Abstract
The immunosuppressants rapamycin and FK506 are known to relax smooth muscle despite facilitating Ca2+ release through ryanodine-receptors of the sarcoplasmic reticulum (SR). The apparent contradiction was studied in isolated guinea-pig urinary bladder myocytes.
Modulation of spontaneous SR Ca2+ release was monitored by means of spontaneous transient outward currents (or STOCs) in isolated smooth muscle cells voltage-clamped to −20 mV. Rapamycin (10 μM, n=18) significantly increased amplitude (50±12%, mean±s.d.), life time (77±19%), and time integral of STOCs (113±22%), and it reduced the interval between STOCs (20±7%). FK506 (20 μM, n=24) increased amplitude (15±7%), life time (50±7%), time integral (104±26%). Cyclosporin A (20 μM, n=18) had no significant effects on STOCs.
The basal cytoplasmic Ca2+ concentration ([Ca2+]c) measured by Indo1-fluorescence was insensitive to rapamycin or FK506. Pretreatment with rapamycin (20 μM, 2 min) did not impair the SR Ca2+ load as can be concluded from caffeine-induced Ca2+-transients.
As it was expected from the enhanced STOC activity, the non-clamped membrane was hyperpolarized by rapamycin (15±2 mV) or by FK506 (15±3 mV).
The data are consistent with the idea that rapamycin and FK506 augment spontaneous SR Ca2+ release by removal of FK-binding proteins from the RyR-complex. Smooth muscle relaxation is interpreted as negative Ca2+ feedback: augmented Ca2+ activation of STOCs induces membrane hyperpolarization that reduces Ca2+ influx through voltage gated channels.
Keywords: STOCs, smooth muscle cells, relaxation, Ca2+ release, Ca2+ feedback, FKBP
Introduction
Rapamycin and FK506 are drugs used as immunosuppressants that may cause intestinal and cardiovascular side-effects (e.g. Drake et al., 1996; Bielefeldt et al., 1997; Lo Rŭsso et al., 1997; for structure of FK506 see Figure 1). Some of these side-effects may be independent of the immunosuppression and result from an interaction with the FK binding proteins. FK-binding proteins (FKBPs) are a family of peptidylprolyl cis-trans-isomerases that modulate the function of a variety of intracellular targets. In the cytosol, the total concentration of FKBPs may range between 5 and 25 μM (Zarnt et al., 1995). Rapamycin or FK506 have been reported to remove FKBP12 from the ryanodine receptor (RyR) Ca2+ release channel (Wagenknecht et al., 1996). Single channel analysis of RyR1 (skeletal isoform) and RyR2 (cardiac isoform), reconstituted into lipid bilayer membranes, indicated that supramaximal concentration of rapamycin (10 μM) or FK506 (20 μM) prolonged the RyR channel open time and suggested that binding of FKBP12 to RyR1 accelerates RyR channel inactivation (Ahern et al., 1997; Chen et al., 1994; Mayrleitner et al., 1994; Kaftan et al., 1996; Xiao et al., 1997). The in-vitro results are in line with in-vivo confocal Ca2+ imaging studies of spontaneous SR Ca2+ release events (sparks), demonstrating that FK506 prolonged the life time and increased the frequency of sparks as can be expected from the longer RyR2 open state (rat ventricular myocytes: Xiao et al., 1997).
Figure 1.
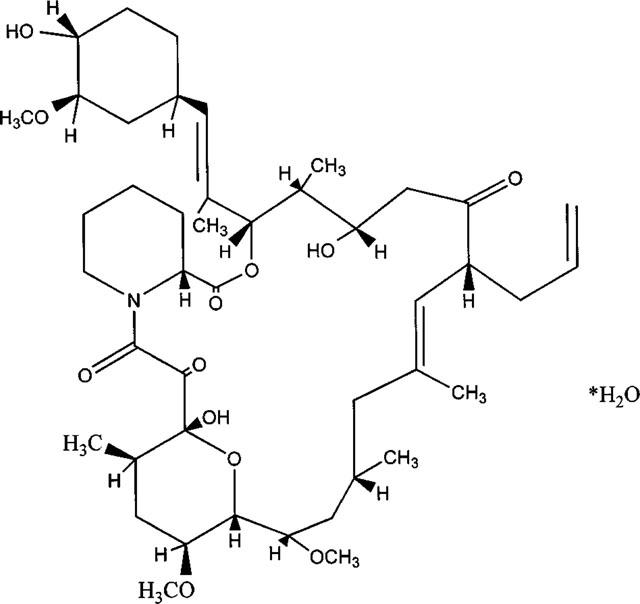
Structure of FK506.
For RyR from smooth muscle, the importance of FKBPs for RyR gating is essentially unknown. Although one may extrapolate the results from skeletal and cardiac muscle and assume that also the Ca2+ release events of smooth muscle cells may be prolonged, such an extrapolation is questionable because smooth muscle cells express both isoforms RyR2 and RyR3 (for bladder smooth muscle see Wendt-Gallitelli et al., 1998). Both in-vitro and in-vivo studies on the effects of rapamycin and FK506 on individual SR Ca2+ release events are missing.
