Abstract
Concentration-dependent effects of bimoclomol, the novel heat shock protein coinducer, on intracellular calcium transients and contractility were studied in Langendorff-perfused guinea-pig hearts loaded with the fluorescent calcium indicator dye Fura-2. Bimoclomol had a biphasic effect on contractility: both peak left ventricular pressure and the rate of force development significantly increased at a concentration of 10 nM or higher. The maximal effect was observed between 0.1 and 1 μM, and the positive inotropic action disappeared by further increasing the concentration of bimoclomol. The drug increased systolic calcium concentration with a similar concentration-dependence. In contrast, diastolic calcium concentration increased monotonically in the presence of bimoclomol. Thus low concentrations of the drug (10–100 nM) increased, whereas high concentrations (10 μM) decreased the amplitude of intracellular calcium transients.
Effects of bimoclomol on action potential configuration was studied in isolated canine ventricular myocytes. Action potential duration was increased at low (10 nM), unaffected at intermediate (0.1–1 μM) and decreased at high (10–100 μM) concentrations of the drug.
In single canine sarcoplasmic calcium release channels (ryanodine receptor), incorporated into artificial lipid bilayer, bimoclomol significantly increased the open probability of the channel in the concentration range of 1–10 μM. The increased open probability was associated with increased mean open time. The effect of bimoclomol was again biphasic: the open probability decreased below the control level in the presence of 1 mM bimoclomol.
Bimoclomol (10 μM–1 mM) had no significant effect on the rate of calcium uptake into sarcoplasmic reticulum vesicles of the dog, indicating that in vivo calcium reuptake might not substantially be affected by the drug.
In conclusion, the positive inotropic action of bimoclomol is likely due to the activation of the sarcoplasmic reticulum calcium release channel in mammalian ventricular myocardium.
Keywords: Heat shock protein, heart, ion currents, action potential duration, intracellular calcium transient, sarcoplasmic reticulum, ryanodine receptor, calcium release, calcium uptake
Introduction
Bimoclomol, the recently developed cytoprotective hydroxylamine derivative (N-2-hydroxy-3-(1-piperidinyl) propoxy]-3-pyridine-carboximidoyl chloride maleate, BRLP-42; see in Figure 1), was shown to have a wide variety of effects in healthy and diseased animals. Chronic treatment with the compound was effective against several types of diabetic dysfunction, including neuropathy (Bíró et al., 1994), retinopathy (Hegedüs et al., 1994) and abnormal vascular reactivity (Jednákovits et al., 1994). Bimoclomol exerted beneficial effects in rat and canine hearts exposed to coronary artery occlusion and vasospasm (Jaszlits et al., 1993), effects associated probably with enhanced expression of the heat shock protein HSP70 (Vígh et al., 1997). In addition, the drug showed strong dose-dependent acute antiarrhythmic action in isolated rat heart and also in anaesthetized animals. Recent electrophysiological studies indicate that bimoclomol may activate calciumdependent outward currents in mammalian ventricular cardiomyocytes (Magyar et al., 1999). Currently, the compound is in human phase 2 clinical trial. The present study was aimed to characterize the effects of the drug on calcium handling (i.e. on calcium release, calcium uptake and [Ca2+]i transients) in mammalian ventricular myocardium.
Figure 1.
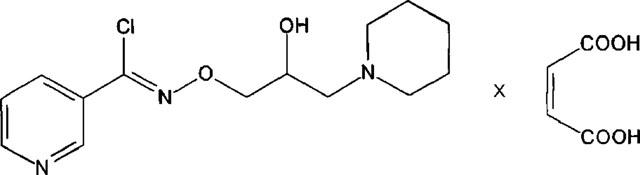
Chemical structure of bimoclomol, applied as maleate salt.
Methods
Recording of contractility and [Ca2+]i transients in Langendorff-perfused guinea-pig heart
Male guinea-pigs (weighing 300–500 g) were i.v. heparinized and anaesthetized with pentobarbitone-Na (150 mg kg−1). After opening the chest the heart was rapidly removed and fixed to the cannula of a Langendorff-perfusion device. The heart was perfused with modified Krebs solution containing (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, Na2EDTA 0.5, KH2PO4 0.23 and glucose 5.5. The pH of this perfusate was set to 7.4 when gassed with a mixture of 5% CO2 and 95% O2 at 37°C. The coronary flow rate, controlled by a peristaltic pump, was adjusted to 10 ml min−1 g−1. Left ventricular pressure (LVP) was continuously monitored using a Braun 2021-02 arterial pressure transducer that was connected to the left ventricular cavity (Edes & Kranias, 1990). Heart rate was maintained 200 beats min−1 by left atrial pacing. Experiments were performed in a cumulative concentration-dependent manner. After taking control records, bimoclomol was applied at 1 nM, 10 nM, 100 nM, 1 μM, 3 μM and 10 μM concentration, each for 10 min. This protocol was performed in six guinea-pig hearts.
