mechanism by which the steroid enhanced adenylyl cyclase activity in these studies was not determined, however, and the possible involvement of PKC in the steroid-induced stimulatory effect was not investigated.
Here, we have demonstrated a significant inhibition of oestradiol-induced stimulation of PKA activity by the specific PKC inhibitor, bisindolylmaleimide I. In contrast, cyclic AMP-stimulated PKA activity was unaffected by bisindolylmaleimide I. 17β-Oestradiol directly and specifically stimulated PKCδ activity in a cell-free assay system (17α-oestradiol and aldosterone were without effect). From the results of this and subsequent experiments, we conclude that the indirect stimulation of PKA activity, via adenylyl cyclase activation, is due to an initial stimulation of PKCδ by 17β-oestradiol. The involvement of the Ca2+/phospholipid-dependent PKC isoform, PKCα, was ruled out as we observed a stimulation of PKCα activity by both 17α-oestradiol and aldosterone as well as 17β-oestradiol, but neither 17α-oestradiol nor aldosterone had any stimulatory effect on PKA activity. The involvement of either PKCε or PKCζ was also ruled out as no stimulation of either of these PKC isoforms was observed with 17β-oestradiol.
An indirect stimulation of PKA activity by oestradiol is also supported by the lack of stimulatory effect of 17β-oestradiol on basal PKA activity of commercially available type I or type II PKA isoforms.
We propose, therefore, that the cytosolic protein responsible for the rapid non-genomic PKA response to oestradiol is the Ca2+-independent, phospholipid-dependent PKC isoform, PKCδ. The PKCδ isoform may be the elusive non-genomic ‘receptor'.
The 17β-oestradiol effect on PKA activity is gender-specific, as neither 17β-oestradiol nor testosterone had any effect on PKA activity in cytosolic fractions from male Sprague-Dawley rats. The reason for this gender specificity is as yet unknown. However, Sylvia et al. (1998) have reported a rapid non-genomic stimulation of PKC activity, specifically by 17β-oestradiol, in a gender-specific manner, in female rat costochondral chondrocytes. One possible explanation for these gender-specific results is that there may be a difference in PKC isoform distribution between male and female tissue.
In the second part of this study the effects of sex steroid hormones were examined on [Ca2+]i in single isolated rat distal colonic crypts. Involvement of the cyclic AMP second messenger system and PKC in this response were also investigated
A rapid increase in basal free [Ca2+]i was measured in the base and lower middle region (M1) of the rat distal colonic crypt following 17β-oestradiol addition. The rise in [Ca2+]i was immediate upon oestradiol addition and was not inhibited by 4-hydroxytamoxifen, indicating an incompatibility with the classical genomic response. Oestradiol stimulated a Ca2+ influx in rat colonic crypts which was blocked in the presence of zero Ca2+ externally and by verapamil and nifedipine. Pre-incubation of the crypts with chelerythrine chloride or with either SQ22536 or (Rp)cAMPS inhibited the oestradiol-induced stimulatory effect on [Ca2+]i. We conclude that oestradiol stimulates an influx of extracellular Ca2+ by a PKC/cyclic AMP/PKA-dependent mechanism. These results are similar to those previously reported for vitamin D3 (DeBoland & Norman, 1990), where rapid non-genomic stimulation of intestinal Ca2+ transport in chick duodenal loops induced by 1,25-dihydroxyvitamin D3 was completely abolished in the presence of either PKA or PKC inhibitors. Taken together, the results of these studies indicate that more than one second messenger pathway is operational during rapid non-genomic responses to steroid hormones. Whether these protein kinases share the same phosphate acceptor remains to be determined.
The effect on [Ca2+]i is also sex steroid-specific, as neither 17α-oestradiol, testosterone nor progesterone induced an increase in [Ca2+]i. The oestradiol-induced stimulatory effect on [Ca2+]i is also gender-specific, as 17β-oestradiol had no effect on [Ca2+]i in isolated colonic crypts from male Sprague-Dawley rats. The reason for this gender specificity is unknown and is currently under investigation.