Recently, Bielefeldt et al. (1997) reported for smooth muscle strips from rat jejunum that 10 μM FK506 suppressed the spontaneous contractions as well as the tension induced by 100 μM carbachol. In addition, for cultured human jejunal smooth muscle cells FK506 was reported to reduce the amount of caffeine-releasable Ca2+. The authors postulated that FK506 would prolong the RyR open time with the consequences of augmentation of Ca2+ release, deprivation of SR Ca2+ load and impairment of contractile activation.
In smooth muscle cells, SR Ca2+ load and SR Ca2+ release are under the control of the membrane potential (e.g. Ganitkevich & Isenberg, 1991; 1993). Hence, we have re-investigated Bielefeldt's hypothesis under the conditions of the voltage-clamp. Individual Ca2+ release events and their change by FK506 or rapamycin were monitored by spontaneous transient outward currents (STOCs) that flow through clusters of high conductance Ca2+ activated K+ channels (synonymous BKCa-channels). STOCs have been described to be a good and convenient indicator of the SR Ca2+ release (Benham et al., 1985; 1986; Trieschmann & Isenberg 1989; Ganitkevich & Isenberg, 1990) and of the local Ca2+ concentration in the subsarcolemmal space (Stehno-Bittel & Sturek, 1992; Ganitkevich & Isenberg, 1996; Bolton & Imaizumi, 1996; Nelson et al., 1995; Porter et al., 1998). The old speculation that STOCs arise from spontaneous release of Ca2+ from SR localized close to the sarcolemma (Benham et al., 1985; 1986; Trieschmann & Isenberg, 1989) has been supported by the recent studies on Ca2+ sparks of smooth muscle cells that were attributed to brief focal releases of SR Ca2+ (Nelson et al., 1995; Gordienko et al., 1998; Porter et al., 1998).
The results of the present study indicate that rapamycin and FK506 (but not cyclosporin A) augment the activity of STOCs by an increase in the STOC amplitude, frequency and duration. They do not confirm that rapamycin and FK506 deprive the SR of releasable Ca2+, i.e. the drugs did not reduce but facilitated the caffeine-induced Ca2+ release. Hence, we suggest that relaxation of smooth muscle by rapamycin and FK506 may be caused by a rather indirect mechanism, that is, augmentation of STOCs hyperpolarizes the membrane thereby closing L-type Ca2+ channels and reducing the cellular Ca2+ load.
Methods
Cell preparation and solutions
Guinea-pigs of ca. 250 g were killed by cervical dislocation, and then the urinary bladder was removed. Single bladder smooth muscle cells were enzymatically isolated according to Klöckner & Isenberg (1985). In brief, chunks of smooth muscular tissue were stirred for 10 min in a Ca2+ free medium composed of (in mM): NaCl 140, KCl 5, MgCl2 1.2, glucose 10, taurine 20, HEPES 5, adjusted with NaOH to pH 7.4. After wash out of Ca2+, the chunks were incubated in the same Ca2+ free medium complemented with 5 mg% collagenase (Boehringer, Mannheim), 2 mg% pronase (Serva, Heidelberg) and 100 μM CaCl2. Cells released after 20 min stirring were discarded, those for the experiments were released after an additional 20 min stirring in fresh enzyme medium.
The acutely isolated cells were continuously superfused with a physiological salt solution (PSS) containing (mM) NaCl 150, CaCl2 3.6, MgCl2 1.2, KCl 5.4, glucose 20, HEPES 10, adjusted to pH 7.4 with NaOH. The pipette solution was composed of (in mM) KCl 140, MgCl2 0.5, EGTA 0.01, HEPES 5, adjusted with NaOH to pH 7.2. When ratiometric [Ca2+]c measurements were performed, 0.1 mM Na5Indo-1 was added to the pipette solution (see Ganitkevich & Isenberg, 1991). The Ca2+ signals were measured by a pair of photomultipliers and an analogue divider that delivered the ratio R=F410/F470 where at F410 and F470 are the Ca2+ sensitive and the Ca2+ insensitive fluorescence intensities of Indo-1 (for details see Ganitkevich & Isenberg, 1991). The Ca2+ signals will be presented as normalized signals ΔR/Ro where Ro is the baseline and ΔR=R−Ro is the caffeine-induced deviation from Ro. All experiments were performed at room temperature (22°C).
Voltage clamp
Whole-cell membrane currents were recorded with patch pipettes of 2–3 MΩ resistance connected to a RK-400 patch-clamp amplifier (Biologic, Echirolles, France). The currents were filtered at 1 kHz and recorded with a thermo-writer (Gould, TA 550, Cleveland, Ohio, U.S.A.). The currents were not corrected for leakage. A microcomputer in combination with a CED-1401 interface (Science Park, Cambridge, U.K.) was used for generating the voltage-clamp commands and for storing the digitized data (digitizing frequency 2 kHz). For measurements of the membrane potential, the RK-400 amplifier was switched into the current-clamp mode. To make sure that cell dialysis was not a severe problem, measurements were repeated by using nystatin-perforated instead of ruptured patches (n=4).