To record [Ca2+]i transients, the heart was loaded with the acetoxymethylester of the fluorescent dye, Fura-2 (5 mM), added to the superfusate together with probenecid (0.6 mM), Synperonic (1.25 g l−1) and albumin (50 g l−1). Probenecid was used to inhibit the unspecific anion-exchanger of the cell membrane in order to avoid the extrusion of Fura-2 from the cells. Synperonic and albumin enhanced loading with Fura-2-AM. Under these conditions, stable calcium signals were obtained for periods as long as 120 min, allowing concentration-dependent studies (Kristof et al., 1998) to be performed. Fluorescence was excited at both 340 and 380 nm wavelengths. The emitted light was collected at 510 nm using a trifurcated quartz fibre optic bundle connected to a Deltascan device (Photon Technology International, New Brunswick, NJ, U.S.A.). [Ca2+]i was measured as a fluorescent ratio (F340/F380). The analogue signals of fluorescence and LVP were sampled at 1 kHz. In each case ten subsequent beats were averaged and stored for analysis. The following parameters were analysed: peak left ventricular pressure, maximum and minimum values of the first time derivative of pressure, systolic and diastolic [Ca2+]i (expressed as fluorescent ratios), and amplitude of the [Ca2+]i transient (defined as the difference between systolic and diastolic values of [Ca2+]i).
Recording of action potentials in single canine cardiomyocytes
Single canine ventricular myocytes were obtained from hearts of adult mongrel dogs using the segment perfusion technique (Magyar et al., 1999). The animals (10–20 kg) were anaesthetized with i.v. injection of 10 mg kg−1 ketamine hydrochloride (Calypsolvet) plus 1 mg kg−1 xylazine hydrochloride (Rometar). After opening the chest the heart was rapidly removed and the left anterior descending coronary artery was perfused using a Langendorff apparatus. Ca2+-free JMM solution (Minimum Essential Medium Eagle, Joklik modification), supplemented with taurine (2.5 g l−1), pyruvic acid (175 mg l−1), ribose (750 mg l−1), allopurinol (13.5 mg l−1) and NaH2PO4 (200 mg l−1), was used during the initial 5 min of perfusion to remove Ca2+ and blood from the tissue. After addition of NaHCO3 (1.3 g l−1), the pH of this perfusate was 7.0 when gassed with 5% CO2 and 95% O2. Cell dispersion was performed for 30 min in the same solution containing also collagenase (660 mg l−1, Worthington CLS-1), bovine albumin (2 g l−1) and CaCl2 (50 μM). During the isolation procedure the solutions were gassed with carbogene and the temperature was maintained at 37°C. 40–60% of the cells were rod shaped and showed clear striation when the external calcium was restored. Before use, the cells were stored overnight at 14°C in modified JMM solution (pH=7.4).
The viable cells were sedimented in a Plexiglass chamber allowing continuous superfusion (10 ml min−1) with modified Krebs solution containing (in mM): NaCl 120, KCl 5.4, CaCl2 2.7, MgCl2 1.1, NaH2PO4 1.1, NaHCO3 24 and glucose 6. The solution was equilibrated with 95% O2 plus 5% CO2 at a temperature of 37°C, and pH was adjusted to 7.4±0.05. Transmembrane potentials were recorded using glass microelectrodes filled with 3 M KCl and having tip resistance between 20 and 40 MΩ. These electrodes were connected to the input of an Axoclamp-2B amplifier (Axon Instruments, Foster City, CA, U.S.A.). The cells were continuously paced through the recording electrode at steady cycle length of 1000 ms using 1 ms wide rectangular current pulses with 120% threshold amplitude. Action potentials were digitized at 100 kHz using Digidata 1200 A/D card (Axon Instruments, Foster City, CA, U.S.A.) and stored for later analysis.
Recording of single channel currents of canine ryanodine receptor (RyR) incorporated into artificial lipid bilayer
Heavy sarcoplasmic reticulum (SR) vesicles were isolated from ventricular free wall of dogs, and the solubilized RyR was purified using the method described previously (Laver et al., 1995) with slight modifications. Single channel bilayer measurements were carried out using CHAPS-solubilized RyR incorporated into planar lipid bilayer formed from phosphatidyl-ethanolamine, phosphatidyl-serine and L-phosphatidyl-choline in a ratio of 5 : 4 : 1, and dissolved in n-decane up to final lipid concentration of 20 g l−1 (Herrmann-Frank et al., 1996; Csernoch et al., 1999). Bilayers were formed across a 150–200 μm aperture of a nolrene cap using a symmetrical buffer solution (containing 250 mM KCl, 100 μM EGTA, 150 μM CaCl2 and 20 mM PIPES) at pH 7.2. The chamber into which the small aliquot of solubilized RyR has been added was designated as the cis (cytoplasmic) side, while the other chamber was labelled as trans (luminal) side and was kept on ground potential. At the end of each experiment 2 μM ryanodine was added to the cis side in order to test the orientation of the channel being measured, and in case of opposite incorporation (5–10% of the cases) data were discarded. Current signals, obtained under voltage clamp conditions, were filtered at 1 kHz using an 8 pole low pass Bessel filter and digitized at 3 kHz using Axopatch 200 amplifier and pClamp 6.02 software (Axon Instruments, Foster City, CA, U.S.A.), which was also used to determine mean open times and open probabilities from the records. Total recording time was 5–10 min for each concentration tested. After changing solution, minimum 5–6 min was allowed for equilibration, which appeared to be enough to reach the new equilibrium of the parameters (Szegedi et al., 1999). Single channel measurements were carried out at 26±1°C, Ca2+ concentration was calculated using the computer program and stability constants published by Fabiato (1988).