The Ca2+ results presented here are compatible with those previously reported in duodenal enterocytes from female Wistar rats, in which Picotto et al. (1996) demonstrated a rapid non-genomic (1–10 min) stimulation by 17β-oestradiol of a cyclic AMP-mediated 45Ca influx that was verapamil- and nitrendipine-sensitive. Other sex steroids were inactive. In a more recent study, Picotto et al. (1999) have proposed that oestrogen-dependent stimulation of both PKA and PKC may contribute to the stimulation of Ca2+ influx in enterocytes. In chicken and pig granulosa cells (Morley et al., 1992) 17β-oestradiol has been reported to stimulate a release of Ca2+ from intracellular stores, with the oestradiol response being unaffected by pre-treatment with Ca2+ channel antagonists or in the presence of zero external Ca2+. Therefore, 17β-oestradiol appears to stimulate an increase in free [Ca2+]i with Ca2+ sources differing in various cell types.
‘Cross-talk' between second messenger pathways may be important in the regulation of membrane ion transport processes, particularly those involved in Cl− secretion (Levine et al., 1991; Warhurst et al., 1991). Studies using colonic epithelial cell line T84 have demonstrated that Ca2+-mobilizing agents can induce a potentiation of the secretory response to cyclic AMP-mediated agonists (Cartwright et al., 1985; Warhurst et al., 1991). Increases in [Ca2+]i appear to be the primary mediator of this interaction. However, the site at which the synergistic interaction between Ca2+ and cyclic AMP pathways takes place remains to be defined. It is possible that the linkage occurs at the second messenger level or, alternatively, there may be a co-operative interaction between ion channels activated by either Ca2+ or cyclic AMP (Barrett & Dharmasthaphorn, 1990). Intracellular Ca2+ is a known signal in the regulation of epithelial sodium absorption and chloride secretion. Previous studies from our laboratory have demonstrated a down-regulation of Cl− secretion and an up-regulation of Na+ reabsorption by 17β-oestradiol in human distal colon (McNamara et al., 1995; 1997). Whether these responses are dependent on the involvement of the cyclic AMP second messenger pathway remains to be defined. Results from other laboratories have also demonstrated a reduction in sodium excretion (i.e. increased sodium retention) in humans in response to oestrogens (Thorn & Engel, 1938; Preedy & Aitken, 1961; Katz & Kappas, 1967) and during pregnancy (Freyberg et al., 1938; Taylor et al., 1939; Thompson & Pommerenke, 1939; Chesley, 1944). Based on our data, we propose a model for the cellular mechanism of oestradiol-induced salt and water retention (Figure 10). In this model, salt retention is caused by inhibition of basolateral Ca2+-sensitive K+ channels (Perez & Toro, 1994; McNamara et al., 1995) which reduces the driving force for Cl− secretion.
Figure 10.
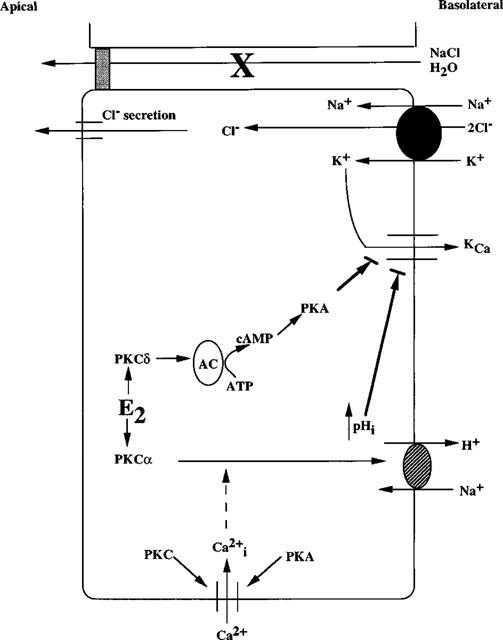
Model for the cellular mechanism of oestradiol-induced salt and water retention in female distal colon. (1) 17β-Oestradiol (E2) directly activates PKCδ activity. (2) PKCδ activates adenylyl cyclase activity (the isoform is as yet unknown), resulting in (3) an increase in PKA activity, followed by an inhibition of KCa channel activity (Perez & Toro, 1994). (4) 17β-Oestradiol also directly stimulates PKCα activity, resulting in an activation of Na+/H+ exchanger activity and intracellular alkalization followed by an inhibition of KCa channel activity. (5) Inhibition of KCa channel activity results in a down-regulation of chloride secretion, resulting in salt and water retention. Both PKC and PKA activation are required for Ca2+ entry (inhibited by chelerythrine chloride, (Rp)cAMPS and SQ22536).