Drugs
Rapamycin was obtained from Sigma (St. Louis, U.S.A.). It was prepared as a 10 mM stock solution in DMSO and frozen until use. Before the experiment, the stock solution was diluted by adding PSS to give a final concentration of 10 μM. FK506 was a gift from Fujisawa, Japan. A 10 mM stock solution of FK506 was prepared by using a mixture of 82% ethanol plus 18% Tween 80 as solvent, it was diluted with PSS to a final concentration of 20 μM. The 10 mM cyclosporin A stock solution used the same mixture of 82% ethanol plus 18% Tween 80, it was diluted with PSS to a final concentration of 20 μM.
Possible effects of the solvents
The final concentrations of the solvents were 0.2% DMSO, or 0.018% Tween 80 plus 0.08% ethanol. Since the solvents may modulate the membrane currents of interest, in a series of control experiments guinea-pig urinary bladder myocytes were clamped to −20 mV and the STOC activity was measured for 2 min of superfusing control PSS. Thereafter, the superfusate was changed to PSS containing either 0.4% DMSO (n=5). When the STOC activity in absence and presence of the solvents was compared, no significant differences could be detected (paired t-test, P<10%). That is, the presence of DMSO insignificantly increased the life time of the STOCs by 4.1±5.3%, the interval between the STOCs by 1.3±5.2%, the amplitude by 6.4±7.3% and the time integral over the STOCs by 3.7±4.4%. The presence of 0.03% Tween 80 plus 0.2% ethanol (n=5) insignificantly increased the life time of the STOCs 5.0±1.9%, the interval by 1.1±2.9%, the amplitude by 8.1±7.2% and the area by 4.1±4.8%.
Statistics
The results are presented as means with the standard deviation (s.d.). For statistical evaluation of significance, we compared data that were recorded from the same cell before and after addition of the drug with a paired t-test. Significance of the drug effects was tested at the 5% level, when other significance levels were tested this will be indicated in the text.
Results
Spontaneous transient outward currents (STOCs) at −20 mV
STOCs that flow through clusters of Ca2+ activated 200 pS K+ channels (BKCa-channels) have been described for a variety of smooth muscle cells, including those from the urinary bladder of the guinea-pig (for references see Bolton & Imaizumi, 1996). The STOC activity is significantly enhanced after depolarization of the membrane from the resting potential (ca. −40 mV, Klöckner & Isenberg, 1985) to −20 mV, hence the present study analysed the STOC activity at −20 mV after a 2 min period of stabilization. Examples are illustrated in Figure 2.
Figure 2.
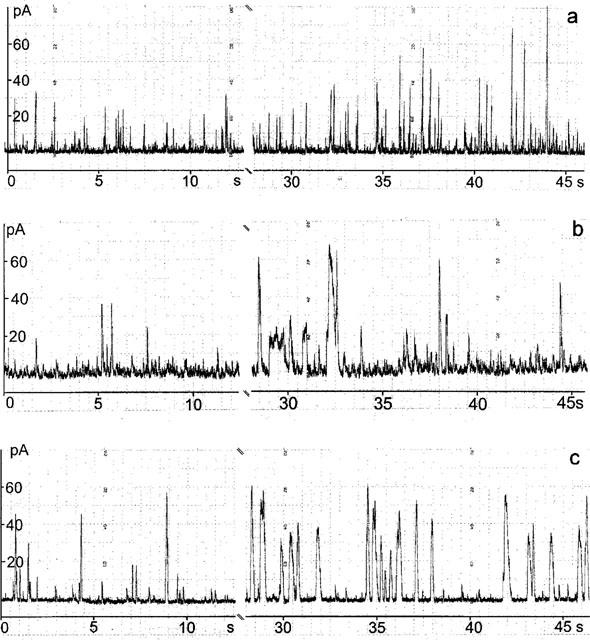
Modulation of spontaneous transient outward currents (STOCs) by 20 μM rapamycin (a) or 20 μM FK506 (b). Holding potential −20 mV. The gap after the control represents the 10 s period of the solution change.
The amplitudes of STOCs broadly distributed around an average of 65 pA. The fit with a Gaussian distribution yielded a standard deviation (s.d.) of 27 pA (59 cells). The ‘STOC life time' was defined as the period during which the STOC amplitude exceeded the 10 pA threshold value. The individual life times were grouped in bins of 8 ms and plotted in a life time histogram (e.g. Figure 3b). The fit with a Gaussian distribution provided a life time of 32 ms (36±3 ms, n=59). Similarly, the interval between the STOCs was evaluated as the period during which the current did not exceed the 10 pA threshold, fits with the Gaussian distribution yielded for the interval between the STOCs a value of 920±60 ms. The averaged STOC activity was calculated by integrating the STOCs over a 100 s period of time and dividing the result by 100 s. The value of averaged STOC activity was 2.5±0.2 pA (n=59). Superfusion of a solution that contained the solvents for rapamycin (DMSO) or for FK506 (Tween 80 plus ethanol) without the drugs did not change the life-time, the interval between the STOCs or the STOC activity (see Methods).