Measurement of uptake and release of calcium
Calcium uptake of heavy SR vesicles of the dog were tested at 37°C following the changes in the Ca2+ concentration of the extravesicular space calculated from antipyrilazo-III absorbance as described earlier (Sárközi et al., 1996). Measurements were performed in 1×1 cm cuvettes in a SPEX Fluoromax single photon counting spectrometer (Jobin-Yvon, Spex Industries, Edison, NJ, U.S.A.) configured for absorbance measurement. Data were collected at a rate of 1 Hz and stored digitally for later analysis. The rate of calcium uptake and release from the SR vesicles was determined from the slope of the Ca2+ transient evoked by the addition of calcium, using a modified procedure described in the literature (Lam et al., 1995). At the beginning of the uptake measurement the medium contained 100 mM KCl, 1 mM MgCl2, 180 μM antipyrilazo-III, 54 μM CaCl2, 4 mM ATP, while protein concentration was adjusted to 0.65 g l−1. Two calcium injections were applied. The rising slope after the first Ca2+ transient corresponds to the maximal speed of calcium uptake (i.e. maximal pumping velocity) at optimal conditions, while the terminal portion of the curve–since all the available ATP were converted to ADP by that time–reflects the release of calcium through RyR (in the presence of 1 mM Mg2+, 4 mM ADP, ≈10 μM Ca2+ at cis and ≈7 mM Ca2+ at trans side).
Chemicals
Bimoclomol was synthetized by Biorex Research and Development Co., and was freshly dissolved in distilled water on the day of the experiment. Lipids were obtained from Avanti Polar Lipids (Alabaster, AL, U.S.A.), [3H]-ryanodine was from Dupont (Boston, MA, U.S.A.), Fura-2-AM from Teflabs (Austin, TX, U.S.A.), Antipyrilazo-III from ICN Biomedicals (Aurora, OH, U.S.A.) and JMM from Sigma (St. Louis, MO, U.S.A.). Protease inhibitors were purchased from Boehringer (Mannheim, Germany) and Merck (Darmstadt, Germany), all other chemicals from Sigma (St. Louis, MO, U.S.A.).
Statistics
All data presented are arithmetic means±s.e.mean. Statistical significance was determined using Student's t-test for paired or unpaired data. Differences were considered significant when the P value was less than 0.05.
The entire investigation conformed to the guidelines for the care and use of laboratory animals published by the U.S. National Institutes of Health, as well as the principles outlined in the Declaration of Helsinki.
Results
Effects of bimoclomol in Langendorff-perfused guinea-pig hearts
In Langendorff-perfused isolated guinea-pig hearts (n=6) bimoclomol displayed a biphasic, concentration-dependent action both on contractile parameters and [Ca2+]i transients (Figure 3). Analogue records, obtained from a representative experiment, are presented in Figure 2. Peak left ventricular pressure (peak LVP) and the maximum rate of pressure development during systole (+dP/dt) increased significantly (P<0.05) by low concentrations (10–100 nM) of bimoclomol. This increase began to decline at concentrations higher than 1 μM, and no significant change was observed at 10 μM bimoclomol. Qualitatively similar results were obtained for the maximum rate of fall in pressure during diastole (−dP/dt), these changes, however, failed to reach the level of statistical significance (Figure 3a–c).
Figure 3.
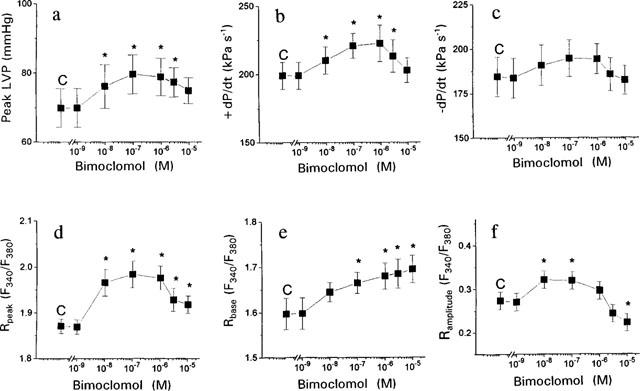
Cumulative concentration-dependent effects of bimoclomol on the contractile parameters and [Ca2+]i in Langendorff-perfused guinea-pig hearts. (a) Peak left ventricular pressure (peak LVP). (b) Maximum rate of pressure development during systole (+dP/dt). (c) Maximum rate of fall in pressure during diastole (−dP/dt). (d) Peak value of [Ca2+]i during systole (Rpeak). (e) Baseline value of [Ca2+]i during diastole (Rbase). (f) Amplitude of the [Ca2+]i transient. [Ca2+]i was expressed as a fluorescent ratio (F340/F380). Each concentration of bimoclomol was applied for 10 min. Symbols and bars represent mean data±s.e.mean obtained in six preparations. Asterisks denote significant (P<0.05) differences compared to pre-drug control (C) determined using Student's t-test for paired data.
Figure 2.