Thus, we propose that the physiological role for the non-genomic action of oestradiol is to reduce fluid and electrolyte secretion in the distal colon, thus enhancing fluid retention.
Acknowledgments
We extend our gratitude to Dr Leo Quinlan, Department of Physiology, National University of Ireland, Galway, for technical assistance. This work was funded by a Wellcome Trust Programme Grant 040067/Z/93 and a grant from the Health Research Board of Ireland.
Abbreviations
- 8-AHA
8-(6-aminohexyl)aminoadenosine 3′ : 5′-cyclic monophosphate
- 8-S-CH3
8-thiomethyladenosine 3′ : 5′-cyclic monophosphate
- ER
(o)estrogen receptor
- N6BZL
N6-benzoyladenosine 3′ : 5′-cyclic monophospate
- (Rp)cAMPS
adenosine 3′ : 5′-cyclic phosphorothioate
References
- ARONICA S.M., KRAUS W.L., KATZENELLENBOGEN B.S. Estrogen action via the cAMP signalling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUKEMA H.M., DAVIDSON L.A., CHANG W.C., LUPTON J.R., DERR J.N., CHAPKIN R.S. Dietary modulation of rat colonic cAMP-dependent protein kinase activity. Biochim. Biophys. Acta. 1994;1224:51–60. doi: 10.1016/0167-4889(94)90112-0. [DOI] [PubMed] [Google Scholar]
- BARRETT K.E., DHARMSATHAPHORN K.Mechanisms of chloride secretion in a colonic epithelial cell line Textbook of Secretory Diarrhea 1990New York: Raven Press; 59–66.In: Lebenthal, E. & Duffey, M. (eds) [Google Scholar]
- BEEBE S.J., BLACKMORE P.F., CHRISMAN T.D., CORBIN J.D. Use of synergistic pairs of site selective cAMP analogs in intact cells. Methods Enzymol. 1988;159:118–139. doi: 10.1016/0076-6879(88)59013-4. [DOI] [PubMed] [Google Scholar]
- BINDER H.J., SANDLE G.I., RAJENDRAN V.M.Colonic fluid and electrolyte transport in health and disease The Large Intestine 1991New York: Raven Press; 141–168.In: Phillips, S.F., Shorter, R. & Pemberton, W. (eds) [Google Scholar]
- BRAUN T., DODS R.F. Development of an Mn2+-sensitive, ‘soluble' adenylate cyclase in rat testis (seminiferous tubules/cytosol/epididymal sperm/membrane associated) Proc. Natl. Acad. Sci. U.S.A. 1975;72:1097–1101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN J.B., PEPPERELL R.J., EVANS J.H.Disorders of ovulation The Infertile Couple 1987Churchill Livingstone; 2–60.In: Pepperell, R.J., Hudson, B. & Wood, C. (eds) [Google Scholar]
- CARTWRIGHT C.A., MCROBERTS J.A., MANDEL K.G., DHARMSATHAPHORN K. Synergistic action of cyclic AMP and calcium mediated chloride secretion in a colonic epithelial cell line. J. Clin. Invest. 1985;76:1837–1842. doi: 10.1172/JCI112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHESLEY L.C. Weight changes and water balance in normal and toxic pregnancy. Am. J. Obstet. Gynecol. 1944;48:565–593. [Google Scholar]
- CONDLIFFE S.B., DOOLAN C.M., HARVEY B.J.17β-oestradiol stimulates whole cell potassium currents in T84 cells J. Physiol. 1998511P30–P31.Abstract [Google Scholar]
- CUTLER R.E., MAIZELS E.T., HUNZICKER-DUNN M. Delta protein kinase C in the rat ovary: oestrogen regulation and localization. Endocrinology. 1994;135:1669–1678. doi: 10.1210/endo.135.4.7925131. [DOI] [PubMed] [Google Scholar]