Figure 3.
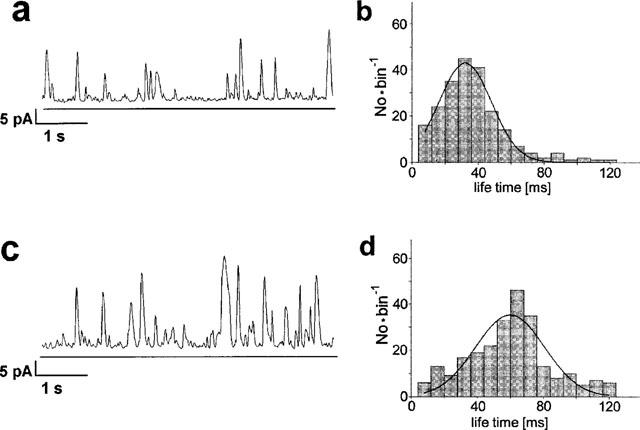
Modulation of STOCs by 10 μM rapamycin. Left: Original traces showing STOCs before (a) and 2–5 min after addition of 10 μM rapamycin (c). Right: Lifetime histograms representing the duration of the STOCs at control (b) and in presence of rapamycin (d). The life time was defined as period during which the STOC exceeds a threshold value of 10 pA. Observed life times were grouped in bins of 8 ms. Distributions were fitted with Gaussian curves, the peak of these curves occurred at 38 ms in absence and at 62 ms in presence of rapamycin.
Augmentation of STOCs by rapamycin
The effects of rapamycin on STOC activity were analysed at 10 μM, a concentration that has been shown to be supramaximal (Bielefeldt et al., 1997; Xiao et al., 1997). The experiments started with a 2 min control period. Application of rapamycin increased the STOC activity without changing the current between them (Figure 2a). Similar rapamycin effects were recorded with nystatin perforated patches (Figure 2b). The augmentation of STOCs stabilized within 30 s and was stable for at least 5 min. The rapamycin-effects were quantified by comparing in the same cell the STOC activity before and 2–5 min after addition of the drug.
Rapamycin increased frequency, amplitude and duration of the STOCs. Figure 3 illustrates the effects by records from a typical experiment by a computer play-back. Paired comparison (n=20) indicates that rapamycin significantly increased the STOC amplitude by 50±12%. The histogram of a typical experiment shows a life-time of 32 ms before and of 56 ms after addition of rapamycin (Figure 3b), this increase in life time was by 77±19% on average. Figure 3c shows that rapamycin increases the STOC frequency by reducing the interval between the STOCs (20±7%). The results were reproduced by those from experiments with nystatin perforated patches (n=4) where rapamycin increased the STOC amplitude by 48±22%, the life time by 81±32% and reduced the interval between the STOCs by 31±19%.
Augmentation of STOCs by FK506
FK506 was applied at 20 μM a concentration that had been shown to be supramaximal in terms of relaxation of vascular smooth muscle strips (Bielefeldt et al., 1997). Original traces from a representative experiment (Figure 2c) indicate that FK506 increased duration and amplitude of the STOCs but had no influence on the current between the individual STOC events.
Figure 4 compares the STOC activity before and 2–5 min after addition of FK506, longer STOCs can be attributed to 15±7% (n=24) longer life times (from 40 to 56 ms in Figure 4b). FK506 increased the STOC-amplitude by 15±7%. FK506 did not reduce the interval between the STOCs (insignificant increase by 20±16%). FK506 increased the averaged STOC activity from 1.7 to 3.4 pA or by 104±26% (calculated from the STOC time integral).
Figure 4.
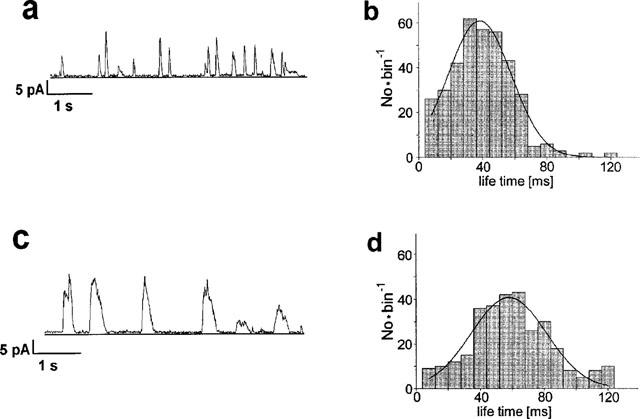
Modulation of STOCs by 20 μM FK506. Left: Original traces showing STOCs before (a) and 2–5 min after addition of FK506 (c). Right: Life time histograms, the fitting Gaussian curves peaked at a life time of 35 ms before (b) and of 54 ms after addition of FK506 (d).