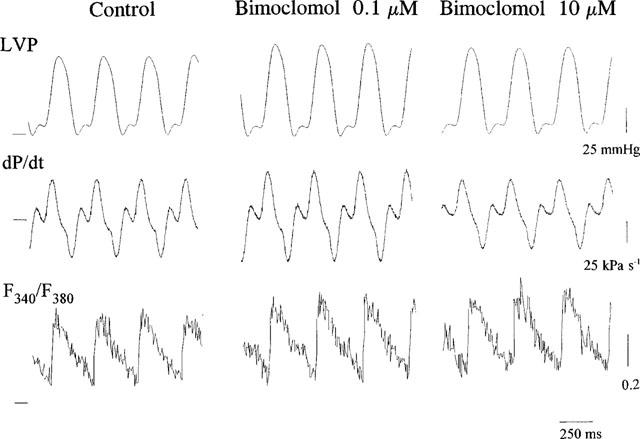
Representative analogue records showing the effect of 0.1 and 10 μM bimoclomol on left ventricular pressure (LVP), its first time derivative (dP/dt), and the fluorescent ratio (F340/F380) in guinea-pig heart. In each trace the horizontal line represents zero level.
Bimoclomol had also a biphasic effect on the parameters of [Ca2+]i transients, recorded from the same preparations. Systolic [Ca2+]i was increased significantly at concentrations of 10 nM or higher. The maximal effect was observed at 0.1 μM bimoclomol, above this concentration the increase in systolic [Ca2+]i was less but still significant. In contrast to systolic [Ca2+]i, diastolic [Ca2+]i increased monotonically at concentrations higher than 10 μM. Thus low concentrations of the drug (10–100 nM) significantly increased, whereas higher concentrations (10 μM) significantly decreased the amplitude of [Ca2+]i transients, calculated as a difference of systolic and diastolic fluorescent ratios (Figure 3d–f).
Time-dependence of the effect of bimoclomol was studied by recording of contractile parameters and [Ca2+]i transients for 30 min following the application of 0.1 μM bimoclomol (Figure 4a–e). All effects of bimoclomol developed fully within 5 min and were constant during the recording period. In this single experiment the heart was not driven electrically allowing the spontaneous heart rate to be monitored (Figure 4f). The stability of the effects of 0.1 μM bimoclomol suggests that the biphasic concentration-dependent nature of action of bimoclomol may not be due to time-dependent changes in drug-effect, it may rather reflect true concentration dependence.
Figure 4.
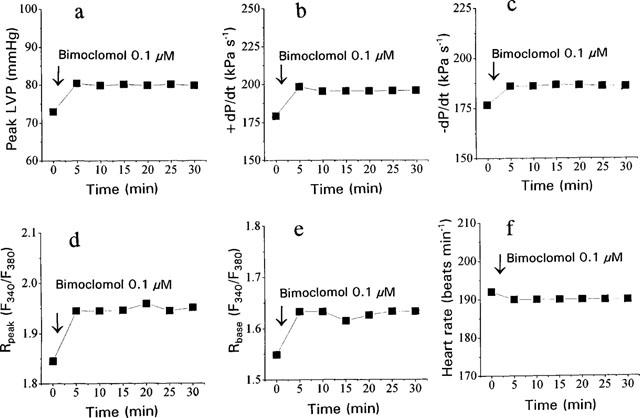
Time-dependence of the effect of 0.1 μM bimoclomol in a single guinea-pig heart. Displayed parameters are identical to those shown in Figure 2, except panel (f), where the heart rate is presented (external pacing was not applied in this experiment so that the heart was beating in sinus rhythm).
Effects of bimoclomol on action potential configuration in isolated canine ventricular myocytes
Effects of bimoclomol on action potential configuration was studied in isolated canine ventricular cells, paced with a constant frequency of 1 Hz. Low concentration of bimoclomol (10 nM) caused a moderate but statistically significant lengthening of action potential duration measured both at 50 and 90% of repolarization (APD50 and APD90, respectively). APD50 increased from 175.8±5 to 182.6±6.6 ms by 10 nM bimoclomol (P<0.05, n=5). Further increases in bimoclomal concentration to 0.1 and then to 1 μM caused a decline in APD-lengthening (179.2±5.2 and 173.4±5.7 ms, respectively). Similar changes were observed in APD90, values of 222.6±8.3 ms (in control), and 229.8±8.9, 225.4±7.4 and 222.6±6.8 ms (in the presence of 10 nM, 0.1 μM and 1 μM bimoclomol, respectively) were obtained. Lengthening of APD90 was significant statistically (P<0.05) only at 10 nM bimoclomol. No significant effects of bimoclomol were observed on the resting membrane potential, action potential amplitude or maximum rate of depolarization up to a concentration of 1 μM. In another set of experiments effects of high bimoclomol concentrations were studied. APD50 was reduced by 27.9±5.2, 79.2±21.4 and 117.7±17.2 ms in the presence of 10, 30 and 100 μM bimoclomol, respectively (P<0.05, n=6). Similar changes were observed in APD90 values (reduction by 22.8±4.5, 65.8±17.9 and 81.3±13.3 ms were obtained). These high concentrations of bimoclomol also caused a marked and progressive reduction of action potential amplitude and maximum rate of depolarization. In summary, bimoclomol displayed a biphasic action on action potential duration similarly to contractile parameters and [Ca2+]i transients.