- DAVIDSON L.A., JIANG Y.H., DERR J.N., AUKEMA H.M., LUPTON J.R., CHAPKIN R.S. Protein kinase C isoforms in human and rat mucosa. Arch. Biochem. Biophys. 1994;312:547–553. doi: 10.1006/abbi.1994.1344. [DOI] [PubMed] [Google Scholar]
- DE BOLAND A.R., NORMAN A. Evidence for involvement of protein kinase C and cyclic adenosine 3′,5′ monophosphate-dependent protein kinase in the 1,25-dihydroxy-vitamin D3-mediated rapid stimulation of intestinal calcium transport (transcaltachia) Endocrinology. 1990;127:39–45. doi: 10.1210/endo-127-1-39. [DOI] [PubMed] [Google Scholar]
- DESSAUER D.W., GILMAN A.G. Purification and characterization of a soluble form of mammalian adenylyl cyclase. J. Biol. Chem. 1996;28:16967–16974. doi: 10.1074/jbc.271.28.16967. [DOI] [PubMed] [Google Scholar]
- DOOLAN C.M., HARVEY B.J. Modulation of cytosolic protein kinase C activity and calcium ion activity by steroid hormones in rat distal colon. J. Biol. Chem. 1996;271:8763–8767. doi: 10.1074/jbc.271.15.8763. [DOI] [PubMed] [Google Scholar]
- DOOLAN C.M., KEENAN A.K. Inhibition by fatty acids of cAMP-dependent protein kinase activity in brush border membranes isolated from human placental vesicles. Br. J. Pharmacol. 1994;111:509–514. doi: 10.1111/j.1476-5381.1994.tb14766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN F.G., JONES I.V., FIFE R. Malignant hypertension associated with use of oral contraceptives. Br. Heart J. 1975;37:336–338. doi: 10.1136/hrt.37.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARHAT M.Y., ABI-YOUNES S., RAMWELL P.W. Oestradiol increases cyclic adenosine monophosphate in rat pulmonary vascular smooth muscle cells by a nongenomic mechanism. Biochem. Pharmacol. 1996;51:571–576. [PubMed] [Google Scholar]
- FERNANDEZ L.M., MASSHEIMER V., DE BOLAND A.R. Cyclic AMP-dependent membrane protein phosphorylation and calmodulin binding are involved in the rapid stimulation of muscle calcium uptake by 1,25-dihydroxyvitamin D3. Calcif. Tissue Int. 1990;47:314–319. doi: 10.1007/BF02555915. [DOI] [PubMed] [Google Scholar]
- FINIDORI-LEPICARD J., SCHORDERET-SLATKINE S., HANOUNE J., BAULIEU E.-E. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981;292:255–257. doi: 10.1038/292255a0. [DOI] [PubMed] [Google Scholar]
- FISCH I.R., FRANK J. Oral contraceptives and hypertension. JAMA. 1977;237:2499–2503. [PubMed] [Google Scholar]
- FREYBERG R.H., REEKIE R.D., FOLSOME C. A study of water, sodium and energy exchange during the latter part of pregnancy. Am. J. Obstet. Gynecol. 1938;36:200–207. [Google Scholar]
- GIEMBYCZ M.A., DIAMOND J. Evaluation of kemptide, a synthetic serine-containing heptapeptide, as a phosphate acceptor for the estimation of cAMP-dependent protein kinase activity in respiratory tissues. Biochem. Pharmacol. 1990;39:271–283. doi: 10.1016/0006-2952(90)90026-h. [DOI] [PubMed] [Google Scholar]
- HAM J., PARKER M.G. Regulation of gene expression by nuclear hormone receptors. Curr. Opin. Cell. Biol. 1989;1:503–511. doi: 10.1016/0955-0674(89)90012-4. [DOI] [PubMed] [Google Scholar]