STOCs are insensitive to cyclosporin A
Cyclosporin A is an immunosuppressive drug like rapamycin or FK506 that interacts with calcineurin phosphatases. It also interacts with peptidylprolyl cis-trans-isomerases, however, not like rapamycin or FK506 with the subfamily of the FK-binding proteins but with the cyclo-philine family. Hence, the analysis of a possible cyclosporin A effects on STOCs was performed to test whether the effects of rapamycin and FK506 were specific for their interaction with the FK binding proteins. A concentration of 10 μM cyclosporin A was used because this concentration was reported to be supramaximal in terms of vascular relaxation (Lo Rŭsso et al., 1997). The traces of Figure 5 suggest that cyclosporin A rather reduced than increased the STOC activity, paired comparison indicated an insignificant reduction by 8±12% (n=18, P=0.83). The interval between the STOCs was insignificantly increased (42±25%, P=0.36). In the experiment of Figure 5, the lifetime of the STOCs was 32 ms before and 32 ms after addition of cyclosporin A (insignificant increase by 8±12%, P=0.36). The average activity of STOCs did not significantly change either (increase by 13±32%, P=0.26).
Figure 5.
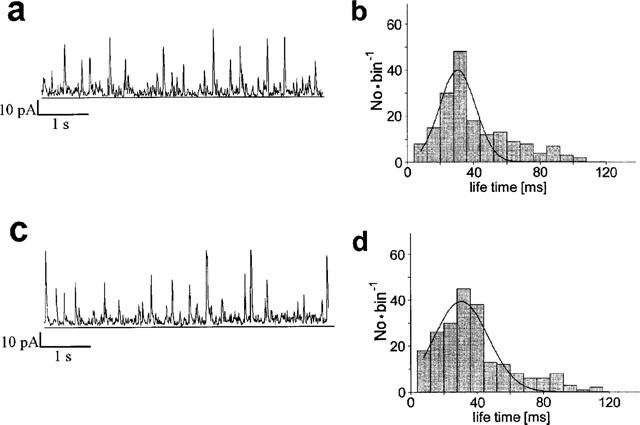
STOCs are insensitive to 20 μM cyclosporin A. Left: Original traces showing STOCs before (a) and 2–5 min after addition of cyclosporin (c). Right: Life time histograms, the fitting Gaussian curves peaked at a life time of 39 ms in absence (b) and of 33 ms in presence of cyclosporin A (d).
Rapamycin-pretreatment does not abolish caffeine induced SR Ca2+ release and IK(Ca)
It has been suggested that rapamycin and FK506 relax vascular smooth muscle cells by depriving the SR of releasable Ca2+-ions (Bielefeldt et al., 1997). The conclusion was based on the result that FK506 blocked caffeine-induced force and Ca2+ transients, hence, we repeated this type of experiments in acutely isolated bladder smooth muscle cells under the conditions of the voltage-clamp.
Figure 6 illustrates the effects of rapamycin on the caffeine induced outward currents (trace labelled IK) and Ca2+ signals. In the absence of 20 μM rapamycin, the first application of 20 mM caffeine induced an outward current that peaked within 0.6 s more than 80 pA and then decayed (Figure 6a). Caffeine increased the Ca2+ fluorescence ΔR/Ro along the following time course: from the baseline Ro, ΔR/Ro rose at a maximal rate of 0.52±0.14 s−1, peaked within 1.22±0.22 s to 2.54±0.55 and then fell at a maximal rate of −0.18±0.05 s−1. After a 5 min period of wash, caffeine was applied a second time (n=5), these second responses to caffeine did not differ from those evaluated from the first caffeine application (Figure 6b).
Figure 6.
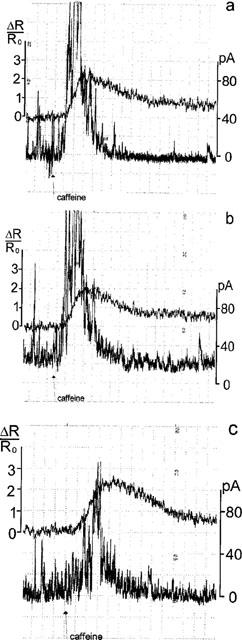
Rapamycin does not block the caffeine-induced Ca2+ signals. Membrane currents (STOCs and outward currents) are calibrated on the left, normalized Ca2+ signals ΔR/Ro at the right. (a) Control. First application of 20 mM caffeine. STOCs added up to a Ca2+ activated K+ current IK(Ca) that peaked values slightly above 80 pA. ΔR/Ro increased at a maximal rate of 0.55 s−1 within 1.1 s to a peak of 2.0 and then decayed. (b) Second caffeine-application after a wash-out period of 5 min. Effects on IK(Ca) and on ΔR/Ro resemble those during the first caffeine-application. (c) Caffeine-application 2 min after continuous superfusion of 20 μM rapamycin. IK(Ca) peaked to 60 pA. ΔR/Ro increased at a rate of 0.6 s−1 within 2.0 s to a peak of 2.2 and then decayed.