Effects of bimoclomol in single canine ryanodine receptors (RyR) incorporated into lipid bilayer
Channels were incorporated in 50 μM Ca2+ at both cis and trans sides in order to obtain open probabilities high enough (Po=0.78±0.08) and to show several full openings (Figure 5, trace a). Channels having conductance higher than 475 pS (in the presence of 250 mM K+ used as current carrier) were identified as RyR current. The channels revealed ohmic characteristics with an average conductance of 502±13 pS (n=15). Using constant 50 μM Ca2+ trans the channel parameters were determined at 0.9 μM cis Ca2+ (Po=0.11±0.043) in order to give enough room to see the activation or inactivation of the channel. (Figure 5, trace b).
Figure 5.
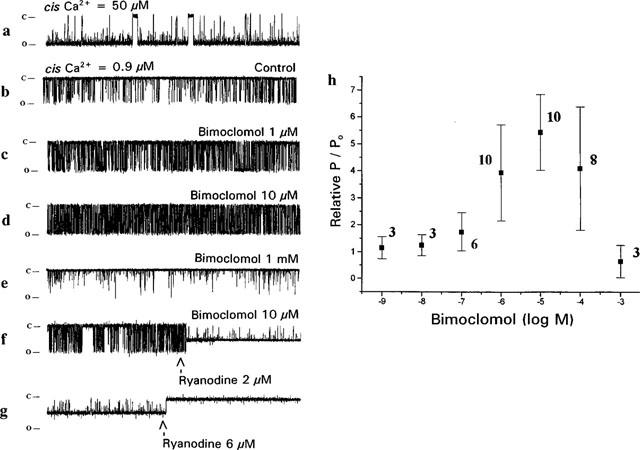
Current traces and open probabilities obtained from single canine RyR channels incorporated into lipid bilayer. Current was measured at −80 mV holding potential in 20 mM HEPES-buffered symmetrical 250 mM KCl solution (pH=7.2). The open and closed state of the channel is indicated at the left side by markers, openings are downward deflections. Current difference between open and closed channel state (amplitude of events) represent 40 pA, duration of each trace is 5 s. (a) Representative current record obtained at 50 μM cis Ca2+ and 50 μM trans Ca2+ in absence of bimoclomol. (b) Same as trace a, but cis Ca2+ was adjusted to 0.9 μM. Since all further records were taken under these circumstances, this configuration is considered to control. (c–e) Records taken in the presence of 1, 10 μM and 1 mM bimoclomol, respectively, applied at cis side. (f) Bimoclomal concentration is 10 μM, Ca2+ concentration is 0.9 μM cis/50 μM trans, and at middle of the trace 2 μM ryanodine was applied. (g) Same as trace f, just in the middle of the record the ryanodine concentration was raised to 6 μM. (h) Concentration-dependent effect of bimoclomol (1 nM–1 mM) on the relative open probability of RyR channel. In each experiment open probability was determined in the presence of bimoclomol and normalized to the bimoclomal-free control value of the respective channel (relative P/P0). Each point represents the average of several measurements obtained from different bilayers, symbols and bars represent mean±s.e.m. values, number of measurements is indicated in parentheses.
Thus the effect of bimoclomol on RyR channel activity was studied in the presence of 0.9 μM cis and 50 μM trans Ca2+. Bimoclomol was applied at the cis side of the bilayer. Open probability was calculated from single channel records at different bimoclomol concentrations after reaching equilibrium and normalized to the Po value measured on the same channel in the absence of the drug. These relative P/Po values are presented in Figure 5h. The solubilized and incorporated RyR was activated by micromolar concentrations of bimoclomol, the highest open probability was observed at 10 μM. Above this concentration the channel became less active (the open probability continuously decreased), however, the relative open probability of the channel was still elevated at 100 μM bimoclomol. Finally, the open probability fell below its control value in the presence of 1 mM bimoclomol (Figure 5, traces c–e). Based on this biphasic curve, half-activation and half-blocking concentrations of bimoclomol (K0.5a and K0.5i) were estimated to be 630 nM and 185 μM, respectively. Fitting the open probabilities using the Hill-equation, Hill-constants of close to unity were obtained for both the ascending and descending limbs indicating the presence of one activatory and one inhibitory bimoclomol binding site.
The bimoclomol activated RyR channel could be closed by ryanodine. This is demonstrated in traces f and g of Figure 5, where the channel, being previously activated by 10 μM bimoclomol, was locked by 2 μM ryanodine in a characteristic half-open state, whereas application of 6 μM ryanodine resulted in a complete closure of the channel. These results indicate that the ryanodine binding sites of RyR is still accessible in the presence of bimoclomol.
Mean open time histograms of the current records could be fitted as a sum of two exponentials, with T1 and T2 being time constants (Figure 6). Both time constants were significantly (P<0.05) increased by bimoclomol concentrations ranging from 0.1–10 μM, showing a concentration-dependence similar to that of the open probability curve (shown in Figure 5h), suggesting that the bimoclomol-induced increase in Po may be–at least partially–due to the longer mean open time. In spite of the similarities in concentration-dependence, half-activation and half-blocking concentrations estimated for T1, T2 and Po were not identical. In case of T1, K0.5a and K0.5i were 100 nM and 30 μM, respectively, while for T2, values of 48.5 nM and 100 μM were obtained. These differences indicate that the effect of bimoclomol on canine RyR is more complex than a simple allosteric modulation. Further indepth studies are required to contemplate for the differences in the effects of bimoclomol on Po, T1 and T2.