- IYENGAR R. Molecular and functional diversity of mammalian Gs-stimulated adenylyl cyclases. FASEB J. 1993;7:768–775. doi: 10.1096/fasebj.7.9.8330684. [DOI] [PubMed] [Google Scholar]
- JACOBOWITZ O., CHEN J., PREMONT R.T., IYENGAR R. Stimulation of specific types of Gs-stimulated adenylyl cyclases by phorbol ester treatment. J. Biol. Chem. 1993;268:3829–3832. [PubMed] [Google Scholar]
- JACOBOWITZ O., IYENGAR R. Phorbol ester-induced stimulation and phosphorylation of adenylyl cyclase 2. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10630–10634. doi: 10.1073/pnas.91.22.10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG Y.H., AUKEMA H.M., DAVIDSON L.A., LUPTON J.R., CHAPKIN R.S. Localization of protein kinase C isozymes in rat colon. Cell Growth Differ. 1995;6:1381–1386. [PubMed] [Google Scholar]
- KATZ F.H., KAPPAS A. The effects of estradiol and estriol on plasma levels of cortisol and thyroid hormone-binding globulins and on aldosterone and cortisol secretion rates in man. J. Clin. Invest. 1967;46:1768–1777. doi: 10.1172/JCI105667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARAGH J.H. Oral contraceptive-induced hypertension nine years later. Am. J. Obstet. Gynecol. 1976;126:141–147. doi: 10.1016/0002-9378(76)90480-4. [DOI] [PubMed] [Google Scholar]
- LEVINE S.A., DONOWITZ M., WATSON A.J.M., SHARP G.W.G. Characterization of the synergistic interaction of Escherichia coli heat stable toxin and carbachol. Am. J. Physiol. 1991;261:G592–G601. doi: 10.1152/ajpgi.1991.261.4.G592. [DOI] [PubMed] [Google Scholar]
- LIEBERHERR M., GROSSE B., KACHKACHE M., BALSAN S. Cell signaling and estrogens in female rat osteoblasts: a possible involvement of unconventional nonnuclear receptors. J. Bone Miner. Res. 1993;8:1365–1376. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- LIN W.W., CHEN B.C. Distinct PKC isoforms mediate the activation of cPLA2 and adenylyl cyclase by phorbol ester in RAW264.7 macrophages. Br. J. Pharmacol. 1998;125:1601–1609. doi: 10.1038/sj.bjp.0702219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOHMANN S., WALTER U. Subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv. Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAIZELS E.T., MILLER J.B., CUTLER R.E., JACKIW V., CARNEY E.M., MIZUNO K., OHNO S., HUNZICKER-DUNNE E. Estrogen modulates Ca2+-independent lipid-stimulated kinase in the rabbit corpus luteum of pseudopregnancy: identification of luteal estrogen-modulated lipid-stimulated kinase as protein kinase C δ. J. Biol. Chem. 1992;267:17061–17068. [PubMed] [Google Scholar]
- MAIZELS E.T., SHANMUGAM M., LAMM M.L.G., HUNZICKER-DUNN M. Hormonal regulation of PKCδ protein and mRNA levels in the rabbit corpus luteum. Mol. Cell. Endocrinol. 1996;122:213–221. doi: 10.1016/0303-7207(96)03885-3. [DOI] [PubMed] [Google Scholar]
- MCNAMARA B., WINTER D.C., CUFFE J., O'SULLIVAN G., GEIBEL J., HARVEY B.J. Real time imaging of the rapid nongenomic oestrogen effects on cellular ion transport. Surgical Forum. 1997;83:522–524. [Google Scholar]
- MCNAMARA B., WINTER D.C., CUFFE J., O'SULLIVAN G., HARVEY B.J. Mechanism of oestrogen-induced salt and water retention. Surgical Forum. 1995;81:560–562. [Google Scholar]