Rapamycin was applied during the first wash out of caffeine. Five minutes after wash out of caffeine and 2 min after addition of rapamycin, the caffeine-induced Ca2+ signal was measured again (Figure 6c): ΔR/Ro rose at a maximal rate of 1.16±0.15 s−1 (n=6), peaked within 1.0±0.14 s to 2.55±0.36 and decayed at a rate of −0.26±0.08 s−1. The comparison of the caffeine-induced Ca2+ signals in absence and presence of rapamycin indicates that rapamycin increased the rate of rise and shortened the time to peak whilst it had no effect on the amplitude of the peak or the rate of the decay (P<0.05). In four cells superfused with rapamycin, caffeine could be applied a second time. Then, the Ca2+ signal increased at lower rate (0.72±0.1 s−1) to a similar peak (2.75±0.41) that occurred at later times (2.5±0.26 s) and decayed more slowly (−0.13±0.03 s−1).
Rapamycin and FK506 hyperpolarize the membrane
In vivo, the cell membrane is not voltage-clamped, and the activation of K+ channels (underlying the increased STOC activity) is expected to shift the membrane potential towards the K+-equilibrium potential. Since membrane hyperpolarization has been reported to cause smooth muscular relaxation (see Discussion), we analysed the effect rapamycin and FK506 on the membrane potential under current clamp conditions (external current clamped to zero).
Figure 7a illustrates that 10 μM rapamycin hyperpolarized the membrane from ca. −37 mV to ca. −53 mV. The membrane hyperpolarization of 15±2 mV (n=5) was stable for at least 5 min. Figure 7b shows a similar hyperpolarization induced by 20 μM FK506 (15±3 mV, n=4).
Figure 7.
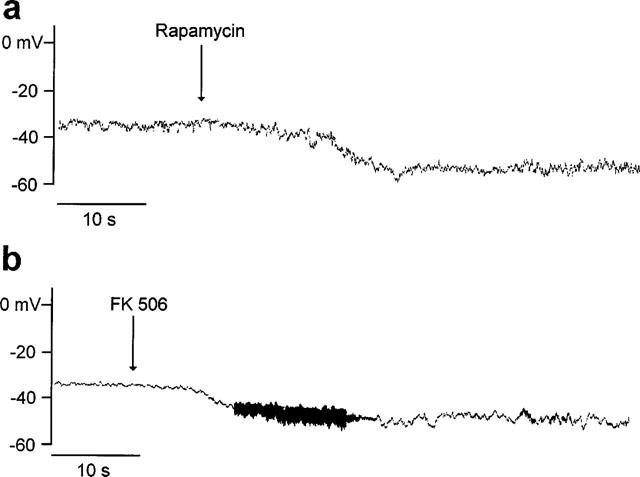
FK506 and rapamycin hyperpolarize bladder smooth muscle cells. Whole-cell recording, amplifier in current-clamp mode. (a) 10 μM; (b) 20 μM. Note: the high frequency noise is an artifact of the solution change.
Discussion
The results of this study indicate that rapamycin and FK506 modulate the events of spontaneous SR Ca2+ release as they can be monitored by STOCs. The missing influence of cyclosporin A on STOC activity suggests that the rapamycin- and FK506-induced modulation of STOCs is not related to the immunosuppressant effect of these drugs and that it is specific for their interaction with the FK binding proteins.
Our analysis attributed the rapamycin- and FK506-mediated increase in STOC activity to an increase in both STOC amplitude and STOC life time. We speculate that both effects result from a prolonged RyR open state (reconstituted RyR1 and RyR2: Ahern et al., 1997; Mayrleitner et al., 1994; Xiao et al., 1997). The Ca2+ release flux is thought to generate a cloud of elevated [Ca2+] in the ‘fuzzy' space between the peripheral SR and the sarcolemma (e.g. Yoshikawa et al., 1996; for review see Bolton & Imaizumi, 1996), prolongation of the Ca2+ release flux should increase [Ca2+] in this cloud and increase its spatial dimensions. With this hypothesis we interpret the observed elevation of STOC amplitude as a recruitment of BKCa channels to the cluster of BKCa channels generating the single STOC. The high local [Ca2+]c may propagate and activate Ca2+ induced Ca2+ release from neighboured SR tubules, a mechanism that could explain the observed increase in STOCs frequency. Alternatively, the increase in STOC frequency could be caused by a direct activation of RyR due to removal of FKBP12 by rapamycin (Ahern et al., 1997).
The present results do not clarify the functional importance of the RyR3 isoform for spontaneous SR Ca2+ release in urinary bladder smooth muscle cells. If Ca2+ release through RyR3 would significantly contribute to the activation of STOCs then one should postulate that interaction of the FKBP12 with the RyR3 would accelerate the inactivation of the RyR3 channel isoform similarly as reported for the skeletal and cardiac isoform of the ryanodine-receptor (Chen et al., 1994; Mayrleitner et al., 1994; Kaftan et al., 1996; Xiao et al., 1997). Alternatively, the present results could be explained with the assumption that Ca2+ release flux through RyR3 does not significantly contribute to the generation of STOCs.