Figure 6.
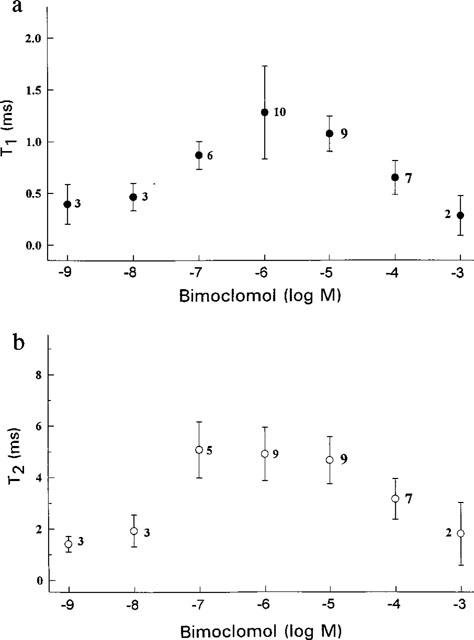
Mean open time of the channel as a function of bimoclomol concentration. Open time histograms (not shown) were fitted as a sum of two exponentials, and the estimated faster and slower time constants (T1 and T2, respectively) were plotted against the concentration of bimoclomol, applied at cis side. Symbols and bars represent mean±s.e.mean values, numbers in parentheses indicate the number of experiments.
Effect of bimoclomol on active calcium uptake of heavy SR vesicles
Effect of bimoclomol on the Ca2+ ATPase was tested by monitoring extravesicular Ca2+ concentration following stepwise increases of external calcium in the absence or in the presence of 10, 100 μM or 1 mM bimoclomol. The two calcium injections, indicated by arrows in Figure 7, resulted in sudden increases in Ca2+ concentration of the medium, which activated the Ca2+ ATPase. This enhanced calcium influx decreased the extravesicular Ca2+ concentration thereby causing the slow ascending phase of the curve (increase in absorbance) following the calcium injection. The rate of calcium influx (activity of pumping) is proportional to the slope of the ascending phase following the first calcium injection. Results indicate that the maximum velocity of calcium uptake, expressed as μmol Ca2+ (mg protein * min)−1, was not influenced by bimoclomol: values of 0.263±0.074, 0.237±0.014, 0.231±0.062 and 0.243±0.055 were obtained in control, in the presence of 10, 100 μM and 1 mM bimoclomol, respectively (not significant, n=5–8 for each point). The decline of the terminal portion of the curve observed in the presence of 1 mM bimoclomol indicates that this concentration of bimoclomol can further activate RyR under peculiar experimental conditions (in the presence of 1 mM Mg2+, ≈10 μM Ca2+ cis, ≈7 mM Ca2+ trans and 4 mM ADP. This is an opposite effect compared to the one measured at the same bimoclomol but at drastically different calcium, magnesium and nucleotide concentrations (see Figure 5), and are likely attributable to the complex regulatory mechanism of RyR.
Figure 7.
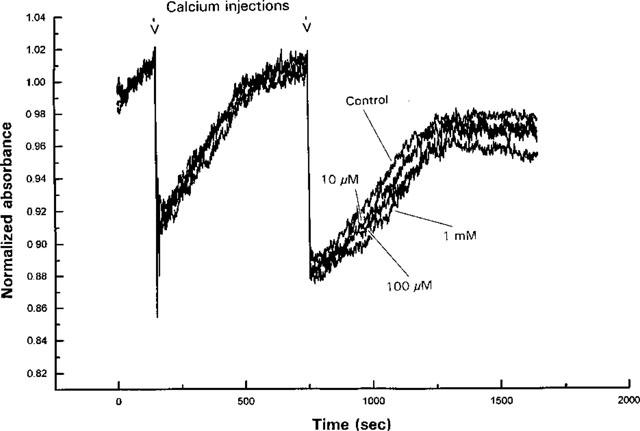
Effect of bimoclomol (10, 100 μM and 1 mM) on the calcium uptake and release of dog heart heavy SR vesicles. Extravesicular Ca2+ concentration was monitored as described in Methods. HSR vesicles were actively loaded with calcium in the presence of 4 mM ATP initial ATP concentration. Absorbance changes (proportional with the Ca2+ concentration) were monitored at 710 nm using 790 nm light as internal reference. The two calcium injections applied resulted in sudden increase in the Ca2+ concentration of the medium, which activated the Ca2+ ATPase, which in turn, decreased extravesicular Ca2+ concentration (slow ascending phase following calcium injections). The rate of active calcium uptake was determined from the slope of the ascending phase following the first calcium injection.