- MEGGOUH F., LOINTIER P., SAEZ S. Sex steroid and 1,25-dihydroxyvitamin D receptor in human colorectal adenocarcinoma and normal mucosa. Cancer Res. 1991;51:1227–1233. [PubMed] [Google Scholar]
- MORLEY P., WHITFIELD J.F., VANDERHYDEN B.C., TSANG B.K., SCHWARTZ J.L. A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology. 1992;131:1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- MUGGE A., RIEDEL M., BARTON M., KUHN M., LICHTLEN P.R. Endothelium independent relaxation of human coronary arteries by 17β-oestradiol in vitro. Cardiovasc. Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- MURONO E.P., KIRDANI R.Y., SANDBERG A.A. Specific estradiol-17β binding component in adult rat kidney. J. Steroid Biochem. 1979;11:1347–1351. doi: 10.1016/0022-4731(79)90105-5. [DOI] [PubMed] [Google Scholar]
- NEER E.J. Physical and functional properties of adenylate cyclase from mature rat testis. J. Biol. Chem. 1978;253:5808–5812. [PubMed] [Google Scholar]
- PEREZ G., TORO L. Differential modulation of large-conductance KCa channels by PKA in pregnant and nonpregnant myometrium. Am. J. Physiol. 1994;266:C1459–C1463. doi: 10.1152/ajpcell.1994.266.5.C1459. [DOI] [PubMed] [Google Scholar]
- PICOTTO G., MASSHEIMER V., BOLAND R. Acute stimulation of intestinal cell calcium influx induced by 17β-estradiol via the cAMP messenger system. Mol. Cell. Endocrinol. 1996;119:129–134. doi: 10.1016/0303-7207(96)03799-9. [DOI] [PubMed] [Google Scholar]
- PICOTTO G., VAZQUEZ G., BOLAND R. 17β-Oestradiol increases intracellular Ca2+ concentration in rat enterocytes: potential role of phospholipase C-dependent store-operated Ca2+ influx. Biochem. J. 1999;339:71–77. [PMC free article] [PubMed] [Google Scholar]
- PREEDY J.R.K., AITKEN E.H. The determination of estrone, estradiol 17-β, and estriol in urine and plasma with column partition chromatography. J. Biol. Chem. 1961;236:1300–1311. [PubMed] [Google Scholar]
- ROCHEFORT H. Non steroidal antiestrogens are estrogen-receptor-targeted growth inhibitors that can act in the absence of estrogens. Horm. Res. 1987;28:196–201. doi: 10.1159/000180944. [DOI] [PubMed] [Google Scholar]
- ROY E.J., MACKAY R. The concentration of oestrogens in blood during pregnancy. J. Obstet. Gynaecol. Br. Commonw. 1962;69:13–17. doi: 10.1111/j.1471-0528.1962.tb00002.x. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-BUENO A., SANCHO M.J., COBBOLD P.H. Progesterone and oestradiol increase cytosolic Ca2+ in single rat hepatocytes. Biochem. J. 1991;280:273–276. doi: 10.1042/bj2800273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER S.J., KELLY M.B., TADDEO F.J., LARKIN J.D., YEAGER M.D., MCLANE J.A., HO C., STUBBS C.D. Direct activation of protein kinase C by 1α-25-dihydroxyvitamin D3. J. Biol. Chem. 1995;270:6639–6643. doi: 10.1074/jbc.270.12.6639. [DOI] [PubMed] [Google Scholar]
- STEFANO G.B., PREVOT V., BEAUVILLAIN J.-C., FIMIANI C., WELTERS I., CADET P., BRETON C., PESTEL J., SALZET M., BILFINGER T.V. Estradiol coupling to human monocyte nitric oxide release is dependent on intracellular calcium transients: evidence for an estrogen surface receptor. J. Immunol. 1999;163:3758–3763. [PubMed] [Google Scholar]