Our main finding, rapamycin and FK506 augmenting spontaneous Ca2+ release, is in line with the suggestion of Bielefeldt et al. (1997) that these drugs would relax smooth muscle by promoting Ca2+ release flux. However, the present results do not support the interpretation that rapamycin and FK506 would relax the smooth muscle cell by depleting the SR of releasable Ca2+. Firstly, the depletion of releasable Ca2+ should have reduced or abolished the activity of STOCs with the consequence of membrane depolarization, just contrary to the effects demonstrated here. Secondly and different to the report of Bielefeldt et al. (1997), we did not find that rapamycin abolished the caffeine-induced SR Ca2+ release, i.e. in absence or presence of rapamycin the amplitudes of the caffeine-induced signals did not differ. We interpret that the effects of augmented spontaneous SR Ca2+ release (STOCs) on the SR Ca2+ filling (peak of the caffeine-induced Ca2+ signal) were compensated by Ca2+ reuptake via the SR Ca2+ ATPase (SERCA). The rate of rise of the caffeine-induced Ca2+ transient was increased by rapamycin, as if rapamycin had increased the rate of the Ca2+ release flux via a higher RyR open probability (see above).
The present results and those from Bielefeldt et al. (1997) are not necessarily exclusive because they were obtained in smooth muscle cells from different specimen, in the present study from guinea-pig urinary bladder, in the study of Bielefeldt et al. (1997) from the jejunum. Different smooth muscular specimens are known to deprive their releasable Ca2+ after application of acetylcholine more rapidly or more slowly depending on the balance between the Ca2+ release flux and RyR Ca2+ reuptake via SERCA (Missiaen et al., 1992). Hence, it might be possible to generalize the consequences of rapamycin-augmented SR Ca2+ release from one smooth muscle to the other. Thus, we do not exclude the Ca2+ deprivation hypothesis as an alternative mechanism that mediates relaxation of smooth muscle cells.
The present data suggest that rapamycin or FK506 could relax smooth muscle cells by an additional mechanism which is called ‘negative Ca2+ feedback'. Our current clamp measurements demonstrated that rapamycin and FK506 induced a 15 mV membrane hyperpolarization. The mechanism, augmented SR Ca2+ release leading to Ca2+ activation of K+ channels and membrane hyperpolarization, has been described in a variety of smooth muscular specimen (Klöckner & Isenberg, 1989; Brayden & Nelson, 1992; for references see Nelson & Quayle, 1995); the membrane hyperpolarization is called ‘negative Ca2+ feedback' because it turns down Ca2+ influx through voltage-operated L-type Ca2+ channels (see also Porter et al., 1998). The contribution of this Ca2+ influx to the concentration of Ca2+ ions that finally activate contraction varies between smooth muscle cells from different tissues. The present results suggest that this negative Ca2+ feedback on Ca2+ influx can have a larger influence on Ca2+ homeostasis and SR Ca2+ filling than the stimulation of spontaneous SR Ca2+ release, at least in non-stimulated smooth muscle cells from the guinea-pig urinary bladder.
Abbreviations
- [Ca2+]c
concentration of Ca2+ ions in the cytosol
- DMSO
dimethyl sulphoxide
- FKBP
FK binding protein (target for FK506)
- FK506
macrocyclic triene antibody from Streptomyces hydroscopicus
- HEPES
N-[2-Hydroxyethylpiperazine-N′-[2-ethanesulphonic acid]
- IK(Ca)
Ca2+ activated potassium current
- PSS
physiological salt solution
- Rapamycin
C44H69NO12, r-INN Tacrolimus
- RyR
Ryanodin-receptor Ca2+-release channel
- SR
sarcoplasmic reticulum
- STOC
spontaneous transient outward current
- Tween 80
Polyoxyethylenesorbitan monooleate
References
- AHERN G.P., JUNAKAR P.R., DULHUNTY A.F. Ryanodine receptors from rabbit skeletal muscle are reversibly activated by rapamycin. Neurosci. Lett. 1997;225:81–84. doi: 10.1016/s0304-3940(97)00193-6. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B., LANG R.J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B., LANG R.J., TAKEWAKI T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J. Physiol. 1986;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIELEFELDT K., SHARMA R.V., WHITEIS C., YEDIDAG E., ABBOUD F.M. Tacrolimus (FK506) modulates calcium release and contractility of intestinal smooth muscle. Cell Calcium. 1997;22:507–514. doi: 10.1016/s0143-4160(97)90078-6. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B., IMAIZUMI Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/s0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- BRAYDEN J.E., NELSON M.T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- CHEN S.R.W., ZHANG L., MACLENNAN D.H. Asymmetrical blockade of the Ca2+ release channel (ryanodine receptor) by 12-kDa FK506 binding protein. Proc. Nat. Acad. Sci. U.S.A. 1994;91:11953–11957. doi: 10.1073/pnas.91.25.11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAKE M., FRIBERG H., BORIS-MÖLLER F., SAKATA K., WIELOCH T. The immunosuppressant FK506 ameliorates ischemic damage in rat brain. Acta Physiol. Scand. 1996;158:155–159. doi: 10.1046/j.1365-201X.1996.535298000.x. [DOI] [PubMed] [Google Scholar]