Discussion
In this study we have shown that the heat shock protein coinducer bimoclomol exerts biphasic actions on action potential duration, contractility, intracellular [Ca2+]i transients and ryanodine receptor function in mammalian myocardiac preparations. Low concentrations (10–100 nM) of the drug increased peak left ventricular pressure and the amplitude of [Ca2+]i transients in working guinea-pig heart, lengthened action potential duration in isolated canine ventricular cells, and increased the open probability of solubilized and lipid incorporated canine RyR channels. In contrast, higher concentrations of the drug had opposite effects: the positive inotropic action of submicromolar bimoclomol concentrations was attenuated, action potential duration was shortened and the [Ca2+]i transient was suppressed by 10 μM bimoclomol. Inhibition of the RyR required extremely high (1 mM) concentration. Analysis of the concentration dependence of bimoclomol action on open probabilities and mean open time histograms of the RyR yielded K0.5a and K0.5i values for each parameter differing from one another by 2.5–3 orders of magnitude. In addition, the Hill coefficient, obtained for the open probability function, was close to unity for both ascending and descending limbs suggesting the presence of two distinct (one activatory and one inhibitory) bimoclomol binding sites on RyR.
The concentration-dependent biphasic effect of bimoclomol on the [Ca2+]i transients in guinea-pig can be explained–at least in part–with the existence of an activatory and an inhibitory binding site on the RyR, however, the monotonic increase of diastolic [Ca2+]i with increasing bimoclomol concentration is also likely involved. Elevation of diastolic [Ca2+]i may not be attributed to the inhibition of calcium uptake into the SR (since the pumping rate was not affected up to 1 mM bimoclomol), it may rather be a consequence of the calcium leak through the RyR during diastole. The possibility, that transsarcolemmal elimination of Ca2+ may be impaired by higher concentrations of the drug, cannot be ruled out either. The biphasic effect of bimoclomol on action potential duration (i.e. low concentrations lengthened, whereas high concentrations shortened canine action potentials) may give some support for this mechanism. At low concentrations the increased Na+/Ca2+ exchange current may prolong action potential duration as a consequence of increased systolic [Ca2+]i (Egan et al., 1989; Noble et al., 1991). At higher concentrations–as the Na+/Ca2+ exchange mechanism may be more and more suppressed–the biomoclomol-induced elevation of [Ca2+]i may activate calcium dependent Cl− and K+ currents (Magyar et al., 1999), both of which tend to shorten action potential duration (Zygmunt & Gibbons, 1992; Sipido et al., 1993; Krause et al., 1993). In addition, high [Ca2+]i may also accelerate Ca2+-dependent inactivation of L-type Ca2+ current (Lee et al., 1985).
In spite of the biphasic nature of bimoclomol action observed in all experimental arrangements studied, there is a discrepancy in the relevant concentrations. In canine ventricular cells as well as in the guinea-pig heart, activation and inhibition occurred at 10 nM and 10 μM bimoclomol, respectively. In the solubilized RyR preparations these concentrations were obtained at 1–2 orders of magnitude higher. Although the experimental conditions were not identical in the working heart and RyR studies (see e.g. differences in [Ca2+]i), it is possible that the sensitivity of the solubilized RyR preparations is different from that of in situ RyR. The single channel conductance of 500 pS, estimated in our experiments, is, however, very close to that observed by others (Brillantes et al., 1994; Copello et al., 1997) in RyR-containing vesicles incorporated into lipid bilayer. Interspecies differences in bimoclomol-sensitivity between guinea-pig and canine myocardium cannot be ruled out either.
Acknowledgments
This study was supported by grants to P.P. Nánási (OTKA-T025577 and ETT-098/1996), to I. Jóna (T-22313), and to L. Kovács (OTKA-T030245, FKFP-1289 and AKP98-75 3,2/44) from the Hungarian Research Found, Hungarian Ministry of Health, Hungarian Ministry of Education and Hungarian Academy of Sciences. Further support was obtained from Biorex Research and Development Co.
Abbreviations
- APD
action potential duration
- [Ca2+]i
intracellular calcium ion concentration
- +dP/dt and −dP/dt
maximum rate of rise and fall of pressure
- K0.5a and K0.5i
half activation and half-blocking concentrations
- LVP
left ventricular pressure
- P/Po
relative open probability
- Rpeak and Rbase
systolic and diastolic fluorescent ratios
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
References
- BÍRÓ K., JEDNÁKOVITS A., JASZLITS L., HEGEDÜS E., KUKORELLI T. Improvement of streptozotocin-diabetic neuropathy by BRLP-42. Diabetologia. 1994;37 Suppl 1:282. [Google Scholar]