- STUMPF W.E., SAR M., NARBAITZ R., REID R.A., DE LUCA H.F., TANAKA Y. Cellular and subcellular localization of 1,25(OH)2-vitamin D3 in rat kidney: comparison with localization of parathyroid hormone and oestradiol. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1149–1153. doi: 10.1073/pnas.77.2.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYLVIA V.L., HUGHES T., DEAN D.D., BOYAN B.D., SCHWARTZ Z. 17β-Estradiol regulation of protein kinase C activity in chrondrocytes is sex-dependent and includes non genomic mechanisms. J. Cell. Physiol. 1998;176:435–444. doi: 10.1002/(SICI)1097-4652(199808)176:2<435::AID-JCP22>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- SZEGO C., DAVIS J. Inhibition of estrogen-induced cyclic AMP elevation in rat uterus by glucocorticoids. Life Sci. 1969;8:1109–1116. doi: 10.1016/0024-3205(69)90164-7. [DOI] [PubMed] [Google Scholar]
- TANG W.-J., GILMAN A.G. Construction of a soluble adenylyl-cyclase activated by Gsα and forskolin. Science. 1995;268:1769–1772. doi: 10.1126/science.7792604. [DOI] [PubMed] [Google Scholar]
- TAYLOR H.C., WARNER R.C., WELSH C.A. The relationship of the estrogens and other placental hormones to sodium and potassium balance at the end of pregnancy and in the puerperium. Am. J. Obstet. Gynecol. 1939;838:748–777. [Google Scholar]
- THOMAS M.L., XU X., NORFLEET A.M., WATSON C.S. The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology. 1993;132:426–430. doi: 10.1210/endo.132.1.8419141. [DOI] [PubMed] [Google Scholar]
- THOMPSON H.E., POMMERENKE W.T. Electrolyte and nitrogen metabolism in pregnancy. J. Nutr. 1939;17:383–392. [Google Scholar]
- THORN G.W., ENGEL L.L. The effect of sex hormones on the renal excretion of electrolytes. J. Exp. Med. 1938;68:299–312. doi: 10.1084/jem.68.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERSTOVEK G., BYRD A., FREY M.R., PETRELLI N.J., BLACK J.D. Colonocyte differentiation is associated with increased expression and altered distribution of protein kinase isozymes. Gastroenterology. 1998;115:75–85. doi: 10.1016/s0016-5085(98)70367-1. [DOI] [PubMed] [Google Scholar]
- WARHURST G., HIGGS N.B., TONGE A., TURNBERG L.A. Stimulatory and inhibitory actions of carbachol on chloride secretory responses in human colonic cell line, T84. Am. J. Physiol. 1991;261:G220–G228. doi: 10.1152/ajpgi.1991.261.2.G220. [DOI] [PubMed] [Google Scholar]
- WHISNANT R.E., GILMAN A.G., DESSAUER C.W. Interaction of the two cytosolic domains of mammalian adenylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6621–6625. doi: 10.1073/pnas.93.13.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITT J.J., ROSKOSKI R. Rapid protein kinase assay using phosphocellulose-paper absorption. Anal. Biochem. 1975;66:253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA M., COOPER D.M. Type-specific stimulation of adenylyl cyclase by protein kinase C. J. Biol. Chem. 1993;268:4604–4607. [PubMed] [Google Scholar]