- GANITKEVICH V., ISENBERG G. Isolated guinea pig coronary smooth muscle cells: Acetylcholine induces hyperpolarization due to sarcoplasmic reticulum calcium release activating potassium channels. Circ. Res. 1990;67:525–528. doi: 10.1161/01.res.67.2.525. [DOI] [PubMed] [Google Scholar]
- GANITKEVICH V., ISENBERG G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J. Physiol. 1991;435:187–205. doi: 10.1113/jphysiol.1991.sp018505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANITKEVICH V., ISENBERG G. Membrane potential modulates inositol 1,4,5-triphosphate mediated Ca2+ transients in guinea pig coronary myocytes. J. Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANITKEVICH V., ISENBERG G. Dissociation of subsarcolemmal from global cytosolic [Ca2+] in myocytes from guinea-pig coronary artery. J. Physiol. 1996;490:305–318. doi: 10.1113/jphysiol.1996.sp021145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDIENKO D.V., BOLTON T.B., CANNELL M.B. Variability in spontaneous subcellular calcium release in guinea-pig ileum smooth muscle cells. J. Physiol. 1998;507:707–720. doi: 10.1111/j.1469-7793.1998.707bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAFTAN E., MARKS A.R., EHRLICH B.E. Effects of rapamycin on ryanodine receptor Ca2+-release channels from cardiac muscle. Circ. Res. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- KLÖCKNER U., ISENBERG G. Action potential and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflügers Arch. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- KLÖCKNER U., ISENBERG G. The dihydropyridine niguldipine modulates calcium and potassium currents in vascular smooth muscle cells. Br. J. Pharmacol. 1989;97:957–967. doi: 10.1111/j.1476-5381.1989.tb12037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LO RŭSSO A., PASSAQUIN A.C., COX C., RUEGG U.T. Cyclosporin A potentiates receptor-activated [Ca2+]c increase. J. Recept. Signal Transduct. Res. 1997;17:149–161. doi: 10.3109/10799899709036600. [DOI] [PubMed] [Google Scholar]
- MAYRLEITNER M., TIMERMAN A.P., WIEDERRECHT G., FLEISCHER S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506 binding protein: Effect of FKBP-12 on single channel activity of the skeletal muscle ryanodine receptor. Cell Calcium. 1994;15:99–108. doi: 10.1016/0143-4160(94)90048-5. [DOI] [PubMed] [Google Scholar]
- MISSIIAEN L., DESMEDT H., DROOGMANS G., HIMPENS B., CASTEELS R. Ca2+ homeostasis in smooth muscle. Pharmac. Ther. 1992;56:191–231. doi: 10.1016/0163-7258(92)90017-t. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., CHENG H., RUBART M., SANTANA L.F., BONEV A.D., KNOT H.J., LEDERER W.J. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. (Cell Physiol.) 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- PORTER V.A., BONEV A.D., KNOT H.J., HEPPNER T.J., STEVENSON A. S., KLEPPISCH T., LEDERER W. J., NELSON M. T. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am. J. Physiol. (Cell Physiol.) 1998;274:C1346–C1355. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- STEHNO-BITTEL L., STUREK M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J. Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRIESCHMANN U., ISENBERG G. Ca2+-activated K+ channels contribute to the resting potential of vascular myocytes. Ca2+-sensitivity is increased by intracellular Mg2+-ions. Pflügers Arch. 1989;414:S183–S184. doi: 10.1007/BF00582296. [DOI] [PubMed] [Google Scholar]
- WAGENKNECHT T., GRASSUCCI R., BERKOWITZ J., WIEDERRECHT G.J., XIN H.B., FLEISCHER S. Cryoelectron microscopy resolves FK506-binding protein sites on the skeletal muscle ryanodine receptor. Biophys. J. 1996;70:1709–1715. doi: 10.1016/S0006-3495(96)79733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENDT-GALLITELLI M.F., MEISSNER G., LANDGRAF G., FRANZINI-ARMSTRONG C. Organization of the Ca2+ release apparatus in smooth muscle of the urinary bladder. Pflügers Arch. 1998;435:P31. [Google Scholar]
- XIAO R.P., VALDIVIA H.H., BOGDANOV K., VALDIVIA C., LAKATTA E.G., CHENG H.P. The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. J. Physiol. 1997;500:343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA A., VAN BREEMEN C., ISENBERG G. Buffering of plasmalemmal Ca2+ current by sarcoplasmic reticulum of guinea pig urinary bladder myocytes. Am. J. Physiol. 1996;271:C833–C841. doi: 10.1152/ajpcell.1996.271.3.C833. [DOI] [PubMed] [Google Scholar]
- ZARNT T., LANG K., BURTSCHER H., FISCHER G. Time-dependent inhibition of peptidylprolyl cis-trans-isomerases by FK506 is probably due to cis-trans isomerization of the inhibitor's imide bond. Biochem. J. 1995;305:159–164. doi: 10.1042/bj3050159. [DOI] [PMC free article] [PubMed] [Google Scholar]