- BRILLANTES A.-M., ONDRIAS K., SCOTT A., KOBRINSKY E., ONDRIASOVÁ E., MOSCHELLA M.C., JAYARAMAN T., LANDERS M., EHRLICH B.E., MARKS A.R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- COPELLO J.A., BRAG S., ONOUE H., FLEISCHER S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys. J. 1997;73:141–156. doi: 10.1016/S0006-3495(97)78055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSERNOCH L., SZENTESI P., SÁRKÖZI S., SZEGEDI Cs., JÓNA I., KOVÁCS L. Effects of tetracaine on sarcoplasmic calcium release in mammalian skeletal muscle fibers. J. Physiol. 1999;515:843–857. doi: 10.1111/j.1469-7793.1999.843ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDES I., KRANIAS E.G. Phospholamban and troponin I are substrates for protein kinase C in vitro but not in intact beating guineapig hearts. Circ. Res. 1990;67:394–400. doi: 10.1161/01.res.67.2.394. [DOI] [PubMed] [Google Scholar]
- EGAN T.M., NOBLE D., NOBLE S.J., POWELL T., SPINDLER A.J., TWIST V.W. Sodium-calcium exchange during the action potential in guineapig ventricular cells. J. Physiol. 1989;411:639–661. doi: 10.1113/jphysiol.1989.sp017596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABIATO A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–147. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- HEGEDÜS E., BÍRÓ K., JASZLITS L., JEDNÁKOVITS A., KÜRTHY M., BÁCSY E. Protective effect of BRLP-42 on experimental retinopathy. Diabetologia. 1994;37 Suppl 1:137. [Google Scholar]
- HERRMANN-FRANK A., RICHTER M., SÁRKÖZI S., MOHR U., LEHMANN-HORN F. 4-chloro-m-cresol, a potent and specific activator of the skeletal muscleryanodine receptor. Biochim. Biophys. Acta. 1996;1289:31–40. doi: 10.1016/0304-4165(95)00131-x. [DOI] [PubMed] [Google Scholar]
- JASZLITS L., JEDNÁKOVITS A., KOLTAI M.Zs., BÍRÓ K., HEGEDÜS E., UDVARDY É., VERECZKEY L., LITERÁTI-NAGY P., POGÁTSA G. Antiischaemic efficacy of BRLP-42. J. Mol. Cell. Cardiol. 1993;25 Suppl 1:20. [Google Scholar]
- JEDNÁKOVITS A., JASZLITS L., BÍRÓ K., POGÁTSA G., HEGEDÜS E., KÜRTHY M. Effect of BRLP-42 on vascular reactivity of diabetic arterial beds. Diabetologia. 1994;37 Suppl 1:19. [Google Scholar]
- KRAUSE P.C., RARDON D.P., MILES W.M., KLEIN L.S., MAHOMED Y., KING R.D., ZIPES D.P. Characteristics of Ca2+-activated K+ channels isolated from the left ventricle of a patient with idiopathic long QT syndrome. Am. Heart J. 1993;126:1134–1141. doi: 10.1016/0002-8703(93)90665-v. [DOI] [PubMed] [Google Scholar]
- KRISTOF E., SZIGETI G., PAPP Z., BODI A., FACSKO A., KOVACS L., PAPP J.G., KRANIAS E.G., EDES I. Cardiac responses to calcium sensitizers and isoproterenol in intact guinea pighearts. Ann. NY. Acad. Sci. 1998;853:316–319. doi: 10.1111/j.1749-6632.1998.tb08288.x. [DOI] [PubMed] [Google Scholar]
- LAM E., MARTIN M.M., TIMERMAN A.P., SABERS C., FLEISCHER S., LUKAS T., ABRAHAM R.T., O'KEEFE S.J., O'NEILL E.A., WIEDERRECHT G.J. A novel FK506 binding protein can mediate the immunosuppressive effects of FK506 and is associated with the cardiac ryanodine receptor. J. Biol. Chem. 1995;270:26511–26512. doi: 10.1074/jbc.270.44.26511. [DOI] [PubMed] [Google Scholar]
- LAVER D.R., RODEN L.D., AHERN G.P., EAGER K.R., JUNANKAR P.R., DUNLHUNTY A.F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J. Membr. Biol. 1995;147:7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- LEE K.S., MARBAN E., TSIEN R.W. Inactivation of Ca channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J. Physiol. 1985;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGYAR J., BÁNYÁSZ T., SZIGLIGETI P., KÖRTVÉLY Á., JEDNÁKOVITS A., NÁNÁSI P.P.Electrophysiological effects of bimoclomol in canine ventricular myocytes Naunyn-Schmiedberg's Arch. Pharmacol 2000. In press [DOI] [PubMed]
- NOBLE D., NOBLE S.J., BETT G.C.L., EARM Y.E., HO W.-K., SO I.-S. The role of sodium-calcium exchange during the cardiac action potential. Ann. NY. Acad. Sci. 1991;639:334–353. doi: 10.1111/j.1749-6632.1991.tb17323.x. [DOI] [PubMed] [Google Scholar]
- SÁRKÖZI S., SZENTESI P., JÓNA I., CSERNOCH L. Effects of cardiac glycosides on excitation-contraction coupling in frog skeletal muscle fibres. J. Physiol. 1996;495:611–626. doi: 10.1113/jphysiol.1996.sp021620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIPIDO K.R., CALLEWAERT G., CARMELIET E. [Ca2+]i transients and [Ca2+]i-dependent chloride current in single Purkinje cells from rabbit heart. J. Physiol. 1993;468:641–667. doi: 10.1113/jphysiol.1993.sp019793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZEGEDI Cs., SÁRKÖZI S., HERZOG A., JÓNA I., VARSÁNYI M. Calsequestrin: more than only a luminal Ca2+ buffer inside the sarcoplasmic reticulum. Biochem. J. 1999;337:19–22. [PMC free article] [PubMed] [Google Scholar]
- VÍGH L., LITERÁTI-NAGY P., HORVÁTH I., TÖRÖK Z., BALOGH G., GLATZ A., KOVÁCS E., BOROS I., FERDINÁNDY P., FARKAS B., JASZLITS L., JEDNÁKOVITS A., KORÁNYI L., MARESCA B. Bimoclomol: a nontoxic hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nature Medicine. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT A.C., GIBBONS W.R. Properties of the calcium-activated chloride current in heart. J. Gen. Physiol. 1992;99:391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]


